Abstract
STUDY QUESTION
Are overall and central obesity associated with reduced fecundability in US black women?
SUMMARY ANSWER
Overall and central obesity—based on self-reported measures of body mass index (BMI, kg/m2), waist circumference and waist-to-hip ratio—were independent risk factors for subfertility in our cohort.
WHAT IS KNOWN ALREADY
Overall obesity (BMI ≥30 kg/m2) has been associated with infertility in several studies. The role of central obesity is less clear. There are no previous studies of time-to-pregnancy (TTP) in black women.
STUDY DESIGN, SIZE, DURATION
Data were derived from the Black Women's Health Study, a prospective cohort study. During 1995–2011, there were 2239 planned pregnancy attempts reported by 1697 women, resulting in 2022 births. Cohort retention was greater than 80%.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Eligible women were aged 21–40 years and reported at least one planned pregnancy attempt during 1995–2011. Height and weight were reported in 1995, with weight updated every two years; waist and hip circumferences were reported in 1995 and updated in 2003. A validation study within the cohort showed high correlations between self-reported and technician-measured weight (r = 0.97), height (r = 0.93), waist circumference (r = 0.75) and hip circumference (r = 0.74). In 2011, TTP was reported in months. Proportional probabilities regression models were used to estimate fecundability ratios (FRs) and 95% confidence intervals (CI), adjusting for covariates.
MAIN RESULTS AND THE ROLE OF CHANCE
High BMI was associated with delayed conception: relative to BMI 18.5–24.9, FRs for BMI categories of <18.5, 25.0–29.9, 30.0–34.9 and ≥35.0 were 0.92 (CI: 0.64–1.32), 0.93 (CI: 0.84–1.03), 0.92 (CI: 0.79–1.06) and 0.73 (CI: 0.61–0.87), respectively. Associations were stronger among nulliparous women (P-interaction = 0.003). After controlling for BMI, reduced fecundability was observed among women with large waist circumferences (≥33 versus <26 inches: FR = 0.73, CI: 0.60–0.88) and large waist-to-hip ratios (≥0.85 versus <0.71: FR = 0.83, CI: 0.71–0.97).
LIMITATIONS, REASONS FOR CAUTION
TTP was reported retrospectively and error in recall is likely, particularly as time since the pregnancy increases. However, results were similar when based on the most recent versus first pregnancies. Confounding may have been introduced by the lack of control for important determinants of TTP. Nevertheless, control for maternal age and education, which are highly correlated with TTP determinants such as paternal age and persistence in trying, should reduce the extent of confounding. The analysis was confined to planned pregnancies. If pregnancy intention was related both to body size and fecundability, our results could be biased. Bias is likely to be small because we found little difference in body size and other measured characteristics between pregnancy planners and non-planners.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings add to the growing body of literature showing that excess BMI is associated with reduced fecundability and further suggest that central obesity is an important independent risk factor for infertility. The relation of obesity to infertility is especially relevant to US black women because they have higher rates of obesity and infertility. Reductions in overall and central obesity may offer the potential to improve fertility outcomes.
STUDY FUNDING/COMPETING INTEREST(S)
This work was funded by National Cancer Institute grant CA58420. We have no competing interests to report.
Keywords: fertility, obesity, body mass index, cohort study, African Americans
Introduction
The prevalence of overweight and obesity has been increasing in the United States (US) in recent decades, especially among African-American women of childbearing age (Ogden et al., 2006; Kim et al., 2007). High body mass index (BMI, kg/m2), a measure of overall body fat (Willett, 1998), has been associated with lower fecundability—the per cycle probability of conception—in several studies of women (Zaadstra et al., 1993; Jensen et al., 1999; Bolumar et al., 2000; Diamanti-Kandarakis and Bergiele, 2001; Hassan and Killick, 2004; Gesink Law et al., 2007; Ramlau-Hansen et al., 2007; Nohr et al., 2009; Wise et al., 2010). Underweight has also been associated with reduced fecundability (Zaadstra et al., 1993; Bolumar et al., 2000; Hassan and Killick, 2004; Gesink Law et al., 2007; Wise et al., 2010). Several mechanisms may be involved. Overweight (BMI 25–29 kg/m2) and obesity (BMI≥30 kg/m2) are associated with anovulation (Grodstein et al., 1994; Rich-Edwards et al., 1994; Rich-Edwards et al., 2002), biochemical alterations in the pre-ovulatory follicular environment (Robker et al., 2009), a greater propensity for intrauterine infections leading to tubal infertility (Sherman et al., 1987; Grodstein et al., 1993; Cates et al., 1994) and lower follicular-phase estradiol levels (Ziomkiewicz et al., 2008). Underweight is associated with increased FSH levels (Cramer et al., 1994), secondary amenorrhea (Frisch, 1987), shortened luteal phase (Frisch, 1987) and lower follicular-phase estradiol levels (Ziomkiewicz et al., 2008).
Central adiposity, the distribution of excess fat in the upper trunk region, is often measured by waist-to-hip ratio (WHR) or waist circumference (Evans et al., 1984; Kirschner et al., 1990; Huang et al., 1999). Data are extremely limited on the role of central adiposity in fertility. Of the three studies that have assessed the influence of central adiposity or body fat distribution on female fertility, two reported evidence of inverse associations (Zaadstra et al., 1993; Wass et al., 1997) and one found no association (Wise et al., 2010). Independent of BMI, central adiposity may deleteriously affect fertility through altered estrogen metabolism (Kirschner et al., 1990), insulin resistance or hyperinsulinemia (Falkner et al., 1999; Moran et al., 1999), oligomenorrhea (De Pergola et al., 2009) and low pH of endocervical mucus (Jenkins et al., 1995).
Studies investigating the determinants of fecundability in black women are scarce. Two nationally representative studies suggest that the prevalence of self-reported infertility is significantly higher in black women than that in white women (Wellons et al., 2008; Kelly-Weeder, 2010). Previous studies of body size in relation to fertility have not included appreciable numbers of black women, nor have they presented data stratified by race or ethnicity. To address these gaps in the literature, we assessed the association of selected anthropometric factors—BMI (measure of overall adiposity), waist circumference (measure of central adiposity) and WHR (measure of body fat distribution)—with fecundability in participants from the Black Women's Health Study (BWHS), a US prospective cohort study. The present study contributes novel data on predictors of fecundability in black women and the importance of central adiposity beyond that of overall adiposity.
Methods
Study population
The BWHS is an ongoing prospective cohort study of 59 000 African-American women aged 21 to 69 at entry in 1995 (Rosenberg et al., 1995). The baseline questionnaire elicited information on demographic and behavioral characteristics, reproductive and contraceptive histories, anthropometric factors, health care utilization and medical history. BWHS respondents live in various states across the country, with the majority residing in California, New York, Illinois, Michigan, Georgia and New Jersey. Every two years, participants are mailed a follow-up questionnaire to update their health information; follow-up through 2011 is ∼80%. The study protocol was approved by the Institutional Review Board at Boston University Medical Center.
Starting in 2003, we sent BWHS participants who provided us with an e-mail address a message containing a link to a web-based version of the follow-up questionnaire before the first mailing for each follow-up cycle (Russell et al., 2010). All those who did not respond to the web-based questionnaire within the first 6 weeks were then mailed a paper questionnaire. In the cover letter of the paper questionnaire, there was a reference to the web-based questionnaire, informing participants of the option to complete the questionnaire using either method. This allowed women who did not previously provide us with an e-mail address an opportunity to complete the web-based questionnaire. Every 2–3 months, a follow-up mailing was sent to all non-respondents, again listing the web-based questionnaire as an option.
The proportion of respondents who completed a web-based questionnaire doubled from 2003 (10%) to 2007 (20%). Web response was greater at younger ages and declined with increasing age (Russell et al., 2010). The proportion of missing data was lower on web-based questionnaires than paper questionnaires, regardless of the sensitivity of a question (Russell et al., 2010). Of the first 41 600 respondents to the 2011 follow-up questionnaire, 40% completed the web-based version (n = 16 462). After adjustment for differences in age between respondents to the web-based versus paper questionnaire (36.7 versus 40.4 years), there was little difference between the two groups at baseline with respect to mean BMI (27.7 versus 27.9 kg/m2), mean waist circumference (31.9 versus 32.0 inches), mean WHR (0.78 versus 0.79), education (15.2 versus 14.6 years), smoking (current: 13% versus 15%, former: 21% versus 19% and never: both 66%) and childbearing history (61.7% versus 65.7% parous; mean births: 2.1 versus 2.2; mean age at first birth: 22.8 versus 22.2 years).
Assessment of time-to-pregnancy
On the 2011 web-based follow-up questionnaire, women reported whether they had given birth to a child, the calendar year of each birth, whether the pregnancy was planned, whether they used any fertility medications to conceive that pregnancy, and the number of months it took to get pregnant if the pregnancy was planned. To identify women who never succeeded in becoming pregnant, we also asked: ‘Have you ever tried for 12 or more months to become pregnant without success?’ and, if yes, ‘How old were you at that time?’
Assessment of maternal anthropometric factors
In 1995, BWHS participants reported their height (feet and inches), current weight (pounds), weight at age 18 (pounds), waist circumference (inches) at the level of the umbilicus and hip circumference (inches) at its widest location. Current weight was updated every two years. Waist and hip circumferences were updated in 2005. Because taller women tend to have larger waist circumferences, we created a measure of height-adjusted waist circumference by regressing waist on height and adding the residuals to the average waist size for a woman of average height in our cohort (Giovannucci et al., 1996). We used BMI (weight (kg) divided by height squared (m2)) to measure overall obesity, waist circumference to estimate total abdominal fat and WHR to estimate the relative distribution of body fat (Giovannucci et al., 1995). In time-varying analyses, BMI data were taken from the questionnaire preceding the calendar year on which a woman reported a birth, unless the woman reported already being pregnant on that questionnaire, in which case data were taken from an earlier questionnaire.
Validation of anthropometric measures
In a validation study conducted in 2001 among 115 BWHS participants from the Washington, D.C. area (Carter-Nolan et al., 2006), the Spearman correlations between self-reported and technician-measured weight, height, waist circumference and hip circumference were 0.97 (P < 0.001), 0.93 (P < 0.001), 0.75 (P < 0.001) and 0.74 (P < 0.001), respectively (Wise et al., 2005).
Assessment of covariates
Data on age at pregnancy attempt, gravidity (number of pregnancies), parity (number of births), smoking (cigarettes per day), alcohol consumption (drinks per week), physician-diagnosed polycystic ovary syndrome (PCOS), physical activity (vigorous and moderate) and state of residence were obtained from the baseline and follow-up questionnaires. Information on education was elicited in 1995, and household income was ascertained in 2003. We estimated total metabolic equivalents (METs) per week by summing the METs from moderate physical activity (hours per week multiplied by 3.5) and vigorous exercise (hours per week multiplied by 7.0) (Jacobs et al., 1993).
Exclusions
Of the 16 462 BWHS participants who completed the 2011 web-based questionnaire, we excluded women based on the following criteria: age >40 in 1995 (n = 5599), menopausal in 1995 (n = 425), history of infertility prior to 1995 (n = 1259), currently pregnant in 1995 (n = 253) or missing/implausible BMI in 1995 (n = 74). These remaining 8852 women contributed 10 420 births. We then excluded unplanned births (n = 6106), births conceived after the women became >40 years (n = 151), births conceived before 1995 (n = 1943), births with missing data on TTP (n = 69) and births with TTP < 12 that resulted from fertility treatment (n = 102). Twenty-seven multiple gestations were recorded only once in the analysis (because we considered them a single pregnancy experience). This left 2022 births contributed by 1480 women; to this group, we added pregnancy attempts from 217 nulliparous women who reported having tried to conceive for ≥12 months without success. Thus, there were 2239 planned pregnancy attempts reported by 1697 women during 1995–2011, resulting in 2022 births; 83% (1853/2239) of conceptions occurred within 12 months.
Planned pregnancies comprised 43% of the reported pregnancies on the web-based questionnaire. Pregnancies were more likely to be planned if the respondent was older at index conception (mean: 34.1 years for planned versus 32.8 years for unplanned), older at first birth (mean: 23.8 versus 22.7 years) and nulliparous (55% versus 38%). However, after accounting for age, minor differences were found in education (mean: 15.7 versus 15.2 years), BMI in 1995 (mean: 25.0 versus 25.6 kg/m2), BMI at time of pregnancy attempt (mean: 26.7 versus 27.5 kg/m2), waist circumference (mean: 29.7 versus 30.4 inches), WHR (mean: 0.77 versus 0.78) and current smoking (5 versus 7%).
Data analysis
We assessed the relation between self-reported body size and TTP using anthropometric variables that were updated over time. BMI categories were based on World Health Organization standards (WHO, 1995) with obesity defined as BMI ≥30 kg/m2. Waist circumference and WHR were categorized into quintiles. We used proportional probabilities regression models to derive fecundability ratios (FRs) and 95% CI (Weinberg et al., 1994), using a generalized estimating equations procedure in SAS (PROC GENMOD, link = log, dist = bin), with an unstructured correlation structure, to account for multiple pregnancies per woman (SAS, 2008). The FR represents the cycle-specific probability of conception among the exposed divided by that among the unexposed. TTP was censored after 12 months if the woman had not conceived within 12 months, or the woman was nulliparous after having tried ≥12 months to conceive without success.
Multivariable models were adjusted for known or suspected confounders of the association between body size and infertility, including age at pregnancy attempt (<25, 25–29, 30–34 and ≥35), calendar year of pregnancy attempt (1995–1999, 2000–2004 and ≥2005), years of education (≤12, 13–15, 16 and ≥17), household income in 2003 (<$50 001$–$100 000, >$100 000), smoking history (current, former and never), alcohol consumption (none, <1, 1–6 and ≥7 drinks/week), physical activity (<5, 5–9, 10–14, 15–19, 20–39 and ≥40 MET-hours/week) and geographic region (Northeast, Midwest, West, South and Other). We conducted separate models that further controlled for PCOS, a potential causal intermediate. We conducted our primary analyses without controlling for parity but then performed secondary analyses with control for parity (Weinberg, 1993). BMI was positively correlated with waist circumference (r = 0.70, P<0.001) and WHR (r = 0.28, P < 0.001), and waist circumference and WHR were positively correlated with each other (r = 0.58, P < 0.001). To assess the independent contribution of overall and central adiposity, we constructed models that included both types of anthropometric variables simultaneously.
We assessed the association of body size with fecundability within strata of parity, age and WHR. Formal tests for interaction were conducted using the likelihood ratio test comparing models with and without cross-product terms between each exposure variable and the effect modifier. Tests for trend were performed by inserting the ordinal categorical variable into the regression model and calculating the associated Wald test statistic. All the analyses were conducted using SAS version 9.2 (SAS, 2008).
Results
BMI at baseline was positively associated with age at baseline, waist circumference, PCOS, current and former smoking, and residence in the South and inversely associated with year of pregnancy attempt, age at menarche, physical activity, alcohol consumption, educational attainment, income >$100 000 (in 2003) and residence in the West (Table I). Patterns were similar for WHR, with the exception of year of pregnancy attempt (positive association), age at menarche (no association), former smoking (no association) and alcohol consumption (no association). Waist circumference was similar to WHR in its relation to the baseline characteristics (data not shown).
Table I.
Characteristics of participants (pregnancies) by body mass index and waist-to-hip ratio at baseline, Black Women's Health Study, 1995–2011.
| Characteristic | Body mass index in 1995 |
Waist-to-hip ratio in 1995 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 (n = 76) | 18.5–24 (n = 1322) | 25–29 (n = 523) | 30–34 (n = 189) | ≥35 (n = 123) | <0.71 (n = 420) | 0.71–0.74 (n = 384) | 0.75–0.77 (n = 369) | 0.78–0.84 (n = 404) | ≥0.85 (n = 318) | |
| Age at baseline (years), mean | 25.8 | 26.7 | 27.6 | 27.3 | 27.3 | 26.8 | 27.7 | 27.0 | 27.2 | 26.4 |
| Age at pregnancy attempt (years), mean | 34.7 | 34.0 | 34.0 | 33.7 | 34.3 | 33.8 | 34.5 | 34.0 | 33.9 | 33.9 |
| Year of pregnancy attempt: 1995–99, % | 22 | 35 | 46 | 43 | 42 | 36 | 42 | 39 | 41 | 34 |
| Year of pregnancy attempt: 2000–04, % | 41 | 41 | 34 | 36 | 35 | 42 | 38 | 37 | 37 | 37 |
| Year of pregnancy attempt: 2005–11, % | 37 | 25 | 20 | 21 | 23 | 22 | 20 | 24 | 22 | 29 |
| Parous at time of attempt, % | 45 | 46 | 46 | 45 | 44 | 44 | 45 | 45 | 47 | 46 |
| Current smoker, % | 4 | 4 | 5 | 6 | 10 | 2 | 5 | 5 | 5 | 4 |
| Former smoker, % | 5 | 6 | 9 | 6 | 17 | 6 | 8 | 6 | 9 | 6 |
| ≥7 drinks/week, % | 5 | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 2 | 3 |
| Waist circumference (inches), mean | 25.2 | 27.7 | 32.0 | 34.6 | 39.2 | 26.7 | 27.9 | 29.4 | 31.3 | 34.3 |
| Waist to hip ratio, mean | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 | 0.8 | 0.8 | 0.9 |
| BMI (kg/m2), mean | 17.9 | 22.0 | 27.1 | 32.1 | 40.4 | 23.8 | 23.4 | 24.1 | 25.6 | 27.6 |
| Polycystic ovary syndrome, % | 3 | 2 | 2 | 3 | 5 | 1 | 2 | 3 | 2 | 4 |
| Age at menarche (years), mean | 12.8 | 12.4 | 12.1 | 11.6 | 11.8 | 12.2 | 12.3 | 12.1 | 12.2 | 12.3 |
| Education (years), mean | 15.8 | 15.8 | 15.7 | 15.6 | 15.4 | 15.8 | 15.8 | 15.9 | 15.7 | 15.5 |
| Northeast, % | 33 | 28 | 32 | 25 | 35 | 29 | 27 | 26 | 28 | 33 |
| West, % | 14 | 19 | 14 | 13 | 8 | 18 | 22 | 17 | 12 | 14 |
| Midwest, % | 26 | 18 | 21 | 20 | 22 | 21 | 17 | 19 | 22 | 16 |
| South, % | 27 | 35 | 33 | 43 | 35 | 32 | 35 | 38 | 38 | 37 |
| Total MET-hours/week, mean | 25 | 28 | 28 | 24 | 21 | 30 | 29 | 26 | 22 | 26 |
| Income in 2003: ≤$50 000, % | 32 | 14 | 19 | 16 | 33 | 14 | 17 | 14 | 14 | 24 |
| Income in 2003: $50 001–$100 000, % | 30 | 41 | 45 | 47 | 39 | 39 | 39 | 42 | 48 | 42 |
| Income in 2003: >$100 000, % | 35 | 36 | 27 | 25 | 23 | 38 | 37 | 32 | 27 | 26 |
Characteristics were ascertained at baseline (1995) unless otherwise noted. Means or percentages are standardized to the age distribution of cohort in 1995 (with the exception of age). Percentages do not sum to 100% for variables with missing data. Participants are included more than once if they had several births during 1995–2011. There were 344 (15%) missing values for waist-to-hip ratio in 1995. MET, metabolic equivalents.
From 1995 through 2011, there were 2239 planned pregnancy attempts reported by 1697 women resulting in 2022 births; 83% (1853/2239) of attempted pregnancies occurred within 12 months (Figure 1). There was evidence of digit preference in the reporting of TTP at 6 and 12 months. The three percent of women who reported a TTP = 0 were re-assigned to TTP = 1 because we assumed that they had conceived within their first cycle of trying (Joffe et al., 2005); sensitivity analyses in which these women remained coded as TTP = 0 made little difference in the effect estimates, as did using different cut points for censoring, including 10 and 14 months (data not shown).
Figure 1.
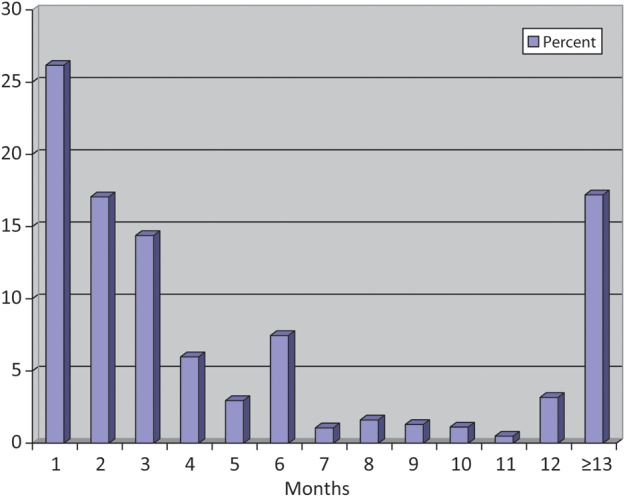
Distribution of reported time-to-pregnancy (months) in the Black Women's Health Study.
Relative to BMI 18.5–24.9 (normal weight), FRs for BMI categories of < 18.5, 25.0–29.9, 30.0–34.9 and ≥35.0 were 0.92 (95% CI: 0.64–1.32), 0.93 (95% CI: 0.84–1.03), 0.92 (95% CI: 0.79–1.06) and 0.73 (95% CI: 0.61–0.87), respectively (Table II). After controlling for BMI, fecundability was lower among women with large waist circumferences (≥33 versus <26 inches: FR = 0.73, 95% CI: 0.60–0.88; P-trend = 0.008) and women with high WHR (≥0.85 versus <0.71: FR = 0.83, 95% CI: 0.71–0.97; P-trend = 0.104).
Table II.
Anthropometric measures and time-to-pregnancy in the Black Women's Health Study, 1995–2011.
| Pregnancies | cycles | Age-adjusted model |
Multivariable modela |
Multivariable modelb |
||||
|---|---|---|---|---|---|---|---|---|
| FR | 95% CI | FR | 95% CI | FR | 95% CI | |||
| BMI, kg/m2 | ||||||||
| <18.5 | 29 | 150 | 0.94 | 0.65–1.34 | 0.94 | 0.66–1.34 | 0.92 | 0.64–1.32 |
| 18.5–24 | 957 | 4821 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 25–29 | 515 | 3206 | 0.88 | 0.80–0.98 | 0.91 | 0.83–1.00 | 0.93 | 0.84–1.03 |
| 30–34 | 219 | 1416 | 0.85 | 0.74–0.98 | 0.89 | 0.77–1.03 | 0.92 | 0.79–1.06 |
| ≥35 | 133 | 1163 | 0.67 | 0.56–0.79 | 0.71 | 0.59–0.84 | 0.73 | 0.61–0.87 |
| Waist circumference, inches | ||||||||
| <26 | 273 | 1270 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 26–27 | 357 | 1773 | 0.97 | 0.84–1.12 | 0.95 | 0.82–1.10 | 0.95 | 0.82–1.10 |
| 28–29 | 360 | 1957 | 0.92 | 0.79–1.06 | 0.90 | 0.77–1.04 | 0.90 | 0.78–1.04 |
| 30–32 | 312 | 1834 | 0.86 | 0.74–1.01 | 0.89 | 0.76–1.04 | 0.91 | 0.77–1.07 |
| ≥33 | 336 | 2737 | 0.66 | 0.57–0.77 | 0.68 | 0.58–0.80 | 0.73 | 0.60–0.88 |
| Waist-to-hip ratio | ||||||||
| <0.71 | 340 | 1799 | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| 0.71–0.74 | 305 | 1717 | 0.97 | 0.83–1.12 | 0.96 | 0.83–1.11 | 0.96 | 0.83–1.11 |
| 0.75–0.77 | 319 | 1616 | 1.02 | 0.88–1.18 | 1.02 | 0.88–1.18 | 1.03 | 0.89–1.19 |
| 0.78–0.84 | 371 | 2244 | 0.91 | 0.79–1.04 | 0.93 | 0.81–1.07 | 0.95 | 0.82–1.10 |
| ≥0.85 | 261 | 1962 | 0.75 | 0.64–0.87 | 0.78 | 0.67–0.91 | 0.83 | 0.71–0.97 |
Waist circumference is adjusted for height.
FR, fecundability ratio; CI, confidence interval.
aAdjusted for cycle number, age, calendar year of birth, smoking history, alcohol intake, physical activity, education, household income and geographic region.
bAdjusted for all variables in footnote a, plus waist-to-hip ratio (in models for BMI) and BMI (in models for waist-to-hip ratio and waist circumference).
Analyses that jointly stratified the data according to BMI and WHR did not reveal any evidence of effect modification (P-interaction = 0.180; data not shown). There were no appreciable differences in results across age at conception (<35 versus ≥35 years; P-interaction = 0.499). FRs for waist circumference and WHR were also relatively uniform across strata of age (waist: P-interaction = 0.953, WHR: P-interaction = 0.285).
Results for BMI were somewhat stronger among nulliparous than parous women (Table III). For the comparison of BMI ≥35.0 versus 18.5–24.9, the FRs were 0.63 among nulliparous women and 0.79 among parous women (P-interaction = 0.003). FRs for waist circumference and WHR were relatively uniform across strata of parity (waist: P-interaction = 0.116, WHR: P-interaction = 0.650). Control for parity slightly attenuated the effect estimates for BMI (≥35.0 versus 18.5–24.9: FR = 0.74, 95% CI: 0.62–0.87) but strengthened the effect estimates for waist circumference (≥33 versus <26 inches: FR = 0.72, 95% CI: 0.60–0.86) and WHR (≥0.85 versus <0.71: FR = 0.78, 95% CI: 0.67–0.91). Although PCOS was strongly associated with fecundability (FR = 0.52, 95% CI: 0.37–0.73), control for PCOS had minimal impact on the associations (BMI ≥35.0 versus 18.5–24.9: FR = 0.74, 95% CI: 0.62–0.89; waist ≥33 versus <26 inches: FR = 0.72, 95% CI: 0.60–0.87; and WHR ≥0.85 versus <0.71: FR = 0.84, 95% CI: 0.72–0.98).
Table III.
Anthropometric measures and time-to-pregnancy by parity history in the Black Women's Health Study, 1995–2011.
| Nulliparous women |
Parous women |
P-value, test for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancies | cycles | Multivariable modela |
Pregnancies | cycles | Multivariable modela |
||||
| FR | 95% CI | FR | 95% CI | ||||||
| BMI, kg/m2 | |||||||||
| <18.5 | 11 | 39 | 0.91 | 0.62–1.32 | 18 | 111 | 0.85 | 0.64–1.83 | 0.003 |
| 18.5–24 | 465 | 1607 | 1.00 | (ref.) | 492 | 3214 | 1.00 | (ref.) | |
| 25–29 | 279 | 1029 | 0.84 | 0.73–0.97 | 236 | 2177 | 0.99 | 0.87–1.13 | |
| 30–34 | 127 | 466 | 0.76 | 0.61–0.94 | 92 | 950 | 0.99 | 0.83–1.17 | |
| ≥35 | 75 | 364 | 0.63 | 0.48–0.81 | 58 | 799 | 0.79 | 0.63–0.99 | |
| Waist circumference, inches | |||||||||
| <26 | 133 | 735 | 1.00 | (ref.) | 181 | 1345 | 1.00 | (ref.) | 0.116 |
| 26–27 | 179 | 605 | 1.01 | 0.83–1.24 | 150 | 1111 | 0.80 | 0.67–0.96 | |
| 28–29 | 177 | 682 | 0.98 | 0.81–1.20 | 153 | 1067 | 0.71 | 0.58–0.86 | |
| 30–32 | 171 | 616 | 0.94 | 0.75–1.18 | 183 | 1532 | 0.75 | 0.61–0.93 | |
| ≥33 | 179 | 816 | 0.71 | 0.55–0.91 | 112 | 1308 | 0.66 | 0.52–0.83 | |
| Waist-to-hip ratio | |||||||||
| <0.71 | 159 | 454 | 1.00 | (ref.) | 140 | 895 | 1.00 | (ref.) | 0.650 |
| 0.71–0.74 | 155 | 606 | 1.03 | 0.85–1.25 | 178 | 1168 | 0.73 | 0.60–0.89 | |
| 0.75–0.77 | 166 | 549 | 1.02 | 0.83–1.25 | 183 | 1275 | 0.83 | 0.70–0.99 | |
| 0.78–0.84 | 188 | 712 | 0.98 | 0.81–1.19 | 141 | 1218 | 0.78 | 0.64–0.95 | |
| ≥0.85 | 149 | 654 | 0.82 | 0.66–1.03 | 157 | 1921 | 0.69 | 0.57–0.83 | |
Waist circumference is adjusted for height.
FR, fecundability ratio; CI, confidence interval.
aAdjusted for cycle number, age, calendar year of birth, smoking history, alcohol intake, physical activity, education, household income and geographic region, plus baseline BMI (in models for waist-to-hip ratio and waist circumference) and waist-to-hip ratio (in models for BMI).
Spearman correlations were 0.47 between the first and second TTP (n = 477 births), 0.40 between the first and third TTP (n = 59) and 0.65 between the second and third TTP (n = 59), all with P-values of <0.001. Associations were similar when analyses were confined to the first pregnancy attempt contributed by each woman (BMI ≥35.0 versus 18.5–24.9: FR = 0.70; waist ≥33 versus <26 inches: FR = 0.76; and WHR ≥0.85 versus <0.71: FR = 0.84) and the most recent pregnancy attempt (BMI ≥35.0 versus 18.5–24.9: FR = 0.79; waist ≥33 versus <26 inches: FR = 0.72; and WHR ≥0.85 versus <0.71: FR = 0.88). After excluding the nulliparous women who tried to conceive for ≥12 months without success, BMI and waist circumference associations with fecundability were attenuated (FRs for BMI categories of <18.5, 25.0–29.9, 30.0–34.9 and ≥35.0 versus 18.5–24.9 were 0.97, 0.95, 0.92 and 0.81, respectively; waist ≥33 versus <26 inches: FR = 0.77), but there was little change in the WHR association (≥0.85 versus <0.71: FR = 0.81). After excluding women using fertility treatments to conceive (67 births; TTP range: 12–108 months), results were not appreciably different (BMI ≥35.0 versus 18.5–24.9: FR = 0.74; waist ≥33 versus <26 inches: FR = 0.76; and WHR ≥0.85 versus <0.71: FR = 0.87). Finally, baseline (1995) analyses showed similar results to the updated analyses for all anthropometric variables (data not shown).
Discussion
In the present study of US black women, women who were very obese experienced delayed conception relative to normal weight women. Independent of BMI, women with larger waist circumferences (≥33 inches) and greater WHRs (≥0.85) also experienced reduced fecundability. Results were similar across strata of age and parity, with the exception of BMI for which results were somewhat stronger among nulliparous women. We found little evidence of an association between underweight and TTP.
Previous studies of anthropometric factors and fertility have largely been based on white women. Our results for BMI agree with that of previous studies in white women that have shown reduced fertility in overweight and obese women (Zaadstra et al., 1993; Jensen et al., 1999; Bolumar et al., 2000; Diamanti-Kandarakis and Bergiele, 2001; Hassan and Killick, 2004; Gesink Law et al., 2007; Ramlau-Hansen et al., 2007; Nohr et al., 2009; Wise et al., 2010), but not with those that found an inverse association among underweight women (Zaadstra et al., 1993; Bolumar et al., 2000; Hassan and Killick, 2004; Gesink Law et al., 2007) or the sole study that found the BMI–fertility association varied by parity status (Wise et al., 2010).
Our results for central adiposity are consistent with some (Zaadstra et al., 1993; Wass et al., 1997) but not all (Wise et al., 2010) previous studies on this topic. In a prospective study of women attending a donor insemination clinic, a 0.1-unit increase in WHR was associated with a 30% decrease in the probability of conception per cycle (FR = 0.71; 95% CI = 0.56–0.89) after adjustment for covariates (Zaadstra et al., 1993). A cross-sectional study based on an IVF population found that a WHR ≥0.80, independent of BMI, was associated with a reduced rate of IVF embryo transfer (Wass et al., 1997). In contrast, a Danish population-based prospective cohort study found no overall association of waist circumference or WHR with fecundability (Wise et al., 2010). Thus, the BWHS provides the first evidence from a population-based study of an association between central obesity and reduced fecundability.
BMI is a good measure of overall obesity in reproductive-aged women, being strongly correlated with percent body fat (r = 0.84) among women aged 20–39 (Flegal et al., 2009). In the present study, anthropometric and covariate data were reported prospectively, before the occurrence of pregnancy, thereby reducing potential for differential exposure misclassification. Repeated measurements of anthropometric factors permitted the updating of exposures over time, which showed nearly identical results to analyses using baseline measurements only. A validation study in our cohort indicated high accuracy of self-reported height, weight and waist circumference (Wise et al., 2005). However, because heavier women tend to underreport their weight, FRs for the highest BMI categories may be attenuated.
TTP is easily obtained by self-administered questionnaire and recall has been shown to be adequate (Joffe et al., 2005; Cooney et al., 2009), although accuracy declines over time, with only 70% of women reporting their TTP within ±3 months after 10 years (Cooney et al., 2009). We were unable to validate reported TTP, and there was some evidence of digit preference in the reporting of TTP. Considering that TTP was reported retrospectively, up to a decade after the occurrence of the conception, misclassification of TTP is likely and we cannot rule out the possibility that error was differential with respect to anthropometric factors (e.g. if obese women experienced irregular menses, inaccuracies in their reporting of TTP could have caused overestimation of the FRs).
Retrospective TTP studies are typically confined to women who achieve pregnancy. However, the present study incorporated pregnancy attempts from nulliparous women who tried to conceive for ≥12 months without success. Associations with BMI in the present study were somewhat weaker after the exclusion of these women, indicating that previous studies may have underestimated FRs by not including women along the full spectrum of fertility. Because our analysis did not capture nulliparous women who tried for <12 months to conceive, there was potential for bias due to differential persistence in trying (Basso et al., 2000).
TTP is highly correlated across pregnancy attempts (Basso et al., 1997; Basso, 2007; Ferrari et al., 2007; McLain et al., 2011). Many retrospective TTP studies have included only the first or most recent pregnancy (due to improved recall of TTP), but these studies make the inherent assumption that a single pregnancy is representative of all pregnancies from each woman. Methods for appropriately analyzing more than one pregnancy per woman—e.g. the discrete-time Cox frailty model—have been developed to avoid the important assumptions when restricting to a single pregnancy (Keiding et al., 1997; Scheike and Jensen, 1997; Ecochard and Clayton, 2000). Unfortunately, none of these methods permit the direct estimation of the FR, the parameter of interest in this study. Thus, we used proportional probabilities regression models with a generalized estimating equations procedure to incorporate all pregnancies reported by each woman while accounting for within-woman correlation of TTP (Joffe et al., 2005). Results were similar when confined to the first or most recent pregnancy attempt per woman, indicating that the amount of bias due to inadequately controlled correlation of TTPs was not as large as the bias due to overestimation of the FR from using a discrete-time Cox frailty model.
Whether to control for parity in analyses of fecundability is controversial (Weinberg, 1993; Howards et al., 2012). NHANES found that women tended to gain body fat with succeeding pregnancies and that increasing parity was associated with a decrease in hip and thigh circumferences, and an increase in waist circumference, independent of age and BMI (Lassek and Gaulin, 2006). Thus, childbearing promotes greater storage of fat in central versus peripheral depots, resulting in a relative increase in upper-body fat. Most previous studies on body size and fertility have controlled for parity, but adjusting for parity may represent over-adjustment: factors that affect the pregnancy under study may have had similar effects on previous pregnancy attempts (Weinberg, 1993) and parity can be thought of as a marker of underlying fecundability. Nonetheless, results of our analyses with and without control of parity were similar.
We did not collect data on several important determinants of TTP (e.g. timing of intercourse during the fertile period, age of male partner, partner BMI, change in partner, or persistence in trying) and differences in these factors by exposure may have introduced confounding. We also were unable to capture women who may have tried for less than 12 months to conceive without success and never had a birth. However, control for maternal age and education, which are highly correlated with TTP determinants such as paternal age (Ruder, 1985) and persistence in trying (Basso et al., 2000), should reduce the extent of confounding. Because the BMIs of female and male partners are positively correlated and some (Sallmen et al., 2006; Nguyen et al., 2007; Ramlau-Hansen et al., 2007) but not all (Magnusdottir et al., 2005; Wise et al., 2010) studies show an inverse association between male BMI and fecundability, lack of control for male BMI in the present study may have overestimated the association between female BMI and fecundability.
The BWHS is a convenience sample of US black women. The percentage of women who conceived within one year (83%) fell within the range of percentages found in studies of white women (range: 70–92%) (Zinaman et al., 1996; Gnoth et al., 2003; Wise et al., 2010). The few studies that provided data on black women did not directly compute cumulative pregnancy rates (Wellons et al., 2008; Butts and Seifer, 2010; Kelly-Weeder, 2010).
Given that up to 22% of early pregnancies are lost before clinical detection (Wilcox et al., 1988), we acknowledge that our findings may apply only to clinically recognized pregnancy. In addition, the analysis was confined to planned pregnancies. Although we found little difference in body size and other measured characteristics between pregnancy planners and non-planners, if pregnancy intention was related jointly to body size and fertility, our results could be biased.
Selection bias from internet participation is unlikely given that completion of the web-based 2011 questionnaire was unrelated to anthropometric factors. Although there were differences in the age demographics of women who completed the questionnaire online, we did not observe any effect modification of our associations by age.
The association of excess body fat with reduced fecundability has biologic plausibility. Obesity may affect fertility via anovulation (Grodstein et al., 1994; Rich-Edwards et al., 1994; Rich-Edwards et al., 2002) and alterations in the pre-ovulatory follicular environment (Robker et al., 2009). Obese women have abnormally high levels of fats and inflammation in the fluid surrounding their oocytes, which can adversely influence oocyte development (Robker et al., 2009). Obesity is associated with increasing follicular fluid levels of insulin, lactate, triglycerides and C-reactive protein and decreasing levels of sex hormone-binding globulin, and obesity-related differences in insulin-regulated genes in granulosa cells have also been found (Robker et al., 2009).
The relation of obesity to infertility is especially relevant to US black women because they are disproportionately affected by both conditions (Ogden et al., 2006; Kim et al., 2007; Wellons et al., 2008; Kelly-Weeder, 2010). Our data suggest that both overall and central adiposity contribute to delayed conception in black women. Reductions in obesity may offer the potential to improve fertility outcomes.
Authors' roles
J.R.P and L.R. designed the parent study and directed its overall implementation, including quality assurance and control. L.A.W. conducted the literature review, performed the statistical analyses and took the lead in drafting the manuscript for publication. All authors made contributions to interpretation of the results, drafting the article and revising the article critically for intellectual content.
Funding
This work was funded by National Cancer Institute grant CA58420.
Conflict of interest
None declared.
Acknowledgements
The authors acknowledge the technical assistance of Cordelia Russell, MPH, and Carolyn Conte, MPH, as well as the ongoing contributions of BWHS participants and staff.
References
- Basso O. Options and limitations in studies of successive pregnancy outcomes: an overview. Paediatr Perinat Epidemiol. 2007;21(Suppl 1):8–12. doi: 10.1111/j.1365-3016.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- Basso O, Olsen J, Bisanti L, Bolumar F, Kuppers-Chinnow M. Repeating episodes of low fecundability. A multicentre European study. The European Study Group on Infertility and Subfecundity. Hum Reprod. 1997;12:1448–1453. doi: 10.1093/humrep/12.7.1448. [DOI] [PubMed] [Google Scholar]
- Basso O, Juul S, Olsen J. Time to pregnancy as a correlate of fecundity: differential persistence in trying to become pregnant as a source of bias. Int J Epidemiol. 2000;29:856–861. doi: 10.1093/ije/29.5.856. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L the European Study Group on Infertility and S. Body mass index and delayed conception: A European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–1079. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- Butts SF, Seifer DB. Racial and ethnic differences in reproductive potential across the life cycle. Fertil Steril. 2010;93:681–690. doi: 10.1016/j.fertnstert.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Carter-Nolan PL, Adams-Campbell LL, Makambi K, Lewis S, Palmer JR, Rosenberg L. Validation of physical activity instruments: Black Women's Health Study. Ethn Dis. 2006;16:943–947. [PubMed] [Google Scholar]
- Cates W, Jr, Wasserheit JN, Marchbanks PA. Pelvic inflammatory disease and tubal infertility: the preventable conditions. Ann N Y Acad Sci. 1994;709:179–195. doi: 10.1111/j.1749-6632.1994.tb30397.x. [DOI] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20:56–59. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer DW, Barbieri RL, Xu H, Reichardt JKV. Determinants of basal follicle-stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79:1105–1109. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Tartagni M, d'Angelo F, Centoducati C, Guida P, Giorgino R. Abdominal fat accumulation, and not insulin resistance, is associated to oligomenorrhea in non-hyperandrogenic overweight/obese women. J Endocrinol Invest. 2009;32:98–101. doi: 10.1007/BF03345694. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bergiele A. The influence of obesity on hyperandrogenism and infertility in the female. Obes Rev. 2001;2:231–238. doi: 10.1046/j.1467-789x.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Clayton DG. Multivariate parametric random effect regression models for fecundability studies. Biometrics. 2000;56:1023–1029. doi: 10.1111/j.0006-341x.2000.01023.x. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of body fat topology to insulin sensitivity and metabolic profiles in premenopausal women. Metabolism. 1984;33:68–75. doi: 10.1016/0026-0495(84)90164-1. [DOI] [PubMed] [Google Scholar]
- Falkner B, Sherif K, Sumner A, Kushner H. Hyperinsulinism and sex hormones in young adult African Americans. Metabolism. 1999;48:107–112. doi: 10.1016/s0026-0495(99)90018-5. [DOI] [PubMed] [Google Scholar]
- Ferrari RM, Cooney MA, Vexler A, Liu A, Buck Louis GM. Time to pregnancy and multiple births. Hum Reprod. 2007;22:407–413. doi: 10.1093/humrep/del374. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch RE. Body fat, menarche, fitness and fertility. Hum Reprod. 1987;2:521–533. doi: 10.1093/oxfordjournals.humrep.a136582. [DOI] [PubMed] [Google Scholar]
- Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–420. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Godehardt D, Godehardt E, Frank-Herrmann P, Freundl G. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18:1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Goldman MB, Cramer DW. Relation of tubal infertility to history of sexually transmitted diseases. Am J Epidemiol. 1993;137:577–584. doi: 10.1093/oxfordjournals.aje.a116711. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Hassan MAM, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–392. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. ‘Toward a clearer definition of confounding’ revisited with directed acyclic graphs. Am J Epidemiol. 2012;176:506–511. doi: 10.1093/aje/kws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, Speizer FE, Hankinson SE. Waist circumference, waist: hip ratio, and risk of breast cancer in the Nurses’ Health Study. Am J Epidemiol. 1999;150:1316–1324. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jenkins JM, Brook PF, Sargeant S, Cooke ID. Endocervical mucus pH is inversely related to serum androgen levels and waist to hip ratio. Fertil Steril. 1995;63:1005–1008. doi: 10.1016/s0015-0282(16)57538-4. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–428. doi: 10.1097/00001648-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- Keiding N, Andersen PK, Klein JP. The role of frailty models and accelerated failure time models in describing heterogeneity due to omitted covariates. Stat Med. 1997;16:215–224. doi: 10.1002/(sici)1097-0258(19970130)16:2<215::aid-sim481>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kelly-Weeder S. Impaired fertility in African-American women: an investigation of behavioral risks. J Natl Black Nurses Assoc. 2010;21:9–15. [PubMed] [Google Scholar]
- Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity. 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab. 1990;70:473. doi: 10.1210/jcem-70-2-473. [DOI] [PubMed] [Google Scholar]
- Lassek WD, Gaulin SJC. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol. 2006;131:295–302. doi: 10.1002/ajpa.20394. [DOI] [PubMed] [Google Scholar]
- Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005;20:208–215. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- McLain AC, Sundaram R, Cooney MA, Gollenberg AL, Buck Louis GM. Clustering of fecundability within women. Paediatr Perinat Epidemiol. 2011;25:460–465. doi: 10.1111/j.1365-3016.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- Moran C, Hernandez E, Ruiz JE, Fonseca ME, Bermudez JA, Zarate A. Upper body obesity and hyperinsulinemia are associated with anovulation. Gynecol Obstet Invest. 1999;47:1–5. doi: 10.1159/000010052. [DOI] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J. Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Hum Reprod. 2009;24:226–232. doi: 10.1093/humrep/den357. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, Manson JE. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, Willett WC, Wand H, Manson JE. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13:184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared to moderate-weight women. J Clin Endocrinol Metab. 2009;94:533–540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Adams-Campbell LL, Palmer JR. The Black Women's health study: a follow-up study for causes and preventions of illness. J Am Med Women’s Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- Ruder A. Paternal-age and birth-order effect on the human secondary sex ratio. Am J Hum Genet. 1985;37:362–372. [PMC free article] [PubMed] [Google Scholar]
- Russell CW, Boggs DA, Palmer JR, Rosenberg L. Use of a web-based questionnaire in the Black Women's Health Study. Am J Epidemiol. 2010;172:1286–1291. doi: 10.1093/aje/kwq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- SAS. 2008. SAS Institute Inc. SAS/STAT® 9.2 User's Guide. Cary, NC, 2008 SAS Institute, Cary, NC.
- Scheike TH, Jensen TK. A discrete survival model with random effects: an application to time to pregnancy. Biometrics. 1997;53:318–329. [PubMed] [Google Scholar]
- Sherman KJ, Daling JR, Weiss NS. Sexually transmitted diseases and tubal infertility. Sex Transm Dis. 1987;14:12–16. doi: 10.1097/00007435-198701000-00003. [DOI] [PubMed] [Google Scholar]
- Wass P, Waldenstrom U, Rossner S, Hellberg D. An android body fat distribution in females impairs the pregnancy rate of in-vitro fertilization-embryo transfer. Hum Reprod. 1997;12:2057–2060. doi: 10.1093/humrep/12.9.2057. [DOI] [PubMed] [Google Scholar]
- Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;137:1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Baird DD, Wilcox AJ. Sources of bias in studies of time to pregnancy. Stat Med. 1994;13:671–681. doi: 10.1002/sim.4780130528. [DOI] [PubMed] [Google Scholar]
- Wellons MF, Lewis CE, Schwartz SM, Gunderson EP, Schreiner PJ, Sternfeld B, Richman J, Sites CK, Siscovick DS. Racial differences in self-reported infertility and risk factors for infertility in a cohort of black and white women: the CARDIA Women's Study. Fertil Steril. 2008;90:1640–1648. doi: 10.1016/j.fertnstert.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. 2nd edition. New York: Oxford University Press; 1998. [Google Scholar]
- Wise LA, Palmer JR, Spiegelman D, Harlow BL, Stewart EA, Adams-Campbell LL, Rosenberg L. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16:346–354. doi: 10.1097/01.ede.0000158742.11877.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, Karbaat J. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. Bmj. 1993;306:484–487. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
- Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Hum Reprod. 2008;23:2555–2563. doi: 10.1093/humrep/den213. [DOI] [PubMed] [Google Scholar]


