Abstract
STUDY QUESTION
Does obesity influence the chance of pregnancy after IVF in donor oocyte recipients?
SUMMARY ANSWER
The chance of pregnancy after IVF is no different in obese donor oocyte recipients versus those in the normal BMI range.
WHAT IS KNOWN ALREADY
Obesity is associated with decreased chances of pregnancy in women undergoing IVF with autologous oocytes. Prior studies have investigated the impact of obesity on IVF outcomes in donor oocyte recipients, with disparate results. This is the first systematic review and meta-analysis to address this topic.
STUDY DESIGN, SIZE, DURATION
A systematic review and meta-analysis of published literature identified in Medline, EMBASE and Scopus through December of 2011 were performed to address the association between BMI and outcomes for donor oocyte recipients. The primary outcome of this study was implantation.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Two authors conducted the searches independently, selected the studies and abstracted the data. Studies in English of first donor oocyte cycles with reported recipient BMI were included. Primary data collected from the IVF program at Washington University were also included as one study (n = 123 donor oocyte recipients). Studies limited to frozen embryo transfer were excluded. Data were synthesized using DerSimonian–Laird random effects models for implantation, clinical pregnancy, miscarriage and live birth.
MAIN RESULTS AND THE ROLE OF CHANCE
Of 475 screened articles, 7 were reviewed and 5 were included together with primary data from Washington University, giving a total of 4758 women who were included for the assessment of the primary outcome. No associations between obesity (BMI ≥ 30 kg/m2) and chance of pregnancy after IVF were noted in women using donor oocytes [risk ratio (RR): 0.98, 95% confidence intervals (CI): 0.83–1.15, I2: 61.6%]. Additional analyses assessing associations between recipient obesity and embryo implantation (RR: 0.93, 95% CI: 0.80–1.07, I2: 0%), miscarriage (RR: 1.12, 95% CI: 0.83–1.50, I2: 0%) and live birth (RR: 0.91, 95% CI: 0.65–1.27, I2 47.9%) also failed to show a negative effect.
LIMITATIONS, REASONS FOR CAUTION
Included studies were small and they were performed in a variety of locations and practice settings where stimulation and laboratory protocols may differ, and extremes of BMI may also differ. Furthermore, included studies had different inclusion and exclusion criteria. These factors could not be controlled for in this meta-analysis and statistical heterogeneity was noted for some outcomes.
WIDER IMPLICATIONS OF THE FINDINGS
These data suggest obesity does not affect IVF outcomes in women using donor oocytes. Oocyte quality rather than endometrial receptivity may be the overriding factor influencing IVF outcomes in obese women using autologous oocytes.
STUDY FUNDING/COMPETING INTEREST(S)
E.S.J. and M.G.T receive support from the Women's Reproductive Health Research Program sponsored by the National Institutes of Health (K12 HD063086). The authors do not have any competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: BMI, female infertility, gamete donation, donor oocyte recipients
Introduction
Obesity is associated with adverse reproductive outcomes including subfertility and increased risk of miscarriage (Boots and Stephenson, 2011). Obese women who conceive naturally are at increased risk of adverse pregnancy outcomes, such as pre-eclampsia, gestational diabetes and stillbirth (Chu et al., 2007). Children born to obese women are at increased risk of congenital and growth abnormalities (Stothard et al., 2009). Obese women who conceive with the assistance of IVF face similar risks in pregnancy as women who conceive naturally (Dokras et al., 2006; Rittenberg et al., 2011). What is different is that the precise timing of events and close follow up involved in the care of women undergoing IVF provide a unique opportunity to observe associations between preconception exposures and various steps of the reproductive process including implantation, clinical pregnancy, miscarriage and live birth.
There has been a longstanding debate about which components of the reproductive process are affected most by obesity. Whereas some have focused on adverse effects of obesity on oocyte quality, others have focused on the endometrium (Jungheim and Moley, 2010). A number of studies have evaluated associations between obesity and adverse reproductive outcomes in women undergoing donor oocyte IVF and embryo transfer as a way to separate out the effects of obesity on the oocyte versus the endometrium and implantation. Taken alone, these studies are limited by sample size. Our objective was to estimate the associations between BMI and IVF outcomes in donor oocyte recipients through a systematic review and meta-analysis.
Methods
The conduct and reporting of this systematic review closely adhered to guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2009). We used a predesigned protocol for the literature search, study selection and data synthesis.
Search strategy
Our literature search included Medline, EMBASE and Scopus through 2011 using MeSH headings: (In vitro Fertilization (IVF) OR assisted reproductive techniques) AND ‘Donor Oocytes’ AND (Obesity OR BMI) AND Human. The reference list was screened and relevant articles retrieved. If there was a question about an article's relevance it was also retrieved for further review. Reference lists in identified articles were manually searched for other potentially relevant publications.
Study selection criteria
Studies were limited to those published in English analyzing the first cycle of donor IVF. Studies were required to report BMI for the donor oocyte recipients. Studies including the use of frozen embryos were excluded. If more than one study included overlapping data, the largest of the studies was kept and the other was excluded. Study design was not limited.
Primary data collection
Institute Review Board approval was obtained and primary data from the IVF program at Washington University were also collected and included as one of the studies. Medical and laboratory charts were reviewed to collect patient BMI, age and IVF outcome including embryo implantation, clinical pregnancy and live birth. Our program has strict selection criteria for anonymous oocyte donors in that they must be <35 years of age, non-smokers, have a BMI in the normal range and no significant medical issues. Our donors all undergo standard gonadotrophin stimulation protocols as previously described (Jungheim et al., 2009) and our oocyte recipients receive GnRH agonists for ovarian suppression, where appropriate, along with oral estrogen and i.m. progesterone for uterine lining preparation.
Study selection and data abstraction
Each eligible article was reviewed and data were extracted for study design and location, year of publication, for the number of patients in each of the four standard World Health Organization (WHO) BMI categories: (i) <20 kg/m2, (ii) 20–24.9 kg/m2, (iii) 25–29.9 kg/m2 and (iv) ≥30 kg/m2. Data were also extracted for donor oocyte recipient age, oocyte donor age, embryo implantation, clinical pregnancy, miscarriage and live birth. Information regarding selection and preparation for oocyte donors and preparation for oocyte donor recipients was also collected along with relevant study inclusion and exclusion criteria. The published data for two studies did not specify the outcomes for first donor oocyte cycles using the BMI groups described above (Bodri et al., 2010; DeUgarte et al., 2010). The authors of these studies were contacted and were able to provide the relevant data necessary for incorporation into this meta-analysis. The primary outcome for this study was implantation (defined as the number of gestational sacs seen on first trimester ultrasound divided by the total number of embryos transferred) while clinical pregnancy, miscarriage and live birth were secondary outcomes. The quality of each study was assessed using methods described by Downs and Black (1998). Briefly, this consists of a checklist of 27 items aimed at assessing the methodological quality of observational studies with regard to the quality of reporting, internal validity, power and external validity. Only 19 of the items from the Downs and Black (1998) checklist were relevant to the studies we assessed. All steps were performed independently by E.S.J. and S.B.S.
Data analysis
Abstracted data and primary data were analyzed using STATA 12 (Stata, College Station, TX, USA). Heterogeneity was assessed using χ2 test for heterogeneity (Cochran's Q statistic), and the magnitude of heterogeneity quantified using I2 [(I2 = Q− degrees of freedom) × 100/Q], where degrees of freedom = k− 1, Q = Cochran's Q statistic and k the number of studies) (Higgins and Thompson, 2002). Pooled risk ratios (RR) and 95% confidence intervals (CI) from the studies were calculated for implantation, clinical pregnancy, miscarriage and live birth using a random effects model (DerSimonian and Laird, 1986).
Results
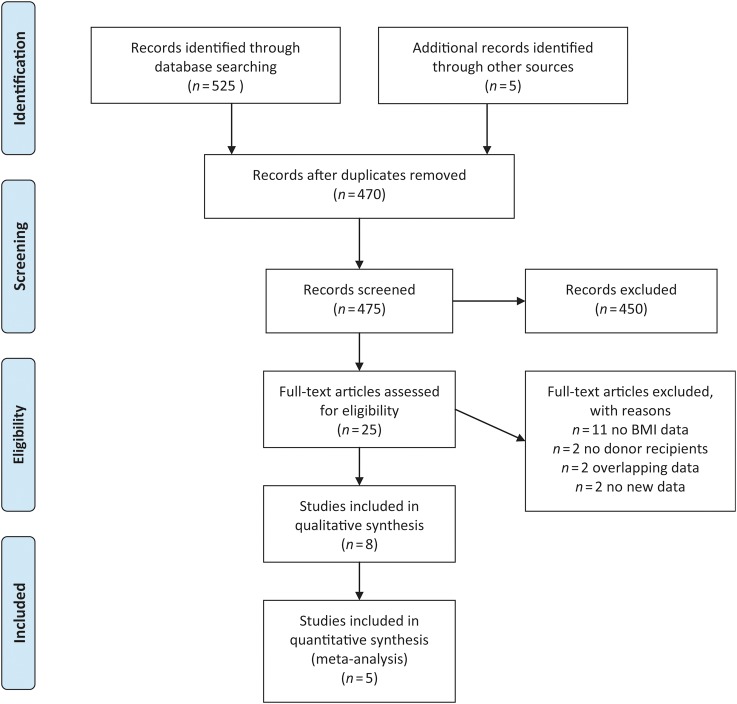
A flow diagram of study identification for the meta-analysis is shown in Fig. 1. Twenty-five publications were identified and reviewed from our initial search (Carrell et al., 2001; Bellver et al., 2003; Thum et al., 2003; Wattanakumtornkul et al., 2003; Zenke and Chetkowski, 2004; Styne-Gross et al., 2005; Bellver et al., 2007; Budak et al., 2007; Maheshwari et al., 2007; Nelson and Fleming, 2007; Soares et al., 2007; Campos et al., 2008; Howards and Cooney, 2008; Metwally et al., 2008; Soares et al., 2008; Dessolle et al., 2009; Lash and Armstrong, 2009; Bodri et al., 2010; Brewer and Balen, 2010; DeUgarte et al., 2010; Huddleston et al., 2010; van der Hoorn et al., 2010; Rittenberg et al., 2011; Luke et al., 2011a, b). Eleven of these studies were excluded because they did not have useable BMI data (Thum et al., 2003; Zenke and Chetkowski, 2004; Budak et al., 2007; Soares et al., 2007; Campos et al., 2008; Howards and Cooney, 2008; Soares et al., 2008; Lash and Armstrong, 2009; Brewer and Balen, 2010; Huddleston et al., 2010; van der Hoorn et al., 2010) and two were excluded because they did not involve oocyte recipients (Maheshwari et al., 2007; Nelson and Fleming, 2007). Two additional studies were excluded because they incorporated data that overlapped with other included publications (Bellver et al., 2003; Luke et al., 2011b). Two more articles were excluded because they were review articles containing no previously unidentified publications or data (Metwally et al., 2008; Rittenberg et al., 2011). Eight articles were reviewed in depth. One of the eight articles was excluded from the meta-analysis because it included multiple cycles per donor oocyte recipient (Luke et al., 2011a). Another was excluded because it used frozen donor embryo cycles only (Dessolle et al., 2009) and a third was excluded because it used unconventional BMI categories (Carrell et al., 2001). Five of the eight articles were included in the meta-analysis along with our primary data (Wattanakumtornkul et al., 2003; Styne-Gross et al., 2005; Bellver et al., 2007; et al., 2010; DeUgarte et.al., 2010) The characteristics of these five studies and the primary data collected in our IVF unit are outlined in Table I along with tabulated quality scores (Downs and Black, 1998). All five studies specified the characteristics of the donors in their programs, all specified the uterine preparation protocols for oocyte recipients and all had useable data for the four standard WHO categories for BMI. Three studies did not report their oocyte donor-controlled ovarian hyperstimulation protocols (Styne-Gross et al., 2005; Bodri et al., 2010; DeUgarte et al., 2010). All five studies had data for embryo implantation. Four had data available for clinical pregnancies and for miscarriage (Styne-Gross et al., 2005; Bellver et al., 2007; Bodri et al., 2010; DeUgarte et al., 2010), and two had information regarding live births (Wattanakumtornkul et al., 2003; DeUgarte et al., 2010).
Figure 1.
PRISMA 2009 flow diagram. From Moher et al. (2009). For more information, visit www.prisma-statement.org.
Table I.
Characteristics and quality scores of studiesa comparing BMI and IVF outcomes in women receiving donor oocytes.
| Study | Year | Location | Design | Inclusion and exclusion criteria | Characteristics of oocyte donors described | COH protocol described | Donor oocyte recipient protocol described | Quality score (out of 19 possible points) | Population BMI (kg/m2) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <20 | 20–24.9 | 25–29.9 | ≥30 | |||||||||
| Bellver et al. | 2007 | Spain | RC | I: Donor oocyte recipients, recent BMI, good embryo quality E: RPL, APA, no uterine pathology or severe sperm pathology |
Yes | Yes | Yes | 18 | 471 | 1613 | 450 | 122 |
| Bodri et al. | 2010 | Spain | RC | I: Donor oocyte recipients, Black, East Asian and Caucasian E: Frozen embryo cycles |
Yes | No | Yes | 14 | 195 | 544 | 92 | 67 |
| DeUgarte et al. | 2010 | CA, USA | RC | I: Donor oocyte recipients E: Smokers, PCOS, RPL, DM, HTN, rescue ICSI |
Yes | No | Yes | 16 | 9 | 125 | 112 | 103 |
| Styne-Gross et al. | 2005 | NJ, USA | RC | I: Donor oocyte recipients E: None specified |
Yes | No | Yes | 15 | 101 | 284 | 74 | 77 |
| Wattanakumtornkul et al. | 2003 | MN, USA | RC | I: Donor oocyte recipients E: No BMI data |
Yes | Yes | Yes | 15 | 7 | 52 | 25 | 12 |
| Jungheim | 2012 | MO, USA | RC | I: Donor oocyte recipients E: None |
Yes | Yes | Yes | – | 8 | 47 | 39 | 29 |
RC, retrospective cohort; COH, controlled ovarian hyperstimulation; I, inclusion criteria; E, exclusion criteria; RPL, recurrent pregnancy loss; APA, antiphospholipid antibodies; PCOS, polycystic ovary syndrome; DM, diabetes mellitus; HTN, hypertension; ICSI, intracytoplasmic sperm injection.
aAs the original search was in 2011, an additional search was performed and no further suitable papers were identified for inclusion.
Study quality scores ranged from 14 to 18 out of a total of 19 possible points. Points were deducted from the studies for failing to report details of stimulation protocols that the donors underwent and/or details regarding how the endometrium was prepared in the donor oocyte recipients. Several studies also lost points for failing to adequately describe characteristics of their donor oocyte recipients. On the other hand, given that there are only a handful of different methods for stimulating oocyte donors and for preparing the uterine endometrium for embryo transfer and given that donors are typically young and chosen for good reproductive potential, we believe the studies were of similar methodological quality.
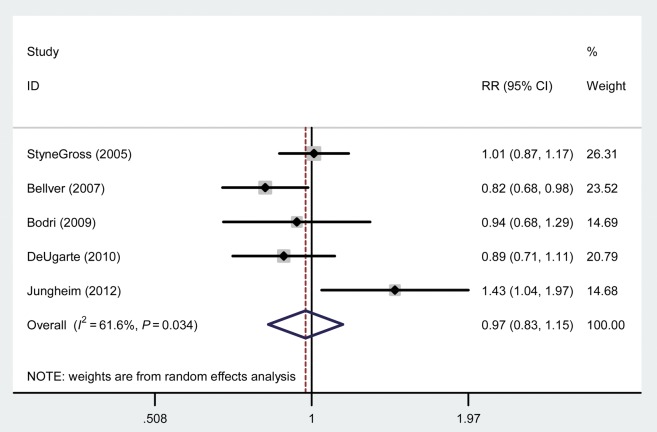
From the meta-analysis, obesity (BMI > 30 kg/m2) was not associated with a difference in implantation (RR: 0.93, 95% CI: 0.80–1.07) or clinical pregnancy (RR: 0.98, 95% CI: 0.83–1.15) rates compared with a BMI in the normal range. However, overall there was significant heterogeneity among studies for clinical pregnancy (I2 value: 61.6%) (Fig. 2). Primary data collected from our center for this meta-analysis were the only data to show a beneficial effect of obesity on clinical pregnancy rates (RR: 1.43, 95% CI: 1.04–1.97). Given this, we performed the analysis again excluding our primary data and still found no association between obesity and clinical pregnancy rates (RR: 0.92, 95% CI: 0.83–1.02). Excluding our data resulted in a dramatic reduction of the heterogeneity seen previously (I2 value: 12.2%). In addition, we found that obesity was not associated with a difference in the chances of miscarriage or live birth compared with normal BMI. Characteristics of underweight and overweight were also not associated with differences in IVF outcomes (implantation, clinical pregnancy, miscarriage, live birth) compared with women of normal BMI. The results for all BMI groups and each of the outcomes evaluated are outlined in Table II.
Figure 2.
Forest plot comparing clinical pregnancy in obese versus normal weight women using donor oocytes.
Table II.
Meta-analysis of included data (to 2011): BMI and IVF outcomes in women using donor oocytes.
| BMI category (kg/m2) | Number of studiesa | Sample size | Statistical model | RR (95%CI) | Statistical heterogeneity (I2, %) |
|---|---|---|---|---|---|
| Implantation (n = 4758) | |||||
| <20 | 6 | 791 | Random effects | 1.01 (0.91–1.12) | 0 |
| 20–24.9 | 2665 | – | Reference | ||
| 25–29.9 | 6 | 892 | Random effects | 0.92 (0.822–1.02) | 0 |
| >30 | 6 | 410 | Random effects | 0.93 (0.80–1.07) | 0 |
| Clinical pregnancy (n = 4662) | |||||
| <20 | 5 | 784 | Random effects | 1.03 (0.93–1.15) | 44.8 |
| 20–24.9 | 2613 | – | Reference | ||
| 25–29.9 | 5 | 867 | Random effects | 0.94 (0.88–1.01) | 0 |
| >30 | 5 | 398 | Random effects | 0.98 (0.83–1.15) | 61.6 |
| Miscarriage (n = 4662) | |||||
| <20 | 5 | 784 | Random effects | 0.92 (0.73–1.17) | 0 |
| 20–24.9 | 2613 | – | Reference | ||
| 25–29.9 | 5 | 867 | Random effects | 0.90 (0.59–1.34) | 45.3 |
| >30 | 5 | 398 | Random effects | 1.12 (0.83–1.50) | 0 |
| Live birth (n = 568) | |||||
| <20 | 3 | 24 | Random effects | 0.83 (0.57–1.21) | 0 |
| 20–24.9 | 224 | – | Reference | ||
| 25–29.9 | 3 | 176 | Random effects | 1.02 (0.82–1.25) | 0 |
| >30 | 3 | 144 | Random effects | 0.91 (0.65–1.27) | 47.9 |
RR, risk ratio; CI, confidence intervals.
aData were available for each outcome from the following studies as listed:
Implantation: Wattanakumtornkul et al. (2003); Styne-Gross et al. (2005); Bellver et al. (2007); Bodri et al. (2010); DeUgarte et al. (2010; Washington University IVF center data; Clinical pregnancy and miscarriage: Styne-Gross et al. (2005); Bellver et al. (2007); Bodri et al. (2010); DeUgarte et al. (2010), Washington University IVF center data; Live birth: Wattanakumtornkul et al. (2003); DeUgarte et al. (2010), Washington University IVF center data.
Discussion
Obesity is common among women of reproductive age and it is associated with significant reproductive sequelae and adverse pregnancy outcomes, although the mechanisms involved are largely unknown. Significant debate exists as to which components of the reproductive process are affected most by obesity. Some have focused on the adverse effects of obesity on oocyte quality, whereas others have focused research efforts on a study of the endometrium (Jungheim and Moley, 2010). The donor oocyte model has been proposed as a model to investigate the effects of various patient characteristics and exposures on endometrial receptivity and contribution to adverse outcomes of assisted reproduction techniques (ARTs), as oocytes are often obtained from healthy young women with no known reproductive problems (Check, 1994). In this meta-analysis, we pooled the results of five studies investigating obesity in donor oocyte recipients with the primary data collected from our donor IVF program. Two of these published studies demonstrate a mild effect of obesity on embryo implantation, clinical pregnancy, miscarriage and live birth in women receiving donor oocytes, whereas the other two published studies show no effect. Collectively, the pooled results of these studies in our meta-analysis show that obesity does not significantly affect embryo implantation or the chance of clinical pregnancy. There was also no significant effect of obesity on miscarriage and live birth rates.
The two studies demonstrating a mild effect of obesity on ART outcomes in women receiving donor oocytes differed from the other two included studies showing no effect in that they excluded women with recurrent pregnancy loss and they had other exclusion criteria (Bellver et al., 2007; DeUgarte et al., 2010). It is possible that these exclusions biased the outcomes in the two studies toward showing an effect by improving the outcomes in the women without obesity or other co-morbidities. It would be beneficial if these studies had provided information on the distribution of the excluded women among the BMI groups in order to make this determination.
While this meta-analysis demonstrates no significant effect of obesity on measured ART outcomes in women receiving donor oocytes, it is important to note that live birth rates were not reported by all of the studies thus limiting our power to investigate this outcome, and adverse pregnancy outcomes were not reported in any of the studies. This is not uncommon in published clinical studies on ART. Ultimately, a healthy live birth is the goal of any ART intervention. Admittedly, we do not have adverse pregnancy event outcomes for our own patient population either. The larger body of obstetric literature demonstrates a significant negative impact of obesity on the chance of live birth and this should not be ignored when counseling obese women on the potential risks of pregnancy using donor oocytes (Chu et al., 2007).
Whereas we did not find an association between donor oocyte recipient obesity and adverse ART outcomes in our meta-analysis, large cohort and cross-sectional studies of obese women using their own oocytes have shown an association (Luke et al., 2011a,b). Meta-analyses on this topic have concurred (Metwally et al., 2008; Rittenberg et al., 2011). Because ART offers the opportunity to separate out events of the reproductive process and donor oocyte cycles offer the opportunity to isolate preconception exposures on oocytes and the endometrium, the donor oocyte model would seem the ideal model for investigating the effect of obesity on the endometrium. On the other hand, one needs to consider how, and if, this model can be used to draw conclusions regarding what happens in natural conception or even in fresh ART cycles among women using their own oocytes. In fresh ART cycles among women receiving their own oocytes, the monitoring and preparation or stimulation are usually focused on the goal of achieving optimal ovarian follicle size, whereas in donor oocyte recipients the goal is reaching optimal endometrial thickness and morphology. It is possible that the way in which the endometrium is prepared and monitored in donor oocyte cycles may override any adverse effects that obesity may have on it.
Another difficulty in using published studies to investigate the donor oocyte recipient model is that most women using donor oocytes are doing so because they are older and not eligible to go through IVF using their own oocytes. Older women often have other medical conditions that place them at higher risk for pregnancy complications and, as a result, many IVF programs place restrictions on who they will accept. For fear that obesity may decrease their published IVF success statistics, some clinics also place BMI and weight restrictions on who they will accept. Consequently, women who are morbidly obese or who have co-morbidities associated with their obesity may not be represented in the published data.
It may be argued that the studies included here are not only evaluating recipient characteristics. Donor BMI may indeed affect oocyte quality and could have biased the results presented here. Although specific data were not available for oocyte donors we would argue that, in general, most clinical practices limit oocyte donation to healthy young women who are likely to have good quality oocytes. This is the assumption we made for our meta-analysis and was based on the fact that all of the studies included in our meta-analysis stated that oocytes were from healthy young women. Thus, a difference in recipient characteristics is not likely to be a confounding factor among the studies —rather it is consistent and controlled for.
In conclusion, the pooled results of studies in our meta-analysis show that obesity is not associated with decreased embryo implantation or clinical pregnancy. There was also no effect on miscarriage and live birth rates. However, it is difficult to apply the results of this work to women conceiving naturally or through IVF using their own oocytes. A prospective study of obesity and its associations with outcomes in natural and ART conceptions will be helpful in moving this forward.
Authors’ roles
E.S.J. was involved in conception and design, acquisition of data, analysis and interpretation of data, drafting and revision of article and final approval of submitted manuscript. S.B.S., M.B.S., D.A.D.U. and S.A.F. were involved in acquisition of data, drafting and revision of article and final approval of submitted manuscript. M.G.T. was involved in conception and design, drafting and revision of article and final approval of submitted manuscript.
Funding
E.S.J and M.G.T. receive support from the Women's Reproductive Health Research Program sponsored by the United States National Institutes of Health (K12 HD063086).
Conflict of interest
None declared.
Acknowledgements
We would like to thank Dr Daniel Bodri for providing additional data for inclusion in this study
References
- Bellver J, Rossal LP, Bosch E, Zuniga A, Corona JT, Melendez F, Gomez E, Simon C, Remohi J, Pellicer A. Obesity and the risk of spontaneous abortion after oocyte donation. Fertil Steril. 2003;79:1136–1140. doi: 10.1016/s0015-0282(03)00176-6. [DOI] [PubMed] [Google Scholar]
- Bellver J, Melo MA, Bosch E, Serra V, Remohi J, Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88:446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- Bodri D, Guillen JJ, Lopez M, Vernaeve V, Coll O. Racial disparity in oocyte donation outcome: a multiethnic, matched cohort study. Hum Reprod. 2010;25:436–442. doi: 10.1093/humrep/dep414. [DOI] [PubMed] [Google Scholar]
- Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29:507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140:347–364. doi: 10.1530/REP-09-0568. [DOI] [PubMed] [Google Scholar]
- Budak E, Garrido N, Soares SR, Melo MA, Meseguer M, Pellicer A, Remohi J. Improvements achieved in an oocyte donation program over a 10-year period: sequential increase in implantation and pregnancy rates and decrease in high-order multiple pregnancies. Fertil Steril. 2007;88:342–349. doi: 10.1016/j.fertnstert.2006.11.118. [DOI] [PubMed] [Google Scholar]
- Campos I, Gomez E, Fernandez-Valencia AL, Landeras J, Gonzalez R, Coy P, Gadea J. Effects of men and recipients’ age on the reproductive outcome of an oocyte donation program. J Assist Reprod Genet. 2008;25:445–452. doi: 10.1007/s10815-008-9255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell DT, Jones KP, Peterson CM, Aoki V, Emery BR, Campbell BR. Body mass index is inversely related to intrafollicular HCG concentrations, embryo quality and IVF outcome. Reprod Biomed Online. 2001;3:109–111. doi: 10.1016/s1472-6483(10)61977-3. [DOI] [PubMed] [Google Scholar]
- Check JH. The use of the donor oocyte program to evaluate embryo implantation. Ann N Y Acad Sci. 1994;734:198–208. doi: 10.1111/j.1749-6632.1994.tb21748.x. [DOI] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, Curtis KM. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197:223–228. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dessolle L, Darai E, Cornet D, Rouzier R, Coutant C, Mandelbaum J, Antoine JM. Determinants of pregnancy rate in the donor oocyte model: a multivariate analysis of 450 frozen-thawed embryo transfers. Hum Reprod. 2009;24:3082–3089. doi: 10.1093/humrep/dep303. [DOI] [PubMed] [Google Scholar]
- DeUgarte DA, DeUgarte CM, Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93:1008–1010. doi: 10.1016/j.fertnstert.2009.07.1005. [DOI] [PubMed] [Google Scholar]
- Dokras A, Baredziak L, Blaine J, Syrop C, VanVoorhis BJ, Sparks A. Obstetric outcomes after in vitro fertilization in obese and morbidly obese women. Obstet Gynecol. 2006;108:61–69. doi: 10.1097/01.AOG.0000219768.08249.b6. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Howards PP, Cooney MA. Disentangling causal paths between obesity and in vitro fertilization outcomes: an intractable problem? Fertil Steril. 2008;89:1604–1605. doi: 10.1016/j.fertnstert.2007.03.093. [DOI] [PubMed] [Google Scholar]
- Huddleston HG, Rosen MP, Lamb JD, Modan A, Cedars MI, Fujimoto VY. Asian ethnicity in anonymous oocyte donors is associated with increased estradiol levels but comparable recipient pregnancy rates compared with Caucasians. Fertil Steril. 2010;94:2059–2063. doi: 10.1016/j.fertnstert.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Lanzendorf SE, Odem RR, Moley KH, Chang AS, Ratts VS. Morbid obesity is associated with lower clinical pregnancy rates after in vitro fertilization in women with polycystic ovary syndrome. Fertil Steril. 2009;92:256–261. doi: 10.1016/j.fertnstert.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203:525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash MM, Armstrong A. Impact of obesity on women's health. Fertil Steril. 2009;91:1712–1716. doi: 10.1016/j.fertnstert.2008.02.141. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011a;96:820–825. doi: 10.1016/j.fertnstert.2011.07.1100. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011b;26:245–252. doi: 10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13:433–444. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Fleming RF. The preconceptual contraception paradigm: obesity and infertility. Hum Reprod. 2007;22:912–915. doi: 10.1093/humrep/del473. [DOI] [PubMed] [Google Scholar]
- Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23:421–439. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Soares SR, Simon C, Remohi J, Pellicer A. Cigarette smoking affects uterine receptiveness. Hum Reprod. 2007;22:543–547. doi: 10.1093/humrep/del394. [DOI] [PubMed] [Google Scholar]
- Soares SR, Velasco JA, Fernandez M, Bosch E, Remohi J, Pellicer A, Simon C. Clinical factors affecting endometrial receptiveness in oocyte donation cycles. Fertil Steril. 2008;89:491–501. doi: 10.1016/j.fertnstert.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- Styne-Gross A, Elkind-Hirsch K, Scott RT., Jr Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril. 2005;83:1629–1634. doi: 10.1016/j.fertnstert.2005.01.099. [DOI] [PubMed] [Google Scholar]
- Thum MY, Gafar A, Wren M, Faris R, Ogunyemi B, Korea L, Scott L, Abdalla HI. Does egg-sharing compromise the chance of donors or recipients achieving a live birth? Hum Reprod. 2003;18:2363–2367. doi: 10.1093/humrep/deg464. [DOI] [PubMed] [Google Scholar]
- van der Hoorn ML, Lashley EE, Bianchi DW, Claas FH, Schonkeren CM, Scherjon SA. Clinical and immunologic aspects of egg donation pregnancies: a systematic review. Hum Reprod Update. 2010;16:704–712. doi: 10.1093/humupd/dmq017. [DOI] [PubMed] [Google Scholar]
- Wattanakumtornkul S, Damario MA, Stevens Hall SA, Thornhill AR, Tummon IS. Body mass index and uterine receptivity in the oocyte donation model. Fertil Steril. 2003;80:336–340. doi: 10.1016/s0015-0282(03)00595-8. [DOI] [PubMed] [Google Scholar]
- Zenke U, Chetkowski RJ. Transfer and uterine factors are the major recipient-related determinants of success with donor eggs. Fertil Steril. 2004;82:850–856. doi: 10.1016/j.fertnstert.2004.03.057. [DOI] [PubMed] [Google Scholar]