Abstract
Saponin SC-2 from Solanum chrysotrichum showed antifungal activity, demonstrated in vitro, which inhibited the growth of dermatophytes, and in vivo, to be effective in the treatment against tinea pedis and pityriasis capitis. Fungistatic and fungicidal activity of saponin SC-2 on Candida albicans and other Candida species, fluconazole and ketoconazole resistaent strains was demostrated. SC-2-associated ultrastructural alterations in several Candida species were observed. An exploratory clinical, randomized, double-blind, and controlled ketoconazole study of ketoconazole was conducted with the aim of assessing the effectiveness and tolerability of an herbal medicinal product containing SC-2, on women with Vulvovaginal candidiasis (VVC). The results exhibited a percentage of therapeutic clinical effectiveness similar to that of ketoconazole (X2, p ≥0.30), but obtained a smaller percentage of mycological effectiveness, and 100% tolerability. In conclusion, saponin SC-2 possesses fungicidale and fungistatic activity on Candida albicans and other multi resistant Candida species, causes morphological changes and fungal death, and it is an alternative therapy for the treatment of VVC.
Keywords: Solanum chrysotrichum, saponins, antifungal activity, vulvovaginal candidiasis, alternative therapy
Introduction
According to numbers of the World Health Organization (WHO), up to 70% of the world population, especially, in some Asian and African countries, depends on traditional medicine for their primary health care. This is in part due to limited access to synthetic drugs for logistic and economic reasons, but also is because of cultural reasons: indigenous persons may also make a positive choice for traditional medicine because they believe in its healing powers. Thus, the WHO has an active policy of integrating traditional medicine into local health care systems. The use of plant preparations can be supported if these are safe and if their activity can be scientifically confirmed. This implies the need for quality control and standardization (Caballero and Gupta, 2011; Xu, et al., 2011).
Medicinal plants remain an endless source of traditional herbal medicine and the approach has led to the discovery of numerous pharmacologically active molecules and drug leads for humans. Evidence of the biomedical potential of herbal drugs employed worldwide in traditional medicine is continually being reported. Biological activities, include those of being antioxidant, antihypertensive agents, antitumor promoter, antimutagenic, cytotoxic for cancer cell lines, possessing an anti-inflammatory effect, managing of Diabetes mellitus (DM), treating of cardiovascular diseases, enhancing the production of selected blood parameters, and having hepatoprotector activities, allergic asthma activities, immune response activities and immunosuppressive activities (Afifi, et al., 2011; Feily and Namazi, 2009; Gertsch and Anavi-Goffer, 2012; Ito, 2011; Knuth, et al., 2011; Koch and Fathi, 2011; Zhou, et al., 2011).
In addition, it has been widely documented that, medicinal plants or their secondary metabolites possess activity against infectious agents such as antiviral, antibacterial, antifungal, antimalarial, etc. (Nakashima et al., 1992; Shiota, et al., 2000; Caballero and Gupta, 2011; Xu, et al., 2011).
The Solanum chrysotrichum plant species has been used in Mexican traditional medicine for the treatment of dermatological infections. Phytochemical and microbiological studies have reported isolation, structural identification, and antifungal activity in dermatophytes and in the Malassezia species of Spirostatic saponin SC-2 (Figure 1) (Lozoya, et al., 1992; Herrera-Arellano, et al., 2003; Herrera-Arellano et al., 2004; López-Villegas, et al., 2009). In this review we analyzed the antifungal activity of SC-2 in vitro on Candida species, and the clinical effectiveness of an S. chrysotrichum herbal medicinal product (Sc-hmp), that was standardized in 1.82 mg of saponin SC-2, in patients with Vulvovaginal candidiasis (VVC). SC-2 reported fungistatic and fungicidal effect in vitro on Candida albicans and other Candida species as such as C. lusitaniae, C. glabrata and C. krusei, which are susceptible or resistant to fluconazole, and also to SC-2, which caused ultrastructural morphological changes such as the digestión of cellular content, evident degradation of yeast, and fungal death. An exploratory clinical study on VVC reported hight percentages of tolerability, clinical cure (elimination of signs and symptoms) and mycologycal effectiveness (negative isolations of etiologic agents) with the use of Sc-hmp (Herrera-Arellano, et al., 2007, Alvarez, et al., 2009; Herrera-Arellano, et al, 2009).
Figure 1.
Antimycotic spirostatic saponins from Solanum chrysotrichum. (Zamilpa, et al., 2002).
Vulvovaginal candidiasis (VVC) and resistance of Candida species to conventional therapy Epidemiology
VVC is the infection caused by Candida spp. It affects 70–75% of women at least once during their lifetimes, most frequently in young women of childbearing age. Adult women (5–8%) will have Recurrent vulvovaginal candidosis (RVVC); this is defined as four or more episodes annually, although, prevalence studies indicate that Candida spp. can be isolated from the vaginas of about 20% (range 10–80%) of asymptomatic, healthy women (Ostrosky-Zeichner, et al., 2002; Consaro, et al., 2004).
Clinical data
The most frequent symptoms of VVC comprise the following: pruritus (56%); vaginal discharge (69%); dyspareunia (25%); vulvar itching or burning (38%); vulvar swelling/edema (25%); white discharge (68%), and vulvar redness (22%). Symptoms may sometimes be aggravated by sexual intercourse (Hoffstter, et al., 2008; Jelovsek, 2010; Martins, et al., 2012; Ray et al., 2011).
Candida species causes VVC
Yeast strains isolated from the vagina are C. albicans in between 85 and 95% of cases and the remainder includes other Candida species the most common of which is Candida glabrata. In many parts of the world, C. glabrata affects 10–20% of women and often causes RVVC. Other species found that caused VVC are as follows: C. parapsilosis, C. tropicalis, C. kefyr, C. lusitaniae and C. krusei. These other Candida species that caused VVC are clinically indistinguishable from those caused by C. albicans; moreover, such species are often more treatment-resistant, and clinical signs and symptoms of infection caused by C. glabrata and C. parapsilosis tend to be associated with milder, and often absent symptoms (Consaro, et al., 2004; Murina, et al., 2011; Ray et al., 2011). A higher cumulative incidence of Candida spp. colonization is reported in some women. Blastoconidia represent the phenotypic form responsible for vaginal transmission and asymptomatic colonization of the vagina. Germinated yeasts, which have produced mycelia (hyphae), are found most commonly in symptomatic vaginitis. Sexual transmission and asymptomatic colonization of the male genitalia with Candida is four times more common in the sexual partners of infected women than in those of non-infected women (Sobel, 2007; Hoffstetter, et al., 2008; Fisher and Bradford, 2011).
Predisposing factors
The principal predisposing factors for VVC are the following: i) therapy with antibacterial drugs, such as systemic broad spectrum antibiotics (tetracycline or amoxicillin clavulinate); these can eliminate the protective bacterial flora. Lactobacillus spp. could provide colonization resistance and prevent germination, maintaining low numbers of yeast; ii) pregnancy, a higher prevalence of vaginal colonization and symptomatic vaginitis are more often observed in pregnant women; iii) contraceptives, these increase vaginal colonization with Candida after the use of oral contraceptives with high estrogen content and in users of intrauterine contraceptive devices, contraceptive sponges, diaphragms, and condoms, with or without spermicides; iv) patients with DM; v) HIV infection, the number of women with HIV grew in the 1980s, vaginal candidiasis was also increasingly reported, and there is a tendency to isolate notably C. glabrata and Candida isolates notably with reduced sensitivity to fluconazole; vi) behavioral factors, the incidence of VVC increases in the second decade of life, corresponding to the onset of sexual activity, cultural differences affect the frequency of Candida species in some groups, women's culture and ethnicity influence their diet, hygiene, and sometimes the use of modern medicine, among other factors; vii) hypersensitivity, chemical contact, atopy, local allergy, or hypersensitivity reactions could alter the vaginal milieu and facilitate transformation from asymptomatic colonization into symptomatic vaginitis (Goswami et al., 2006; Sobel, 2007; Wei, et al., 2010; Fisher and Bradford, 2011).
Resistance of Candida species to conventional therapy
The recommended therapy for VVC is topical vaginal treatment with imidazoles; other therapy comprises topical application of nystatin, amphotericin B, fluconazole, ketoconazole and miconazole, and oral itraconazole. Systemic therapy is recommended when the infection is severe or recurrent or if the patient has major predisposing factors (AIDS or chemotherapy for cancer). Imidazoles have a potential side effect of systemic toxicity, which has dramatically restricted the use of ketoconazole (Kaplanek, 2006; Ray et al., 2011). C. glabrata strains isolated from cases of RVVC demonstrate reduced sensitivity to fluconazole compared with C. albicans. Maintenance of fluconazole prophylaxis could favor the growth of C. glabrata in the vagina. Also, certain strains of C. glabrata are fluconazole_resistant. Minimal inhibitory concentration (MIC) data for these fluconazole_resistant isolates revealed extensive cross-resistance to the other azoles tested, such as itraconazole, ketoconazole, and voriconazole (Ostrosky-Zeichner and Rex, 2002; Sanguinetti et al., 2005; Ray et al., 2011; Wang, et al., 2010). The mechanisms of resistance to azole antifungal agents have been well elucidated in C. albicans and can be mainly categorized as follows: (i) changes in the cell wall or plasma membrane; (ii) alterations in the affinity of the drug target Erg11p (lanosterol 14α-demethylase) to azoles or in the cellular content of Erg11p due to target_site mutation or overexpression of the ERG11 gene; and (iii) the efflux of drugs mediated by membrane transport proteins belonging to the Adenosine triphosphste (ATP)-binding cassette (ABC) transporter family (CDR1 and CDR2) or to the major facilitator superfamily (MDR1 and FLU1). In C. glabrata, increased levels of expression of ABC transporter genes C. glabrata CDR1 (CgCDR1) and C. glabrata CDR2 (CgCDR2) have also been observed in azole_resistant isolates. The role of the upregulation of the ABC efflux transporters is a major mechanism of azole-resistance in C. glabrata, but the multifactorial nature of azole resistance in C. glabrata must not be disregarded (Sanguinetti et al., 2005; Paulitsch, et al., 2006).
Ethnobotanical antecedents of Solanum chrysotrichum and SC-2
The plant species Solanum chrysotrichum (Solanaceae) is employed in the highlands of Chiapas State in Mexico for empirical treatment of different fungus- and yeast-associated skin and mucosal infections. An ethnobotanical field study carried out in 200 rural communities determined that the leaves of Solanum chrysotrichum Schldl. are utilized in Mexican traditional medicine for the treatment of skin mycoses, particularly recommended for curing Tinae pedis. The methanolic extract from this plant inhibited the growth in vitro of Trichophyton mentagrophytes, T. rubrum, Microsporum gypseum (MIC = 15 µg/mL) and Candida genus yeasts (MIC = 200 µg/mL) (Lozoya et al., 1992, Zamilpa et al. 2002). On the other hand, clinical studies have shown that the methanolic extract of S. chrysotrichum leaves in the form of skin cream and shampoo possesses high rates of clinical cure and fungal eradication in local treatment of Tinea pedis and Malassezia genus yeast-associated Pityriasis capitis (Herrera-Arellano, et al., 2003; Herrera-Arellano, et al., 2004). Other phytochemical and microbiological studies, such as bioactivity-directed isolation procedures, have allowed identification, isolation, and structural elucidation of a group of spirostanic saponins to which antifungal activity is attributed; this group was identified with the names comprising SC-2 through -SC-6 (Zamilpa et al., 2002). SC-2 (Figure 1) showed to be the most active molecule because it demonstrated fungistatic and fungicidal activity against 12 different Candida albicans strains and other Candida species, including some isolated multi-resistant clinical yeasts. SC-2 saponin concentrations between 200 and 800 µg/mL caused injuries in all of the yeast's cell membranes, including those of the cytoplasm and nuclear and mitochondrial membranes, which finally led to the disintegration and death of the fungus (Herrera-Arellano, et al., 2007). Moreover, our group demostrates the ultra-structural injuries that SC-2 originates on three dermatophytes, Trichophyton rubrum, T. mentagrophytes and Microsporum gypseum (López-Villegas, et al. 2009).
Chemical isolation and identification of SC-2 saponin from S. chrysotrichum
By means of bioguided phytochemical studies of leaves from S. chrysotrichum, the separation and isolation of two known sterol glycosides (3-O-β-D-sitosterol glycoside and 3-O-β-D-stigmasterol glycoside) and six spirostanol saponins were achieved. For this purification, methanol extract (280 g) was suspended in water and partitioned with chloroform. The organic fraction (138 g) was concentrated to dryness and fractionated by silica gel chromatographic column. n-hexane-chloroformmethanol mixtures of increasing polarity were employed as mobile phase to yied five fractions. Re-purification of the most steroidal saponin concentrate fractions (F3, 0:100:0; F4, 0:95:0 and F5, 0:90:10) by normal and reverse phase chromatography afforded 15 mg of SC-3, 12 mg of SC-4, 60 mg of SC-2, and 3.5 g of a mixture of SC-5 and SC-6 (Zamilpa, et al., 2002). Structural elucidation of these steroidal metabolites was performed by spectroscopic measurements analysis, specially 1D and 2D Nuclear magnetic resonsnce (NMR) data (SC-2, SC-3, SC-4, SC-5, and SC-6). Their 1H and 13C NMR spectra indicated that these saponins consist of a C-27 spirostanol skeletal with different hydroxylation and glycosylation levels. Acid hydrolysis of SC-2, SC-3, SC-4 and SC-5 afforded chlorogenin [(25R)-5α-spirostan-3β-5α-diol] such as aglycone. This sapogenin exhibited a molecular ion M+ at m/z 432 (C27H44O4). The 1H NMR spectrum of 8, displayed four signals at d 0.74 (s), 0.83 (s), 1.31 (d) and 1.31 (d) ppm, which were attributed to the C-18, C-19, C-21 and C-26 methyl groups, respectively. Acetylation of SC-2 produced compound SC-2a, which was obtained as a white amorphous powder. This peracetate derivative displayed the molecular ion at m/z 962.4901 in High resolution fast atom bombardment mass spectrometry (HRFABMS), which corresponded to the molecular formula C50H74O18. The 13C MNR spectrum showed 50 signals: 29 were assigned to the acetylated spirostanol moiety and 21 to the saccharide portion (peracetylated quinovose-xylose). The interglycosidic linkage among sugar units was derived from Heteronuclear multiple bond correlations (HMBC) and Nuclear Overhauser enhancement spectroscopy (NOESY) interactions. The anomeric proton of xylose at δ 4.45 (d, J= 8.0) displays a long range correlation with C-3 of the quinovopyranosyl unit at δ 80.8 ppm, indicating that xylose sugar was the terminal saccharide unit. The position of the sugar residue was at C-6 due to the long-range correlation between the C-6 (δ 80.69) of the aglycon and the H-1 (δ 4.33) of the quinovopyranosyl unit. The natural product SC-2 was identified as 6α-O-β-D-xylopyranosyl-(1→3)-β-D-quinovopyranosyl-(25R)-5α-spirostan-3β-ol. The remaining saponins are: denominated as follows: 6α-O-β-D-xylopyranosyl-(25R)-5α-spirostan-3β-ol (SC-3); 6α-O-β-quinovopyranosyl(25R)-5α-spirostan-3β-ol (SC-4); 6α-O -α-L-rhamnopyranosyl(1→3)-β-D-quinovopyranosyl-(25R)-5α-spirostan-3β-ol (SC-5), and 6α-O-α-L-rhamnopyranosyl(1→3)-β-D-quinovopyranosyl-(25R)-5α-spirostan-3β-,23α-diol (SC-6) (Zamilpa et al., 2002).
Microbiological studies and ultrastructural changes in fluconazole-susceptible or -resistant Candida species by SC-2
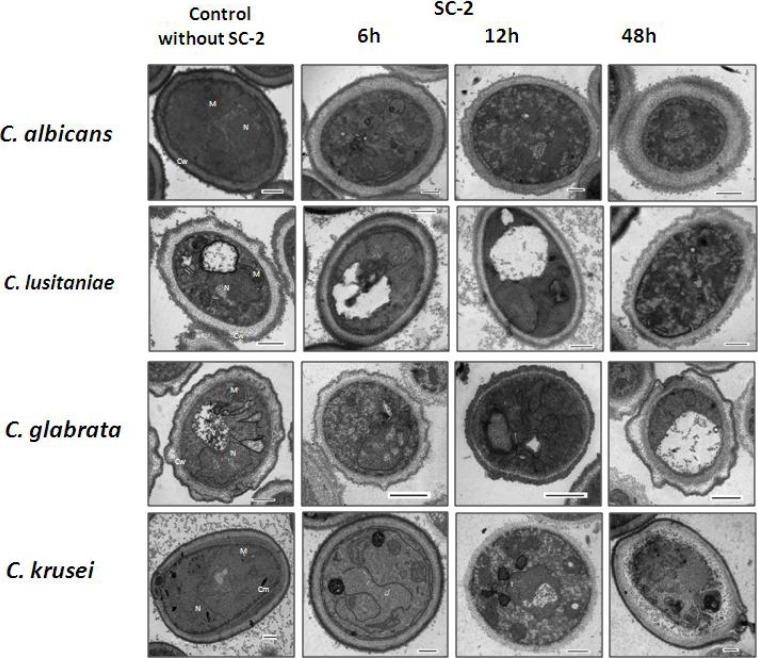
In recent years, an increase has been reported in the number of patients, principally immunocompromised, with local or systemic infections caused by Candida genus yeasts; thus, there is also an increase of cases with candidiasis resistance or multiresistance to conventional antifungals. Susceptibility or resistance profiles to fluconazole, itraconazole, ketoconazole, and amphotericin B for all strains were assessed and compared with the saponin SC-2 effect. SC-2 displayed fungistatic and fungicidal activities against all of the Candida strains assayed. Minimal fungicidal concentration (MFC) was defined as the minimal concentration at which no visible growth was observed and represents the killing of >99% of the original inocula. Growth inhibition and fungicidal activity was observed at a SC-2 concentration between 200 and 800 µg/mL (Herrera-Arellano, et al., 2007). C. albicans was highly susceptible to SC-2 concentration, while C. glabrata (that was resistant to the drugs tested) was less susceptible to SC-2 (IC50 and MFC = 800 µg/mL). SC-2 exhibited fungistatic and fungicidal activities on fluconazole susceptible or not C. albicans and other Candida species. The difference in susceptibility of Candida strains to SC-2 can be related with the chemical composition of the fungal membrane and the cell wall, which vary for each species (Herrera-Arellano et al., 2007). Ultrastructural damages on Candida species were described by Herrera and collegues. The antifungal activity of SC-2 was shown in clinical isolates of C. albicans and, C. lusitaniae (fluconazole-susceptible strains) and in, C. glabrata and, C. krusei (fluconazole-resistant strains). Despite that C. albicans is more susceptible than other species tested in SC-2, all these showed the same alterations between 6 and 12 h postexposure by Transmission electron microscopy (TEM) (Figure 2). Membranes appeared disrupted and organelles such mitochondria and nucleus lost conventional morphology. Damage increase gradually, with the disappeararance of organelle organization, cytoplasm degradation and finally, cellular disintegration. Deformation, degradation and loss of cellular wall were observed as well. The changes observed were dramatically irreversible and causeed cell death at 2448h post exposure in all strains assayed (Herrera-Arellano, et al., 2007).
Figure 2.
Ultrastructural changes caused by SC-2 saponin on Candida species. Cw, cell wall; Cm, Cellular membrane; N, Nucleus; M, Mitochondria; V, Vacuole. Bar represents 500 nm.
Exploratory study on the clinical effectiveness of SC-2 from S. chrysotrichum
With the aim of exploring the effectiveness and tolerability of an herbal medicinal product developed from an extract of S. chrysotrichum, standardized in SC-2, in the treatment of patients with cervical or vaginal infection caused by Candida genus yeasts, we implemented a randomized, double_blind clinical trial controlled with ketoconazole (Herrera-Arellano, et al., 2009). Experimental treatment was formulated with 125 mg of the dry S. chrysotrichum extract, containing 33.72 mg of total saponins and 1.89 mg of SC-2. Control treatment contained 400 mg of ketoconazole United States Pharmacopeia (USP). Both treatments were prepared as vaginal suppositories, with de the same presentation. The inclusion factor in the study was women with clinical diagnoses of VVC wich was confirmed by mycological studies including direct examination, culture, and Candida species identification (Hoffstetter et al., 2008). The study did not include patients with diabets, breast-feeding women, and cases with VVC complicated by parasites and/or bacteria and with local or systemic administration of anti-fungal treatment during the month prior to the study. The experimental procedure consisted of the following four evaluations: before treatment (0); at days 3–4 of treatment initiation (1); after concluding a 7-day treatment (2), and 3 weeks after concluding the treatment (3). These evaluations consisted of clinical assessment (based on the intensity of genital infection-related signs and symptoms) and mycological studies (direct examination and culture, and identification of the Candida species at baseline conditions). Seven suppositories were provided to each study participant for deep vaginal application of one suppository each night before going to bed. For mycological studies, two samples of genital discharge from cervix and vaginal wall were taken to practice direct examination, yeast isolation and identification. Direct examination was positive on observing yeasts and the pseudohyphae characteristic of Candida infection. For isolation, yeasts were grown, purified, and identified. Clinical trial evaluated the following outcomes: a) clinical effectiveness, declared when signs and symptoms attributed to VVC were totally absent; b) mycological effectiveness, achieved upon detection of a negative direct examination and culture; c) therapeutic effectiveness, when clinical and mycological improvement coincided, and d) tolerability, determined by the absence of local and systemic side effects attributed to the assigned treatment. Post-treatment effectiveness was discerned by means of clinical and mycological evaluations, conducted 3 weeks after the treatment ended. The study included 101 women (49 were in the experimental group). On average, patient age was 33 years (range, 17–54 years), clinical-condition evolution time was 9 months, number of pregnancies was two (range, 0–9 pregnancies), and number of sexual partners was 1.2 (range, 1–6 sexual partners). The majority of patients had already received some previous pharmacological treatment, used cotton clothing, and a few utilized tampons; there were no significant differences on comparing these parameters in both treatment groups (p ≥0.21). Under baseline conditions, the intensity of signs and symptoms that the patients in both groups presented was not different (p ≥0.18); a moderate clinical condition predominated. In relation to mycological studies at study initiation, there were no differences between groups (p ≥0.39), direct examination was positive in 70% of cases, the culture was positive in 91%, isolating mainly C. albicans (71.74%) followed by C. glabrata (26.08%), and in only two cases, C. tropicalis (2.17%). At day 4 of treatment initiation, only mycological effectiveness reached statistical differences favorable to the control treatment, because the fungus was eradicated in 79.07% of cases, compared with eradication in 48.78% obtained by S. chrysotrichum treatment (p = 0.004). When finalizing the 7 treatment days, at the end of the administration period, percentages of mycological and therapeutic—but not clinical—effectiveness were higher in the control group (p ≤0.01). Only one half of patients who initiated the study presented for the final evaluation, which was conducted 3 weeks after treatment conclusion. Despite this, outcomes in this study phase showed no significant differences (X2, p ≥0.30); the majority of patients exhibited absence of signs and symptoms related with VVC as well as negative mycological studies. It is noteworthy that in this last evaluation, the percentage of fungus eradication in the S. chrysotrichum-treated group was higher than that on conclusion of the 7-day treatment (62.86 vs. 79.17%), which indicates that this possesses a residual antifungal effect.
Conclusion
SC-2 has fungistatic and/or fungicidal activity, demonstrated by IC50 and MCF; the mechanism is unknown by TEM, but it could possibly be analogous to that of the secondary metabolites, such as the steroidal glycoalkaloids alpha-solanine, alphachaconine, and alpha-tomatine, as well as solandidine aglycone, which interact with the cytoplasmic-membrane sterols, causing structural alterations and permeabilization, all of which lead to cell destruction. Plant glycoalkaloids can act on fungal cell membranes, which includes the following three steps: i) insertion of the membrane bilayer; ii) formation of a sterol-glycoalkaloid complex, and iii) membrane rearrangement. The final effect concludes with the formation of membrane channels with an increase in their permeability that, facilitates the escape of small cytoplasm molecules, causing membrane disruption. These cytoplasmic-membrane lesions cause irreversible damage and intracellular organelle disintegration, which inhibits the growth of the fungus, and finally, death (Herrera-Arellano, et al., 2007). Additionally, the saponins, including SC-2, possess a detergent effect that diminishes superficial tension, causing cell-wall damage and fungal-cell disintegration. Additional studies are necessary to clarify the action mechanism of SC-2.
Our clinical study shows that, when concluding the 7 treatment days of treatment and at the dose employed the S. chrysotrichum herbal medicinal product, containing 125 mg of extract with 33.72 mg of total saponins and standardized in 1.89 mg of SC-2, exhibited a percentage of therapeutic clinical effectiveness that was not different from that obtained with ketoconazole, but it did obtain a smaller percentage of mycological effectiveness, and 100% tolerability. The severe in vitro damage observed in Candida species caused by SC-2 can explain the results observed on clinical study. These results allow consideration of SC-2 as an excellent alternative for treatment of VVC. Clinical studies, with higher doses to SC-2, are necessary to increase its clinical and mycological therapeutic effectiveness in VVC (Herrera-Arellano, et al., 2007; Herrera-Arellano, et al., 2009).
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- (ATP)
Adenosine triphosphate
- (ABC)
binding cassette
- Cm
Cellular membrane
- Cw
Cell wall
- CDR1 and CDR2
Cassette drug resistance 1 and 2
- DM
Diabetes mellitus
- ERG11
the Ergosterol 11 gene
- FLU1
the Fluconazole1-gene
- HMBC
Heteronuclear multiple bond correlations
- HRFABMS
High resolution fast atom bombardment mass spectrometry
- HIV
Human immunodeficiency virus
- MDR1
Multidrug resistance 1
- MFC
Minimal fungicidal concentration
- MIC
Minimal inhibitory concentration
- M
Mitochondria
- N
Nucleus
- NMR
Nuclear magnetic resonance
- NOESY
Nuclear Overhauser enhancement spectroscopy
- RVVC
Recurrent vulvovaginal candidosis
- TEM
Transmission electron microscopy
- USP
United States Pharmacopeia
- V
Vacuole
- VVC
Vulvovaginal candidiasis
- WHO
the World Health Organization
References
- 1.Alvarez L, Herrera-Arellano A, Marquina S, Tortoriello J, Zamilpa A, Gonzalez M, Villarreal ML, Martinez-Rivera MA, Lopez-Villegas EO, Rodriguez-Tovar AV, Puebla-Pérez AM, Villaseñor-Garcia M, Navarro V. Anti-mycotic and anti-inflammatory constituents from four Mexican medicinal Solanum species. Curr Top in Steroid Res. 2009;6:89–104. http://www.cabdirect.org/abstracts/20113031779.html. [Google Scholar]
- 2.Afifi-Yazar FU, Kasabri V, Abu-Dahab R. Medicinal plants from Jordan in the treatment of cancer: traditional uses vs. in vitro and in vivo evaluations — Part 1. Planta Med. 2011;77:1203–1209. doi: 10.1055/s-0030-1270832. https://www.thiemeconnect.com/ejournals/pdf/plantamedica/doi/10.1055/s-0030-1270832.pdf. [DOI] [PubMed] [Google Scholar]
- 3.Caballero-George C, Gupta MP. A quarter century of pharmacognostic research on Panamanian flora: a review. Planta Med. 2011;77:1189–1202. doi: 10.1055/s-0030-1271187. https://www.thieme-connect.com/ejournals/pdf/plantamedica/doi/10.1055/s-00301271187.pdf. [DOI] [PubMed] [Google Scholar]
- 4.Consolaro MEL, Albertoni T A, Yoshida CS, Mazucheli J, Peralta RM, Svidzinski TIE. Correlation of Candida species and symptoms among patients with vulvovaginal candidiasis in Maringá, Paraná, Brazil. Rev Iberoam Micol. 2004;21:202–205. http://www.reviberoammicol.com/2004-21/202205.pdf. [PubMed] [Google Scholar]
- 5.Feily A, Namazi MR. Aloe vera in dermatology: a brief review. G Ital Dermatol Venereol. 2009;144:85–91. http://www.minervamedica.it. [PubMed] [Google Scholar]
- 6.Fischer G, Bradford J. Vulvovaginal candidiasis in postmenopausal women: the role of hormone replacement therapy. J Low Genit Tract Dis. 2011;15:263–267. doi: 10.1097/LGT.0b013e3182241f1a. http://journals.lww.com/jlgtd/pages/articleviewer.aspx-year=2011&issue=10000&article=00003&type=abstract. [DOI] [PubMed] [Google Scholar]
- 7.Gertsch J, Anavi-Goffer S. Methylhonokiol attenuates neuroinflamm: a role for cannabinoid receptors? J Neuroinflammation. 2012;9:135. doi: 10.1186/1742-2094-9-135. (Abstract) http://www.ncbi.nlm.nih.gov/pubmed/22716035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goswami D, Goswami R, Banerjee U, Dadhwal V, Miglani S, Lattif AA, Kochupillai N. Pattern of Candida species isolates from patients with diabetes mellitus and vulvovaginal candidiasis and their response to single dose oral fluconazole therapy. J Infect Dis. 2006;52:111–117. doi: 10.1016/j.jinf.2005.03.005. http://www.ncbi.nlm.nih.gov/pubmed-term=. [DOI] [PubMed] [Google Scholar]
- 9.Ito H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77:1110–1115. doi: 10.1055/s-0030-1270749. https://www.thieme-connect.com/ejournals/pdf/plant. [DOI] [PubMed] [Google Scholar]
- 10.Herrera-Arellano A, Rodríguez-Soberanes A, Martínez-Rivera MA, Martínez-Cruz E, Zamilpa A, Alvarez L, Tortoriello J. Effectiveness and tolerability of a standardized phytodrug derived from Solanum chrysotrichum on Tinea pedis: a controlled and randomized clinical trial. Planta Med. 2003;69:390–393. doi: 10.1055/s-2003-39710. https://www.thieme-connect.com/ejournals/abstract/plantamedica/doi/10.1055/s-2003-39710. [DOI] [PubMed] [Google Scholar]
- 11.Herrera-Arellano A, Jiménez-Ferrer E, Vega-Pimentel AM, Martínez-Rivera MA, Hernández-Hernández M, Zamilpa A, Tortoriello J. Clinical and mycological evaluation of therapeutic effectiveness of Solanum chrysotrichum standardized extract on patients with pytiriasis capitis (dandruff). A double blind and randomized clinical trial controlled with ketoconazole. Planta Med. 2004;70:483–488. doi: 10.1055/s-2004-827145. https://www.thieme-connect.com/ejournals/abstract/plantamedica/doi/10.1055/s-2004-827145. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Arellano A, Martínez-Rivera MA, Hernandez-Cruz M, López-Villegas EO, Rodríguez-Tovar AV, Alvarez L, Marquina-Bahena S, Navarro-García VM, Tortoriello J. Mycological and electron microscopic study of Solanum chrysotrichum saponin SC-2 antifungal activity on Candida species of medical significance. Planta Med. 2007;73:1568–1573. doi: 10.1055/s-2007-993744. https://www.thieme-connect.com/ejournals/abstract/plantamedica/doi/10.1055/s-2007-993744. [DOI] [PubMed] [Google Scholar]
- 13.Herrera-Arellano A, Jiménez-Ferrer E, Zamilpa A, Martínez-Rivera MA, Rodríguez-Tovar AV, Herrera-Álvarez S, Salas-Andonaegui ML, Nava-Xalpa MY, Méndez-Salas A, Tortoriello J. Exploratory study on the clinical and mycological effectiveness of an herbal medicinal product from Solanum chrysotrichum in patients with Candida yeast-associated vaginal infection. Planta Med. 2009;75:466–471. doi: 10.1055/s-0029-1185318. https://www.thieme-connect.com/DOI/DOI?10.1055/s-0029-1185318. [DOI] [PubMed] [Google Scholar]
- 14.Hoffstetter SE, Barr S, LeFevre C, Leong FC, Leet T. Self-reported yeast symptoms compared with clinical wet mount analysis and vaginal yeast culture in a specialty clinic setting. J Reprod Med. 2008;53:402–406. http://www.ncbi.nlm.nih.gov/pubmed/18664056. [PubMed] [Google Scholar]
- 15.Jelovsek FR. Signs and symptoms of vulvovaginal candidiasis (vaginal yeast infections) URL: http://www.wdxcyber.com/npapvg09.htm.
- 16.Kaplanek P, de Boer A, Gross U, de Groot P, Hube B, Weig M. Candida and candidosis today: where are we, and where to go? The Interdisciplinary Forum on Candidosis (IFOCAN), Göttingen (Germany), 23–25 September 2005. FEMS Yeast Res. 2006;6:1290–1294. doi: 10.1111/j.1567-1364.2006.00184.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1567-1364.2006.00184.x/pdf. [DOI] [PubMed] [Google Scholar]
- 17.Knuth S, Schübel H, Hellemann M, Jürgenliemk G. Catechol, a bioactive degradation product of salicortin, reduces TNF-α induced ICAM-1 Expression in human endothelial cells. Planta Med. 2011;77:1024–1026. doi: 10.1055/s-0030-1270722. https://www.thiemeconnect.com/DOI/DOI?10.1055/s-0030-1270722. [DOI] [PubMed] [Google Scholar]
- 18.Koch E, Fathi AM. Standardized extracts from hawthone leaves and flowers in the treatment of cardiovascular disorders. Preclinical and clinical studies. Planta Med. 2011;77:1123–1128. doi: 10.1055/s-0030-1270849. http://naturalpathonline.com/?sn=5383-6. [DOI] [PubMed] [Google Scholar]
- 19.López-Villegas EO, Herrera-Arellano A, Martínez-Rivera MA, Alvarez L, Cano-Nepauseno M, Marquina S, Rodríguez-Tovar AV, Tortoriello J. Ultrastructural changes on clinical isolates of Trichophyton rubrum, Trichophyton mentagrophytes, and Microsporum gypseum caused by Solanum chrysotrichum saponin SC-2. Planta Med. 2009;75:1517–1520. doi: 10.1055/s-0029-1185810. https://www.thieme-connect.com/DOI/DOI?10.1055/s-0029-1185810. [DOI] [PubMed] [Google Scholar]
- 20.Lozoya X, Navarro V, García M, Zurita M. Solanum chrysotrichum (Schldl.), a plant used in Mexico for the treatment of skin mycosis. J Ethnopharmacol. 1992;36:127–132. doi: 10.1016/0378-8741(92)90011-f. http://www.sciencedirect.com/science/article/pii/037887419290011F. [DOI] [PubMed] [Google Scholar]
- 21.Martins HPR, Da Silva MC, Paiva LCF, Svidzinski TIE, Consolaro MEL. Efficacy of fluconazole and nystatin in the treatment of vaginal Candida species. Acta Derm Venereol. 2012;92:78–82. doi: 10.2340/00015555-1194. http://www.medicaljournals.se/acta/content/?doi=10.2340/00015555-1194. [DOI] [PubMed] [Google Scholar]
- 22.Murina F, Graziottin A, Felice R, Radici GL, Di Francesco S. The recurrent vulvovaginal candidiasis: proposal of a personalized therapeutic protocol. Obstet Gynecol. 2011;2011:806065. doi: 10.5402/2011/806065. (Abstract) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3153925/pdf/OBGYN2011-806065.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakashima H, Murakami T, Yamamoto N, Sakagami H, Tanuma S, Hatano T, Yoshida T, Okuda T. Inhibition of human immunodeficiency viral replication by tannins and related compounds. Antiviral Res. 1992;18:91–103. doi: 10.1016/0166-3542(92)90008-s. http://www.ncbi.nlm.nih.gov/pubmed/1416904. [DOI] [PubMed] [Google Scholar]
- 24.Ostrosky-Zeichner L, Rex JH, Bennett J, Kullberg BJ. Deeply invasive candidiasis. Infect Dis Clin North Am. 2002;16:821–835. doi: 10.1016/s0891-5520(02)00034-x. http://www.ncbi.nlm.nih.gov/pubmed/12512183. [DOI] [PubMed] [Google Scholar]
- 25.Ostrosky-Zeichner L, Rex JH. Recurrent vulvo-vaginal candidiasis: some reassuring answers for an old question. Medscape Infectious Dis. 2002 http://www.medscape.org/viewarticle/442211. [Google Scholar]
- 26.Paulitsch A, Weger W, Ginter-Hanselmayer G, Marth E, Buzina W. A 5 year (2002–2004) epidemiological survey of Candida and non-candida yeast species causing vulvovaginal candidiasis in Graz, Austria. Mycoses. 2006;49:471–475. doi: 10.1111/j.1439-0507.2006.01284.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1439-0507.2006.01284.x/pdf. [DOI] [PubMed] [Google Scholar]
- 27.Ray A, Ray S, George AT, Swaminathan N. Interventions for prevention and treatment of vulvovaginal candidiasis in women with HIV infection. Cochrane Database Syst Rev. 2011;10:CD008739. doi: 10.1002/14651858.CD008739.pub2. (Abstract) http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008739.pub2/abstract-systemMessage=Wiley+Online+Library+will+be+disrupted+on+9+June+from+10%3A00-12%3A00+BST+%2805%3A0007%3A00+EDT%29+for+essential+maintenance. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti M, Posteraro B, Fiori B, Ranno S, Torelli R, Fadda G. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob Agents Chemother. 2005;49:668–679. doi: 10.1128/AAC.49.2.668-679.2005. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC547307/pdf/0816-04.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiota S, Shimizu M, Mizusima T, Ito H, Hatano T, Yoshida T, Tsuchiya T. Restoration of effectiveness of βlactams on methicillin-resistant Staphylococcus aureus by tellimagrandin I from red rose. FEMS Microbiol Lett. 2000;185:135–138. doi: 10.1111/j.1574-6968.2000.tb09051.x. http://www.ncbi.nlm.nih.gov/pubmed-term=Restoration%20of%20effectiveness%20of%20%CE%B2lactams%20on%20methicillinresistant%20Staphylococcus%20aureus%20by%20tellimagrandin%20I%20from%20rose%20red. [DOI] [PubMed] [Google Scholar]
- 30.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;9(9577):1961–1971. doi: 10.1016/S0140-6736(07)60917-9. 369. http://www.sciencedirect.com/science/article/pii/S0140673607609179. [DOI] [PubMed] [Google Scholar]
- 31.Wang JL, Chang CH, Young-Xu Y, Chank KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agent Chemother. 2010;54:2409–2419. doi: 10.1128/AAC.01657-09. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2876415/pdf/1657-09.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei YP, Feng J, Luo ZC. Isolation and genotyping of vaginal non-albicans Candida spp. in women from two different ethnic groups in Lanzhou, China. Int J Gynecol Obstet. 2010;110:227–230. doi: 10.1016/j.ijgo.2010.04.026. http://www.sciencedirect.com/science/article/pii/S0020729210002444. [DOI] [PubMed] [Google Scholar]
- 33.Xu YJ, Capistrano I R, Dhooghe L, Foubert K, Lemière F, Maregesi S, Baldé A, Apers S, Pieters L. Herbal medicines and infectious diseases: characterization by LC-SPE-MR of some medicinal plant extracts used against malaria. Planta Med. 2011;77:1139–1148. doi: 10.1055/s-0030-1270719. https://www.thieme-connect.com/ejournals/pdf/plant. [DOI] [PubMed] [Google Scholar]
- 34.Zamilpa A, Tortoriello J, Navarro V, Delgado G, Alvarez L. Five new steroidal saponins from Solanum chrysotrichum leaves and their antimycotic activity. J Nat Prod. 2002;65:1815–1819. doi: 10.1021/np020261h. http://pubs.acs.org/doi/pdf/10.1021/np020261h. [DOI] [PubMed] [Google Scholar]
- 35.Zhou DY, Du Q, Li RR, Huang M, Zhang Q, Wei GZ. Grape seed proanthocyanidin extract attenuates airway inflammation and hyperresponsiveness in a murine model of asthma by downregulating inducible nitric oxide synthase. Planta Med. 2011;77:1575–1581. doi: 10.1055/s-0030-1270957. https://www.thieme-connect.com/DOI/DOI?10.1055/s-0030-1270957. [DOI] [PubMed] [Google Scholar]