Abstract
Projections from the central amygdala (CeA) and lateral hypothalamus (LH) modulate the activity of gustatory brainstem neurons, however, the role of these projections in gustatory behaviors is unclear. The goal of the current study was to determine the effects of electrical stimulation of the CeA or LH on unconditioned taste reactivity (TR) behaviors in response to intra-oral infusion of tastants. In conscious rats, electrical stimulation of the CeA or LH was delivered with and without simultaneous intra-oral infusion of taste solutions via an intra-oral cannula. Immunohistochemistry for the Fos protein was used to identify neurons in the gustatory brainstem activated by the electrical and/or intra-oral stimulation. In the absence of intra-oral infusion of a tastant, electrical stimulation of either the CeA or the LH increased the number of ingestive, but not aversive, TR behaviors performed. During intra-oral infusions of taste solutions, CeA stimulation tended to increase aversive behaviors whereas LH stimulation dramatically reduced the number of aversive responses to quinine hydrochloride (QHCl). These data indicate that projections from the CeA and LH alter TR behaviors. A few of the behavioral effects were accompanied by changes in the number of Fos-immunoreactive neurons in the gustatory brainstem, suggesting a possible anatomical substrate for these effects.
Key words: gustation, nucleus of the solitary tract, oromotor behavior, parabrachial nucleus, reticular formation
Introduction
Taste reactivity (TR) behaviors are the immediate oromotor responses to taste solutions in the oral cavity (Grill and Norgren 1978a). The number and type of TR behaviors performed can be interpreted as an indication of potential solution intake, as a measure of reflexive responses to taste input, and as an overall indication of the palatability of the intra-orally introduced substances (Grill and Norgren 1978a; Grill and Berridge 1985; Spector et al. 1988; Berridge 2000). The neural circuitry required for TR behaviors is in the brainstem and is composed of the rostral nucleus of the solitary tract (rNST), parabrachial nucleus (PBN), medullary reticular formation (Rt), and motor nuclei of the trigeminal, facial, and hypoglossal nerves (Grill and Norgren 1978b; Travers et al. 1997).
The rNST is the first central structure to receive gustatory and other sensory input from the oral cavity (Norgren 1995). In rodents, neurons in the rNST project to 2 main targets in the brainstem, the PBN and the Rt. The PBN receives sensory input from the rNST (Herbert et al. 1990; Halsell et al. 1996) and gives rise to ascending pathways to the gustatory cortex, via a relay in the thalamus, and to the ventral forebrain and hypothalamus (Norgren 1976; Saper and Loewy 1980; Halsell 1992) as well as descending pathways to the rNST and Rt (Herbert et al. 1990; Krukoff et al. 1993; Karimnamazi and Travers 1998). The Rt contains the premotor network that coordinates oromotor output (Travers et al. 1997).
Each of the brainstem gustatory nuclei has been split into subdivisions based on cytoarchitecture and connectivity (Fulwiler and Saper 1984; Travers et al. 1997; King 2007). In addition, some of the subdivisions have been shown to serve different orosensory and oromotor functions. For example, most of the gustatory afferent fibers in the facial, glossopharyngeal, and vagus nerves terminate within the rostral central (RC) subdivision of the rNST (Whitehead 1988) and neurons in the RC give rise to the bulk of the ascending projection to the PBN (Whitehead 1990; Halsell et al. 1996; Gill et al. 1999). Also within the rNST, the ventral (V) subdivision contains the majority of neurons that project to the Rt and therefore serve a premotor function (Travers 1988; Halsell et al. 1996; Beckman and Whitehead 1991). In the PBN, the main taste-responsive area is the waist region (W) that includes the central medial (CM) and ventral lateral (VL) subnuclei (Norgren and Pfaffmann 1975; Fulwiler and Saper 1984). Neurons in W give rise to the gustatory pathway to the thalamus as well as a descending projection to the rNST and Rt (Herbert et al. 1990; Krukoff et al. 1993; Karimnamazi and Travers 1998). Finally, in the Rt, the intermediate reticular formation (IRt) contains neurons that project to cranial nerve motor nuclei, whereas neurons in the parvocellular reticular formation (PCRt) receive projections from orosensory brainstem nuclei and forebrain areas involved in homeostatic, learning, and gustatory processes (Beckman and Whitehead 1991; Shammah-Lagnado et al. 1992; DiNardo and Travers 1997; Hayakawa et al. 1999) and project to the IRt and oromotor nuclei (Holstege et al. 1977; Mizuno et al. 1983; Travers and Norgren 1983; Ter Horst et al. 1991; Fay and Norgren 1997a, 1997b, 1997c; Travers et al. 1997, 2000; Travers and Rinaman 2002).
Several forebrain structures, including the central nucleus of the amygdala (CeA) and lateral hypothalamus (LH), are interconnected with gustatory brainstem structures. Specifically, the CeA receives direct projections from the rNST and PBN (Norgren 1976; Bernard et al. 1993; Krukoff et al. 1993) and provides descending projections back to these nuclei (van der Kooy et al. 1984; Moga et al. 1990; Whitehead et al. 2000; Saggu and Lundy 2008) as well as to the Rt (Shammah-Lagnado et al. 1992). In the rNST, the descending projection from the CeA terminates preferentially in V and the ventral half of RC (Halsell 1998; Whitehead et al. 2000) suggesting a significant role in premotor function in this nucleus. Electrophysiological data demonstrate a functional role of the descending projections from the CeA to the rNST (Li et al. 2002) and the PBN (Lundy and Norgren 2001, 2004; Tokita et al. 2004). Specifically, taste-responsive rNST neurons are primarily excited by CeA stimulation whereas PBN neurons are mainly inhibited but excitation occurs as well (Lundy 2008). In both the rNST and PBN, activation of the CeA increases the selectivity of taste responses (Lundy and Norgren 2001, 2004; Li et al. 2002; Kang and Lundy 2010).
Some neurons in the LH respond to taste stimuli applied to the oral cavity (Norgren 1970) and stimulation of the LH produces increases in food intake (Coons et al. 1965; Frank et al. 1982) whereas lesions cause aphasia and adipsia (Grossman et al. 1978). The LH could influence feeding-related behaviors via its projections to the PBN, rNST, and Rt (Hosoya and Matsushita 1981; Berk and Finkelstein 1982; Villalobos and Ferssiwi 1987; Moga et al. 1990; Shammah-Lagnado et al. 1992; Whitehead et al. 2000). Like the descending pathways from the CeA, activation of projections from the LH leads to both inhibitory and excitatory responses in taste-responsive neurons in the rNST (Matsuo et al. 1984; Murzi et al. 1986; Cho et al. 2002, 2003) and the PBN (Lundy and Norgren 2004; Li et al. 2005). Lesions centered in the LH increase the concentrations of saccharin and quinine necessary to elicit aversive responses in rats (Ferssiwi et al. 1987) suggesting that the LH may alter TR behaviors.
Immunohistochemistry for the Fos protein, the product of the immediate early gene c-fos (Morgan and Curran 1989; Sheng and Greenberg 1990), has been used to identify neurons in the central gustatory system activated by taste stimuli. It has been found that the bitter tastant quinine hydrochloride (QHCl) elicits the most robust increases in the number of Fos-immunorective (Fos-IR) neurons in the gustatory brainstem (Yamamoto et al. 1994; Harrer and Travers 1996; DiNardo and Travers 1997; King et al. 1999; Travers et al. 1999; Travers 2002), and that other tastants elicit different patterns of Fos-IR neurons (Yamamoto et al. 1993, 1994; Harrer and Travers 1996; Streefland et al. 1996; Travers 2002; Tokita et al. 2007). The Fos technique also has been used to evaluate the effects of electrical stimulation of taste nerves (Harrison 2001) and central brain structures including the PBN (Krukoff et al. 1992; Morganti et al. 2007), CeA (Petrov et al. 1996), and LH (Arvanitogiannis et al. 1997).
Although the connections between the CeA and LH and the gustatory brainstem are fairly well defined anatomically and have been investigated electrophysiologically, data on the effects of activating descending projections from these structures on behavioral responses to taste input are limited. Therefore, the current study was designed to determine the role of descending projections originating in the CeA and LH in the control of TR behaviors elicited by intra-oral infusion of taste solutions. Potential mechanisms underlying the behavioral effects of these descending pathways were investigated by identifying neurons in the subdivisions of the rNST, PBN, and Rt activated by CeA or LH stimulation using immunohistochemistry for the Fos protein.
Material and methods
Animals
Data from 84 male Wistar rats (250–350 g) are included in this report (n = 4 in each treatment group). An additional 19 rats were used during the study but did not yield useful data because of misplaced or loose stimulating electrodes (n = 16) or failed histology (n = 3). All rats were housed individually in standard hanging stainless steel cages in a secluded room with a 12 h light:12 h dark cycle and constant access to water and standard block rodent food (Harlan Teklad). The housing conditions and procedures that were performed during this study conform to the guidelines of the National Institutes of Health and were approved by the Stetson University Animal Care and Use Committee.
Surgical procedures
All rats were implemented with an electrode placed within either the right CeA or LH and bilateral intra-oral cannulas. The choice of the right CeA or LH over the left was arbitrary, and electrodes were placed unilaterally instead of bilaterally because preliminary studies indicated that unilateral stimulation of these areas evoked behavioral responses (King et al. 2010, 2012; Riley et al. 2011). The surgical procedures used were similar to those previously described (Grill and Norgren 1978a; King et al. 1999; Lundy and Norgren 2004; Morganti et al. 2007). Briefly, rats were anesthetized by intraperitoneal injection of 60mg/kg sodium pentobarbital and placed in a stereotaxic device with nontraumatic ear bars (Stoelting) so that the top of the skull was horizontal. The scalp was shaved and cleaned with a betadine solution and a 1–2cm incision was made in the scalp. A 1mm burr hole was made in the skull above the right CeA or LH. The bipolar stimulating electrodes consisted of 2 stainless steel Formvar-insulated wires that were twisted around each other and protruded 9mm from a plastic pedastal containing electrical mounts (Plastics One). Each wire plus insulation was 0.15mm in diameter and therefore the bare tips of the wires only were 150 µm apart (allowing stimulation of discrete brain areas). The electrode tip was placed into the CeA at 2.0mm caudal to bregma, 4.1mm lateral to the midline, and 8.3mm ventral to the skull surface and into the LH at 2.0mm caudal to bregma, 1.7mm lateral to the midline, and 8.6mm ventral to the skull (Paxinos and Watson 1998). The electrode was secured with dental acrylic and small screws embedded in the skull and a cap was placed over the electrical mount.
During the same surgical session, intra-oral cannulas were implanted bilaterally. The cannulas were formed from approximately 1.0cm of PE-100 tubing that had a Teflon washer threaded onto one end that was then heat flanged to secure the washer. One side of the washer was cut flat to allow it to sit beside the gum comfortably when in place. The other end of the tubing was connected to a 20-gauge syringe needle that allowed it to be inserted through the temporal muscle just anterolateral to the first maxillary molar and brought up the side of the skull, under the skin, to exit the incision in the scalp. On the top of the skull the PE tubing was cut and connected to about 1.0cm of 19-gauge stainless steel tubing and secured in place with dental acrylic. Finally, a topical antibiotic was applied, the skin sutured shut, and each rat placed back into its home cage after a brief recovery on a heated pad.
Stimulation and behavioral testing
The rats were given 1 week to recover from surgery before behavioral testing. On each day during recovery the wound was examined for infection, the rats weighed to assess recovery, and the intra-oral cannulas flushed with dH2O. For 3 days prior to behavioral testing, each rat was placed into the behavioral arena for 30 min without stimulation to allow for acclimation to the testing environment. The behavioral arena was located in an isolated room and consisted of an opaque cylinder (26cm tall and 26cm diameter) mounted on a Plexiglas stand with a mirror underneath the platform to allow visualization of the rats from below. On testing day, the electrical mount was connected to a stimulator (Grass Instruments S48) through a photoelectric stimulus isolation unit (World Precision Instruments) and 1 intra-oral cannula was attached to tubing connected to a 10-ml syringe that was held within a syringe pump (Harvard Apparatus) and the rat was placed into the arena for 30min before stimulation. Electrical stimulation of the CeA or LH was accomplished by passing current for 5min (100–200 μA pulses of 0.4ms duration at 50 Hz), switching the polarity of the current every 30 s. These stimulation parameters were chosen because they were shown to evoke behavioral responses and the expression of Fos protein in previous studies (Galvin et al. 2004; Morganti et al. 2007). Electrical stimulation occurred alone or during intra-oral infusion of dH2O, 0.10M NaCl, 0.10M sucrose, 0.03M HCl, 0.003M QHCl, or 0.16M monosodium glutamate (MSG) (0.233 mL/min). These concentrations were selected based on previous reports (Spector et al. 1988; Harrer and Travers 1996; Tokita et al. 2007). Control rats did not receive electrical stimulation but still endured the same surgical procedures including having electrodes positioned within the CeA or LH. During the 5-min stimulation period TR behaviors were videotaped with S-VHS equipment.
Histology and Fos immunohistochemistry
Following behavioral testing and a 45-min period to allow the expression of the Fos protein, the rats were sacrificed with an overdose of sodium pentobarbital (80mg/kg). Once unresponsive to toe pinch, the rats were perfused intracardially with about 200mL of cold heparinized 0.15M NaCl followed by about 500mL of sodium phosphate-buffered 4% paraformaldehyde. The brains then were removed and postfixed overnight at 4 ºC and then cut into 75 μm coronal sections using a vibratome. Every other section was processed for Fos immunohistochemistry as previously described (Morganti et al. 2007). Briefly, the sections were treated with 1% sodium borohydride in potassium phosphate-buffered saline (KPBS) for 20min. Following rinses in KPBS, the brain sections were incubated in a Fos primary antibody raised in rabbit (Santa Cruz Biotech) diluted at 1:10 000 in KPBS with 0.4% Triton X-100 for 72h at 4 ºC. After incubation in the primary antibody, the sections were rinsed with KPBS and incubated in biotinylated goat antirabbit IgG (Vector Labs) at 1:600 in KPBS with 0.4% Triton X-100 for 4h at room temperature. The sections then were rinsed using KPBS and incubated in the reagents of an ABC kit (Vector Labs) overnight at 4 ºC. Finally, the sections were rinsed and reacted in 0.1M sodium phosphate buffer containing 0.03% diaminobenzidine, 0.008% nickel ammonium sulfate, 0.008% cobalt chloride, and 0.0075% H2O2 for 9min at room temperature. Following a final rinse in KPBS, the sections were mounted on gelatin- and chrome alum-coated glass slides, let to dry overnight, and then coverslips mounted using Permount (Fisher Scientific). The alternate sections that were not processed for the Fos protein were mounted on slides and Nissl-stained with 0.1% thionin.
Data analysis
TR behaviors were viewed frame by frame and counted for the entire 5-min stimulation period using previously described criteria (Grill and Norgen 1978a; Spector et al. 1988) by an investigator who was unaware of the tape sequence being analyzed. Ingestive behaviors counted were mouth movements, lip flares, tongue protrusions, and lateral tongue protrusions. Aversive behaviors were gapes, chin rubs, headshakes, and forelimb flails. The number, type, and timing of each behavior were recorded. Total ingestive and aversive scores reflect the sum of the occurrences of each individual oromotor behavior.
Fos-IR neurons were counted bilaterally in the rNST, PBN, and Rt. These nuclei and their subregions were identified in the Nissl-stained tissue viewed on a Zeiss Axioskop light microscope equipped with a video camera. The corresponding Fos-labeled sections then were video captured and the nuclei and associated subregions outlined, and the number of Fos-IR neurons in each subregion counted manually. The neuron counts were performed by an investigator who was unaware of the behavioral response outcomes. The rNST and Rt were examined in 7 coronal sections beginning where the NST first moves lateral to the 4th ventricle and ending where the dorsal cochlear nucleus forms. Neuron counts were made within the medial (M), RC, rostral lateral (RL), and V subdivisions for the rNST, and the PCRt and IRt. The numbers of Fos-IR neurons reported for the rNST and Rt are the total from the 7 sections. Fos-IR neurons in the PBN were examined in 6 sections and counted within the CM and VL subnuclei (that make up the waist area), as well as the dorsal lateral (DL), external lateral (EL), and external medial (EM) subdivisions. Each subdivision typically was present in 4 sections with the CM and VL being in the caudal 4 sections, the EL and EM being in the rostral 4 sections, and the DL being in the 4 middle sections.
Statistical analysis was accomplished by performing single-factor analysis of variance (ANOVA) followed by post hoc Fisher’s Least Significance Difference tests. Specifically, ANOVAs were performed to determine if the number of behaviors or Fos-IR neurons counted were different for each intra-oral infusion condition (none, water, NaCl, sucrose, HCl, QHCl, and MSG). If the ANOVA revealed a significant treatment effect (P < 0.05), then the post hoc tests were used to determine differences between each treatment. This analysis procedure also was used to compare the effects of the 3 brain stimulation conditions under the same intra-oral infusion condition (e.g., the effect of CeA, LH, or no stimulation during QHCl infusion). Finally, potential relationships between the number of TR behaviors performed and the number of Fos-IR neurons in a particular brain region under each stimulation condition were investigated using linear regression analysis.
Results
TR behaviors and Fos-IR neurons without CeA or LH stimulation
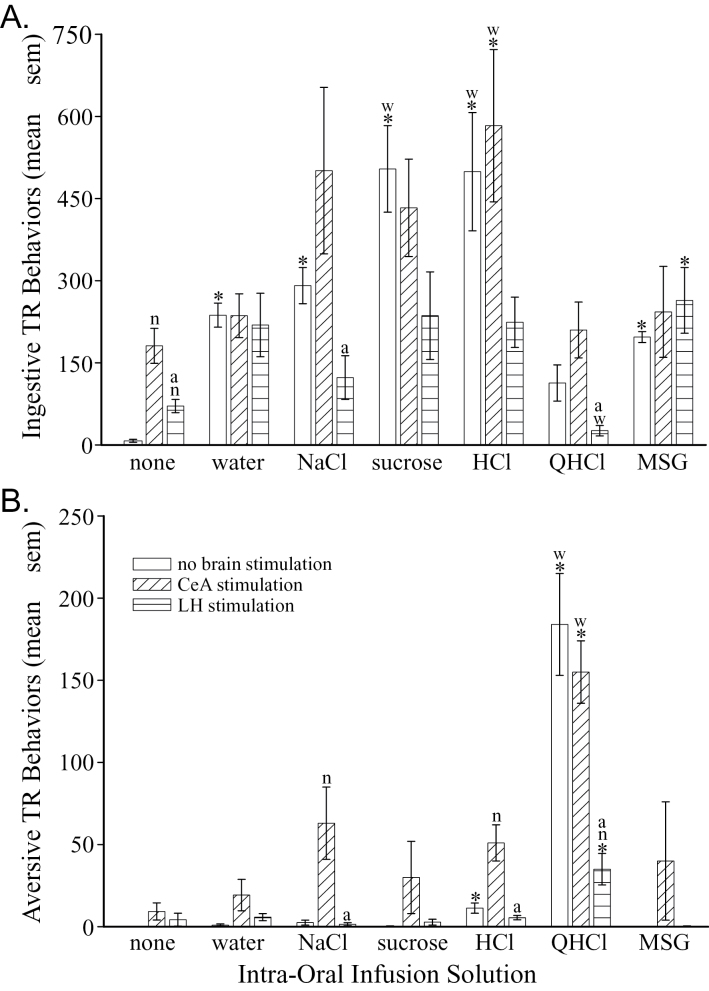
In the absence of electrical stimulation, the number of ingestive TR behaviors varied depending on the solution infused (F (6,21) = 11.70, P = 0.00001). Intra-oral infusion of water (P = 0.000001) and each taste solution (P < 0.0001), except QHCl (P = 0.185), significantly increased the number of ingestive TR behaviors performed (Figure 1A, first bar in each triplet). Sucrose and HCl elicited the most ingestive responses compared with the other tastants (P < 0.013) and water (P < 0.002). The number of aversive behaviors also differed among the tastants (F (6,21) = 33.24, P = 1 × 10− 9, Figure 1B). More aversive TR behaviors were observed in response to intra-oral infusion of HCl (P = 0.001) and QHCl (P = 0.00003) in comparison to controls that did not receive an infusion. However, only QHCl increased the number of aversive TR behaviors over intra-oral infusion of water (P = 0.0006), an effect mainly due to an increased number of gapes and chin rubs (P < 0.001).
Figure 1.
Graphs of the behavioral effects of an intra-oral infusion and CeA or LH stimulation. (A) Graph of the total number (±SEM, standard errors of mean) of ingestive TR behaviors performed during the 5-min stimulation period. (B) Graph of the total number (±SEM) of aversive TR behaviors performed during the 5-min stimulation period. The first bar of each triplet shows the results in the unstimulated condition (neither the CeA nor LH were stimulated). The second bar of each triplet shows the results when the CeA was stimulated. And, the third bar in each triplet is the results in rats that received LH stimulation. Statistical differences from the control group that did not receive an intra-oral infusion (first triplet) and the group that received infusion of water (second triplet) are indicated with an asterisks (*) and a “w,” respectively. These comparisons are only within a brain stimulation condition (comparing the same bar in different triplets). Statistical differences among the 3 groups receiving the same intra-oral infusion (within each triplet of bars) are indicated with an “n” (difference from the no brain stimulation group, i.e., the first bar) and an “a” (difference from the CeA stimulation group, i.e., the second bar).
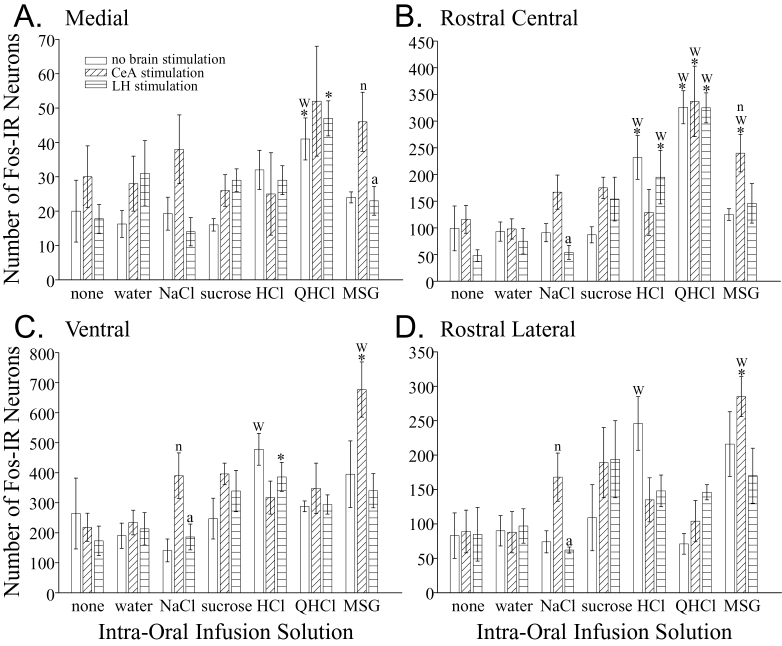
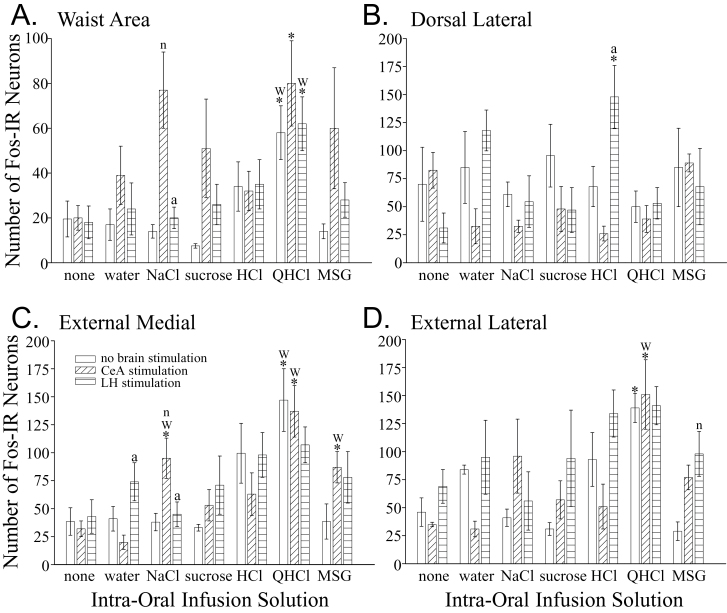
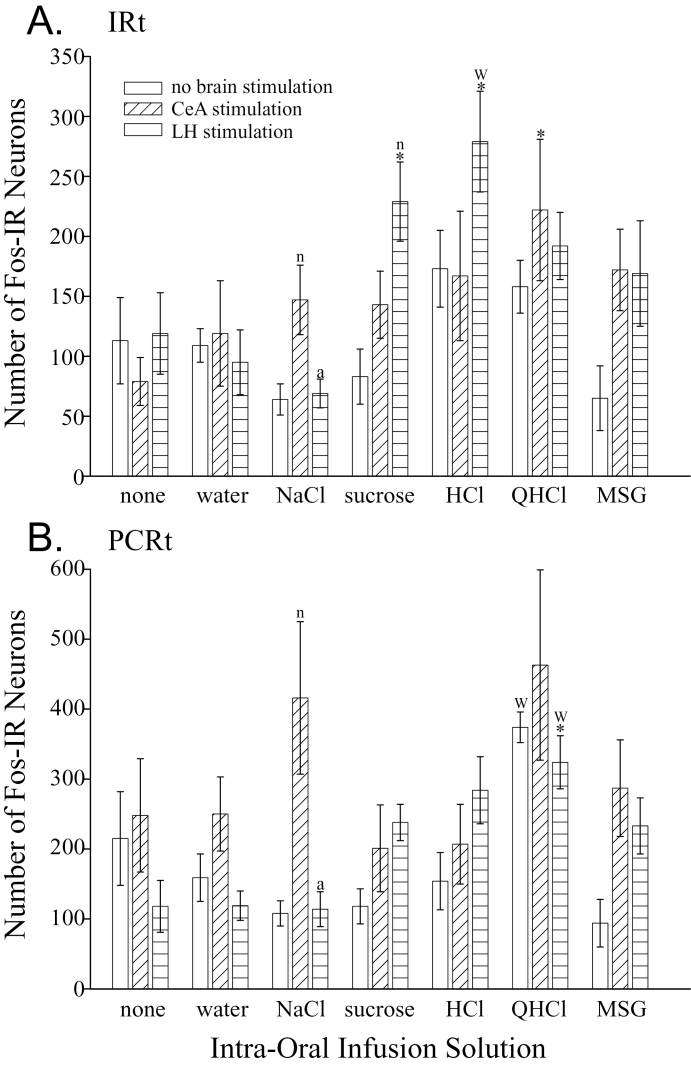
The numbers of Fos-IR neurons in the rNST (F (6,21) = 4.24, P = 0.006; Figures 2 and 3), PBN (F (6,21) = 3.96, P = 0.008; Figures 2 and 4), and Rt (F (6,21) = 4.39, P = 0.005, Figures 2 and 5) were affected differently depending on the solution infused. Generally speaking, only the intra-oral infusion of HCl or QHCl yielded more Fos-IR neurons compared with controls not receiving an infusion. In the rNST, in comparison to no taste stimulation, infusion of HCl increased the total number of Fos-IR neurons (P = 0.004). In this nucleus, HCl also increased the total number of Fos-IR neurons compared with water (P = 0.0014), NaCl (P = 0.0006), and sucrose (P = 0.004). In the medial subdivision, only QHCl increased the number of Fos-IR neurons compared with the uninfused controls and water (Figure 3A). Both HCl and QHCl increased the number of Fos-IR neurons in the RC subdivision over all other tastants and water (P < 0.0025; Figure 3B). Finally, HCl was the only tastant that increased the number of Fos-IR neurons in the RL and V subnuclei compared with water (P < 0.006; Figure 3C,D). Within the PBN, intra-oral infusion of QHCl or HCl increased the total number of Fos-IR neurons in comparison to controls not receiving an intra-oral infusion (P < 0.018). Within the waist area of the PBN, QHCl increased the number of Fos-IR neurons over the controls as well as all other tastants except HCl (P < 0.02; Figure 4A). No other tastant altered the expression of Fos within W over controls not receiving an intra-oral infusion. The increase in Fos-IR neurons caused by QHCl occurred in both the CM and VL subdivisions that make up W. No tastant altered the number of Fos-IR neurons in the dorsal lateral PBN subdivision (Figure 4B); however, QHCl increased the number of Fos-IR neurons over controls in the EM and EL subdivisions (Figures 4C,D). Within the Rt, only intra-oral infusion of QHCl significantly increased the number of Fos-IR neurons overall (P = 0.0057) as well as within the PCRt (P = 0.0005) compared with the intra-oral infusion of water (Figure 5).
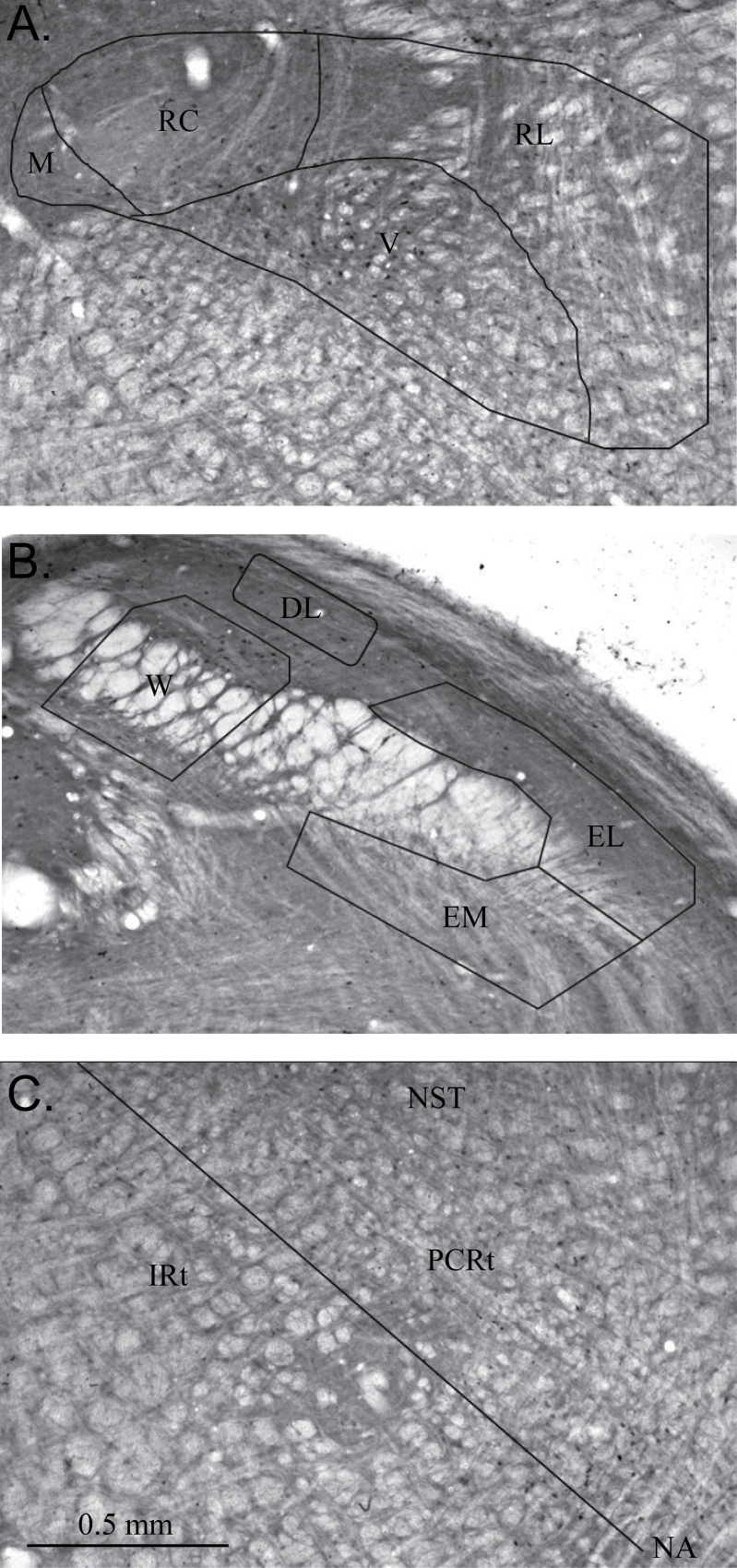
Figure 2.
Images of coronal sections through the rostral nucleus of the solitary tract (A), caudal parabrachial nucleus (B), and medullary reticular formation (C) showing Fos-IR neurons and the subdivisions of each area.
Figure 3.
Graphs of the number of Fos-IR neurons (mean ± SEM) in the medial (A), rostral central (B), ventral (C), and rostral lateral (D) rNST subdivisions elicited by each treatment. The first bar of each triplet shows the results in the unstimulated condition (neither the CeA nor LH were stimulated). The second bar of each triplet shows the results when the CeA was stimulated. And, the third bar in each triplet is the results in rats that received LH stimulation. Statistical differences from the control group that did not receive an intra-oral infusion (first triplet) and the group that received infusion of water (second triplet) are indicated with an asterisks (*) and a “w,” respectively. These comparisons are only within a brain stimulation condition (comparing the same bar in different triplets). Statistical differences among the 3 groups receiving the same intra-oral infusion (within each triplet of bars) are indicated with an “n” (difference from the no brain stimulation group, i.e., the first bar) and an “a” (difference from the CeA stimulation group, i.e., the second bar).
Figure 4.
Graphs of the number of Fos-IR neurons (mean ± SEM) in the waist area of the PBN (A), as well as the dorsal lateral (B), external medial (C), and external lateral (D) PBN subnuclei elicited by each treatment. The first bar of each triplet shows the results in the unstimulated condition (neither the CeA nor LH were stimulated). The second bar of each triplet shows the results when the CeA was stimulated. And, the third bar in each triplet is the results in rats that received LH stimulation. Statistical differences from the control group that did not receive an intra-oral infusion (first triplet) and the group that received infusion of water (second triplet) are indicated with an asterisks (*) and a “w,” respectively. These comparisons are only within a brain stimulation condition (comparing the same bar in different triplets). Statistical differences among the 3 groups receiving the same intra-oral infusion (within each triplet of bars) are indicated with an “n” (difference from the no brain stimulation group, i.e., the first bar) and an “a” (difference from the CeA stimulation group, i.e., the second bar).
Figure 5.
Graphs of the number of Fos-IR neurons (mean ± SEM) in the intermediate (A) and parvocellular (B) reticular formation elicited by each treatment. The first bar of each triplet shows the results in the unstimulated condition (neither the CeA nor LH were stimulated). The second bar of each triplet shows the results when the CeA was stimulated. And, the third bar in each triplet is the results in rats that received LH stimulation. Statistical differences from the control group that did not receive an intra-oral infusion (first triplet) and the group that received infusion of water (second triplet) are indicated with an asterisks (*) and a “w,” respectively. These comparisons are only within a brain stimulation condition (comparing the same bar in different triplets). Statistical differences among the 3 groups receiving the same intra-oral infusion (within each triplet of bars) are indicated with an “n” (difference from the no brain stimulation group, i.e., the first bar) and an “a” (difference from the CeA stimulation group, i.e., the second bar).
Effects of CeA or LH stimulation on TR behaviors and Fos-IR neurons
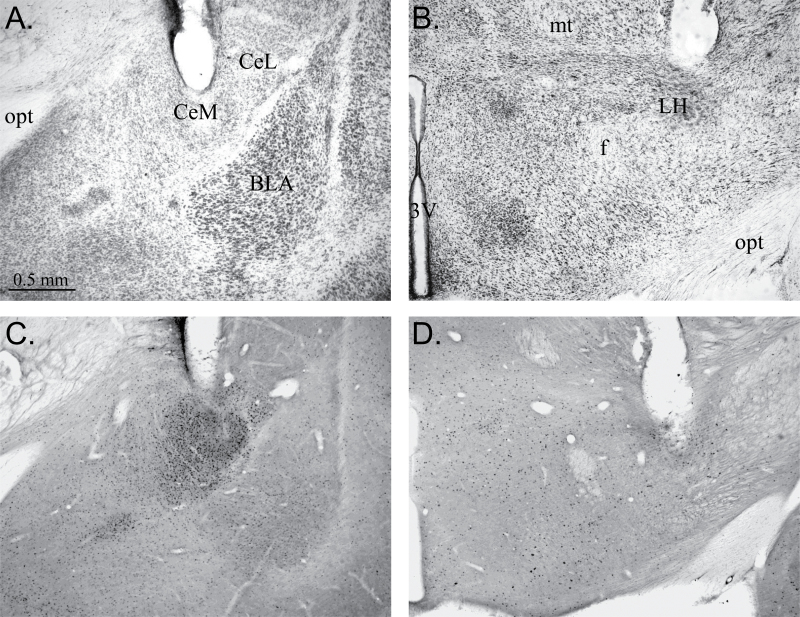
In the rats included in this study, the stimulation site in the amygdala always included the central amygdalar complex and dramatically increased the number of Fos-IR neurons in both the medial and lateral CeA with relatively minor increases in the number of labeled neurons in adjacent structures (Figure 6A,C). The hypothalamic stimulation site was centered in the LH just lateral and dorsal to the fornix and was confirmed by the relatively localized increase in Fos-IR neurons (Figure 6B,D).
Figure 6.
Images of coronal sections through the amygdalar complex and hypothalamus showing electrode placement into the CeA (A and C) and LH (B and D). (A) Nissl-stained section showing the end of the electrode track in the central medial amygdala (CeM). Also labeled are the central lateral amygdala (CeL), basolateral amygdala (BLA), and the optic tract (opt). (B) Nissl-stained section showing the end of the electrode track in the LH. Also labeled are the third ventricle (3V), fornix (f), mammillothalamic tract (mt), and the optic tract (opt). (C) Coronal section through the amygdala showing Fos-IR neurons at the stimulation site primarily within CeM and CeL. (D) Coronal section through the hypothalamus showing Fos-IR neurons near the LH stimulation site.
Both CeA and LH stimulation increased ingestive, but not aversive, TR behaviors in conscious rats that did not receive an intra-oral infusion (Figure 1A; P < 0.01). Although CeA stimulation did not alter the number of ingestive responses to water or the tastants (F (5,18) = 2.46, P = 0.073), it tended to increase the number of aversive responses (Figure 1B). In particular, the aversive TR responses to intra-oral infusion of NaCl and HCl were increased significantly by stimulation of the CeA (P < 0.016). LH stimulation tended to decrease the number of ingestive behaviors performed to the tastants, but none of these changes were significantly different from the groups receiving the tastants without brain stimulation. However, there were significantly different effects of CeA and LH stimulation with the latter causing fewer ingestive TR behaviors during NaCl (P = 0.015) and QHCl (P = 0.006) infusions. The clearest behavioral effect of LH stimulation was a significant reduction in the number of aversive TR behaviors to QHCl compared with controls that received that tastant without brain stimulation (P < 0.002).
On their own, CeA and LH stimulation did not alter the total number of Fos-IR neurons in the rNST (F (2,9) =0.32, P = 0.73), PBN (F (2,9) = 0.76, P = 0.50), or Rt (F (2,9) = 0.33, P = 0.72) compared with unstimulated controls. However, there were a few significant effects of CeA or LH stimulation on the expression of Fos in response to intra-oral infusion of a tastant. In particular, CeA stimulation increased the number of Fos-IR neurons elicited by intra-oral infusion of NaCl in RL and V of the rNST (P < 0.013; Figure 3), W and EM in the PBN (P < 0.015; Figure 4), as well as in the PCRt and IRt (P < 0.0.15; Figure 5). Stimulation of the LH did not alter the number of Fos-IR neurons in the rNST to any taste solution (Figure 3), but did increase Fos-IR neurons in EL of the PBN to MSG (P = 0.01; Figure 4) and the IRt to sucrose (P = 0.008; Figure 5). When comparing the effects of CeA and LH stimulation, the latter did not increase the number of Fos-IR neurons in the rNST, PBN or Rt to NaCl as CeA stimulation did, LH stimulation increased Fos-IR neurons elicited by water in the EM of the PBN compared with CeA stimulation (P = 0.013), and LH stimulation increased the number of Fos-IR neurons in DL of the PBN elicited by HCl (P = 0.015).
The results of a linear regression analysis to detect a relationship between the number of Fos-IR neurons in the gustatory brainstem and TR behaviors revealed a few weak relationships and one good one. The best relationship was between the number of Fos-IR neurons in the ventral subdivision of the rNST and the total TR behaviors performed in the LH stimulated group (R = 0.62, P = 0.0005).
Discussion
The goal of the current study was to determine the effects of stimulation of the CeA or LH in conscious rats on TR behaviors. Stimulation of these forebrain regions elicited ingestive TR behaviors without intra-oral stimulation and altered some TR responses to taste solutions. In addition, the investigation of the neural substrate underlying these behavioral effects was begun by locating and counting neurons activated by forebrain and taste stimulation using Fos immunohistochemistry.
Technical considerations
The main advantage of the Fos immunohistochemistry technique is that the number and location of neurons activated by a particular treatment can be identified in brain tissue. Clearly this technique was useful in the current study because some of the behavioral effects reported were accompanied by changes in Fos-IR (active) neurons in the gustatory brainstem. However, many of the behavioral changes reported were not accompanied by changes in the number and location of Fos-IR neurons. This failure of the pattern of Fos-IR neurons in the gustatory brainstem to reflect behavioral changes may indicate that the total number of active neurons remains the same under the different stimulation parameters used or it may indicate the importance of indirect or multisynaptic pathways to the gustatory brainstem originating in the CeA and LH. On the other hand, the lack of a change in the number of Fos-IR neurons may be the result of limitations of the Fos immunohistochemical technique, that include only a subset of active neurons being labeled (Dragunow and Faull 1989). These issues can be addressed by examining other brain areas in the tissue generated in the current study (like the gustatory thalamus and cortex) and using alternate immunostaining techniques in subsequent studies. In addition, the identification of Fos-IR neurons associated with a particular behavioral change only suggests a possible neural substrate for the behaviors, additional studies more directly investigating the role of the identified areas would be necessary.
Also, it is necessary to consider the stimulation parameters used in the current study when interpreting the results. For example, although the volume and rate of the intra-oral infusion (0.233 mL/min for 5min) were chosen to stimulate gustatory receptors adequately with minimal ingestion, it is likely that some of the palatable stimuli were consumed. Therefore, visceral input could have influenced Fos expression and behavioral responses late in the stimulation period. It should be noted that the volume and/or rate used in the current study were similar to some previous studies (Harrer and Travers 1996; DiNardo and Travers 1997; King et al. 1999; Travers 2002) and considerable less than others (Yamamoto et al. 1994; Tokita et al. 2007). It is possible that some of the differences in the results among studies, like NaCl infusion eliciting Fos in the DL subdivision of the PBN (Yamamoto et al. 1994), are due to the volume or rate of intra-oral infusion and variability in consumption of infused solution. The concentrations of taste stimuli used in the current study were chosen because they have been shown to elicit TR behaviors and Fos expression (Spector et al. 1988; Harrer and Travers 1996; Tokita et al. 2007) but are low enough to allow detection of potential augmentation by brain stimulation. Finally, the brain stimulation parameters were chosen to stimulate the CeA or LH discretely and to elicit TR behaviors (Galvin et al. 2004; Morganti et al. 2007). Because stimulation of exactly the same location on both sides of the brain would have been technically challenging and stimulation of slightly different locations within the CeA and LH would have confounded the interpretation of results, only one side of these nuclei, arbitrarily the right, was stimulated in the current study. Clearly, more robust effects might be elicited by bilateral stimulation or by using different stimulation parameters (DiLorenzo et al. 2003).
TR behaviors and Fos-IR neurons without CeA or LH stimulation
Rats performed TR behaviors when water or a taste solution was infused into the oral cavity. As previously reported (Grill and Norgren 1978a), the specific taste solution infused influenced the number and type of behaviors performed with sweet and sour tastes eliciting more ingestive TR behaviors (mainly mouth movements and lateral tongue protrusions) and bitter eliciting more aversive behaviors (mainly gapes and chin rubs). Also as previously reported (Yamamoto et al. 1994; Harrer and Travers 1996; King et al. 1999), different taste solutions elicited a different pattern of Fos-IR neurons in gustatory brainstem structures, with intra-oral infusion of QHCl having the most robust and consistent effects. The different behavioral responses to bitter reported in the current study may be due to increased activation of neurons in the rNST (mainly RC), PBN (W, EL, and EM), and Rt (mainly PCRt) caused by QHCl compared with other taste solutions.
Effects of CeA or LH stimulation on TR behaviors and Fos-IR neurons
In general, activation of neurons in the CeA or LH via direct electrical stimulation in conscious rats increased ingestive TR behaviors in the absence of intra-oral stimulation without significantly altering aversive behaviors. Therefore, projections originating in these nuclei are capable of activating the brainstem neurons responsible for generating ingestive, but not aversive, TR behaviors without afferent taste input stimulation. Given these behavioral effects, it is surprising that electrical stimulation of the CeA or LH did not consistently alter the number of Fos-IR neurons in the rNST, PBN, or Rt compared with unstimulated controls. This finding possibly reflects a limitation of the Fos immunohistochemical technique or it may mean that the descending projections have effects by modulating ongoing activity, but not elicited new activity, or by activating different, and not necessarily more, neurons in the gustatory brainstem.
CeA stimulation during intra-oral infusion did not alter ingestive TR responses to any taste solution used but tended to increase the aversive responses to all taste solutions except QHCl (significantly so to NaCl and HCl). It is interesting that the increase in ingestive TR behaviors seen during CeA stimulation without intra-oral infusion did not occur when taste solutions were present in the oral cavity, and instead aversive TR behaviors to taste solutions tended to increase. Therefore, activation of gustatory brainstem centers by afferent taste input altered the behavioral effect of the pathway descending from the CeA. The different behavioral effects could be due to alteration of the sensitivity of gustatory neurons to tastants by the descending pathway (Lundy and Norgren 2001, 2004) or due to activation of a different ensemble of neurons within the gustatory brainstem when electrical and intra-oral stimulation occurred concurrently. Unfortunately, there was no clear difference in the number and location of Fos-IR neurons in gustatory brainstem structures that can explain all of the behavioral effects of CeA stimulation. However, the increase in aversive TR responses to NaCl caused by CeA stimulation was accompanied by an increase in Fos-IR neurons in the rNST, PBN and Rt, particularly V, W, and the PCRt. These data imply that projections from the CeA increase the number of neurons in these areas that are activated by NaCl and could modulate both premotor and sensory processing of salt taste in the brainstem. Some of these findings are consistent with the known anatomy of the descending projections from the CeA (particularly the prevalence of terminations in V; Halsell 1998) as well as electrophysiological data that show modulatory effects of CeA stimulation on the processing of NaCl input in the PBN (Lundy and Norgren 2001, 2004).
The most striking behavioral effect of LH stimulation was a decrease in the number of aversive behaviors to QHCl (mainly gapes and chin rubs). This behavioral effect was not accompanied by a change in the number of Fos-IR neurons in the rNST, PBN, or Rt. The lack of effect on Fos-IR neurons does not rule out the possibility that LH stimulation had this behavioral effect by altering neural activity in the gustatory brainstem elicited by QHCl, as suggested by previous electrophysiological studies (Cho et al. 2002, 2003; Lundy and Norgren 2004; Li et al. 2005). The number of active neurons may remain the same when the LH is stimulated during QHCl infusion, but the activity pattern in these neurons, which would not be detected using the Fos technique, may be different. In addition, the results might be due to altered neuron activation in other, possibly forebrain, areas. In other words, the behavioral effect of LH stimulation may be due to multisynaptic pathways originating in the LH, the activation of which may not be detected in brainstem structures using Fos immunohistochemistry. Future studies will investigate the changes in Fos expression in the forebrain under the stimulation conditions used in the current study.
There were a few differences between the effects of CeA and LH stimulation on TR behaviors and the number and location of Fos-IR neurons in the gustatory brainstem that may indicate different roles for these forebrain areas in modulating behavioral responses to taste input. Specifically, stimulation of the CeA elicited more ingestive behaviors without intra-oral infusion, as well as to NaCl and QHCl, than LH stimulation. In addition, CeA stimulation increased aversive responses to NaCl and HCl, whereas LH stimulation dramatically reduced aversive TR responses to QHCl. So, the data suggest that descending pathways originating in the CeA generally act to increase both ingestive and aversive TR responses whereas pathways from the LH tend to reduce TR behaviors. Perhaps, these generally opposing effects of descending pathways from the CeA and LH combine, probably with those of projections from other forebrain areas, to generate the behavioral responses caused by conditioning (Spector et al. 1988). Only in rats receiving intra-oral infusion of NaCl were there differences in the number of Fos-IR neurons elicited by CeA and LH stimulation, with LH stimulation eliciting fewer Fos-IR neurons throughout the rNST, PBN, and Rt. Other than for NaCl, the current data do not reveal changes in Fos-IR neurons in the gustatory brainstem that may account for the behavioral differences caused by CeA and LH stimulation. This lack of association between changes in behavior and Fos-IR neurons was confirmed by the failure of linear regression analyses to detect a strong relationship between the number of Fos-IR neurons in the rNST, PBN, or Rt and the number of TR behaviors performed.
Conclusions
In conclusion, the most striking behavioral effects of electrical stimulation of the CeA or LH in conscious rats found in the current study were the elicitation of ingestive TR behaviors without intra-oral infusion of a taste solution, the increase in aversive TR responses to NaCl and HCl caused by CeA stimulation, and the reduction of aversive TR responses to QHCl during LH stimulation. These results are the first demonstration that the pathways descending from the CeA and LH can alter TR behaviors, and they suggest that these pathways have different roles in modulating the behavioral responses to taste input. Simply put, activation of pathways from the CeA tended to increase aversive responses to tastants whereas activation of pathways from the LH tended to decrease ingestive response to tastants and decreased the aversive TR responses to QHCl. Some of the behavioral effects of intra-oral infusion of taste solutions and brain stimulation were accompanied by changes in the number of Fos-IR neurons in the rNST, PBN, and/or Rt providing a starting point for the identification of the neural substrate underlying them. On the other hand, other behavioral effects of brain stimulation were not accompanied by changes in Fos-IR neurons supporting the idea that descending projections act by modulating responses in neurons already activated by taste input, as suggested by previous electrophysiological studies.
Funding
This work was supported by the National Institutes of Health [R01 DC007854 to M.S.K.]; the National Science Foundation [RUI IOS-1145132 to M.S.K.]; and Stetson University [SURE to C.A.R.].
Acknowledgements
Several other students contributed to this work, including Paige Angelson, Matthew Clayman, Joshua Hargrove, Kristen Krolick, Nicole Nagrani, Elizabeth Pernicone, Trevor Tobin, and Carmen Torres. We are grateful to Dr Camille T. King for reviewing previous versions of this manuscript. Preliminary data from this study have been presented in abstract form (King et al. 2010, 2012; Riley et al. 2011).
References
- Arvanitogiannis A, Flores C, Shizgal P. 1997. Fos-like immunoreactivity in the caudal diencephalon and brainstem following lateral hypothalamic self-stimulation. Behav Brain Res. 88(2):275–279 [DOI] [PubMed] [Google Scholar]
- Beckman ME, Whitehead MC. 1991. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res. 557(1-2):265–279 [DOI] [PubMed] [Google Scholar]
- Berk ML, Finkelstein JA. 1982. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 8(5):511–526 [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. 1993. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 329(2):201–229 [DOI] [PubMed] [Google Scholar]
- Berridge KC. 2000. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 24(2):173–198 [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. 2002. Taste responses of neurons of the hamster solitary nucleus are enhanced by lateral hypothalamic stimulation. J Neurophysiol. 87(4):1981–1992 [DOI] [PubMed] [Google Scholar]
- Cho YK, Li CS, Smith DV. 2003. Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses. 28(2):155–171 [DOI] [PubMed] [Google Scholar]
- Coons EE, Levak M, Miller NE. 1965. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 150(3701):1320–1321 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Hallock RM, Kennedy DP. 2003. Temporal coding of sensation: mimicking taste quality with electrical stimulation of the brain. Behav Neurosci. 117(6):1423–1433 [DOI] [PubMed] [Google Scholar]
- DiNardo LA, Travers JB. 1997. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. J Neurosci. 17(10):3826–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. 1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 29(3):261–265 [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. 1997a. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. I. Masticatory muscle motor systems. Brain Res Brain Res Rev. 25(3):255–275 [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. 1997b. Identification of rat brainstem multisynaptic connections to the oral motor nuclei in the rat using pseudorabies virus. II. Facial muscle motor systems. Brain Res Brain Res Rev. 25(3):276–290 [DOI] [PubMed] [Google Scholar]
- Fay RA, Norgren R. 1997c. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus. III. Lingual muscle motor systems. Brain Res Brain Res Rev. 25(3):291–311 [DOI] [PubMed] [Google Scholar]
- Ferssiwi A, Cardo B, Velley L. 1987. Gustatory preference-aversion thresholds are increased by ibotenic acid lesion of the lateral hypothalamus in the rat. Brain Res. 437(1):142–150 [DOI] [PubMed] [Google Scholar]
- Frank RA, Preshaw RL, Stutz RM, Valenstein ES. 1982. Lateral hypothalamic stimulation: stimulus-bound eating and self-deprivation. Physiol Behav. 29(1):17–21 [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. 1984. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 319(3):229–259 [DOI] [PubMed] [Google Scholar]
- Galvin KE, King CT, King MS. 2004. Stimulation of specific regions of the parabrachial nucleus elicits ingestive oromotor behaviors in conscious rats. Behav Neurosci. 118(1):163–172 [DOI] [PubMed] [Google Scholar]
- Gill CF, Madden JM, Roberts BP, Evans LD, King MS. 1999. A subpopulation of neurons in the rat rostral nucleus of the solitary tract that project to the parabrachial nucleus express glutamate-like immunoreactivity. Brain Res. 821(2):251–262 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Berridge KC. 1985. Taste reactivity as a measure of the neural control of palatability. In: Sprague JM, Epstein, AN, editors. Progress in psychobiology and physiological psychology. Vol.11 New York: Academic Press; p. 2–61 [Google Scholar]
- Grill HJ, Norgren R. 1978a. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 143(2):263–279 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. 1978b. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 143(2):281–297 [DOI] [PubMed] [Google Scholar]
- Grossman SP, Dacey D, Halaris AE, Collier T, Routtenberg A. 1978. Aphagia and adipsia after preferential destruction of nerve cell bodies in hypothalamus. Science. 202(4367):537–539 [DOI] [PubMed] [Google Scholar]
- Halsell CB. 1992. Organization of parabrachial nucleus efferents to the thalamus and amygdala in the golden hamster. J Comp Neurol. 317(1):57–78 [DOI] [PubMed] [Google Scholar]
- Halsell CB. 1998. Differential distribution of amygdaloid input across rostral solitary nucleus subdivisions in rat. Ann N Y Acad Sci. 855:482–485 [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. 1996. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience. 72(1):185–197 [DOI] [PubMed] [Google Scholar]
- Harrer MI, Travers SP. 1996. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 711(1-2):125–137 [DOI] [PubMed] [Google Scholar]
- Harrison TA. 2001. Chorda tympani nerve stimulation evokes Fos expression in regionally limited neuron populations within the gustatory nucleus of the solitary tract. Brain Res. 904(1):54–66 [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Zheng JQ, Seki M. 1999. Direct parabrachial nuclear projections to the pharyngeal motoneurons in the rat: an anterograde and retrograde double-labeling study. Brain Res. 816(2):364–374 [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. 1990. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 293(4):540–580 [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG, Dekker JJ. 1977. The organization of the bulbar fibre connections to the trigeminal, facial and hypoglossal motor nuclei. II. An autoradiographic tracing study in cat. Brain. 100(2):264–286 [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. 1981. Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neurosci Lett. 24(2):111–116 [DOI] [PubMed] [Google Scholar]
- Kang Y, Lundy RF. 2010. Amygdalofugal influence on processing of taste information in the nucleus of the solitary tract of the rat. J Neurophysiol. 104(2):726–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. 1998. Differential projections from gustatory responsive regions of the parabrachial nucleus to the medulla and forebrain. Brain Res. 813(2):283–302 [DOI] [PubMed] [Google Scholar]
- King CT, Travers SP, Rowland NE, Garcea M, Spector AC. 1999. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 19(8):3107–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MS. 2007. Anatomy of the rostral nucleus of the solitary tract. In: Bradley RM, editor. The Role of the Nucleus of the Solitary Tract in Gustatory Processing. Boca Raton, FL: CRC Press; p. 17–38 [Google Scholar]
- King MS, Angelson P, Hargrove J, Clayman M. 2010. Central amygdala stimulation activates neurons in the gustatory brainstem and increases the number of taste reactivity behaviors in conscious rats [abstract 292]. Annual Meeting of the Association for Chemoreception Sciences [Google Scholar]
- King MS, Riley CA, Nagrani NK. 2012. Differential effects of electrical stimulation of the central amygdala and lateral hypothalamus on taste reactivity behaviors in conscious rats [abstract 877.09]. Annual Meeting of the Society for Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. 1993. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 30(1-2):163–172 [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Morton TL, Harris KH, Jhamandas JH. 1992. Expression of c-fos protein in rat brain elicited by electrical stimulation of the pontine parabrachial nucleus. J Neurosci. 12(9):3582–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. 2002. Taste responses of neurons in the hamster solitary nucleus are modulated by the central nucleus of the amygdala. J Neurophysiol. 88(6):2979–2992 [DOI] [PubMed] [Google Scholar]
- Li CS, Cho YK, Smith DV. 2005. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol. 93(3):1183–1196 [DOI] [PubMed] [Google Scholar]
- Lundy RF., Jr 2008. Gustatory hedonic value: potential function for forebrain control of brainstem taste processing. Neurosci Biobehav Rev. 32(8):1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. 2001. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol. 85(2):770–783 [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Norgren R. 2004. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol. 91(3):1143–1157 [DOI] [PubMed] [Google Scholar]
- Matsuo R, Shimizu N, Kusano K. 1984. Lateral hypothalamic modulation of oral sensory afferent activity in nucleus tractus solitarius neurons of rats. J Neurosci. 4(5):1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, Yasui Y, Nomura S, Itoh K, Konishi A, Takada M, Kudo M. 1983. A light and electron microscopic study of premotor neurons for the trigeminal motor nucleus. J Comp Neurol. 215(3):290–298 [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. 1990. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 295(4):624–661 [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. 1989. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 12(11):459–462 [DOI] [PubMed] [Google Scholar]
- Morganti JM, Odegard AK, King MS. 2007. The number and location of Fos-like immunoreactive neurons in the central gustatory system following electrical stimulation of the parabrachial nucleus in conscious rats. Chem Senses. 32(6):543–555 [DOI] [PubMed] [Google Scholar]
- Murzi E, Hernandez L, Baptista T. 1986. Lateral hypothalamic sites eliciting eating affect medullary taste neurons in rats. Physiol Behav. 36(5):829–834 [DOI] [PubMed] [Google Scholar]
- Norgren R. 1970. Gustatory responses in the hypothalamus. Brain Res. 21(1):63–77 [DOI] [PubMed] [Google Scholar]
- Norgren R. 1976. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 166(1):17–30 [DOI] [PubMed] [Google Scholar]
- Norgren R. 1995. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. Orlando: Academic Press; p. 751–771 [Google Scholar]
- Norgren R, Pfaffmann C. 1975. The pontine taste area in the rat. Brain Res. 91(1):99–117 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The Rat Brain in Stereotaxic Coordinates. 4th ed San Diego: Academic Press [Google Scholar]
- Petrov T, Jhamandas JH, Krukoff TL. 1996. Connectivity between brainstem autonomic structures and expression of c-fos following electrical stimulation of the central nucleus of the amygdala in rat. Cell Tissue Res. 283(3):367–374 [DOI] [PubMed] [Google Scholar]
- Riley CA, Tobin TW, King MS. 2011. Electrical stimulation of the central amygdala activates neurons in the gustatory brainstem and alters taste reactivity behaviors in conscious rats [abstract 100]. Annual Meeting of the Association for Chemoreception Sciences [Google Scholar]
- Saggu S, Lundy RF. 2008. Forebrain neurons that project to the gustatory parabrachial nucleus in rat lack glutamic acid decarboxylase. Am J Physiol Regul Integr Comp Physiol. 294(1):R52–R57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. 1980. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 197(2):291–317 [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Costa MS, Ricardo JA. 1992. Afferent connections of the parvocellular reticular formation: a horseradish peroxidase study in the rat. Neuroscience. 50(2):403–425 [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. 1990. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 4(4):477–485 [DOI] [PubMed] [Google Scholar]
- Spector AC, Breslin P, Grill HJ. 1988. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci. 102(6):942–952 [DOI] [PubMed] [Google Scholar]
- Streefland C, Farkas E, Maes FW, Bohus B. 1996. C-fos expression in the brainstem after voluntary ingestion of sucrose in the rat. Neurobiology (Bp). 4(1-2):85–102 [PubMed] [Google Scholar]
- Ter Horst GJ, Copray JC, Liem RS, Van Willigen JD. 1991. Projections from the rostral parvocellular reticular formation to pontine and medullary nuclei in the rat: involvement in autonomic regulation and orofacial motor control. Neuroscience. 40(3):735–758 [DOI] [PubMed] [Google Scholar]
- Tokita K, Karádi Z, Shimura T, Yamamoto T. 2004. Centrifugal inputs modulate taste aversion learning associated parabrachial neuronal activities. J Neurophysiol. 92(1):265–279 [DOI] [PubMed] [Google Scholar]
- Tokita K, Shimura T, Nakamura S, Inoue T, Yamamoto T. 2007. Involvement of forebrain in parabrachial neuronal activation induced by aversively conditioned taste stimuli in the rat. Brain Res. 1141:188–196 [DOI] [PubMed] [Google Scholar]
- Travers JB. 1988. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res. 457(1):1–11 [DOI] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. 1997. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 21(5):631–647 [DOI] [PubMed] [Google Scholar]
- Travers JB, DiNardo LA, Karimnamazi H. 2000. Medullary reticular formation activity during ingestion and rejection in the awake rat. Exp Brain Res. 130(1):78–92 [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. 1983. Afferent projections to the oral motor nuclei in the rat. J Comp Neurol. 220(3):280–298 [DOI] [PubMed] [Google Scholar]
- Travers JB, Rinaman L. 2002. Identification of lingual motor control circuits using two strains of pseudorabies virus. Neuroscience. 115(4):1139–1151 [DOI] [PubMed] [Google Scholar]
- Travers JB, Urbanek K, Grill HJ. 1999. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol. 277(2 Pt 2):R384–R394 [DOI] [PubMed] [Google Scholar]
- Travers SP. 2002. Quinine and citric acid elicit distinctive Fos-like immunoreactivity in the rat nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 282(6):R1798–R1810 [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. 1984. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 224(1):1–24 [DOI] [PubMed] [Google Scholar]
- Villalobos J, Ferssiwi A. 1987. The differential descending projections from the anterior, central and posterior regions of the lateral hypothalamic area: an autoradiographic study. Neurosci Lett. 81(1-2):95–99 [DOI] [PubMed] [Google Scholar]
- Whitehead MC. 1988. Neuronal architecture of the nucleus of the solitary tract in the hamster. J Comp Neurol. 276(4):547–572 [DOI] [PubMed] [Google Scholar]
- Whitehead MC. 1990. Subdivisions and neuron types of the nucleus of the solitary tract that project to the parabrachial nucleus in the hamster. J Comp Neurol. 301(4):554–574 [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Bergula A, Holliday K. 2000. Forebrain projections to the rostral nucleus of the solitary tract in the hamster. J Comp Neurol. 422(3):429–447 [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sakai N, Ozaki N. 1994. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav. 56(6):1197–1202 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Sakai N, Tanimizu T, Wakisaka S. 1993. c-Fos expression in the parabrachial nucleus after ingestion of sodium chloride in the rat. Neuroreport. 4(11):1223–1226 [DOI] [PubMed] [Google Scholar]