Abstract
Objective
In the present study, we compared the effects of treatment with the novel soluble epoxide hydrolase (sEH) inhibitor (c-AUCB) with those of the AT1 receptor antagonist losartan on blood pressure (BP), autoregulation of renal blood flow (RBF) and on glomerular filtration rate (GFR) and the pressure–natriuresis relationship in response to stepwise reduction in renal arterial pressure (RAP) in Cyp1a1-Ren-2 transgenic rats.
Methods
Hypertension was induced in Cyp1a1-Ren-2 rats through dietary administration for 11 days of the natural xenobiotic indole-3-carbinol (I3C) which activates the renin gene. Treatment with c-AUCB and losartan was started 48 h before initiating administration of the diet containing I3C. Rats were prepared for renal functional studies to evaluate in-vivo renal autoregulatory efficiency when RAP was gradually decreased by an aortic clamp.
Results
I3C administration resulted in the development of severe hypertension which was associated with markedly lower basal RBF and GFR and substantially impaired autoregulatory efficiency as well as a suppression of the pressure–natriuresis relationship when compared with noninduced rats. Treatment with c-AUCB significantly decreased BP, improved autoregulatory efficiency of RBF and GFR and the slope of pressure–natriuresis relationship. Treatment with losartan completely prevented the impaired autoregulation and pressure–natriuresis relationship as well as the development of hypertension in I3C-induced rats.
Conclusion
Our present findings indicate that chronic treatment with the sEH inhibitor c-AUCB substantially attenuates the development of malignant hypertension in I3C-induced rats likely via improvement of the renal autoregulatory efficiency and the pressure–natriuresis relationship.
Keywords: AT1 receptor antagonist, cytochrome P-450 metabolites, epoxyeicosatrienoic acids, malignant hypertension, renal autoregulation, renin-angiotensin system, sodium excretion, soluble epoxide hydrolase
Introduction
It is well recognized that the renin-angiotensin system (RAS) physiologically plays a central role in the regulation of blood pressure (BP) and it has also been demonstrated that the inappropriate activation of this powerful system critically contributes to the pathophysiology of hypertension [1,2]. Nevertheless, emerging evidence indicates that the role of the RAS in the regulation of BP and the pathophysiology of hypertension is more complex than so far thought and remains still incompletely understood [1,3,4].
According to the concept originally proposed by Guyton et al. [5] and supported by many other groups [2,6,7], the kidney’s pressure–natriuresis mechanism plays a dominant role in the long-term BP regulation. It has also been shown that activation of the RAS – when not adequately counteracted by other physiological systems – can lead to a marked suppression of the pressure–natriuresis relationship which has been implicated in the pathophysiology of hypertension, especially in the model of angiotensin II (ANG II)-dependent hypertension [1,2,8–13]. However, the role of counteracting compensatory mechanisms that dampen the effects of ANG II-induced vasoconstriction under conditions of excessive activation of the RAS remains poorly understood, especially in models of an ANG II-dependent malignant form of hypertension. Methodological limitations related to the mode of induction of this form of hypertension have so far hampered the approach of how to study this issue.
Recently, an inbred transgenic rat line [strain name: TGR(Cyp1a1-Ren-2)] was generated, which offers a new possibility to precisely control the development of an ANG II-dependent malignant form hypertension [14]. The Cyp1a1-Ren-2 transgenic rat was generated by inserting the mouse Ren-2 renin gene, fused to the cytochrome P-450 (Cyp1a1) promoter, into the genome of the Fischer 344 rat. The Cyp1a1 promoter is not constitutively expressed in the liver; however, after exposure to various natural xenobiotics such as indole-3-carbinol (I3C) the expression of the Cyp1a1 promoter is rapidly enhanced with a marked increase of the expression of the Ren-2 renin gene in the liver with a subsequent increase in ANG II levels [14]. We and others [15–18] have demonstrated that the endogenous activity of the RAS and subsequently the degree of BP up to a level of malignant hypertension and associated end-organ damage can be precisely regulated in a dose-dependent and time-dependent way.
Increasing evidence indicates that cytochrome P450 (CYP)-dependent metabolites of arachidonic acid, such as epoxyeicosatrienoic acids (EETs), play an important role in the regulation of renal tubular ion transport and renal and systemic vascular tone and it has been suggested that EETs serve as a compensatory system with protective effects against enhanced RAS activity [19–23]. In addition, results from our recent study have revealed that substantial increases in the intrarenal level of endogenous EETs significantly attenuated the development of malignant hypertension in Cyp1a1-Ren-2 transgenic rats [17]. In view of these findings, we hypothesized that the antihypertensive actions of the inhibition of soluble epoxide hydrolase (sEH) – an enzyme responsible for the conversion of EETs to biologically inactive dihydroxyeicosatrienoic acids (DHETEs) – in this ANG II-dependent model of malignant hypertension are mediated by an improvement of the impaired renal autoregulatory efficiency of the pressure–natriuresis mechanism.
To test this hypothesis, the first aim of the present study was to characterize the effects of chronic treatment with the novel sEH inhibitor cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyl-oxy]-benzoic acid (c-AUCB) [24] on the autoregulation of renal hemodynamics, the pressure–natriuresis relationship and the development of the associated hypertension-induced cardiac hypertrophy and renal injury after induction of the renin gene in Cyp1a1-Ren-2 transgenic rats.
As recent studies have demonstrated that activation of ANG II type 1 receptors (AT1) contributes to the augmented BP and the impairment of renal function in Cyp1a1-Ren-2 transgenic rats with malignant hypertension [15,25], the second aim of the present study was to compare the effects of chronic AT1 receptor blockade with those of chronic sEH inhibition on BP, renal function and hypertension-associated end-organ damage in this model of ANG II-dependent malignant hypertension.
Methods
The studies were performed in accordance with guidelines and practices established by the Institute for Clinical and Experimental Medicine Animal Care and Use Committee and are in accordance with laws in the Czech Republic. All animals used in the present study were housed in facilities accredited by the Czech Association for Accreditation of Laboratory Animal Care.
Animals and diets
Please see the online Data Supplement, http://links.lww.com/HJH/A98 for details.
Experimental design
Series 1: responses of glomerular filtration rate, renal blood flow and renal sodium excretion to decreases in renal arterial pressure
To induce malignant hypertension, Cyp1a1-Ren-2 rats were fed a diet containing 0.3% I3C for 11 days, which results in the development of malignant hypertension with markedly elevated circulating and tissue ANG II levels as we and others [14–18] have demonstrated. The sEH inhibitor c-AUCB was given at a dose of 26 mg/l in drinking water that was prepared freshly every third day as described previously [17]. Treatment with c-AUCB was started 48 h before feeding the diet with or without I3C. This dose of c-AUCB and the same treatment protocol for c-AUCB were used in our recent study in which we found that it significantly attenuated the development of malignant hypertension in Cyp1a1-Ren-2 transgenic rats and substantially increased tissue concentrations of EETs [17]. Animals who were exposed to AT1 receptor blockade received losartan in their drinking water (100 mg/l; Lozap, Zentiva, Prague, Czech Republic); previous studies have demonstrated that this dose of losartan prevents the development of hypertension in this model [25,26]. On the day of the experiment (day 11 after induction of the renin gene) rats were prepared for acute renal functional studies and for details see the online Data Supplement, http://links.lww.com/HJH/A98.
The following experimental groups of Cyp1a1-Ren-2 transgenic rats were examined:
Group 1: noninduced + untreated + control protocol (n =6).
Group 2: noninduced + untreated + experimental protocol (n =13).
Group 3: noninduced + c-AUCB + control protocol (n =7).
Group 4: noninduced + c-AUCB + experimental protocol (n =11).
Group 5: noninduced + losartan + control protocol (n =6).
Group 6: noninduced + losartan + experimental protocol (n =10).
Group 7: induced + untreated + control protocol (n =7).
Group 8: induced + untreated + experimental protocol (n =13).
Group 9: induced + c-AUCB + control protocol (n =7).
Group 10: induced + c-AUCB + experimental protocol (n =13).
Group 11: induced + losartan + control protocol (n =6).
Group 12: induced + losartan + experimental protocol (n =11).
Series 2: effects of chronic soluble epoxide hydrolase inhibition and AT1 receptor blockade on cardiac hypertrophy, proteinuria, renal glomerular damage and kidney tubulointerstitial injury as well as concentrations of epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids in renal tissue
Animals were divided into the following experimental groups and were exposed to the same chronic experimental protocol as in series 1:
Group 1: noninduced + untreated (n =7).
Group 2: noninduced + c-AUCB (n =6).
Group 3: noninduced + losartan (n =6).
Group 4: induced + untreated (n =8).
Group 5: induced + c-AUCB (n =8).
Group 6: induced + losartan (n =8).
Urine collections were performed prior (72 h before) and 10 days after initiating the administration of the diet with or without I3C to assess daily proteinuria as described previously [17]. On day 11, rats were sacrificed by decapitation and always one kidney was used for evaluation of renal glomerular damage and renal cortical tubulointerstitial injury using the following method, which is now routinely employed in our laboratory [27,28] and for details see the online Data Supplement, http://links.lww.com/HJH/A98.
The level of the arachidonic acid metabolites, EETs and DHETEs, were measured in the kidney cortex. Samples were extracted, separated by reverse-phase, high-performance liquid chromatography, and analyzed by negative-mode electrospray ionization and tandem mass spectroscopy as described previously [17].
Statistical analyses
All values are expressed as means ± SEM. With Graph-Pad Prism software (Graph Pad Software, San Diego, California, USA), statistical analysis was performed using Student’s t-test, Wilcoxon’s signed-rank test for unpaired data, or one-way analysis of variance (ANOVA) when appropriate. ANOVA for repeated measurements, followed by Student–Newman–Keuls test was performed for the analysis within groups [e.g. for the analysis of autoregulation capacity of renal blood flow (RBF) and glomerular filtration rate (GFR)]. Values exceeding the 95% probability limits (P <0.05) were considered statistically significant.
Results
Series 1: responses of glomerular filtration rate, renal blood flow and renal sodium excretion to decreases in renal arterial pressure
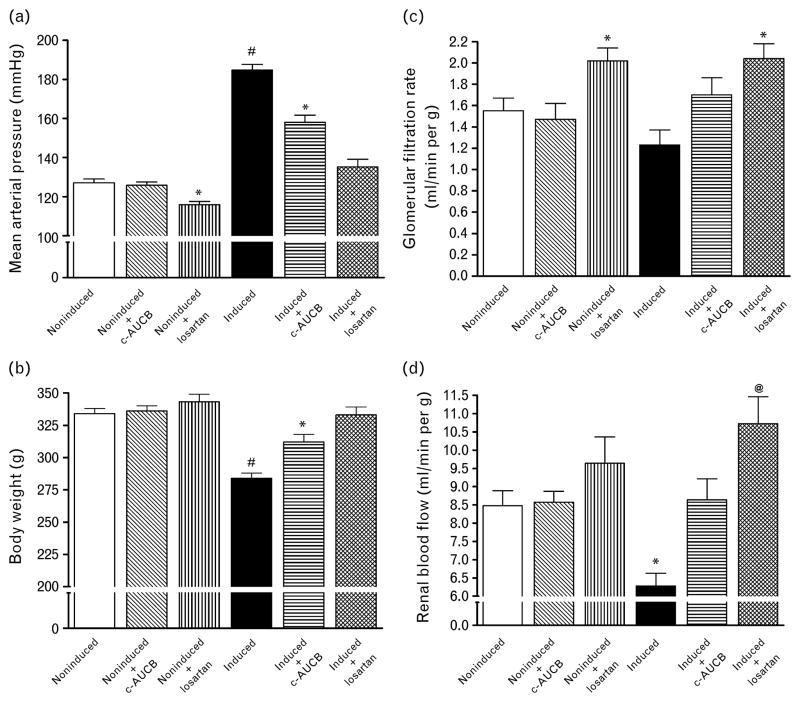
Basal values [average values obtained from the first clearance periods performed at a physiological level of renal arterial pressure (RAP) were pooled from groups exposed to control and experimental protocols] of mean arterial pressure (MAP), body weight, RBF and GFR are summarized in Fig. 1. Treatment with the sEH inhibitor c-AUCB did not alter MAP, body weight, RBF and GFR in noninduced rats when compared with untreated non-induced normotensive Cyp1a1-Ren-2 rats. In contrast, treatment with the AT1 receptor antagonist losartan significantly decreased MAP and GFR, but did not change body weight and RBF in noninduced normotensive Cyp1a1-Ren-2 rats. Administration of the diet containing 0.3% I3C resulted in severe hypertension (Fig. 1a), which was associated with a profound loss of body weight (Fig. 1b) and a marked reduction in RBF (Fig. 1c) as compared with noninduced animals. In addition, untreated I3C-induced Cyp1a1-Ren-2 rats demonstrated serious lethargy, hunched posture and piloerection, which are manifestations of a malignant form of hypertension in rats [15,18,34]. Treatment with c-AUCB attenuated the development of hypertension and the loss in body weight and prevented the decreases in RBF in I3C-induced Cyp1a1-Ren-2 transgenic rats as compared with untreated I3C-induced Cyp1a1-Ren-2 rats. Treatment with losartan abolished the increases in MAP and decreases in body weight and not only prevented the decreases in RBF in I3C-induced Cyp1a1-Ren-2 transgenic rats, but even significantly increased it as compared with noninduced normotensive Cyp1a1-Ren-2 rats (Fig. 1d).
Fig. 1.
Basal values of mean arterial pressure (a), body weight (b), renal blood flow (d) and glomerular filtration rate (c) in Cyp1a1-Ren-2 transgenic rats fed either a normal rat chow (noninduced) or a diet containing 0.3% indole-3-carbinol (I3C-induced). c-AUCB indicates treatment with the sEH inhibitor c-AUCB, losartan indicates treatment with AT1 receptor antagonist. *P < 0.05 vs. unmarked values. @P <0.05 vs. untreated noninduced rats.
#P <0.05 vs. all values.
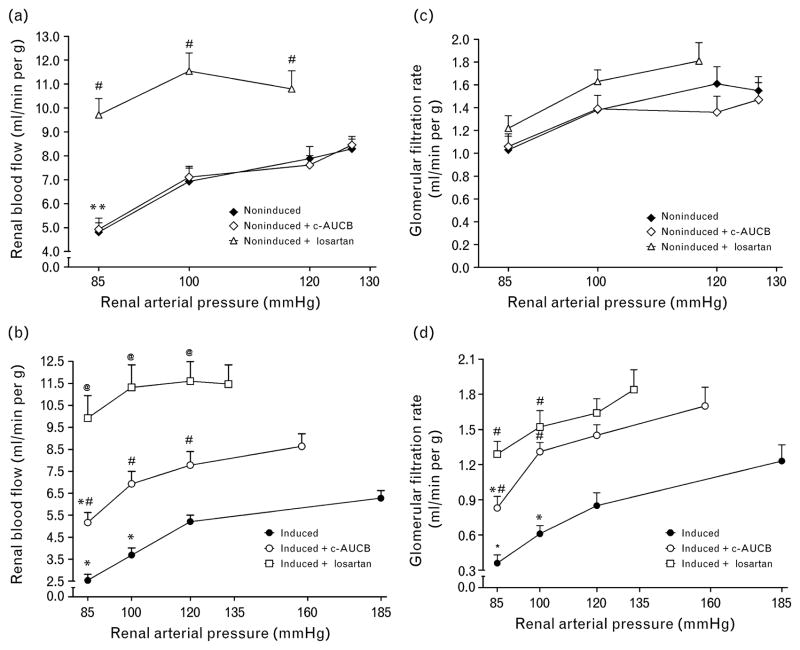
As shown in Fig. 2a and c, noninduced rats maintained autoregulatory efficiency of RBF and GFR in response to reductions in RAP without any effect of treatment with c-AUCB. Treatment with losartan did not alter autoregulatory efficiency of GFR in noninduced rats; however, it increased not only basal values of RBF, but as well improved the autoregulation of RBF at the lowest level of RAP (P <0.05) (Fig. 2a). As shown in Fig. 2b at the basal level of RAP RBF was significantly lower in untreated I3C-induced Cyp1a1-Ren-2 rats as compared with noninduced animals (6.28 ± 0.35 vs. 8.29 ± 0.41 ml/min per g, P <0.05) and reduction of RAP resulted in significant decreases in RBF already at a level of RAP of 100 mmHg. In contrast, the treatment with c-AUCB in I3C-induced Cyp1a1-Ren-2 transgenic rats not only prevented the decrease in RBF at the basal level of RAP, but also markedly improved the autoregulary efficiency of RBF to levels observed in noninduced animals. Treatment with losar-tan further increased RBF at the basal level of RAP (11.47 ± 0.88 ml/min per g) and the autoregulatory efficiency was maintained even at the lowest level of RAP (Fig. 2b). Similarly, as shown in Figure 2d, untreated I3C-induced Cyp1a1-Ren-2 transgenic rats responded to reductions in RAP by significant decreases in GFR and the treatment either with c-AUCB or with losartan markedly improved the autoregulatory efficiency of GFR in these animals.
Fig. 2.
Relationship between renal arterial pressure and renal blood flow (a and b) and glomerular filtration rate (c and d) in Cyp1a1-Ren-2 transgenic rats fed either a normal rat chow (noninduced) or a diet containing 0.3% indole-3-carbinol (I3C-induced). c-AUCB indicates treatment with the sEH inhibitor c-AUCB, losartan indicates treatment with AT1 receptor antagonist. *P < 0.05 vs. basal values. #P <0.05 vs. corresponding values from untreated rats. @P <0.05 vs. corresponding values from c-AUCB-treated rats.
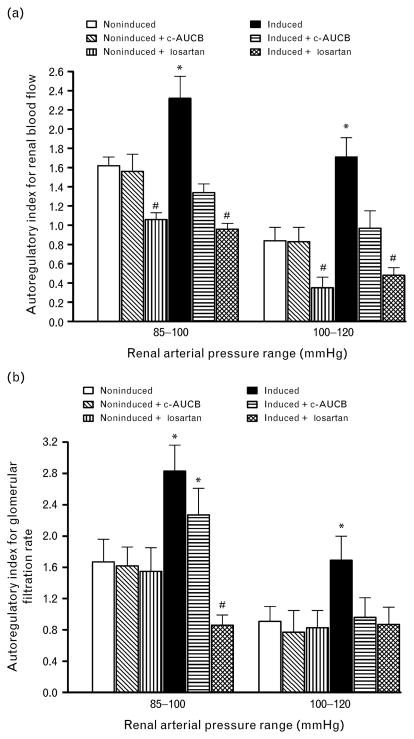
Figure 3 shows the autoregulatory efficiency of RBF and GFR using autoregulatory indices calculated by the method of Semple and de Wardener [29]. Results of autoregulatory indices show that untreated I3C-induced Cyp1a1-Ren-2 transgenic rats exhibited substantial impairment of the autoregulatory efficiency of RBF as well as GFR already by the reduction of RAP from 120 to 100 mmHg. This was even more pronounced by the reduction of RAP to the final level of 85 mmHg. The treatment with c-AUCB did not alter autoregulatory index in noninduced animals but restored autoregulatory index of RBF in I3C-induced Cyp1a1-Ren-2 transgenic rats and normalized in these rats autoregulatory index of GFR for the reduction of RAP from 120 to 100 mmHg. However, treatment with c-AUCB did not improve the impaired autoregulatory index of GFR at the reduction of RAP from 100 to 85 mmHg in I3C-induced Cyp1a1-Ren-2 transgenic rats. In contrast, treatment with losartan not only restored autoregulatory indices of RBF and GFR in I3C-induced Cyp1a1-Ren-2 transgenic rats, but even improved autoregulatory index of RBF in noninduced animals as compared with untreated noninduced Cyp1a1-Ren-2 transgenic rats.
Fig. 3.
Autoregulatory indexes calculated by the method of Semple and de Wardener for RBF (a) and GFR (b) responses to reductions of renal arterial pressure from 120 to 100 and from 100 to 85 mmHg. *P <0.05 vs. unmarked values. #P <0.05 vs. all values.
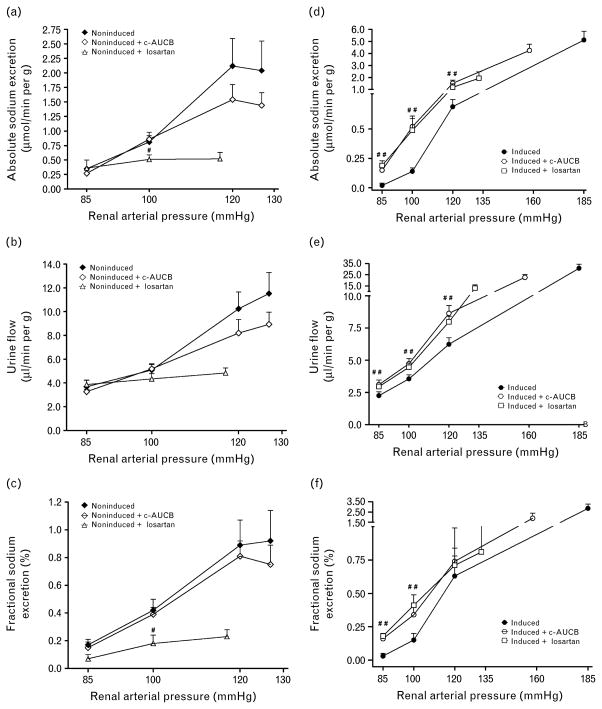
As shown in Fig. 4a, b and c, absolute sodium excretion, urine flow, and fractional sodium excretion at the spontaneous levels of RAP were not significantly different between untreated and c-AUCB-treated noninduced Cyp1a1-Ren-2 transgenic rats and the reduction in RAP elicited similar responses in both groups of animals. In contrast, treatment with losartan resulted in substantial decreases in absolute sodium excretion, urine flow, and fractional sodium excretion at the spontaneous levels of RAP as compared with untreated noninduced Cyp1a1-Ren-2 transgenic rats.
Fig. 4.
Relationship between renal arterial pressure and absolute (a and d) and fractional sodium excretion (c and f) and urine flow (b and e) in Cyp1a1-Ren-2 transgenic rats fed either a normal rat chow (noninduced) or a diet containing 0.3% indole-3-carbinol (I3C-induced). c-AUCB indicates treatment with the sEH inhibitor c-AUCB, losartan indicates treatment with AT1 receptor antagonist. #P <0.05 vs. corresponding values from untreated rats.
As shown in Fig. 4d–f, absolute sodium excretion, urine flow, and fractional sodium excretion at basal level of RAP were not significantly different between untreated and c-AUCB and losartan-treated I3C-induced Cyp1a1-Ren-2 transgenic rats. The reduction in RAP resulted in significantly greater decreases in absolute, fractional sodium excretion and in urine flow in untreated I3C-induced Cyp1a1-Ren-2 transgenic rats than in c-AUCB-treated and losartan-treated I3C-induced Cyp1a1-Ren-2 transgenic rats.
Untreated and c-AUCB-treated or losartan-treated non-induced as well as I3C-induced Cyp1a1-Ren-2 transgenic rats exposed to the control protocol did not show any significant changes in renal hemodynamics or renal sodium excretion throughout the experiment and therefore data are not presented.
Series 2: effects of chronic soluble epoxide hydrolase inhibition and AT1 receptor blockade on cardiac hypertrophy, proteinuria, renal glomerular and tubulointerstitial injury as well as concentrations of epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids in renal tissue
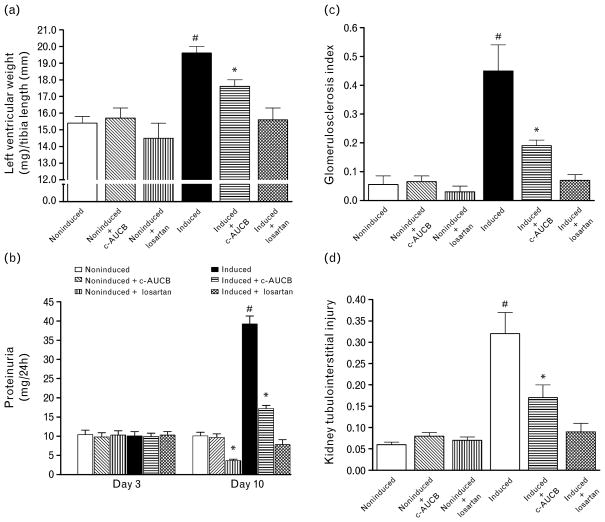
Our results show that untreated I3C-induced Cyp1a1-Ren-2 transgenic rats developed distinct cardiac hypertrophy, expressed as the ratio of left ventricle weight (LVW) to tibial length, pronounced proteinuria and marked degree of renal glomerular and tubulointerstitial injury as compared with noninduced normotensive Cyp1a1-Ren-2 transgenic rats. Treatment with c-AUCB significantly attenuated the development of cardiac hypertrophy, proteinuria and the rise in glomerulosclerosis index (GSI) and tubulointerstitial injury in I3C-induced Cyp1a1-Ren-2 transgenic rats. Treatment with losartan completely prevented the development of cardiac hypertrophy, increases in proteinuria and abolished the rise in GSI and tubulointerstitial injury in I3C-induced Cyp1a1-Ren-2 transgenic rats. Noninduced normotensive Cyp1a1-Ren-2 transgenic rats exhibited minor proteinuria and minimal degree of renal glomerular and tubulointerstitial injury and either treatment with c-AUCB or losartan did not markedly alter these parameters (Fig. 5).
Fig. 5.
Heart left ventricle weight to tibia length ratio (a), proteinuria on basal conditions (day 3) and at the end of experiment (day 10) (b), glomerulosclerosis index (c) and kidney tubulointerstitial injury (d) in Cyp1a1-Ren-2 transgenic rats fed either a normal rat chow (noninduced) or a diet containing 0.3% indole-3-carbinol (I3C-induced). c-AUCB indicates treatment with the sEH inhibitor c-AUCB, losartan indicates treatment with AT1 receptor antagonist. *P <0.05 vs. unmarked values. #P <0.05 vs. all values.
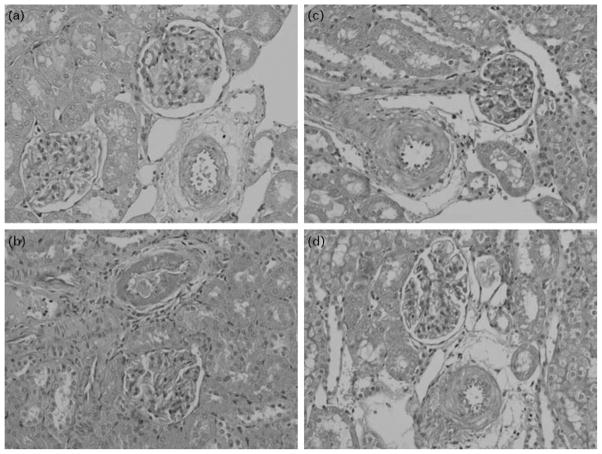
Figure 6 shows representative histological kidney slides from noninduced Cyp1a1-Ren-2 transgenic rats (a), untreated I3C-induced Cyp1a1-Ren-2 transgenic rats (b), and c-AUCB-treated and losartan-treated I3C-induced Cyp1a1-Ren-2 transgenic rats (c and d).
Fig. 6.
Representative histological slides of kidney from noninduced Cyp1a1-Ren-2 transgenic rats (a), untreated I3C-induced Cyp1a1-Ren-2 transgenic rats (b), c-AUCB- and losartan-treated I3C-induced Cyp1a1-Ren-2 transgenic rats (c and d).
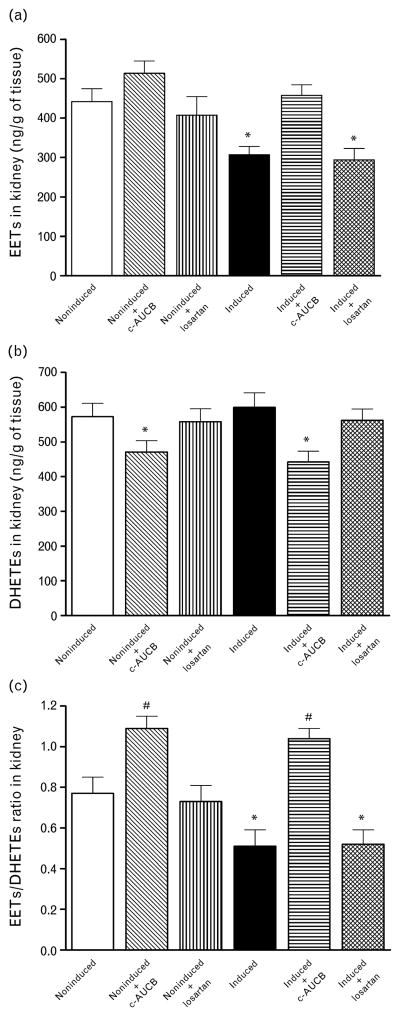
Our results show that untreated and losartan-treated I3C-induced Cyp1a1-Ren-2 transgenic rats exhibited a significant decrease in the concentration of EETs in renal cortex. Treatment with c-AUCB prevented the decrease in renal levels of EETs in I3C-induced Cyp1a1-Ren-2 transgenic rats. In addition, the treatment with c-AUCB in noninduced as well as in I3C-induced Cyp1a1-Ren-2 transgenic rats resulted in a significant decrease of the concentration of DHETEs in renal tissue. When the results are expressed as EETs/DHETEs ratio, which is the best way to describe the intrarenal availability of biologically active epoxygenase metabolites, it is shown that this ratio was markedly lower in untreated I3C-induced rats than in untreated noninduced Cyp1a1-Ren-2 transgenic rats. This ratio was not significantly altered by treatment with losartan, but the treatment with c-AUCB significantly increased this ratio in noninduced as well as in I3C-induced Cyp1a1-Ren-2 transgenic rats (Fig. 7).
Fig. 7.
Epoxyeicosatrienoic acids (EETs) (a) and dihydroxyeicosatrienoic acids (DHETEs) (b) and ratio of EETs/DHETEs in kidney cortex (c) at the end of experimental period in Cyp1a1-Ren-2 transgenic rats fed either a normal rat chow (noninduced) or a diet containing 0.3% indole-3-carbinol (I3C-induced). c-AUCB indicates treatment with the sEH inhibitor c-AUCB, losartan indicates treatment with AT1 receptor antagonist. *P <0.05 vs. unmarked values. #P <0.05 vs. all values.
Discussion
The first major finding of this study is that chronic treatment with the sEH inhibitor c-AUCB attenuated the development of malignant hypertension and prevented decreases in RBF in I3C-induced Cyp1a1-Ren-2 transgenic rats. In addition, autoregulatory indices calculated by the method of Semple and de Wardener [29] for the reductions of RAP from 120 to 100 mmHg and from 100 to 85 mmHg clearly show an impairment of the autoregulatory efficiency of RBF and GFR in untreated I3C-induced Cyp1a1-Ren-2 transgenic rats as compared with noninduced normotensive animals.
As a second major finding of the present study we observed that chronic inhibition of sEH activity by c-AUCB not only prevented the decreases in RBF but also allowed markedly better maintenance of autoregulation of RBF and GFR at any given level of RAP in the I3C-induced rats than in untreated I3C-induced Cyp1a1-Ren-2 transgenic rats. Moreover, we found that chronic treatment with c-AUCB significantly improved the suppressed slope of the pressure–natriuresis relationship in I3C-induced rats as compared with untreated I3C-induced Cyp1a1-Ren-2 transgenic rats. Taken together, our present findings support our original hypothesis that the antihypertensive actions of chronic inhibition of sEH activity by c-AUCB in this model of ANG II-dependent malignant form of hypertension is mediated by the improvement of the impaired autoregulatory efficiency of RBF and GFR and by attenuation of the impairment, that is the shift to the right, of the slope of the pressure–natriuresis relationship.
In this context, it should be pointed out that an important issue of our findings is in fact related to the question as to what are the underlying mechanism(s) responsible for the improvement of the impaired autoregulatory efficiency of RBF and GFR and the suppressed pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats treated chronically by the sEH inhibitor.
In this regard it is of critical importance to recognize that studies performed during the past 25 years have revealed that EETs are not only involved in regulating vascular tone and antagonizing the vasoconstrictor actions of ANG II, but they also directly influence tubular transport of sodium [19–23]. Our previous [17] and present results show that untreated I3C-induced Cyp1a1-Ren-2 transgenic rats exhibit a reduced intrarenal availability of biologically active epoxygenase metabolites – expressed as the ratio of EETs/DHETEs – when compared with noninduced rats. This ratio was significantly increased by the treatment with c-AUCB. It is highly conceivable that the increase in biological availability of EETs is responsible for the improvement of basal RBF, of the auto-regulatory efficiency of RBF and GFR in response to reductions in RAP and of the slope of the pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats. This notion is also supported by our recent observation that the treatment with c-AUCB markedly increased EETs/DHETEs without altering the elevated circulating and renal RAS activity in I3C-induced Cyp1a1-Ren-2 transgenic rats [17].
This suggests that changes in renal function and BP in response to c-AUCB should be primarily ascribed to changes in the bioavailability of EETs rather than alterations of RAS activity. There are two major underlying mechanisms how increased EETs bioavailability could improve the impaired autoregulatory efficiency of renal hemodynamics and the suppressed pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats.
First, it has been shown that at the vascular level EETs elicit vasodilatation through activation of the large-conductance calcium-activated potassium channel which is thought to attenuate calcium entry into vascular smooth muscle cells via voltage-sensitive channels [19,21]. Moreover, EETs have been implicated as the endothelium-derived hyperpolarizing factor mediating nitric oxide and prostaglandin-independent vasodilatation [21,23,30] and it has been also demonstrated that EETs oppose the vasoconstrictor actions of ANG II [22,31]. On the basis of these data we suggest that the first potential mechanism is likely related to vasodilatory effects of increased EETs following c-AUCB treatment and normalization of RBF in I3C-induced Cyp1a1-Ren-2 transgenic rats.
Second, previous studies have shown that at the kidney level EETs inhibit sodium reabsorption in the proximal tubule by blocking the sodium-hydrogen exchanger [32] and also decrease sodium reabsorption in the cortical collecting duct by blocking the epithelial sodium channels (ENaCs) [33]. It has been demonstrated that ENaC plays an important role in mediating the changes in sodium excretion in response to changes in RAP [6,34]. Although we are not able to determine the specific transporter involved in c-AUCB-induced natriureis, an improvement in tubular function was likely responsible for the improvement of the impaired pressure–natriruesis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats.
Taken together, even if the present study does not allow determination of the relative contribution of vasodilatory and tubular effects of c-AUCB-induced increased EET bioavailability on kidney function, it is highly conceivable that combination of both actions are underlying mechanism(s) responsible for the improvement of the impaired autoregulatory efficiency of RBF and GFR and the suppressed pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats treated chronically by the sEH inhibitor. This notion is strongly supported by our recent findings in two-kidney, one-clip (2K1C) Goldblatt hypertensive rats – another model of ANG II-dependent hypertension, in which we found that c-AUCB displays antihypertensive properties that are mediated by an improvement of RBF and sodium excretion [35].
The third major finding of the present study is that chronic treatment with the AT1 receptor antagonist losartan completely prevented the development of hypertension and the impairment of RBF and the autoregulatory efficiency of RBF and GFR and of the pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 rats. In addition, we have found that chronic treatment with losartan did not alter the concentrations of EETs and DHETEs in I3C-induced rats when compared with untreated I3C-induced Cyp1a1-Ren-2 transgenic rats, suggesting that the antihypertensive actions of losartan were not attributed to an increase in biological availability of intrarenal EETs. Taken together, these findings indicate that AT1 receptor-mediated alterations in the autoregulatory efficiency of renal hemodynamics and tubular sodium reabsorption are responsible for the impairment of the pressure–natriuresis relationship in I3C-induced Cyp1a1-Ren-2 transgenic rats. This notion is in good agreement with our recent findings showing that intrarenal augmentation of ANG II and ANG II-mediated derangements of the autoregulation of renal hemodynamics and the pressure–natriuresis relationship precedes the development of the malignant form of hypertension in Cyp1a1-Ren-2 transgenic rats [25].
The fourth major finding is that chronic treatment with c-AUCB not only significantly attenuated the development of hypertension, but also exhibited considerable cardioprotective and nephroprotective effects, that is, reduction of cardiac hypertrophy, of proteinuria and of renal glomerular and tubulointerstitial injury in I3C-induced Cyp1a1-Ren-2 transgenic rats. These observations are in agreement with the results of our recent studies performed in transgenic rats with constitutive expression of the mouse Ren-2 [TGR; strain name TGR(mREn2)] [27] – another commonly used transgenic model of ANG II-dependent hypertension. They have shown that altered production and/or action of CYP-derived eicosanoids contribute to the development of hypertension and of the associated end-organ damage in the monogenetic model of ANG II-dependent hypertension [27,28]. However, our present findings are of particular interest, since to our knowledge this is the first study clearly showing that chronic pharmacological inhibition of sEH, which is able to significantly enhance the concentrations of biologically active epoxygenase metabolites, exerts long-term antihypertensive action and renal and cardiovascular protection in this model of ANG II-dependent malignant hypertension. With regard to the underlying mechanism(s) responsible for the beneficial effects of c-AUCB treatment on the development of end-organ damage two potential mechanisms can be responsible. The first one can be ascribed to BP-lowering effects of chronic inhibition of sEH and previous studies have demonstrated that antihypertensive actions of sEH are always accompanied by marked reductions in end-organ damage [23,36,37] and it is in accordance with our recently published study showing that cardio and renoprotection is predominantly BP-dependent [38]. The second one is based on a recent study in the diabetic Goto-Kaizaki rats infused with ANG II and exposed to high-salt diet, in which Olearczyk et al. [39] have demonstrated that sEH inhibition could provide renal end-organ protection without lowering BP and it was associated with decreased renal macrophage infiltration. However, our current study does not allow determination of the specific mechanism(s) responsible for end-organ protection elicited by sEH inhibition and future studies are needed to resolve this issue.
Of special interest is also our observation that chronic treatment with losartan completely prevented the development of hypertension and of hypertension-induced end-organ in I3C-induced Cyp1a1-Ren-2 transgenic rats. These data further strongly support our notion that inappropriate activation of AT1 receptors by enhanced circulating and renal ANG II concentrations plays a critical role in the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats with malignant hypertension.
In summary, our present findings indicate, first, that chronic treatment with the sEH inhibitor c-AUCB substantially attenuates the development of malignant hypertension and hypertension-induced end-organ damage in I3C-induced Cyp1a1-Ren-2 transgenic rats.
Second, treatment with the sEH inhibitor markedly improves the autoregulatory efficiency of RBF and GFR and the blunted pressure–natriuresis relationship in these transgenic rats with inducible hypertension. Our data also indicate that the improvement of the right-shifted slope of the pressure–natriuresis relationship is likely to be the essential mechanism responsible for the antihypertensive effects of chronic sEH inhibition by c-AUCB in this ANG II-dependent malignant form of hypertension.
In conclusion, the information derived from our present study should be considered in attempts to develop new therapeutic approaches or tools for the treatment of hypertension.
Acknowledgments
This study was supported by Marie Curie Fellowship from the European Commission – Program – PEOPLE (IRG 247847) and postdoctoral fellowship from the Czech Science Foundation (GAČR; 303/10/P170), received by A.S. Z.H. and A.S. are partly supported from the Center for Cardiovascular Research (1M6798582302). L.C. is supported by the institutional financial support of the Institute for Clinical and Experimental Medicine (MZO 00023001) and by grant no. NS/10499-3 and by grant no. NS/10500-3 awarded by the Internal Grant Agency of the Ministry of Health. H.J.K. was supported by grants from the German Research Foundation (DFG), Bonn (Kra 436/14-2 and 436 TSE 113/57/0-1) and the Deutsche Akademische Austauschdienst (DAAD), Bonn-Prague University partnership. L.K. is supported by grant no. NS/9699-4 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic. L.Č. was also supported by grant nos. NS/9703-4 awarded by the Internal Grant Agency of the Ministry of Health of the Czech Republic. This study also received financial support from the European Commission by the Operational Program Prague – Competitiveness; project ‘CEVKOON’ (#CZ.2.16/3.1.00/22126).
Abbreviations
- ANG II
angiotensin II
- AT1
receptors for angiotensin II* type 1
- BP
blood pressure
- c-AUCB
cis-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyl-oxy]-benzoic acid
- CYP
cytochrome P-450
- DHETEs
dihydroxyeicosatrienoic acids
- EETs
epoxyeicosatrienoic acids
- ENaC
epithelial sodium channel
- GFR
glomerular filtration rate
- GSI
glomerulosclerosis index
- I3C
indole-3-carbinol
- LVW
left heart ventricle weight
- MAP
mean arterial pressure
- RAP
renal arterial pressure
- RAS
renin–angiotensin system
- RBF
renal blood flow
- sEH
soluble epoxide hydrolase
References
- 1.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, Brands MW. The renin–angiotensin–aldosterone system: renal mechanisms and circulatory homeostasis. In: Seldin DW, Giebisch G, editors. The kidney: physiology and pathophysiology. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 1009–1046. [Google Scholar]
- 3.Ferreira AJ, Santos RAS, Bradford CN, Mecca AP, Sumners C, Katovich MJ, Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55 :207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010;90:607–673. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 5.Guyton AC, Hall JE, Coleman TG, Manning RD., Jr . The dominant role of the kidneys in the long term regulation of arterial pressure in normal and hypertensive states. In: Laragh JH, Brenner BM, editors. Hypertension: pathophysiology, diagnosis and management. New York, NY: Raven Press, Publishers; 1990. pp. 1029–1052. [Google Scholar]
- 6.Navar LG, Majid DSW. Interactions between arterial pressure and sodium excretion. Curr Opin Nephrol Hypertens. 1996;5:64–71. doi: 10.1097/00041552-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. J Am Med Assoc. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 8.Ploth DW. Angiotensin-dependent renal mechanism in two-kidney, one-clip renal vascular hypertension. Am J Physiol. 1983;245:F131–F141. doi: 10.1152/ajprenal.1983.245.2.F131. [DOI] [PubMed] [Google Scholar]
- 9.Van der Mark J, Kline RL. Altered pressure natriuresis in chronic angiotensin II hypertension in rats. Am J Physiol. 1994;266:F739–F748. doi: 10.1152/ajpregu.1994.266.3.R739. [DOI] [PubMed] [Google Scholar]
- 10.Hall JE, Mizelle HL, Brands MV, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 11.Mattson DL, Raff H, Roman RJ. Influence of angiotensin II on pressure natriuresis and renal hemodynamics in volume-expanded rats. Am J Physiol. 1991;29:R1200–R1209. doi: 10.1152/ajpregu.1991.260.6.R1200. [DOI] [PubMed] [Google Scholar]
- 12.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 13.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol. 1999;10:S258–S265. [PubMed] [Google Scholar]
- 14.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, et al. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 15.Vaňourková Z, Kramer HJ, Husková Z, Vaněčková I, Opočenský M, Čertíková Chábová V, et al. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens. 2006;24:2465–2472. doi: 10.1097/01.hjh.0000251909.00923.22. [DOI] [PubMed] [Google Scholar]
- 16.Husková Z, Vaňourková Z, Erbanová M, Thumová M, Opočenský M, Mullins JJ, et al. Inappropriately high circulating and intrarenal angiotensin II levels during dietary salt loading exacerbate hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2010;28:495–509. doi: 10.1097/HJH.0b013e3283345d69. [DOI] [PubMed] [Google Scholar]
- 17.Honetschlagerová Z, Husková Z, Vaňourková Z, Sporková A, Kramer HJ, Hwang SH, et al. Renal mechanisms contributing to the antihypertensive action of soluble epoxide hydrolase inhibition in Ren-2 transgenic rats with inducible hypertension. J Physiol. 2011;589:207–219. doi: 10.1113/jphysiol.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell KD, Bagatell SJ, Miller CS, Mouton CR, Seth DM, Mullins JJ. Genetic clamping of renin gene expression induces hypertension and elevation of intrarenal II levels of graded severity in Cyp1a1-Ren2 transgenic rats. JRAAS. 2006;7:74–86. doi: 10.3317/jraas.2006.013. [DOI] [PubMed] [Google Scholar]
- 19.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 20.Capdevila JH, Falck JR, Imig JD. Role of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int. 2007;72:683–689. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 21.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 22.Imig JD, Zhao X, Falck JR, Wei S, Capdevila JH. Enhanced renal mircovascular reactivity to angiotensin II in hypertension is ameliorated by the sulfonimide analog of 11,12-epoxyeicosatrienoic acid. J Hypertens. 2001;19:983–992. doi: 10.1097/00004872-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Imig JD. Targeting epoxides for organ damage in hypertension. J Cardiovasc Pharmacol. 2010;56:329–335. doi: 10.1097/FJC.0b013e3181e96e0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erbanová M, Thumová M, Husková Z, Vaněčková I, Vaňourková Z, Mullins JJ, et al. Impairment of the autoregulation of renal hemodynamics and of the pressure-natriuresis relationship precedes the development of hypertension in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2009;27 :575–586. doi: 10.1097/hjh.0b013e32831cbd5a. [DOI] [PubMed] [Google Scholar]
- 26.Williams DE, Minolfa CP, Mullins JJ, Navar LG, Mitchell KD. AT1 receptor blockade prevents the increase in blood pressure and the augmentation of intrarenal ANG II levels in hypertensive Cyp1a1-Ren-2 transgenic rats fed a high salt diet. Am J Med Sci. 2010;339:356–361. doi: 10.1097/MAJ.0b013e3181d2b0a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Čertíková Chábová V, Kramer HJ, Vaněčková I, Vernerová Z, Eis V, Tesař V, et al. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vascul Pharmacol. 2007;47:145–149. doi: 10.1016/j.vph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Čertíková Chábová V, Walkovska A, Kompanowska-Jezierska E, Sadowski J, Kujal P, Vernerová Z, et al. Combined inhibition of 20-hydroxy-eicosatetrenoic acid formation and epoxyeicosaetraenoic degradation attenuates hypertension and hypertension-induced end-organ damage in Ren-2 transgenic rats. Clin Sci. 2010;118:617–632. doi: 10.1042/CS20090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semple SJ, de Wardener HE. Effect of increased renal venous pressure on circulatory autoregulation of isolated dog kidneys. Circ Res. 1959;7:643–648. doi: 10.1161/01.res.7.4.643. [DOI] [PubMed] [Google Scholar]
- 30.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilatation in response to bradykinin. J Vasc Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 31.Kohagure K, Endo Y, Ito O, Arima S, Omata K, Ito S. Endogenous nitric oxide and epoxyeicosatrienoic acids modulate angiotensin II-induced constriction in the rabbit afferent arteriole. Acta Physiol Scand. 2000;168:107–112. doi: 10.1046/j.1365-201X.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 32.Madhun ZT, Goldthwait DA, McKay D, Hopfer U, Douglas JG. An epoxygenase metabolite of arachidonic acid mediates angiotensin II-induced rises in cytosolic calcium in rabbit proximal tubule epithelial cells. J Clin Invest. 1991;88:456–461. doi: 10.1172/JCI115325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakairi Y, Jacobson HR, Noland DT, Capdevila JH, Falck JR, Breyer MD. 5,6-EET inhibits ion transport in collecting duct by stimulating endogenous prostaglandin synthesis. Am J Physiol. 1995;268:F931–F939. doi: 10.1152/ajprenal.1995.268.5.F931. [DOI] [PubMed] [Google Scholar]
- 34.Majid DSA, Navar LG. Blockade of distal nephron sodium transport attenuates pressure natriuresis in dogs. Hypertension. 1994;23:1040–1045. doi: 10.1161/01.hyp.23.6.1040. [DOI] [PubMed] [Google Scholar]
- 35.Sporková A, Kopkan L, Varcabová A, Husková Z, Hwang SH, Hammock BD, et al. Role of cytochrome P450 metabolites in the regulation of renal function and blood pressure in 2-kidney, 1-clip hypertensive rats. Am J Physiol. 2011 doi: 10.1152/ajpregu.00215.2010. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–1253. [PubMed] [Google Scholar]
- 37.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, et al. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46:975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kujal P, Čertíková Chábová V, Vernerová Z, Walkovska A, Kompanowska-Jezierska E, Sadowski J, et al. Similar renoprotection after renin-angiotensin-dependent and –independent antihypertensive therapy in 5/6-nephrectomized Ren-2 transgenic rats: are there blood pressure-independent effects? Clin Exp Pharmacol Physiol. 2010;37:1159–1169. doi: 10.1111/j.1440-1681.2010.05453.x. [DOI] [PubMed] [Google Scholar]
- 39.Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, et al. Administration of a substituted adamantly urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci. 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]