Abstract
Objective
To determine frequency of emotional disorders and sleep disturbances in adolescent migraineurs with episodic and chronic headaches. To determine the relationship of whole blood serotonin, caffeine consumption, and frequency of sleep and mood disorders.
Background
The neurotransmitter serotonin has been implicated to play a role in the initiation and maintenance of sleep and in modulating mood. A putative role in migraine pathophysiology is also known.
Methods
Adolescents from 13 to 17 years of age were identified from our headache clinic with episodic or chronic migraine (according to International Classification of Headache Disorders-Second Edition criteria) and healthy controls enrolled. Psychological rating scales were completed, including Adolescent Symptom Inventory (4th Edition) and Child Depression Inventory. Sleep questionnaires (Pediatric Sleep Questionnaire and Child Sleep Habit Questionnaire) were completed by the teenager’s parents/guardian. Whole blood serotonin levels were drawn and analyzed and caffeine consumption obtained by history.
Results
A total of 18 controls (8 girls) and 15 patients each with episodic migraines (9 girls) and chronic migraine (10 girls) were studied.
Patients with headache had significantly more sleep problems than controls. Patients with chronic migraines had increased daytime sleepiness and dysthymia compared with teenagers with episodic migraines. Serotonin levels were not significantly different, and no association was noted between serotonin levels and sleep abnormalities or emotional rating scales. Increased caffeine intake was related to sleep and depressive complaints.
Conclusions
Sleep and emotional disorders were common in adolescents with migraine. Sleep disorders and dysthymia were more prevalent with increased headache frequency. No correlation was noted with whole blood serotonin levels.
Keywords: migraine, serotonin, sleep, depression, pediatrics
Migraine headaches are common among adolescents with prevalence rates of about 10% with the highest prevalence in older teenagers.1 In a minority of these patients, migraines increase in frequency to become chronic daily headaches (CDH). Frequent headaches can cause significant lifestyle disruption, school absences, and increased psychosocial burden for families. Adolescents with chronic migraine (CM) oftentimes display headaches that persist into adulthood. Many adults have their near daily headache onset in adolescence or young adulthood.
Chronic migraine as a subtype of CDH utilizing the new Appendix International Classification of Headache Disorders-Second Edition (ICHD-II) criteria occurs infrequently in adolescent subtypes.2,3 Recent studies demonstrate prevalence rate of approximately 2% of adolescents with CDH. CM tends to be a persistent diagnosis over time compared with chronic tension headache. However, chronic tension headache predominates in the adolescent population with near daily headaches.
Serotonin function has long been implicated in the pathophysiology of migraine.4,5 The triptan classes of drugs, such as serotonin IB/1D agonists, are standard abortive therapy for migraine attacks.Platelet concentrations of neurotransmitter serotonin (5-HT) are model for central nervous system levels because of the similarities between platelet and serotonergic neurons regarding pharmacologic and biochemical properties.6
Most of the information on the correlation between central serotonin levels (measured through serotonin metabolite 5-hydroxyindoleacetic acid in cerebrospinal fluid) and peripheral platelet serotonin activity has been obtained in depressed patients in the past.7 Studies in adults with migraine have shown decreased platelet serotonin level related to more frequent headaches.8
Whole blood 5-HT, because of its high concentration of platelets, is thought to correlate with central serotonergic activity.9,10 Recent positron emission tomography neuro-imaging advances suggest brain serotonin synthesis is higher in adults with migraine than controls.4 It is postulated that increased central serotonin activity may deplete during frequent migraine attacks, possibly predisposing these patients to recurrent headaches with low serotonin concentrations possibly lowering pain perception thresholds.11
Serotonin also plays an important role in sleep pathophysiology.12 The brainstem structures involving the raphé nuclei are replete with serotonergic neurons and 5-HT levels have long been thought to modulate normal sleep physiology and play a major role in initiation and maintenance of sleep activity.
Neurotransmitter serotonin plays an important role in genesis of emotional and mood disorders.13 With recent advances in pharmacologic treatment of depression, the serotonin reuptake inhibitors are used in treatment to increase central serotonin levels with subsequent modulation of the pharmacophysiologic state involved in depressive disorders.
As some studies have suggested caffeine consumption may negatively influence headache disorders with potentiation to CM; intake may also play a factor in mood and sleep pathophysiology and its effects on exacerbation of migraine in the adult and adolescent population.14 Its stimulant properties may exert effects on sleep pathophysiology and mood.
METHODS
In this study, the frequency of emotional disorders and sleep abnormalities was investigated in adolescent patients with episodic and CM compared with healthy age-matched controls. Whole blood serotonin levels were obtained to elucidate whether changes in whole blood serotonin in headache patients are correlated with sleep and emotional disorders in these patients.
Otherwise healthy adolescents between 13 to 17 years of age prospectively presenting to our headache clinic with the diagnosis of episodic or CM according to ICHD-II criteria were enrolled.3,15 Headache frequency was analyzed from prospective diaries brought to headache clinic appointments. Pediatric Migraine Disability Assessment Survey (PedMIDAS) was obtained.16 Enrollment occurred over a 1-year time interval. Patients with tension-type headaches or medication overuse were excluded. The study approval was obtained by Nationwide Children’s Hospital Institutional Review Board. Healthy age-matched controls were enrolled from local pediatrician offices. History, physical, and neurological examinations were completed and controls were selected without significant neurological, medical, or psychiatric histories or symptoms.
After written informed consent was obtained from parent/guardian, the adolescent was asked to complete the Child Depression Inventory (CDI), and the parent/guardian completed the Adolescent Symptom Inventory-4 (ASI), both of which are validated Diagnostic and Statistical Manual-4-based psychologic rating scales for multiple behavioral and emotional disorders.17-19 Patients with high predictive scores for emotional disorders underwent screening interview to confirm diagnosis.
Two sleep questionnaires, Child Sleep Habits Questionnaire (CSHQ) and Pediatric Sleep Questionnaire (PSQ), were completed by the parent/guardian.The PSQ is a 22-item questionnaire that has been shown to be a validated and reliable instrument in identifying predominately sleep-related breathing disorders in children 2-18 years of age.20 The CSHQ is a 45-item questionnaire that uses an abbreviated version of 35 individual items for scoring that comprise a scale diagnosing common sleep problems in children 4-12 years of age.21 Although our patients were adolescents, the CSHQ was used in conjunction with the PSQ.
Blood was drawn for venipuncture for assessment of whole blood serotonin levels through ARUP National Reference Laboratory, Salt Lake City, Utah, with samples placed on ice and subsequently analyzed using high pressure gas liquid chromatography. Dietary factors and medications known to influence serotonin levels were discussed with patients and families prior to enrollment. Headache patients with exclusionary criteria and features were not enrolled, including less than 1 migraine headache per month, ongoing treatment with serotonin re-uptake inhibitors, and other medications that affect 5-HT levels such as lithium, monoamine oxidase inhibitors, methyldopa, morphine, or cyproheptadine. Cigarette smokers were excluded. Average daily caffeine consumption over the past month, both dietary and medicinal, was determined by history. Statistical analysis using chi-square tests compared demographic variables across groups. Simple paired T-tests compared group differences.
RESULTS
In total, 20 controls (8 girls) and 17 patients with episodic migraine (EM) and 16 with CM were identified over a 12-month period. Completing final data analysis were 15 patients (9 girls) with EM. Five of the 15 patients had migraine with aura. Fifteen completed patients had CM; 1 of 15 patients had CM with aura. Eighteen controls (8 girls) completed data analysis. Five patients and controls did not complete the study. Two refused blood draw, and 3 had serotonin levels processed incorrectly.
Episodic migraine patients had a mean age of 13.9 years, and CM patients had a mean age of 15.2 years; the mean age of controls was 15.9 years. Mean monthly headache days in the EM group was 4.7 (range 1-12). CM patients had mean monthly headache days of 21 (range 15-28). PedMIDAS disability score was significantly elevated in CM patients compared with EM patients (59.0 vs 38.1, P < .05 Wilcoxon rank sums nonparametric test).
Sleep scales evaluated revealed significantly more sleep problems in both groups of headache patients compared with controls. Headache patients compared with controls had worse PSQ total score, more morning headaches, and more sleepiness (P < .05) (Table 1). CSHQ revealed that the headache group compared with controls had more daytime sleepiness, night awakenings, sleep onset delay, parasomnias, and total CSHQ score compared with controls. No differences were noted in PSQ obesity scores and CSHQ sleep disorder breathing scales. CM adolescents differed from EM (EM) patients only regarding increased sleepiness (P < .05) (Table 1).
Table 1.
Relationship Between Adolescent Sleep Behavior and Headaches
| Control vs any headache |
Control vs CM† |
Control vs EM‡ |
Chronic vs episodic |
|||||
|---|---|---|---|---|---|---|---|---|
| Wilcoxon nonparametric rank sums test | Z | P value | Z | P value | Z | P value | Z | P value |
| PSQ total | −2.94 | .00 | −2.98 | .00 | −2.02 | .05 | −1.13 | .26 |
| PSQ sleepiness | −2.69 | .01 | −372 | .00 | −0.84 | .49 | −2.51 | .01 |
| PSQ morning headache | −3.93 | .00 | −4.09 | .00 | −3.22 | .02 | −1.09 | .28 |
| PSQ obesity | −0.26 | .79 | −0.70 | .68 | −0.38 | .87 | −0.99 | .32 |
| CSHQ total | −2.92 | .00 | −3.19 | .00 | −1.78 | .08 | −1.37 | .17 |
| CSHQ bedtime resistance | −1.53 | .13 | −1.81 | .13 | −0.85 | .51 | −1.09 | .28 |
| CSHQ sleep onset delay | −2.84 | .00 | −3.03 | .00 | −1.87 | .09 | −1.06 | .29 |
| CSHQ sleep duration | −0.86 | .39 | −1.30 | .23 | −0.17 | .87 | −1.48 | .14 |
| CSHQ sleep anxiety | −1.07 | .29 | −0.68 | .63 | −1.18 | .38 | −0.49 | .63 |
| CSHQ night wakenings | −2.09 | .04 | −1.50 | .18 | −2.10 | .05 | −0.67 | .50 |
| CSHQ parasomnias | −2.05 | .04 | −2.31 | .03 | −1.23 | .27 | −0.81 | .42 |
| CSHQ sleep disordered breathing | 0.00 | 1.00 | −0.38 | .79 | −0.35 | .79 | −0.81 | .42 |
| CSHQ daytine sleepiness | −2.36 | .02 | −2.75 | .01 | −1.26 | .22 | −1.08 | .28 |
Chronic migraine.
Episodic migraine.
CM = chronic migraine;CSHQ = Child Sleep Habits Questionnaire;EM = episodic migraine;PSQ = Pediatric Sleep Questionnaire.
Bold data indicate significance at P ≤ .05.
Child Depression Inventory and ASI scales showed differences between headache patients and controls. Headache patients, both episodic and CM grouped together, had significantly elevated T-scores on ASI for generalized anxiety, depression, and dysthymia utilizing ASI (Table 2). The depression inventory T-score showed significant elevation on the CDI, T-score mean of 50.9 compared with control mean of 41.6 (P < .05, χ2).
Table 2.
Mean Comparison of Psychological Measures Between Headache Patients and Controls (Mean T-Scores)
| ASI – parent report |
CDI – self-report |
|||
|---|---|---|---|---|
| Generalized anxiety | Depression | Dysthymia | Depression | |
| Headache patients (EM† & CM‡) | 62.4* | 62.4** | 64.8** | 50.9* |
| Controls | 51.8 | 52.6 | 57.2 | 41.6 |
Significance P ≤ .01,
significance P ≤ .05.
Episodic migraine.
Chronic migraine.
ASI = Adolescent Symptom Inventory-4; CDI = Child Depression Inventory; CM = chronic migraine; EM = episodic migraine.
Chronic migraine patients differed from EM patients only with respect to increased dysthymia complaints (x = 69.4 vs x = 60.5, P < .05) (Table 2).
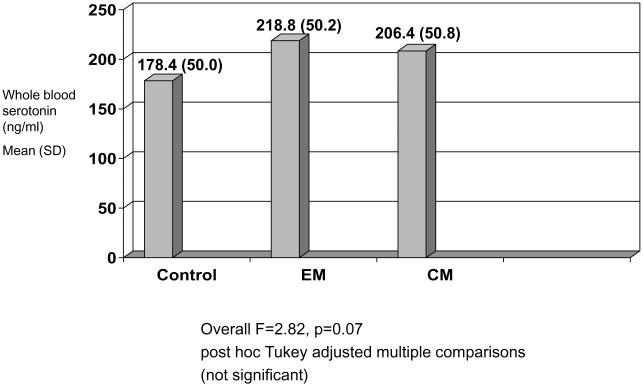
Whole blood serotonin levels were evaluated to determine relationship between sleep problems, emotional disorders, and serotonin levels. There was no correlation between 5-HT and any of the psychologic rating scales or sleep rating scores. Serotonin levels were not significantly different among any of the headache groups or the controls (Fig. 1).
Fig 1.
Whole blood serotonin levels controls, episodic migraine, and chronic migraine.
Patients with headache (both EM and CM) did not differ by questionnaire in caffeine consumption from controls. However, increased caffeine consumption was associated with significant elevation in CDI total score and also several sleep score elevations (PSQ total, morning headache, obesity, CSHQ total, sleep duration, and daytime sleepiness) (Table 3). CM patients, as expected, with more frequent headaches had greater associated headache-related disability.
Table 3.
Daily Caffeine Consumption and Sleep/Mood Disorder Scales (Adolescents With and Without Headache) (n = 48)
| ASI | Caffeine use |
|
|---|---|---|
| Depression | Pearson correlation | 0.18 |
| Sig. 2-tailed | 0.23 | |
| Dysthymia | Pearson correlation | 0.14 |
| Sig. 2-tailed | 0.34 | |
| Anxiety | Pearson correlation | 0.05 |
| Sig. 2-tailed | 0.72 | |
| CDI – depression | Pearson correlation | 0.41 |
| Sig. 2-tailed | 0.00 | |
| PSQ | Pearson correlation | 0.30 |
| Sig. 2-tailed | 0.04 | |
| PSQ sleepiness | Pearson correlation | 0.23 |
| Sig. 2-tailed | 0.11 | |
| PSQ morning headache | Pearson correlation | 0.32 |
| Sig. 2-tailed | 0.03 | |
| PSQ obesity | Pearson correlation | 0.36 |
| Sig. 2-tailed | 0.01 | |
| CSHQ total | Pearson correlation | 0.43 |
| Sig. 2-tailed | 0.00 | |
| CSHQ bedtime | Pearson correlation | 0.08 |
| resistance | Sig. 2-tailed | 0.57 |
| CSHQ sleep onset delay | Pearson correlation | 0.21 |
| Sig. 2-tailed | 0.16 | |
| CSHQ sleep duration | Pearson correlation | 0.31 |
| Sig. 2-tailed | 0.03 | |
| CSHQ parasomnias | Pearson correlation | 0.18 |
| Sig. 2-tailed | 0.23 | |
| CSHQ sleep disordered | Pearson Correlation | −0.17 |
| breathing | Sig. 2-tailed | 0.24 |
| CSHQ daytime | Pearson correlation | 0.50 |
| sleepiness | Sig. 2-tailed | 0.00 |
| CSHQ sleep anxiety | Pearson correlation | −0.18 |
| Sig. 2-tailed | 0.22 | |
| CSHQ night wakenings | Pearson correlation | 0.16 |
| Sig. 2-tailed | 0.29 |
ASI = Adolescent Symptom Inventory-4; CDI = Child Depression Inventory; CSHQ = Child Sleep Habits Questionnaire; PSQ = Pediatric Sleep Questionnaire. Bold data indicate significance at P < .05.
DISCUSSION
While adolescents with migraine had more significant issues with parasomnias, daytime sleepiness, night awakenings, and sleep onset delays than controls, caffeine consumption was not significantly different from controls. Increased caffeine consumption in all adolescents studied (both with and without headache) was associated with significant abnormalities in many sleep parameters, including total PSQ andVSHQ total scores, sleep onset, sleep duration, daytime sleepiness, and morning headaches (Table 3). These results are similar to an adult study by Scher et al, which found no difference in dietary coffee consumption between individuals with CDH and episodic headaches.14 However, their study retrospectively found caffeine consumption to be elevated in an adult CDH group prior to onset of their near daily headaches. This suggests a possible association between caffeine consumption, sleep disturbances, and depression as predisposing factors for genesis of CDH. We found that patients and controls with increased caffeine consumption self-reported more depression complaints and were poorer sleepers in general than their cohorts irrespective of headache state. Minimizing caffeine consumption may benefit sleep and mood in headache patients and secondarily effect susceptibility to migraine attacks.
Neurotransmitter serotonin has been found to be important in migraine pathophysiology. Plasma 5-HT levels have been found to be reduced in patients with migraine with aura during attack-free periods compared with controls without headache.22 However, conflicting evidence for platelet dysfunction has been reported in migraine.23,24 Urinary excretion of the main metabolite of 5-HT, 5-hydroxyindoleacetic acid, decreases with the frequency of migraine attacks. Other investigations have shown decreased whole blood levels of 5-HT in patients with CDH and medication overuse and increase in serotonin levels with improvements in headache frequency and resolution of overuse. Sarchielli et al found platelet 5-HT levels diminished in CDH patients compared with healthy controls.8 5-HT platelet fractions correlate positively with 5-HT uptake in some studies. This suggests change in platelet 5-HT during migraine attacks in patients without aura.24 With chronic headache, platelet levels of 5-HT may decrease, possibly related to 5-HT depletion in control serotonergic pathways.25
Neurotransmitter serotonin levels were mildly, but not significantly, elevated in our headache patients compared with controls. None of our patients suffered from medication overuse as did adult patients in prior CDH studies.
In a previous study involving adolescents with anxiety and depression symptoms, whole blood 5-HT was diminished in patients with anxiety and depression symptoms and increased with conduct disorder.26 Although our patients with CM had more significant dysthymia complaints than controls or EM patients, whole blood serotonin levels did not significantly differ.
While sleep dysfunction was more prevalent in our adolescent CM patients than controls, there was no correlation noted with 5-HT levels. This contrasts with prior information delineating hyposerotonergic state associated with sleep pathophysiology related to metabolism in dorsal raphe nuclei in brainstem.12
In this group of adolescents with migraines, sleep and mood disorder were common, and teenagers with CM had higher predilection to sleep issues and dysthymia than those with episodic headaches. Caffeine consumption appeared to exacerbate sleep issues and depressive symptoms irrespective of headache status. Whole blood 5-HT levels were not related to presence of emotional disorders or sleep dysfunction. The genesis of their psychologic and sleep dysfunction may be multifunctional and not clearly delineated by a distinct alteration in serotonin metabolism. Limitations of this study may include relatively small sample size, discrepancy with parental report of sleep problems in patients and true sleep pathology, and measurement of whole blood serotonin levels. Further studies in larger groups of patients may be helpful, especially in investigating post-pharmacologic treatment effects on migraine frequency, sleep, and emotional disorders.
Acknowledgments
This study was supported in part by a research grant from Nationwide Children’s Hospital Research Institute. Mrs. Julie Campbell prepared the manuscript. John Hayes, PhD, provided the statistical analysis.
Funding support: This study was supported in part by a research grant from Nationwide Children’s Research Institute.
Abbreviations
- ASI
Adolescent Symptom Inventory-4
- CDH
chronic daily headache
- CDI
Child Depression Inventory
- CM
chronic migraine
- CSHQ
Child Sleep Habits Questionnaire
- EM
episodic migraine
- ICHD-II
International Classification of Headache Disorders-Second Edition
- PedMIDAS
Pediatric Migraine Disability Assessment Survey
- PSQ
Pediatric Sleep Questionnaire
- 5-HT
neurotransmitter serotonin
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Split W, Neuman W. Epidemiology of migraine among students from randomly selected secondary schools in lodz. Headache. 1999;39:494–501. doi: 10.1046/j.1526-4610.1999.3907494.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang SJ, Fuh JL, Lu SR, Juang KD. Outcomes and predictors of chronic daily headache in adolescents. Neurology. 2007;68:591–596. doi: 10.1212/01.wnl.0000252800.82704.62. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 4.Chugani DC, Niimura K, Chaturvedi S, et al. Increased brain serotonin synthesis in migraine. Neurology. 1999;53:1473–1483. doi: 10.1212/wnl.53.7.1473. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro RE, Tepper SJ. The serotonin syndrome, triptans, and the potential for drug-drug interactions. Headache. 2007;47:266–269. doi: 10.1111/j.1526-4610.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 6.Cook EH, Jr, Stein MA, Ellison T, Unis A, Leventhal BL. Attention deficit hyperactivity disorder and whole-blood serotonin levels: Effects of comorbidity. Psychiatry Res. 1995;57:13–20. doi: 10.1016/0165-1781(95)02596-o. [DOI] [PubMed] [Google Scholar]
- 7.Pandey GN. Altered serotonin function in suicide—Evidence from platelet and neuroendocrine studies. Ann N Y Acad Sci. 1997;836:182–200. doi: 10.1111/j.1749-6632.1997.tb52360.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarchielli P, Alberti A, Russo S, et al. Nitric oxide pathway, CA2, and serotonin content in platelets from patients suffering from chronic daily headache. Cephalalgia. 1999;19:810–816. doi: 10.1046/j.1468-2982.1999.1909810.x. [DOI] [PubMed] [Google Scholar]
- 9.Cook EH, Jr, Arora RC, Anderson GM, et al. Platelet serotonin studies in hyperserotonemic relatives of children with autistic disorder. Life Sci. 1993;52:2005–2015. doi: 10.1016/0024-3205(93)90685-v. [DOI] [PubMed] [Google Scholar]
- 10.Lesch KP, Wolozin BL, Murphy DL, Riederer P. Primary structure of the human platelet serotonin uptake site: Identity with the brain serotonin transporter. J Neurochem. 1993;60:2311–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwedt TJ. Serotonin and migraine: The latest developments. Cephalalgia. 2007;27:1301–1307. [Google Scholar]
- 12.Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Pine DS, Cohen P, Brook J. The association between major depression and headache: Results of a longitudinal epidemiologic study in youth. J Child Adolesc Psychopharmacol. 1996;6:153–164. doi: 10.1089/cap.1996.6.153. [DOI] [PubMed] [Google Scholar]
- 14.Scher AI, Stewart WF, Lipton RB. Caffeine as a risk factor for chronic daily headache. Neurology. 2004;63:2022–2027. doi: 10.1212/01.wnl.0000145760.37852.ed. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 2nd edn. Cephalalgia. 2004;24(1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 16.Hershey AD, Powers SW, Vockell ALB, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS – Development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034–2039. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs M. Children’s Depression Inventory Manual. Multihealth System; New York: 1992. [Google Scholar]
- 18.Gadow KD, Sprafkin J. Adolescent Symptom Inventory-4: Screening Manual 1997b. Checkmate Plus; Stonybrook, NY: 1997. [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Satistical Manual of Mental Disorders. 4th American Psychiatric Press; Washington, DC: 1994. 1994. [Google Scholar]
- 20.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ) validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 21.Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 22.Nagata E, Shibata M, Hamada J, et al. Plasma 5-hydroxytryptamine (5-HT) in migraine during an attack free period. Headache. 2006;46:592–596. doi: 10.1111/j.1526-4610.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 23.Oxman TE, Hitzemann RJ, Smith R. Platelet membrane lipid composition and the frequency of migraine. Headache. 1982;22:261–267. doi: 10.1111/j.1526-4610.1982.hed2206261.x. [DOI] [PubMed] [Google Scholar]
- 24.Jernej B, Vladic A, Cicin-Sain L, et al. Platelet serotonin measures in migraine. Headache. 2002;42:588–595. doi: 10.1046/j.1526-4610.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari MD, Odink J, Tapparelli C, Van Kempen GM, Pennings EJ, Bruyn GW. Serotonin metabolism in migraines. Neurology. 1989;39:1239–1242. doi: 10.1212/wnl.39.9.1239. [DOI] [PubMed] [Google Scholar]
- 26.Pliszka SR, Rogeness GA, Renner P, Sherman J, Broussard T. Plasma neurochemistry in juvenile offenders. J Am Acad Child Adolesc Psychiatry. 1988;27:588–594. doi: 10.1097/00004583-198809000-00012. [DOI] [PubMed] [Google Scholar]