Abstract
Background
HIV and SIV infections induce NK cell dysfunction and hematopoietic defects in the bone marrow, but the effects of infection on bone marrow NK cell development and function are unknown.
Methods
Bone marrow NK cells were analyzed from both naïve and chronically SIV-infected rhesus macaques using polychromatic flow cytometry.
Results
NK cell frequencies were reduced in infected compared to naïve animals, associated with increased apoptosis. Bone marrow NK cells from SIV-infected macaques upregulated perforin expression, suggesting increased cytotoxicity, and shifted toward a more mature CD16+ NK cell subpopulation phenotype. Unexpectedly, expression of the trafficking markers α4β7, CCR7, and CD62L were unchanged on bone marrow NK cells during SIV infection.
Conclusion
These data demonstrate that during SIV infection bone marrow NK cells are reduced in number, but upregulate cytotoxic functions. Furthermore, our data suggest acquired cytotoxicity and loss may be due to in situ NK cell differentiation and not emigration.
Keywords: Simian immunodeficiency virus, innate immunity, apoptosis, lymphocyte homing
INTRODUCTION
Natural killer (NK) cells are essential components of the innate immune system that play important roles in defense against viruses, bacteria, and other pathogenic infections. NK cells can also contribute significantly to shaping subsequent adaptive immune responses both directly and indirectly [1–4]. Even though NK cells can derive from thymus, liver and lymph nodes [5–7], the bone marrow (BM) is the primary site for NK cell development from which a variety of NK cell subpopulations emerge and are found systemically [8, 9]. In spite of the classical characterization of NK cells as non-specific cytotoxic cells, the functional repertoires and associated phenotypes of NK cell subpopulations are also remarkably heterogeneous and diverse. In humans, NK cells can be divided into two main subsets, CD56highCD16−/dim and CD56dimCD16+ [3, 4]. In healthy individuals, approximately 90% of circulating NK cells are CD56dimCD16+ cells that mediate primarily cytotoxic functions, whereas the minor population of CD56brightCD16− NK cells display little cytotoxic activity but produce large quantities of cytokines and chemokines [3, 10, 11]. An aberrant CD56−CD16+ NK cell subpopulation is generally rare in peripheral blood, but significantly expands during chronic virus infections such as HIV and HCV [12, 13]. Distribution of NK cell subsets is also tissue-specific. Compared to peripheral blood, human lymph node NK cells are primarily CD56brightCD16− cells that are immunomodulatory in nature [10, 11]. A high percentage of CD56bright cells are also localized in the normal intestinal mucosae, female reproductive tracts and tonsils, and these cells express very low levels of perforin and granzymes [14, 15] whereas most lung NK cells are CD56dimCD16+ and more cytotoxic similar to those found in blood [16]. The systemic distribution and functional divisions of labor are highly similar in nonhuman primate species such as rhesus macaques [17]. NK cell subpopulations in macaques are most readily defined by expression of the NK cell-specific marker, NKG2A, and can be further divided into CD56+ and CD16+ NK cells, which are functionally analogous to their human counterparts. Interestingly, a CD56−CD16− (DN) NK cell is highly prevalent in rhesus macaques, has an intermediate functional profile between CD56+ and CD16+ NK cells, but has no obvious human counterpart.
Despite intensive study, the role of NK cells in lentivirus infections remains unclear. Increasing evidence has shown that HIV/SIV infections induce functional perturbations in NK cells beginning in acute infection, characteristically an expansion of CD56dimCD16+ NK cells and an early depletion of CD56brightCD16− NK cells [17–20]. Interestingly, some reports also suggest that HIV/SIV infection induces persistent BM hematopoiesis defects [21–25]. However, little is known about the specific effects of HIV and SIV infection on BM NK cells. In this study using SIV infection of rhesus macaques as a model, we sought to comprehensively analyze the effects of chronic lentivirus infection on BM NK cells. We found that chronic SIV infection not only induced BM NK loss and subset perturbation, but also induced significant functional changes. The findings presented here may serve as a basis for better understanding the roles of BM NK cells in HIV pathogenesis.
MATERIAL AND METHODS
Animals
Fifteen Indian rhesus macaques (Macaca mulatta) were analyzed; 6 SIV-naïve macaques and 9 chronically SIVmac239-infected macaques. All animals were freeof simian retrovirus type D, simian T-lymphotrophic virustype 1, and herpes B virus and were housed at the New England Primate Research Center and maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the Guide for the Care and Use of Laboratory Animals.
Cell processing
BM samples were collected from all animals at time of scheduled necropsy using standard protocols. Briefly, femurs were cut below the hip and above the knee joint and removed. Bone sections were then split lengthwise and bulk BM was removed with forceps. BM samples were then mechanically disrupted in media. Mononuclear cells were isolated from BM by density gradient centrifugation over LSM (MP Biomedicals, Solon, OH) and contaminating red blood cells were lysed using a hypotonic ammonium chloride solution.
Antibodies and flow cytometric analyses
Flow cytometry staining of mononuclear cells was carried out for cell surface and intracellular molecules. LIVE/DEAD Aqua dye (Invitrogen, Carlsbad, CA) and isotype-matched controls and/or fluorescence-minus-one (FMO) controls were included for all assays. Except where noted, all antibodies were obtained from BD Biosciences (La Jolla, CA) and included fluorochrome-conjugated mAbs to the following molecules: α4β7-APC (NHP reagent resource), caspase-3-Alexa647 (clone C92-605), CCR7-Alexa700 (clone 150503, R&D Systems), CD3-APC-Cy7 (clone SP34.2), CD16-Alexa-700 (clone 3G8), CD45-FITC (clone D058-1283), CD45-PerCp-Cy5.5 (clone Tu116), CD62L-FITC (clone SK11), CXCR3-PE-Cy5 (clone 1C6), HLA-DR-PE-Texas Red (clone Immu-357, Beckman-Coulter), NKG2A-PE (clone Z199, Beckman-Coulter), NKG2A-Pacific Blue (clone Z199, in-house custom conjugate, Beckman-Coulter), NKG2D-APC (clone BAT221, Miltenyi), NKp30-PE (clone Z25, Beckman-Coulter), NKp44-PerCp-Cy5.5 (cloneZ231, Beckman-Coulter), Ki67-FITC (cloneB56), Perforin-Pacific Blue (in-house custom conjugate, clone pf-344, Mabtech). Acquisitions were made on an LSR II (BD Biosciences, La Jolla, CA) and analyzed using FlowJo (version 9.5) software (Tree Star Inc., Ashland, OR).
Statistical analyses
All statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA). Nonparametric Mann-Whitney U tests were used where indicated and P < 0.05 were assumed to be significant in all analyses.
RESULTS
Loss and subset perturbation of bone marrow NK cells in SIV-infected macaques
Although SIV-induced perturbations of the NK cell repertoire are well documented, little is known about how SIV specifically affects NK cell subpopulations in BM. To address this deficit, we first analyzed the distribution of NK cells subsets in BM from naïve rhesus macaques. Using our established gating strategy to identify NK cell subsets from blood and other lymphoid organs [17], we first gated on CD45+leukocytes to exclude stromal cells and then excluded dead cells and debris using a vital stain. Among live CD45+CD3−HLA-DR− mononuclear cells, NKG2A+ cells were identified as NK cells (Fig. 1A). Among the total NK cell population we also identified four subpopulations based on CD16 and CD56 expression: CD56+CD16−(CD56+); CD56−CD16+(CD16+); and CD56+CD16+ (double positive [DP]), and CD56−CD16−(double negative [DN]) (Fig. 1B). Interestingly, distinct from peripheral blood (Fig. 1B), where CD16+ NK cells dominate, among total BM NKG2A+ NK cells, DN NK cells were the dominant subset (median frequency 66.9%, range 51.3%–83.2%), CD56+ cell and CD16+ cells have similar percentage (median frequency 15%, range 6.59–21.8%; median frequency 15.35%, range 9.42–23.6%, respectively), while DP NK cells were relatively rare (median frequency 2.27%, range 0.78%–3.4%). As we have reported previously [17], NKG2A+ cells expressed natural cytotoxicity receptors NKp30 and NKG2D (Fig. 1C), further confirming their identity as NK cells.
Fig. 1. Loss and subset perturbation of bone marrow NK cells in SIV-infected macaques.
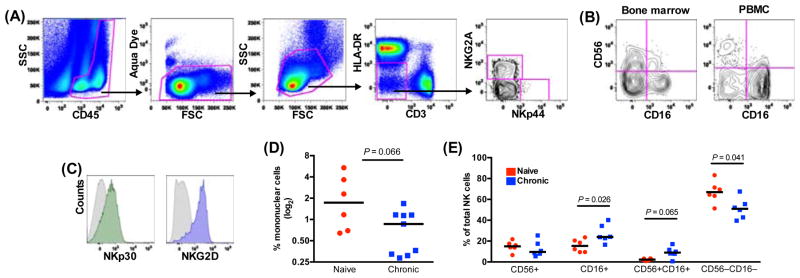
(A) Representative gating strategy to identify NKG2A+ NK cells and NKp44+ innate lymphoid cells among live mononuclear bone marrow cells. (B) Disparate expression of NK subpopulations in bone marrow and PBMC based on CD56 and CD16 expression. (C) Representative histograms of NKp30 and NKG2D expression on NK cells. Gray closed histograms are from isotype-matched controls while colored histograms represent the indicated markers on total NKG2A+ NK cells as gated in A. (D) Frequencies of NK cells among live CD45+CD3−HLA-DR− mononuclear cells in naïve and SIV-infected macaques. (E) Frequencies of NK cell subpopulations among total NK cells as gated in A. Horizontal bars indicate medians. Mann–Whitney U tests were used for naïve-versus-SIV comparisons; P < 0.1 are shown; P < 0.05 are considered significant.
After identifying NK cell in the BM of normal macaques, we next wanted to evaluate whether chronic SIV infection had any impact on the frequencies of NK cell subpopulations. As shown in Figure1D, the frequency of NK cell in BM of chronically SIV-infected macaques was approximately half that of their naïve counterparts (chronic, median 0.99%, range 0.31%–1.16%; naive, median 1.73%, range 0.64%–5.39%, respectively). Interestingly, this loss was subset specific, with a significant decrease in DN NK cells while both CD16+ and DP increased during chronic SIV infection (Fig. 1E). The results suggested subset perturbation of BM NK cells in chronically SIV-infected macaques.
SIV infection induces increased turnover rates and apoptosis in bone marrow NK cells
To address the mechanism(s) of BM NK cell loss and subset perturbation, we next analyzed intracellular expression of the proliferation marker, Ki67, and the apoptotic molecule, caspase-3. As shown in Figure 2, compared with naïve macaques, BM NK cells from SIV-infected macaques had a 3-fold increase in Ki67 expression, and a 4-fold increase in caspase-3 expression. These data indicated higher rates of proliferation and apoptosis, suggestive of an overall increase in NK cell turnover. Furthermore, the disproportionately high increase in apoptosis could account for the generalized loss of NK cells in the BM. Interestingly, SIV infection disparately impacted the four NK cell subpopulations. Proliferation and apoptosis were generally low in the CD16+ subset and remained relatively stable even during infection. However, the other three subpopulations all had increased proliferation and apoptosis. This could suggest that mechanisms of induced turnover and apoptosis have less effect on CD16+ NK cells, or, alternatively, since CD16+ NK cells are thought to be the most terminally differentiated could indicate that SIV infection is driving differentiation in situ.
Fig. 2. SIV infection induces increased turnover rates in bone marrow NK cells.
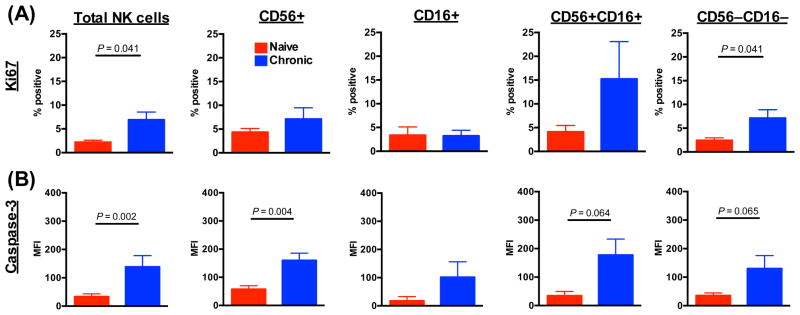
(A) Frequencies of Ki67 expression in total NKG2A+ NK cell and NK cell subpopulations. (B) Mean fluorescence intensities (MFI) of caspase-3 expression in total NK cell and NK cell subpopulations. Bars represent means ± SEM for 5 to 6 animals per group.
Increased perforin expression in bone marrow NK cells during SIV infection
In general peripheral CD16+ NK cells are thought to be more cytotoxic cells containing high levels of perforin and granzymes, whereas CD56+ NK cells are predominantly immunoregulatory cells that produce high levels of cytokines. We next sought to determine if this dichotomous functional pattern also held true in BM NK cells. As shown in Figure 3, BM CD16+ expressed the highest level of intracellular perforin in naïve macaques. However, during chronic SIV infection other subpopulations of NK cells upregulated perforin, most notably the normally noncytolytic CD56+ NK cells (median MFIs 134 and 70, chronic and naïve, respectively). The results suggested abnormal altered function of CD56+ NK subsets in BM. We have previously reported a similar phenomenon in CD56+ NK cells in peripheral blood of SIV-infected macaques [17].
Fig. 3. Increased perforin expression in bone marrow NK cells during SIV infection.
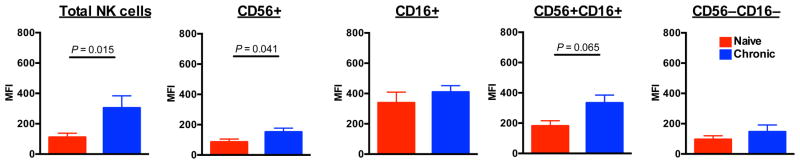
Mean fluorescence intensities (MFI) of perforin expression in NK cells and subpopulations are shown. Bars represent means ± SEM for 6 animals per group.
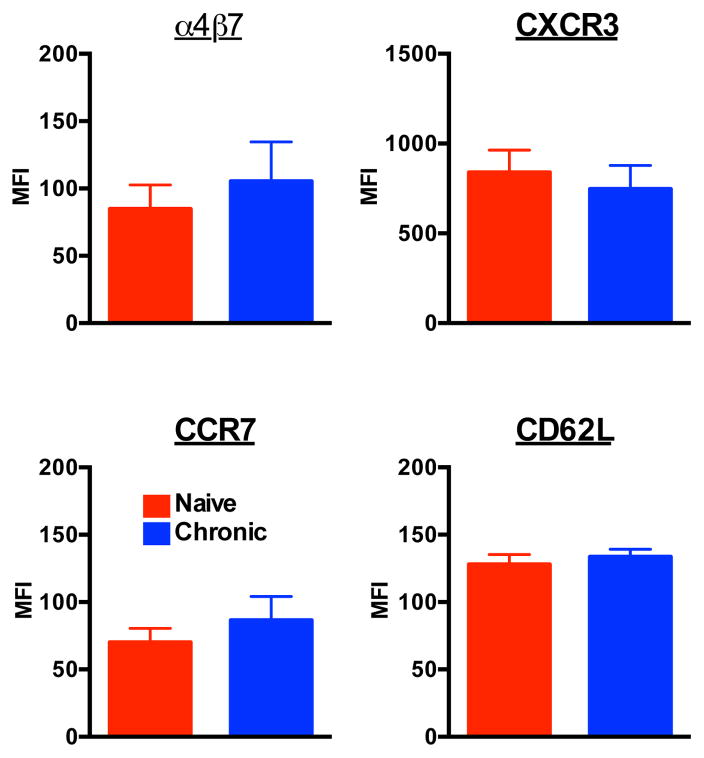
Stable trafficking marker expression on bone marrow NK cells during SIV infection
We and others have previously demonstrated that chronic SIV infection induces significant up-regulation of the gut-homing marker α4β7 on peripheral NK cells, coupled with down-regulation of the lymph node-homing marker, CCR7 and CD62L [26]. This change suggests an overall shift in NK cell repertoire from lymph node to gut-trafficking. CXCR3 is also upregulated on NK cells during SIV infection, suggestive of an overall increased inflammatory response. However, α4β7, CCR7, CXCR3 and CD62L expression were unchanged on BM NK cells during SIV infection. These data suggest that mechanism(s) of altered NK cell homing during SIV infection may occur after emigration from the BM.
DISCUSSION
Herein we present data on the effects of chronic SIV infection on BM NK cells demonstrating: (1) NK cell frequencies are reduced in infected compared to naïve animals associated with increased turnover; (2) remaining BM NK cells are skewed toward a cytolytic CD16+ NK cell phenotype; and (3) SIV infection seemingly has little effect on trafficking repertoires of BM NK cells.
The development of NK cells in the BM of humans and mice is well-documented 18,28,29, but prior to this study little was known about NK cells in the BM of nonhuman primates such as rhesus macaques. In this study we report that ~ 2% of mononuclear cells in the BM of rhesus macaques are NKG2A+ NK cells, a similar frequency to what has been shown for humans[27]. However, more than 50% of BM NK cells in macaques were found to express neither CD56 nor CD16. This result is in contrast to human BM, where 90% of CD3−CD56+ NK cells are CD56dimCD16+ [27, 28]. While this could be strictly a species difference, which is well-documented between human and macaque NK cells in other tissues [17], it could also be explained partially for technical reasons. Human NK cells are defined by CD56 expression, but CD56 appears at a late stage of NK cell development [27]. Because CD56 is expressed on a much lower frequency of macaque NK cells in general, nonhuman primate studies such as ours tend to use NKG2A as the NK cell delineating marker, but NKG2A is expressed early in development [29]. Thus, some differences we see in macaque and human BM subset distribution may be reflective of how we identify NK cells in different species, and these data could suggest in fact that studies of human BM NK cells inadvertently exclude certain subpopulations.
It is generally accepted that progressive HIV infection perturbs circulating NK cell subpopulations by inducing a generalized increase in CD56−CD16+ NK cells but a decline in CD56dim and CD56bright subsets [18, 30-32]. A similar phenomenon occurs in pathogenic SIV infections whereupon DN and CD16+ NK cells expand, while CD56+ NK cells decline in number [17]. While most evidence indicates NK cells are not directly infected, it is unclear whether changes in peripheral blood are reflective of redistribution to other tissues or an alteration in NK cell development and efflux from sites of differentiation, such as the BM. In the present study we found chronic SIV infection caused an overall decline in NKG2A+ NK cells in BM, but disparately affected the four subpopulations — frequencies of CD56+ and DN NK cells declined, while CD16+ and DP NK cells increased. This was coupled to an overall increase in apoptotic NK cells and a selective increase in proliferation (Ki67 expression) in the DN subset. While the increased apoptosis does partially explain the decreased frequency of NK cells in the BM, the proliferation of DN NK cells, the dominant subset, could also suggest expansion and differentiation. Data from our laboratory (Hong et al., submitted) suggests the DN subset is actually a precursor to the CD16 subset, and the BM would undoubtedly be a primary site for such differentiation. The fact that the CD16+ subpopulation exhibited little change in turnover may actually support the hypothesis that they are the most terminally differentiated BM NK cells. This could also explain why we observed an increase in CD16+ NK cells in the BM without an increase in proliferation in SIV-infected macaques. However, because the BM both seeds the periphery with lymphocytes and is conversely vascularized by the blood, it is difficult from these data to determine which NK cells may be developing and maturing in situ and which may be recirculating from other tissues. Van Helden et al. [33] recently demonstrated in mice that phenotypically mature NK cells in the periphery can migrate back to the BM and proliferate there, both homeostatically and in response to infection. Therefore, we cannot exclude the possibility that CD16+ NK cells in circulation are trafficking back to BM during chronic SIV infection.
As has been observed for circulating CD16+ NK cells [17], CD16+ NK cells in the bone marrow expressed the highest level of intracellular perforin, and high levels were maintained in SIV-infected macaques. However, BM CD56+ NK cells in SIV-infected animals also had significantly upregulated perforin, an unexpected change in function for the normally noncytotoxic subset of NK cells that has also been observed in peripheral blood[17]. This could be interpreted in two ways: (1) If the majority of the NK cells in the BM are mature and terminally differentiated and are migrating there via the circulation, then some systemic effect of SIV infection is activating NK cells to upregulate cytoxic functions, as has been shown in peripheral blood; or (2) if a larger proportion of the NK cells in the BM are indeed in situ-differentiating cells, then SIV infection is altering acquisition of cytotoxic functions during NK cell development specific to this compartment, the effects of which may be evident in other tissues. Regardless of the mechanism, we can conclude that SIV infection drives NK cells toward a more cytotoxic phenotype that could be important for HIV/AIDS immunopathogenesis.
Finally, we have previously demonstrated that SIV infection induces circulating NK cells to traffic away from lymph nodes and migrate to the gut mucosae through a loss of CCR7 coupled to upregulation of α4β7 [26], a phenomenon which also seems to occur, at least in part, during HIV infection [32]. In this study, we examined the expression of gut-homing and lymph node-homing markers on BM NK cells and, perhaps unexpectedly, expression of α4β7, CCR7, CXCR3 and CD62L were all indistinguishable in naïve versus SIV-infected macaques. These data suggest that the mechanism(s) of altered NK cell homing during HIV and SIV infections may occur after emigration from the BM. In a more general sense, we could surmise that the overall trafficking repertoires of NK cells during SIV infection are not changed, just that fewer numbers and varied subsets of NK cells, albeit with increased cytotoxicity, are emigrating from the BM. Regardless, the mechanism(s) behind SIV-induced perturbation of the BM NK cell compartment and whether similar mechanisms occur in alternative sites of NK cell development will require further study.
Fig. 4. Stable trafficking marker expression on bone marrow NK cells during SIV infection.
Mean fluorescence intensities (MFI) of α4β7, CXCR3, CCR7 and CD62L expression on total NK cells are shown. Bars represent means ± SEM for 5 to 6 animals per group.
Acknowledgments
Grateful thanks are expressed to Jacqueline Gillis and Michelle Connole for expert technical assistance. This work was supported by a CHAVI/HVTN Early Career Investigator award, grant number U19 AI067854, a Harvard University CFAR grant, number P30 AI060354 (both to RKR), and NIH NEPRC base grant P51 OD011103.
Funding: This work was supported by a CHAVI/HVTN Early Career Investigator award, grant number U19 AI067854, a Harvard University CFAR grant, number P30 AI060354 (both to RKR), and NIH NEPRC base grant P51 OD011103.
References
- 1.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annual Reviews of Immunology. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 5.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 6.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 7.Moroso V, Famili F, Papazian N, Cupedo T, van der Laan LJ, Kazemier G, et al. NK cells can generate from precursors in the adult human liver. Eur J Immunol. 2011;41:3340–3350. doi: 10.1002/eji.201141760. [DOI] [PubMed] [Google Scholar]
- 8.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 9.Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma-based long-term culture system: identification of a CD34+7+ NK progenitor. Blood. 1994;83:2594–2601. [PubMed] [Google Scholar]
- 10.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez VD, Falconer K, Bjorkstrom NK, Blom KG, Weiland O, Ljunggren HG, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009;183:6612–6618. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 13.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114:3822–3830. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chinen H, Matsuoka K, Sato T, Kamada N, Okamoto S, Hisamatsu T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133:559–573. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 16.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 17.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 19.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. J Intern Med. 2008;265:29–42. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat Rev Immunol. 2005 doi: 10.1038/nri1711. epub 10-20-05. [DOI] [PubMed] [Google Scholar]
- 21.Thiebot H, Louache F, Vaslin B, de Revel T, Neildez O, Larghero J, et al. Early and persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J Virol. 2001;75:11594–11602. doi: 10.1128/JVI.75.23.11594-11602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiebot H, Vaslin B, Derdouch S, Bertho JM, Mouthon F, Prost S, et al. Impact of bone marrow hematopoiesis failure on T-cell generation during pathogenic simian immunodeficiency virus infection in macaques. Blood. 2005;105:2403–2409. doi: 10.1182/blood-2004-01-0025. [DOI] [PubMed] [Google Scholar]
- 23.Koka PS, Reddy ST. Cytopenias in HIV infection: mechanisms and alleviation of hematopoietic inhibition. Curr HIV Res. 2004;2:275–282. doi: 10.2174/1570162043351282. [DOI] [PubMed] [Google Scholar]
- 24.Hillyer CD, Klumpp SA, Hall JM, Lackey DA, III, Ansari AA, McClure HM. Multifactorial etiology of anemia in SIV-infected rhesus macaques: Decreased BFU-E formation, serologic evidence of autoimmune hemolysis, and an exuberant erythropoietic response. J Med Primatol. 1993;22:253–256. [PubMed] [Google Scholar]
- 25.Hillyer CD, Lackey DA, 3rd, Villinger F, Winton EF, McClure HM, Ansari AA. CD34+ and CFU-GM progenitors are significantly decreased in SIVsmm9 infected rhesus macaques with minimal evidence of direct viral infection by polymerase chain reaction. Am J Hematol. 1993;43:274–278. doi: 10.1002/ajh.2830430409. [DOI] [PubMed] [Google Scholar]
- 26.Reeves RK, Evans TI, Gillis J, Johnson RP. Simian immunodeficiency virus infection induces expansion of alpha4beta7+ and cytotoxic CD56+ NK cells. J Virol. 2010;84:8959–8963. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freud AG, Caligiuri MA. Human natural killer cell development. Immunological Reviews. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 29.Eissens DN, Spanholtz J, van der Meer A, van Cranenbroek B, Dolstra H, Kwekkeboom J, et al. Defining early human NK cell developmental stages in primary and secondary lymphoid tissues. PLoS One. 2012;7:e30930. doi: 10.1371/journal.pone.0030930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez VD, Falconer K, Michaelsson J, Moll M, Reichard O, Alaeus A, et al. Expansion of CD56- NK cells in chronic HCV/HIV-1 co-infection: reversion by antiviral treatment with pegylated IFNalpha and ribavirin. Clin Immunol. 2008;128:46–56. doi: 10.1016/j.clim.2008.03.521. [DOI] [PubMed] [Google Scholar]
- 31.Hong HS, Eberhard JM, Keudel P, Bollmann BA, Ahmad F, Ballmaier M, et al. Phenotypically and functionally distinct subsets contribute to the expansion of CD56-/CD16+ natural killer cells in HIV infection. AIDS. 2010;24:1823–1834. doi: 10.1097/QAD.0b013e32833b556f. [DOI] [PubMed] [Google Scholar]
- 32.Hong HS, Ahmad F, Eberhard JM, Bhatnagar N, Bollmann BA, Keudel P, et al. Loss of CCR7 expression on CD56(bright) NK cells is associated with a CD56(dim)CD16(+) NK cell-like phenotype and correlates with HIV viral load. PLoS One. 2012;7:e44820. doi: 10.1371/journal.pone.0044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Helden MJ, de Graaf N, Boog CJ, Topham DJ, Zaiss DM, Sijts AJ. The bone marrow functions as the central site of proliferation for long-lived NK cells. J Immunol. 2012;189:2333–2337. doi: 10.4049/jimmunol.1200008. [DOI] [PMC free article] [PubMed] [Google Scholar]