Abstract
Compound K (CK; 20-O-D-glucopyranosyl-20(S)-protopanaxadiol), a major metabolite of ginsenoside, has been shown to possess several biological activities such as potent antitumor properties. However, the effect of CK on the apoptosis of bladder cancer cells and its underlying mechanisms remain poorly understood. Therefore, we examined the effect of CK on the apoptosis of bladder cancer T 24 cells. Cell counts showed that treatment of T24 cells with CK decreased the cell number in a dose- and time-dependent manner. Flow cytometric analysis revealed that CK could significantly induce apoptosis of T24 cells in vitro. Further, cellular glutathione reduction, accumulation of reactive oxygen species (ROS) were also observed in CK-treated T24 cells. Western blot demonstrated the release of cytochrome c, activation of procaspases-3, procaspases-9, and the change of Bax/Bcl-2 proteins ratio. We also found that the phosphorylation of p38MAPK was increased by CK, while treatment with SB203580 inhibited CK-induced cell apoptosis in T24 cells. The blockage of ROS generation by N-acetylcysteine effectively prevented the apoptosis induction in T24 cells with CK treatment, accompanied by the decrease of activation of p38MAPK. These results suggested that CK induced the apoptosis of bladder cancer T24 cells, which is partially due to ROS generation and p38MAPK activation.
Key words: apoptosis, compound K, p38 MAPK, ROS, T24 cell
Introduction
Bladder cancer ranks ninth in worldwide cancer incidence in 2012, and more than 90% of new cases occur in people ≥55 years of age. Approximately, 80% of bladder cancer presents as a superficial tumor that can be easily treated with minimally invasive surgery. Nevertheless, recurrence of superficial transitional cell carcinoma of the bladder after transurethral resection constitutes a major problem in the management of bladder cancer.1 As severe side effects invoked by most of the antitumor agents are the major hurdle limiting the application and therapeutic success, much attention has been focused on natural compounds with minimal or no toxicity and having the capacity to suppress proliferation and induce apoptosis in cancer cells.2
Apoptosis, or programmed cell death, is initiated and completed in an orderly manner. It is proposed that apoptosis is mediated by two strictly intertwined ways. One pathway is via death receptors, and the other pathway is shown to be primarily linked to mitochondrial changes, directly inducing the release of cytochrome c into the cytosol and apoptosome complex formation with activation of caspases. Growing evidence implicated the mitochondrion as a central integrating organelle in apoptosis, with the capacity to directly activate the execution pathways.3 Oxidative stress is associated with cell apoptosis and necrosis implicated in cancer, aging, and neurodegenerative disorders.4,5 The generation of reactive oxygen species (ROS) is part of the mechanism by which most chemotherapeutic agents or ionizing radiation kill tumor cells.6 An increasing number of studies have implicated ROS in anticancer drug-mediated apoptosis and generation of ROS has been related to p38MAPK activation in cell apoptosis.7 Members of the mitogen-activated protein kinase (MAPK) family participate in many signaling pathways. Members of each major MAPK subfamily—the extracellular signal-regulated protein kinases (ERK), c-Jun N-terminal protein kinase (JNK), and p38MAPK—have been shown to be activated in response to proinflammatory and other stress signals.8 Oxidative stress induced by ROS has been demonstrated to activate the MAPKs via phosphorylation cascade, and p38MAPK may be an apoptosis factor mediated by oxidative stress.9
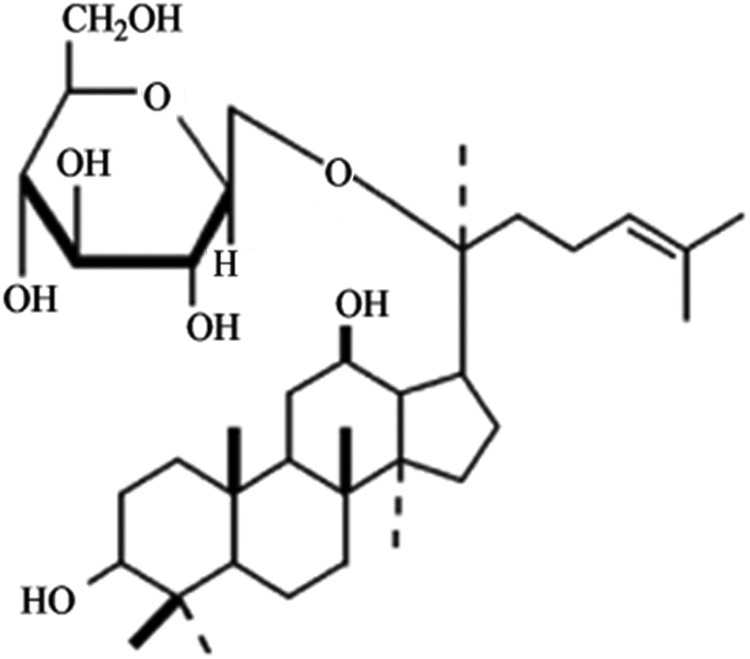
Compound K (CK; 20-O-(β-D-glucopyranosyl)-20(S)-protopanaxadiol (Fig. 1), a protopanaxadiol metabolite derived by biotransformations of intestinal bacteria in humans from ginsenosides Rb1, Rb2, Rc, is one of the major metabolites detected in the urine and blood after administration of total ginsenoside.10 The metabolite CK has been shown to enhance the efficacy of anticancer drugs in cancer cells and induce an anti-metastatic or anti-carcinogenic effect and apoptosis.11–13 However, the effect of CK on the apoptosis of bladder cancer cells is unclear. In this study, we investigated the induction of cell apoptosis in bladder cancer T24 cells by compound K and the mechanism related to ROS accumulation and the activation of p38MAPK.
FIG. 1.
Structure of compound K (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol).
Materials and Methods
Reagents
Ginseng saponin ginsenosides CK, Rd, Rb1 was obtained from the Jin's lab, Dalian Polytechnic University (Dalian, China).14 The purity of the compounds was more than 98% by high-performance liquid chromatography analysis. Cell counting kit-8 (CCK-8) was from Dojindo Laboratories Fetal bovine serum (FBS), penicillin/streptomycin, and RPMI 1640 medium were from Hyclone. Annexin V-FITC apoptosis detection kit was obtained from Biovision. Antibodies against cleaved caspase-3, caspase-9, p38MAPK, phospho-p38MAPK, cytochrome c, Bax, Bcl-2, P53, β-actin, and secondary antibodies were purchased from Santa Cruz Biotechnology. Enhanced chemiluminescence (ECL) kit was from Amersham. p38 inhibitor SB203580 was obtained from Promega Corp. 2′, 7′-dichlorofluorescein diacetate (H2DCFDA) and N-acetyl-cysteine (NAC) were from Beijing Biosynthesis Biotechnology Co. LTD. Other reagents were commercially available in China.
Cell culture
Human bladder T24 cancer cells were obtained from Shanghai institutes for biological sciences and cultured at 37°C in an atmosphere of 95% air and 5% CO2. Cells were grown in RPMI 1640 medium containing 10% FBS, 100 unit/mL penicillin, and 100 μg/mL streptomycin. When the cells became confluent, they were detached by incubation with 0.25% trypsin and 1 mM EDTA at 37°C for 5 min, and then subcultured.
Cell viability assay
Cell counting kit-8 (CCK-8) was used to evaluate the cell viability of T24 cells following the manufacturer's instructions. In brief, the cells were seeded (5×104/well) in 96-well plates. After 24 hours incubation, they were treated with different concentrations of CK, Rd, Rb1 (0, 10, 15, 20, 25 μM) for indicated time periods, respectively. Subsequently, 10 μL of CCK-8 solution was added to each well and the plate was incubated for another 2 hours at 37°C. After that, the absorbance was determined immediately at a wavelength of 450 nm. The cell growth rate was expressed as a percentage compared with the vehicle control. Each measurement was performed in triplicate.
For the inhibition tests, the cells were treated in the presence of NAC (5 mM) or SB203580 (20 μM), respectively. The cell viability was evaluated by CCK-8 method. The cells with treatment in the absence of NAC or SB203580 served as control.
Apoptosis detection by Annexin V-FITC and propidium iodide double staining
After treatment of CK for 24 hours, cell apoptosis was detected by annexin V-FITC apoptosis detection kit following the manufacturer's instructions. Briefly, the cells were gently trypsinized, washed once with serum-containing media, and resuspended in 500 μL of binding buffer. Five microliters of annexin V-FITC and 5 μL of propidium iodide (PI) stocking solutions were added to the suspension and the cells were incubated at room temperature in the dark for 5 minutes. Apoptotic cells were analyzed on a FACScalibur Flow Cytometer (Becton Dickinson) by using FITC signal detector and phycoerythrin emission signal detector and quantified by using Cell Quest Software (BD Biosciences).
Preparation of cytosolic extracts
Cells were harvested after treatment with different concentrations of CK for 24 hours and mixed with ice-cold digitonin solution (325 mM digitonin, 2.5 mM EDTA, 250 mM mannitol, and 17 mM 4-morpholine propane sulfonic acid, pH 7.4). The lysed cells were centrifuged for 10 minutes at 1000 g at 4°C. The supernatant was centrifuged under the same conditions to remove nuclear debris. The supernatant was centrifuged for 20 minutes at 12,000 g at 4°C. The supernatants were stored at −80°C for western analysis of cytochrome c.
Western blot analysis
The cells were collected, washed twice with ice-cold phosphate-buffered saline (PBS), and then lysed with ice-cold lysis buffer (1×PBS, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS, with freshly added 1 mM PMSF and 2 μg/mL leupeptin) on ice for 30 minutes. Samples were centrifuged at 14000 g for 10 minutes at 4°C. 5×sodium dodecyl sulfate (SDS) loading buffer was added to the supernatants, with subsequent incubation for 5 minutes at 97°C. Aliquots of 50 μg proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and electroblotted to polyvinylidene fluoride membranes. Membranes were blocked with 5% fat-free milk in PBS containing 0.05% Tween 20 (PBS-T) and then incubated overnight at 4°C with primary antibodies. After incubation for 1 hour with horseradish peroxidase-conjugated secondary antibodies, membranes were washed thrice with PBS-T and the bands were visualized using an ECL system. The intensities were quantified by densitometry and the expression of proteins was reported as a proportion of β-actin or p38 protein expression. Fold change versus control values was calculated by normalizing densitometric values obtained from the various proteins with those obtained from β-actin or p38 (GeneTools analysis software; Syngene).
Measurement of glutathione levels
The cells were homogenized with an isotonic buffer and then centrifuged at 1000 g at 4°C. After centrifugation, intracellular glutathione (GSH) levels were measured using glutathione reduced assay kit according to the manufacturer's instructions.
Measurement of intracellular ROS
The levels of intracellular ROS were monitored by the oxidation-sensitive probe H2DCFDA. After treatment of cultured cells with CK for the length of time as indicated, the cells were stained with 10 μM of H2DCFDA for 30 minutes at 37°C and then collected and washed twice with PBS. The cellular fluorescence of cells was detected by flow cytometry with an excitation wavelength of 480 nm and an emission wavelength of 525 nm (Becton Dickinson).
Statistical analysis
Statistical significance was assessed by comparing mean (±SD) values of at least three samples per treatment group with Student t-test for independent groups. Significance was assumed for p value less than 0.05. Statistical analysis was performed using SSPS software.
Results
CK inhibits the growth of T24 cells
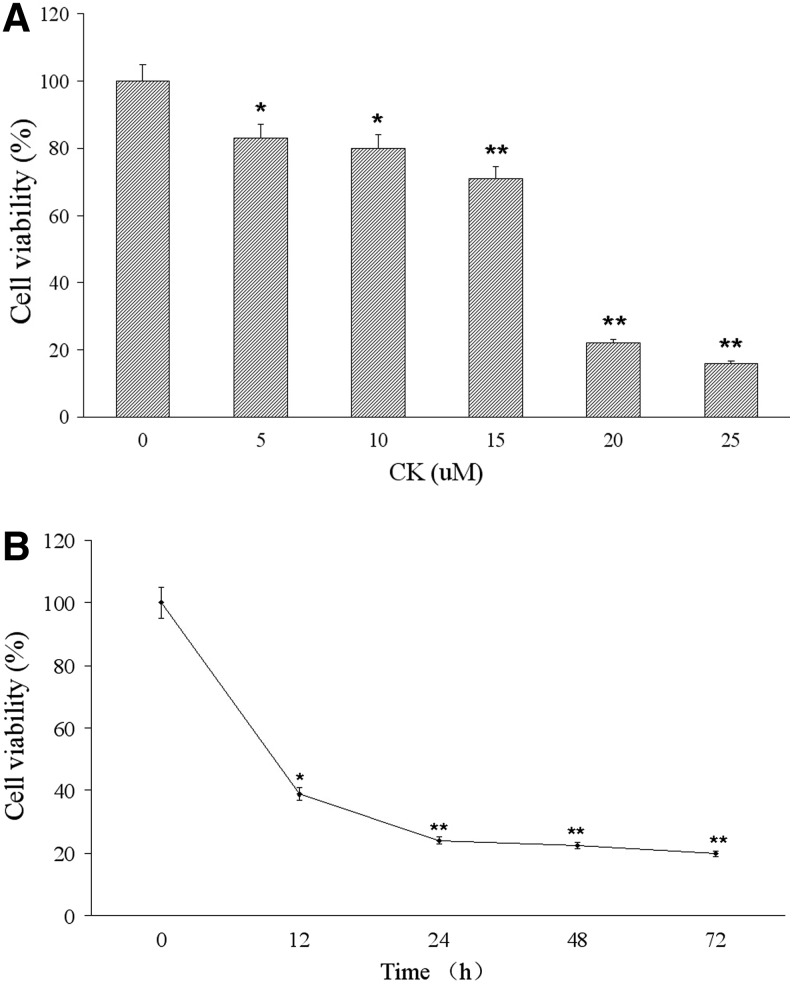
To evaluate the effect of CK on the cell growth of T24 cancer cells, we treated the cells with CK, Rd, Rb1 for indicated time periods. The cells treated with vehicle served as control. Upon the exposure of Rd and Rb1 (0–25 μM) for 24 hours, the cell growth of T24 cells was not significantly changed (data not shown). However, the cell number of T24 treated with different concentrations of CK for 24 hours significantly decreased in a dose-dependent manner. It can be seen from Figure 2A that 5 μM was the minimal dose to inhibit the cell growth of T24, with the percentage of viable cells decreasing to 83.0%. The cell growth of T24 cells dramatically reduced to 16.4% at 25 μM. In addition, the cell number of T24 cells treated with 20 μM CK at various time points decreased in a time-dependent manner, as shown in Figure 2B. The shortest treatment time for the inhibition of T24 cell growth was observed at 12 hours, but the optimal incubation time was about 24 hours.
FIG. 2.
Effect of compound K on cell growth of human bladder cancer T24 cells. (A) T24 cells were treated with different concentrations of compound K (0, 5, 10, 15, 20, 25 μM) or vehicle for 24 hours, respectively. (B) T24 cells were treated with 20 μM compound K for the indicated times (12, 24, 48, and 72 hours). CCK-8 assays were performed and the cell growth rate was expressed as percentages compared with the average of control T24 cells. Data represent mean±SD from four independent experiments. *p<0.05, compared with the control group, **p<0.01, compared with the control group.
CK induces apoptosis in T24 cells
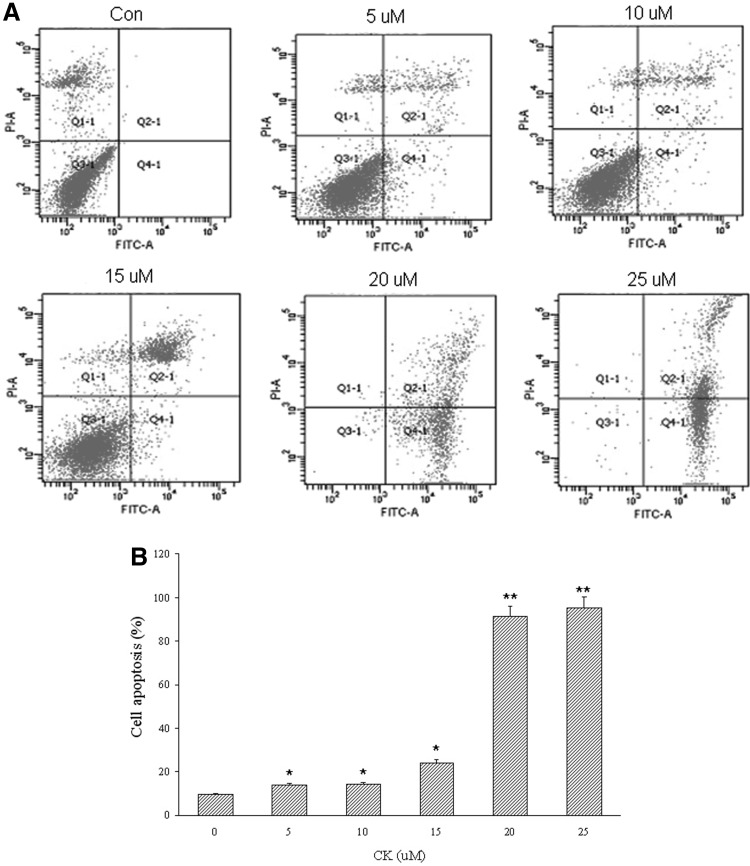
To determine whether the growth inhibitory effect of CK on T24 cells was associated with the process of cell apoptosis, we tested the apoptosis of T24 cells with annexin V-FITC apoptosis detection kit and flow cytometry. Annexin V-FITC binding while remaining PI negative can indicate phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane without membrane damage, which is the feature of an early stage of apoptosis. As shown in Figure 3, after exposure to CK for 24 hours, the proportion of apoptotic cells significantly increased accompanied with the augment of CK concentration. There was at least a nine-fold increase in the percentage of apoptotic cells 24 hours after supplementation with CK in relation to control in T24 cells. We then proceeded to evaluate the effect of CK on cell apoptosis and identified the cellular mechanism involved.
FIG. 3.
Effect of compound K on apoptosis of human bladder cancer T24 cells. (A) Apoptosis of T24 cells incubated with different concentrations of compound K (0, 5, 10, 15, 20, 25 μM) and vehicle (served as control) for 24 hours was examined using flow cytometry with annexin V-FITC apoptosis detection kit. Annexin V-FITC binding while remaining propidium iodide (PI) negative can indicate phosphatidylserine (PS) exposure on the outer leaflet of the plasma membrane without membrane damage, which is the feature of an early stage of apoptosis. Pictures are representative of three independent experiments. (B) Percentage of analysis of annexin V/PI flow cytometry of T24 cells treated with compound K. At least 10,000 cells were analyzed per sample, and quadrant analysis was performed. *p<0.05; **p<0.001.
CK induces apoptosis via mitochondria pathway
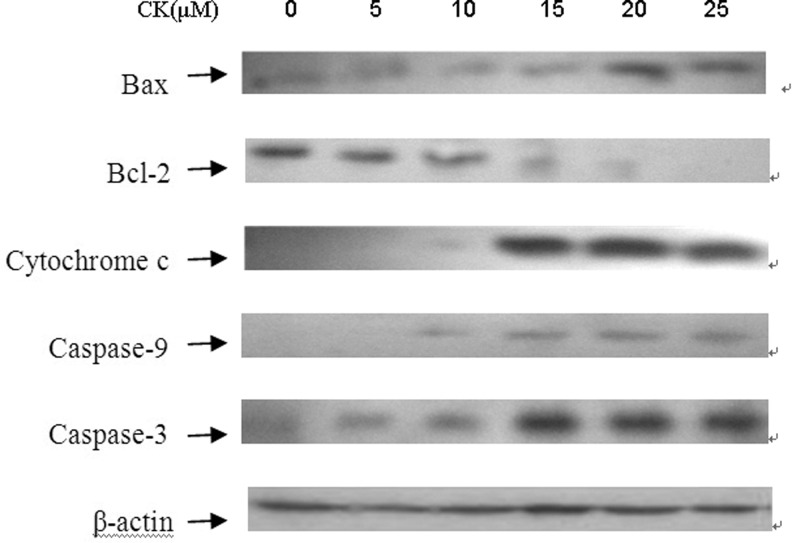
Mitochondria play a central role in the commitment of cells to apoptosis. To ascertain the molecular mechanisms of CK-induced T24 cells apoptosis, the expression of several key proteins relevant to apoptosis were analyzed by western blot. Our data showed that CK increased the levels of Bax and decreased Bcl-2 levels after 24 hours treatment, so that changed the Bax/Bcl-2 expression ratio (Fig. 4). Simultaneously, the amount of cytochrome c in the cytosol significantly increased, suggesting that cytochrome c was released from mitochondria to cytosol. Since the release of cytochrome c from mitochondria can activate caspase cascade, which were important proteins related to the apoptotic process, we examined the cleaved procaspase-9 and procaspase-3 in CK-induced apoptosis. As shown in Figure 4, cleaved procaspase-9 and procaspase-3 markedly increased after 24 hours of CK treatment. These results suggested that CK may induce apoptosis through mitochondria pathway.
FIG. 4.
Expression of apoptosis-related protein in T24 cells after treatment with compound K. Cells were treated with compound K (20 μM) and collected for western blot analysis. Protein contents were analyzed by densitometry and normalized with β-actin. Representative data from three independent experiments are shown.
CK induces apoptosis via p38MAPK activation
It is reported that the activation of p38 MAPK can lead to the induction of apoptosis in many human cells. Therefore, we further investigate whether p38 MAPK pathway was involved in CK-induced apoptosis. As shown in Figure 5, CK markedly stimulated the phosphorylation of p38 MAPK of T24 cells after 24 hours treatment, while the expression level of total p38 MAPK mainly stayed unchanged.
FIG. 5.
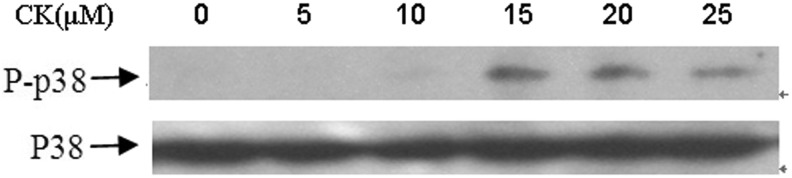
Expression of p38MAPK and phospho-p38MAPK protein in T24 cells after treatment with compound K. Cells were treated with compound K (20 μM) and collected for western blot analysis. Protein contents were analyzed by densitometry and normalized with p38MAPK. Representative data from three independent experiments are shown.
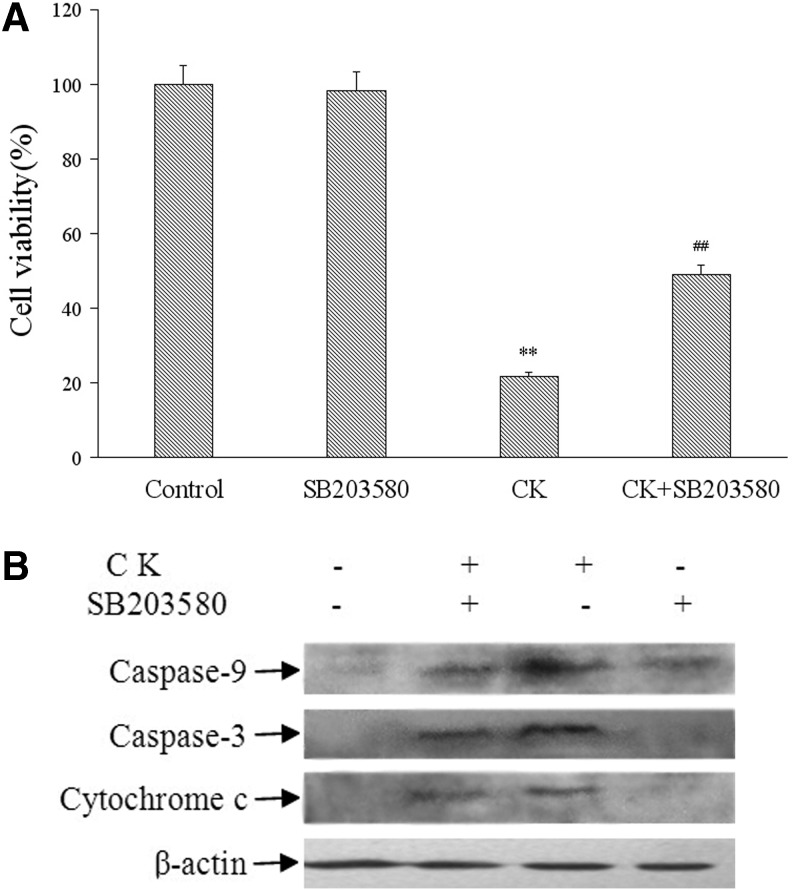
To confirm the involvement of p38 MAPK phosphorylation in the induction of apoptosis by CK, we used SB203580, a p38 MAPK specific inhibitor, to determine the contribution of p38 MAPK pathway in CK-induced cell apoptosis. When added to CK-treated cultures, the p38 inhibitor SB203580 significantly increased the percentage of viable cells as shown in the cell viability assay (Fig. 6A). Moreover, SB203580 attenuated CK-induced cleavage of procaspase 3, procaspase 9, and the release of cytochrome c in the cytosol (Fig. 6B). These results indicated that the activation of p38MAPK was involved in CK-induced apoptosis in T24 cells.
FIG. 6.
Apoptosis of T24 cells after treatment of p38 inhibitor SB203580 and compound K. (A) Cell growth rates of compound K treatment, SB203580 treatment, compound K with SB203580 treatment, and control were measured by CCK-8 assays and the data expressed as a mean percentage compared with that of control from four independent experiments. **p<0.01 versus control group; ##p<0.05 versus CK group. The treatment time for all the cell groups was 24 hours. (B) The expression of cleaved procaspase 3, procaspase 9, and cytochrome c in the cytosol of T24 cells treated with p38 inhibitor SB203580 and compound K were analyzed by western blot. Representative data from three independent experiments are shown.
CK induces ROS generation in T24 cells
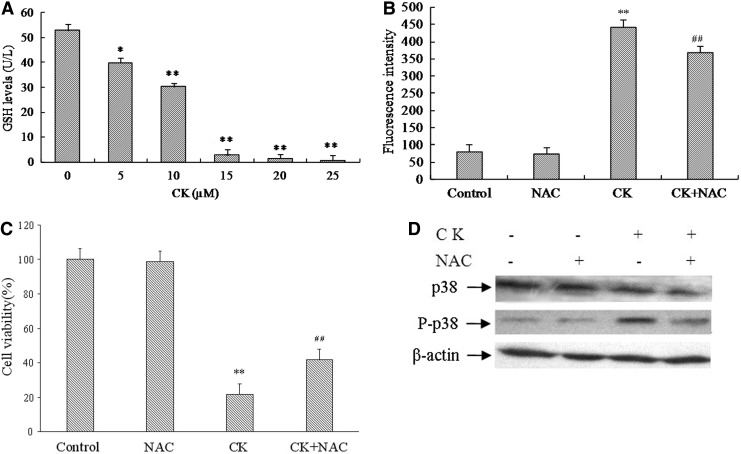
Generation of ROS plays an important role in proapoptotic activities of various anticancer agents. Therefore, we investigated whether generation of intracellular ROS is part of the mechanism by which CK induces apoptosis in T24 cells. Glutathione is an intracellular antioxidant that scavenges ROS directly and indirectly. Whether CK exacerbates oxidative stress by causing depletion of intracellular glutathione reduced was investigated. As shown in Figure 7A, the levels of intracellular glutathione reduced significantly decreased after treatment with CK for 24 hours.
FIG. 7.
N-acetyl-cysteine (NAC) blocks compound K-induced apoptosis and activation of p38MAPK. (A) Reduced GSH levels in T24 cells treated with different concentrations of CK (5, 10, 15, 20, and 25 μM) for 24 hours using glutathione assay kit. (B) After treatment with compound K (20 μM), NAC (5 mM), compound K with NAC, or vehicle in T24 cells, intracellular reactive oxygen species levels were assessed by flow cytometry. (C) The cell growth was assessed by CCK-8 assay, after treatment with compound K (20 μM), NAC (5 mM), compound K with NAC, or vehicle in T24 cells. (D) p38 MAPK and phosphory-p38 MAPK were analyzed by western blot after treatment of NAC (5 mM) for 24 hours. *p<0.05 versus control group; **p<0.01 versus control group; ##p<0.05 versus CK group.
The generation of ROS by CK was further assessed by using fluorescent probe H2DCFDA. Figure 7B showed that after exposure to 20 μM CK for 24 hours, the DCF fluorescence intensity were found to be significantly increased compared with vehicle-treated control. Treatment of cells with antioxidant NAC significantly blocked the CK-induced increase in DCF fluorescence in T24 cells. Collectively, the oxidation of H2DCFDA and depletion of glutathione suggested that ROS generation might play a role in the induction of T24 cell apoptosis.
NAC blocks CK-induced apoptosis and activation of p38MAPK
To further validate the involvement of ROS in mediating the CK apoptotic effect, we measured the effect of antioxidant NAC on the cell apoptosis of T24 cells and the activation of p38MAPK. As shown in Figure 7C, NAC significantly protected T24 cells from growth inhibition by CK. Next, we determined the effect of NAC on cleavage of procaspase-3 and procaspase-9 by CK. Figure 7D clearly demonstrated the cleavage of procaspases by CK in T24 cells. In contrast, treatment with NAC protected the cleavage of procaspase-3 and procaspase-9 by CK, indicating that ROS plays a role in the induction of apoptosis.
To delineate whether CK-induced ROS generation is involved in the p38 MAPK activation in T24 cells, the cells treated with CK for 24 hours in the presence or absence of NAC was analyzed for p38 MAPK and phosphor- p38 MAPK expression. The blockage of ROS generation by the administration of NAC treatment to the cells effectively prevented the CK-induced activation of p38 MAPK (Fig. 7D). These results suggested that ROS may have a role in the promotion of p38 MAPK activation by CK.
Discussion
The saponins of ginseng (ginsenosides) have been used as a traditional medicine in Asian countries for preventive and therapeutic purposes. Compound K, an active metabolite of ginsenside formed by intestinal bacteria after the oral administration of ginseng extract in human and rats,15–17 were found to have many biological activities including enhancing the efficacy of anticancer drugs in cancer cells previously resistant to several anticancer drug, antigenotoxic and anticlastogenic activity in rats, and inducing apoptosis and so on.11–13,18,19 Previous studies have demonstrated the anticancer effects of CK through the induction of apoptosis in myeloma, colon cancer, breast cancer, astrocytoma, leukemia cells, and others.11–13,18–22 However, the effect of CK on the apoptosis of bladder cancer cells and the related mechanism has not been reported. Current studies found that CK was active against bladder cancer T24 cells, and the growth inhibition and killing of T24 cells by CK were attributed to the induction of apoptosis as demonstrated by an increase in annexin V and PI binding.
The release of cytochrome c is a critical process in apoptosis, and is controlled by Bcl-2 family members.23 Cytochrome c released from the mitochondria into the cytosol forms a complex with the apoptosis-acting factor-1 and procapase-9, which results in the maturation of caspase-9 and subsequently activates caspase-3. We found the CK-induced release of cytochrome c from the mitochondria to the cytosol, the changes of Bax/Bcl-2 expression ratio and the enhanced cleavage of procaspase-9 and procaspase-3, therefore provided a direct link between the mitochondria and the CK-induced apoptosis in T24 cells.
It has been reported that there are multiple lines of evidence that MAPK family of serine/threonine kinases plays a critical role in apoptosis signaling pathways, and one of the major MAPK subfamily, p38MAPK, is involved in the activities of CK in many aspects. Jeong et al. revealed that CK downregulated p38 MAPK in a concentration-dependent manner in bFGF-treated HUVECs while activation of p38 MAPK mediates apoptosis in CK-treated cancer cells.22,24,25 Our results showed that the activation of p38MAPK was elevated in T24 cells by CK. Moreover, the blockade of p38MAPK by SB203580 (p38 MAPK inhibitor) effectively and obviously inhibited the effect of CK on the apoptotic enhancement of T24 cells, suggesting that the activation of p38MAPK may be necessary for the induction of apoptosis by CK. These results are in accordance with Choi and Cuong's data.22,25
We also investigated the role of ROS in CK-induced apoptosis in bladder cancer T24 cells. ROS is intracellularly generated as byproducts of normal aerobic metabolism or as second messengers in various signal transduction pathways or in response to environmental stress.26,27 The generation of ROS is part of the mechanism by which most chemotherapeutic agents kill tumor cells.28–30 Our present findings demonstrated that CK caused the oxidation of H2DCFDA in T24 cells, indicating the production of ROS by CK. We also found that CK causes marked depletion of intracellular levels of GSH. The generation of ROS in response to CK was further supported by the finding that treatment with NAC, a general antioxidant blocked the oxidation of H2DCFDA. In addition, data demonstrating prevention of CK-induced cytotoxicity by NAC suggested the involvement of ROS in killing of bladder cancer T24 cells by CK. These results are consistent with the results of previous studies showing participation of ROS in killing of astrocytoma CRT-MG and breast cancer MCF-7 cells.21,22 The role of ROS in CK-induced apoptosis in T24 cells was further confirmed by a decrease of activation of procaspase-3 and −9. The phosphorylation of p38MAPK by CK was also inhibited by NAC, implying that ROS may have a role in the activation of p38MAPK in the apoptotic process induced by CK in T24 cells.
Previous studies have described that CK exhibits cytotoxicity by the induction of apoptosis and cell cycle arrest at the G1 phase, by a caspase-dependent pathway via mitochondria disruption and by inhibition of telomerase activity and others in different cell lines, and various mechanisms are involved in the apoptotic effect of CK.21,22,31 In the present study, we demonstrated that CK could induce the apoptosis of bladder cancer T24 cells. The apoptotic effect of CK was confirmed by the release of cytochrome c from the mitochondria into the cytosol, the changes of Bax/Bcl-2 expression ratio and the increased activation of procaspase-9 and procaspase-3. In addition, both SB203580 and NAC partially rescue the apoptosis induced by CK, indicating that this effect of CK on apoptosis of T24 cells is at least partly due to the activation of p38MAPK via the generation of ROS, while other pathways may also involve in this phenotype. Our results are in line with the results previously reported.21,22 Clearly more work on an in vivo model and the molecular mechanism of action of CK in detail is necessary for the full understanding of the effect of CK on apoptosis of bladder cancer T24 cells.
Conclusions
The present study revealed that compound K could enhance the apoptosis of bladder cancer T24 cells in a dose- and time-dependent manner, and provided an insight into the role of ROS and p38MAPK in apoptosis by compound K in bladder cancer cells. A better understanding of the mechanism of action of compound K could potentially facilitate clinical development of compound K for bladder cancer.
Acknowledgments
We thank Professor Fengxie Jin (Dalian Polytechnic University) for his help in obtaining Ginseng saponin ginsenosides CK, Rd, Rb1. This work was supported by the National Natural Science Foundation of China (Grant No. 31201301), S&T plan project of Liaoning Provincial Education Department (L2011085), Program for Liaoning Excellent Talents in University (LR2011012), and Program for Liaoning Provincial Foundation (201202013).
Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Ehdaie B. Smith SC. Theodorescu D. Personalized medicine in advanced urothelial cancer: When to treat, how to treat and who to treat. Can Urol Assoc J. 2009;3:s232. doi: 10.5489/cuaj.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skommer J. Wlodkowic D. Pelkonen J. Cellular foundation of curcumin-induced apoptosis in follicular lymphoma cell lines. Exp Hematol. 2006;34:463. doi: 10.1016/j.exphem.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 4.Martindale JL. Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 5.Hussain SP. Hofseth LJ. Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 6.Huan P. Feng L. Oldham EA, et al. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang S. Demirs JT. Kochevar IE. p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J Biol Chem. 2000;275:39. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 8.Najjar El. Chatila N. Moukadem M, et al. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 9.Wada T. Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 10.Lee HU. Bae EA. Han MJ, et al. Hepatoprotective effect of ginsenoside Rb1 and compound K on tertbutyl hydroperoxide-induced liver injury. Liver Int. 2005;25:1069. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 11.Oh SH. Lee BH. A ginseng saponin metabolite-induced apoptosis inHepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicol Appl Pharmacol. 2004;194:221. doi: 10.1016/j.taap.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Oh SH. Yin HQ. Lee BH. Role of the FAS/Fas ligand death receptor pathway in ginseng saponin metabolite-induced apoptosis in HepG2 cells. Arch Pharm Res. 2004;27:402. doi: 10.1007/BF02980081. [DOI] [PubMed] [Google Scholar]
- 13.Yim HW. Jong HS. Kim TY, et al. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Res. 2005;65:1952. doi: 10.1158/0008-5472.CAN-04-1740. [DOI] [PubMed] [Google Scholar]
- 14.Yu H. Zhang C. Liu M, et al. Purification and characterization of ginsenosidase hydrolyzing malti-glycosides of protopanaxadiol ginsenoside, Ginsenoside Type I. Chem Pharm Bull. 2007;55:231. doi: 10.1248/cpb.55.231. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa H. Sung JH. Matsumiya S, et al. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 16.Bae EA. Park SY. Kim DH. Constitutive β-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 17.Bae EA. Han MJ. Choo MK, et al. Metabolismof 20 (S)- and 20 (R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ. Sung JH. Lee SJ, et al. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999;144:339. doi: 10.1016/s0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee BH. Lee SJ. Hur JH, et al. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1998;64:500. doi: 10.1055/s-2006-957501. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY. Park MW. Yuan HD, et al. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57:10573. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 21.Kim AD. Kang KA. Zhang R, et al. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ Toxicol Phar. 2010;30:134. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Choi K. Choi C. Proapoptotic ginsenosides compound K and Rh2 enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res Treat. 2009;41:36. doi: 10.4143/crt.2009.41.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desagher S. Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 24.Jeong A. Lee HJ. Jeong SJ, et al. Compound K Inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biol Pharm Bull. 2010;33:945. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- 25.Cuong TT. Yang CS. Yuk JM, et al. Glucocorticoid receptor agonist compound K regulates dectin-1-dependent inflammatory signaling through inhibition of reactive oxygen species. Life Sci. 2009;85:625. doi: 10.1016/j.lfs.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Nohl H. Kozlov AV. Gille L, et al. Cell respiration and formation of reactive oxygen species: Facts and artifacts. Biochem Soc Trans. 2003;31:1308. doi: 10.1042/bst0311308. [DOI] [PubMed] [Google Scholar]
- 27.Raha S. Robinson BH. Mitochondria, oxygen free radicals, and apoptosis. Amer J Med Genet. 2001;106:62. doi: 10.1002/ajmg.1398. [DOI] [PubMed] [Google Scholar]
- 28.Gao J. Liu X. Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci USA. 2005;102:17207. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramanathan B. Jan KY. Chen CH, et al. Resistance to paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 30.Sun Yu. Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: Implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho SH. Chung KS. Choi JH, et al. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9:449. doi: 10.1186/1471-2407-9-449. [DOI] [PMC free article] [PubMed] [Google Scholar]