Abstract
The effects of Duranta repens fruits were investigated on H2O2 induced oxidative cell death to evaluate its antioxidative potential in vitro. HEK293T cells were treated with different concentrations [0–1000 µg/ ml] of ethanol extract (E-Ex) and methanol extract (M-Ex) of D. repens for 24h, and then treated with 100 µM H2O2 for 24h. Cell viability, antioxidant parameters of cells, and antioxidant constituents of the extracts were determined. Treatment with limited dose of E-Ex or M-Ex increased the survival rate of H2O2-treated HEK293T cells, however the extra-high dose showed growth inhibitory effect. Treatment with E-Ex or M-Ex protected cellular lipid per-oxidation. In vitro analyses showed the 2,2-diphenyl-1-picrylhydrazyl and H2O2 scavenging activities as well as reducing potential of the extracts. We report here that the limited dose of E-Ex and M-Ex possess antioxidative potential, which can protect H2O2-induced oxidative cell damage.
Keywords: Duranta repens (Linn.), Oxidative cell death, Lipid per-oxidation, Antioxidant, Phytochemicals
Introduction
Oxidative stress occurs when the generation of free radicals or reactive oxygen species (ROS) exceeds the antioxidant capacity of a living system during normal metabolic courses and other activities (Zima et al., 2001). Excess free radicals and ROS, including hydrogen peroxide (H2O2) and superoxide anion (O2−) attack biomolecules such as lipids, proteins, enzymes, DNA and RNA that lead to tissue or cellular injury (Khan et al., 2010). These oxidative stresses have been implicated in a wide variety of degenerative processes, diseases and syndromes, including mutagenesis and cell transformation; atherosclerosis and heart attacks; strokes and ischaemia/ reperfusion injury; chronic inflammatory diseases; neurodegenerative diseases and a wide variety of age-related disorders (Davies, 1995; Khan et al., 2010). Antioxidants are free-radical scavengers that provide protection to living organisms from ROS inducing cellular damage. Although almost all organisms possess antioxidant defense and repair systems, these systems are insufficient to prevent the damage entirely. Therefore, for human and animals, dietary antioxidant supplementation is a promising way to strengthen the antioxidant defense and repair systems. As, most commonly used synthetic antioxidants such as, butylatedhydoxyanisole, butylatedhydoxytoluene, Propylgallat and butylatedhydroquinone showed side effects like liver damage and carcinogenesis (Sun and Fukuhara, 1997), searching for the natural antioxidants is an important task of present biological research.
Duranta repens (Linn.) [Common name: Sky Flower, Golden Dew Drop, Pigeon Berry] is an evergreen shrub in Verbenaceae family, which is distributed in tropical and sub-tropical regions (Ahmed et al., 2009). Different parts of this plant have been used as traditional medicines, and have been reported to have some therapeutic activities, such as anti-plasmodia and anti-malarial, anti-thrombin, antioxidant, anti-cancer and antiviral activities (Nagao et al., 2001; Anis et al., 2002; Shahat et al., 2005; Abou-Setta et al., 2007; Ahmed et al., 2009; Ijaz et al., 2010). In this study, we have investigated the antioxidant potential of ethanolic and methanolic extracts of D. repens fruits against H2O2 induced cell death from oxidative stress in vitro.
Material and methods
Extracts preparation
The fresh washed fruits of D. repens were subjected to exhaustive extraction using absolute ethanol and methanol as extraction solvents. The ethanol extract (E-Ex) and methanol extract (M-Ex) were then filtered, evaporated to a thick residue at 40°C, and stored at 4°C. These solid extracts were used to prepare the different concentration of treatment solutions along with PBS (phosphate buffered saline, pH 7.4).
Cell culture and treatment
HEK293T (normal human embryonic kidney 293T) cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum at 37°C in an atmosphere with 5% CO2. Cells (initial cell density: 1.0 × 105/ml) were incubated with different concentrations of E-Ex and M-Ex (10, 50, 100, 500 and 1000 µg/ ml) for 24 h, and then treated with 100 µM H2O2 for more 24 h. HEK293T cell culture treated with neither extracts, nor H2O2 was regarded as control, and HEK293T cell culture treated with no extract but H2O2 was regarded with negative control.
Assay of cell viability
Cell viability was assayed by trypan blue (0.8 mM trypan blue in PBS) staining. Cells were trypsinized, detached from culture dish, harvested and mixed with equal volume of trypan blue, and then counted on hemocytometer. Viable cells excluded trypan blue, while dead cells stained blue. The percentage of living cells in control culture was regarded to have 100% survival rate, and the treated cell cultures were compared with control to calculate their survival rates.
Assay of the lipid per-oxidation
Lipid per-oxidation was assayed by using commercial assay kit supplied by Nanjing Jiancheng Bioengineering Institute, China {cat. no: A003} (www.njjcbio.com). The maleic dialdehyde (MDA) in decomposed products of lipid per-oxidation had condensation reaction with thio-barbituric acid (TBA), which generated red color compound, of which absorbance was measured at 532 nm by UV-VIS spectrophotometer. The MDA content has been measured as ‘nmol/ mg protein’ in particular volume of cell homogenate. The protein content was determined by using Bradford method (Bradford, 1976).
Assays of the antioxidative potential
Total reducing potential assay, DPPH (2, 2-diphenyl-1-picrylhydrazyl) scavenging assay and hydrogen peroxide (H2O2) scavenging activity assays were performed to evaluate antioxidative activities of E-Ex and M-Ex.
Assay of total reducing potential: The reducing power of E-Ex and M-Ex were determined according to the method described by Oyaizu (1986). The reducing power of the extracts was expressed as ascorbic acid equivalents (µg of ascorbate equivalent/mg of extract).
Assay of DPPH scavenging activity: DPPH-free radical scavenging activity was measured by slightly modified method described by Braca et al. (2002). DPPH scavenging activity was expressed as the concentration of the extracts necessary to decrease the absorbance of the DPPH by 50% (IC50).
Assay of H2O2 scavenging activity: H2O2 scavenging activity of E-Ex and M-Ex was estimated by replacement titration (Nagulendran et al., 2007). H2O2 scavenging activity was expressed as the concentration of the extracts necessary to decrease the absorbance of the H2O2 by 50% (IC50).
Quantitative estimation of the antioxidative phytochemicals
Quantitative estimation of the antioxidative phyto-chemicals was carried out by the estimation of total polyphenol, total flavonoid, tannin and β-carotene in the ethanol extract (E-Ex) and methanol extract (M-Ex) of D. repens. Total phenolic contents of E-Ex and M-Ex were determined using the modified Folin-Ciocalteu's method (Amin et al., 2006). Total flavonoid contents of E-Ex and M-Ex were measured by aluminum chloride colorimetric assay method (Kumar et al., 2008). Total tannin contents of E-Ex and M-Ex were determined by using the Folin-Ciocalteu's method (Folin and Ciocalteu, 1927). β-carotene contents of E-Ex and M-Ex were determined according to the method described by Nagata and Yamashita (1992). Monomeric anthocyanins contents of E-Ex and M-Ex were determined according to the method described by Lee et al. (2005).
Statistical Analysis
Data were analyzed by one-way ANOVA and then post-hoc comparison (LSD test) by using the statistical program SPSS 16.0 and MS-Excel. Results have been presented as mean ± SEM (standard error of mean). P≤0.01 and P≤0.05 have been considered as levels of significance.
Results
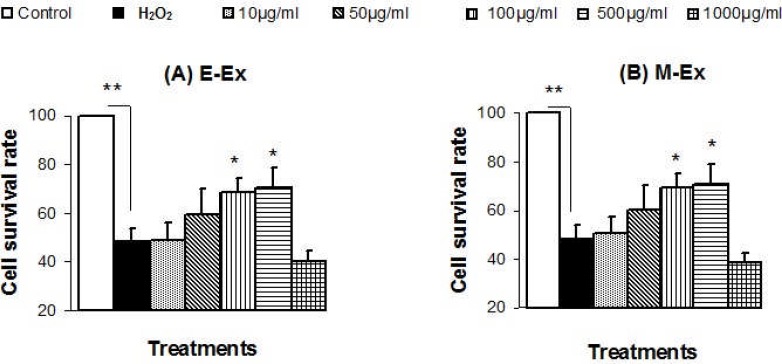
Treatment of H2O2 (100 µM) caused cellular death of HEK293T cells, as the survival rate of H2O2 treated cells was significantly (P≤0.01) lower (by 48.4%) than that of control HEK293T cells (Figure 1). But pre-incubation of H2O2 treated cells with 100 µg/ml and 500 µg/ml of both E-Ex and M-Ex significantly (P≤0.05) increased the cell survival rate. However, lower dose of E-Ex and M-Ex (10 µg/ml or 50µg/ml) showed no significant improvement of cell survival. Again, E-Ex and M-Ex with very high dose (1000 µg/ml) did not show any improvement, rather showed growth inhibitory effect (Figure 1). Both the extracts showed similar pattern of effects on H2O2 treated HEK293T cells.
Figure 1.
Effects of ethanolic and methanolic extracts of D. repens on H2O2 treated HEK293T cells. Cells were pre-treated with different concentrations of ethanolic extract (E-Ex) and methanolic extract (M-Ex) for 24 hours, and then treated with 100 µM H2O2 for more 24h. Negative control cells were treated with H2O2 only but no extract, and there was no treatment in control cells. Results are presented as mean±SEM (n= 5). ** Values are significantly different compared with H2O2 treated cells at P≤0.01 level; *values are significantly different compared with H2O2 treated cells at P≤0.05 level.
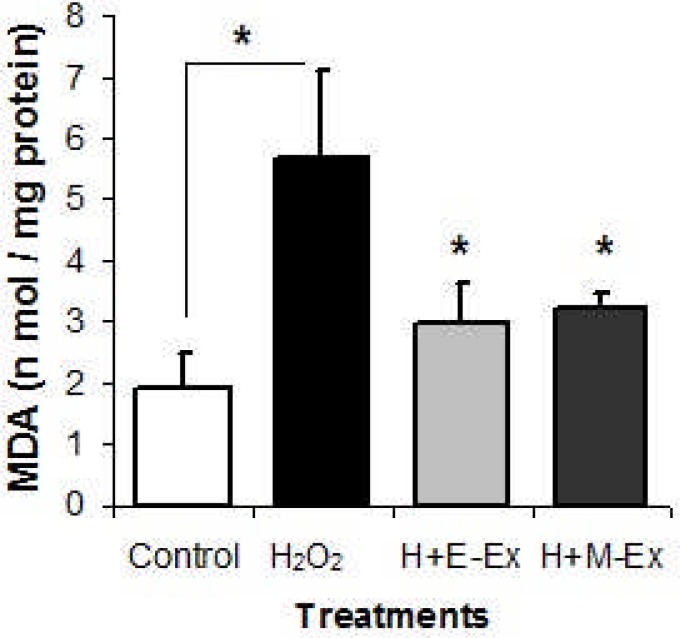
The MDA content (lipid per-oxidation product) was significantly higher in H2O2 treated cells than that in control HEK293T cells (P≤0.05). But the H2O2 treated cells which were pre-incubated with 100 µg/ml E-Ex or M-Ex had significantly (P≤0.05) lower MDA content than H2O2 treated positive control cells (Figure 2). This confirmed the lipid per-oxidation protecting capacity of the extracts.
Figure 2.
Effects of ethanolic and methanolic extracts of D. repens on lipid per-oxidation (MDA content) in H2O2 treated HEK293T cells Cells were pre-treated with 100 µg/ ml of ethanolic extract (E-Ex) and methanolic extract (M-Ex) for 24 hours, and then treated with 100 µM H2O2 for more 24h. Negative control cells were treated with H2O2 only but no extract, and there was no treatment in control cells. Results are mean±SEM (n=5). *values are significantly different compared with that of the H2O2 treated cells at P≤0.05 level.
Total reducing potential of extracts was expressed as ascorbic acid equivalent (AE: µg of ascorbate equivalent/mg of extract), which was found 54.17 AE in E-Ex and 45.77 AE in M-Ex. The reducing potential of E-Ex was found significantly (P ≤ 0.05) higher than that of the M-Ex (Table 1). The IC50 value for DPPH scavenging activity was found as 114.82 µg/ ml and 172.98 µg/ ml in E-Ex and M-Ex, respectively. DPPH-free radical scavenging capacity of the E-Ex and M-Ex was significantly different at P ≤ 0.05 (Table 1). In case of H2O2 scavenging potential of extracts, IC50 value of E-Ex was 250 µg/ ml, and 172.19 µg/ ml of M-Ex. H2O2-scavenging capacity of the two extracts was significantly different at P ≤ 0.05 (Table 1).
Table 1.
Antioxidant activities of ethanol and methanol extracts of D. repens fruits
| Antioxidant activities | E-Ex | M-Ex |
| Reducing Potential (AE) | 54.17±0.20 | 45.77±1.34* |
| IC50 value of DPPH Scavenging activity (µg/ ml) | 114.82±1.5 | 172.98±1.41* |
| IC50 value of H2O2 scavenging activity (µg/ ml) | 250.03±1.0 | 172.19±2.63* |
Results are means±SEM, n=5.
Values in a same row are significantly different at P ≤ 0.05. AE - Ascorbic acid Equivalent (µg of ascorbate equivalent/mg of extract); IC50 - concentration of the extracts necessary to decrease the absorbance of the DPPH or H2O2 by 50%.
The total polyphenol contents were found as 43.21 mg/g in E-Ex and 34 mg/g in M-Ex; total flavonoid contents were 10.22 mg/g in E-Ex and 9.5 mg/g in M-Ex; Tannin contents were found 6.96 mg/g in E-Ex and 6.76 mg/g in M-Ex; β-carotene contents were 20 mg/g in E-Ex and 117.8 mg/g in M-Ex; monomaric anthocyanin contents were found as 2.86 mg/g in E-Ex and 6.40 mg/g in M-Ex. The polyphenol and β-carotene contents between two extracts were significantly different (Table 2).
Table 2.
Antioxidative phyto-chemicals of ethanol and methanol extracts of D. repens fruits
| Phytochemicals | E-Ex (mg/g) | M-Ex (mg/g) |
| Polyphenols | 43.21±0.43 | 34.00±0.19* |
| Flavonoids | 10.22±0.20 | 9.5±0.09 |
| Tannin | 6.96±0.67 | 6.73±0.27 |
| β-carotene | 20.02±0.37 | 117.76±1.19** |
| Monomeric anthocyanin | 2.86±1.0 | 6.40±2.0 |
Results are mean±SEM (n=5).
values are significantly different between two extracts at P≤0.01 level.
values are significantly different between two extracts at P≤0.05 level.
Discussion
A paradox in metabolism is that oxygen is a highly reactive molecule that damages living organisms by producing reactive oxygen species (including H2O2), although the vast majority of the complex process in life on earth requires oxygen for its existence. Natural antioxidant supplementation in food is a promising way to compensate this damage. The antioxidant phyto-chemicals of plant extracts are mainly responsible for observed antioxidant activity (Gupta and Sarma, 2006). The majority of the active antioxidant compounds found in natural foods are polyphenols, flavonoides, anthocyanins, vitamins C, β-carotene, tocopherol etc. while the major cellular defense consists of antioxidative enzymes and bioactive compounds such as glutathione (Sahnoun et al., 1997). In this study, it has been shown that ethanol and methanol extracts of D. repens (in limited dose) could protect HEK293T cells from H2O2 induced oxidative damage. These extracts also protected the cellular lipid per-oxidation; however they showed no significant effect on antioxidant enzymes (data not shown here). The extracts showed in vitro antioxidative potential like DPPH-scavenging activity, reducing power and H2O2-scavenging activity.
H2O2 can easily penetrates cellular membranes; once inside the cell, H2O2 can probably react with Fe2+ and Cu2+ ions to form hydroxyl radical and this may be the origin of many of its toxic effects, like lipid per-oxidation (Halliwell and Gutteridge, 1984; Sokołowska et al., 1999). It is therefore biologically advantageous for cells to control the amount of H2O2 that is allowed to accumulate. H2O2 scavenging assay reflect the extract capacity to neutralize the H2O2 directly. The lipid per-oxidation has been protected by E-Ex and M-Ex probably through the scavenging of the H2O2 at the membrane, as the major H2O2 scavenging system, antioxidant enzyme activities remained unaffected by the treatment.
The antioxidant activity of polyphenols is mainly due to their redox properties, which allows them to act as reducing agents, hydrogen donors, singlet oxygen quenchers and metal chelator (Javanmardi et al., 2003). The DPPH scavenging activity and reducing potential of any agent depend on the capability to donate electron directly (Ribeiro et al., 2008). The polyphenol content in the extracts may provide these antioxidative potential, as the polyphenols are known to be related to the total reducing power, as well as DPPH-scavenging activity (Rohman et al., 2010). The mechanisms of action of flavonoids include the scavenging or chelating process (Schmitt-Schillig et al., 2005). There exist reports that flavonoids and a host of other secondary plant metabolites, exhibit analgesic, anti-inflammatory effects as a result of their membrane stabilizing action in various experimental animal models (Awe et al., 2009). Therefore, it can be speculated that flavonoids and other chemical components are responsible for the observed membrane stabilizing action of the extracts. β-carotene is an excellent scavenger of singlet oxygen (Gupta and Sarma, 2006), which can attenuate the deleterious effect of ROS. Combined with other antioxidant phytochemicals like tannin and monomeric anthocyanin, the antioxidative mechanisms of polyphenols, flavonoids and β-carotene may provide protecting role of D. repens fruit-extracts against oxidative cell death of HEK293T cells. Some other antioxidant phyto-chemicals have also been reported in D. repens, like iridoid glycosides, repenins A–D, coumarino-lignoids, cleomiscosin A, durantin A etc. (from whole plant) (Ahmad et al., 2009; Ijaz et al., 2011).
This study reports that the ethanol and methanol extracts of the fruits of D. repens (Linn.) have antioxidative potential, which can protect H2O2-induced oxidative damage of HEK293T cells in some extents. However, an important point should be noted that the extra-high dose (1000 µg/ ml) of E-Ex or M-Ex did not protect cells against oxidative cell damage; rather they showed growth inhibitory effect. Future researches are suggested to test the extracts against experimentally induced oxidative stress in animal models, or some clinical study after proper authentication about its safety.
Acknowledgement
This work was supported by the Open-End Fund for the Valuable and Precision Instruments of Central South University. The first author (Khan M. A.) has been supported by Chinese Government Scholarship provided by Chinese Scholarship Council.
References
- 1.Abou-Setta LM, Nazif NM, Shahat AA. Phytochemical Investigation and Antiviral Activity of Duranta repens. J Appl Sci Res. 2007;3:1426–1433. [Google Scholar]
- 2.Ahmad N, Zeb F, Ahmad I, Wang F. Repenins A–D, four new antioxidative coumarinolignoids from Duranta repens Linn. Bioorg Med Chem Lett. 2009;19:3521–3524. doi: 10.1016/j.bmcl.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed WS, Mohamed MA, El-Dib RA, Hamed MM. New triterpene saponins from Duranta repens Linn. and their cytotoxic activity. Molecules. 2009;14:1952–1965. doi: 10.3390/molecules14051952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin I, Norazaidah Y, Hainida KIE. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006;94:47–52. [Google Scholar]
- 5.Anis I, Ahmed S, Malik A, Yasin A, Choudary MI. Enzyme inhibitory constituents from Duranta repens. Chem Pharm Bull. 2002;50:515–518. doi: 10.1248/cpb.50.515. [DOI] [PubMed] [Google Scholar]
- 6.Awe EO, Makinde JM, Adeloye OA, Banjoko SO. Membrane stabilizing activity of Russelia equisetiformis, Schlecht & Chan. J Nat Pro. 2009;2:3–9. [Google Scholar]
- 7.Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 10.Folin O, Ciocalteu V. On Tyrosine and tryptophan determinations in proteins. J Biol Chem. 1927;73:627–650. [Google Scholar]
- 11.Gupta VK, Sarma SK. Plants as natural Antioxidant. Nat Prod Rad. 2006;15:326–334. [Google Scholar]
- 12.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijaz F, Ahmad N, Ahmad I, Haq AU, Wang F. Two new anti-plasmodial flavonoid glycosides from Duranta repens. J Enzyme Inhib Med Chem. 2010;25:773–778. doi: 10.3109/14756360903433365. [DOI] [PubMed] [Google Scholar]
- 14.Ijaz F, Haq AU, Ahmad I, Ahmad N, Hussain J, Chen S. Antioxidative iridoid glycosides from the sky flower (Duranta repens Linn) J Enzyme Inhib Med Chem. 2011;26:88–92. doi: 10.3109/14756361003724778. [DOI] [PubMed] [Google Scholar]
- 15.Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. [Google Scholar]
- 16.Khan MA, Tania M, Zhang DZ, Chen H. Antioxidant enzymes and cancers. Chin J Cancer Res. 2010;22:87–92. [Google Scholar]
- 17.Kumar S, Kumar D, Manjusha Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58:215–220. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. [PubMed] [Google Scholar]
- 19.Nagao T, Abe F, Okabe H. Antiproliferative constituents in the plants 7. Leaves of Clerodendron bungei and leaves and bark of C. trichotomum. Biol Pharm Bull. 2001;24:1338–1341. doi: 10.1248/bpb.24.1338. [DOI] [PubMed] [Google Scholar]
- 20.Nagata M, Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaish. 1992;39:925–928. [Google Scholar]
- 21.Nagulendran KR, Velavan S, Mahesh R, Begum VH. In vitro antioxidant activity and total polyphenolic content of Cyperus rotundus rhizomes. E-Journal of Chemistry. 2007;4:440–449. [Google Scholar]
- 22.Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jap J Nutr. 1986;44:307–315. [Google Scholar]
- 23.Ribeiro SMR, Barbosa LCA, Queiroz J, Knodler HM, Schieber A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008;110:620–626. [Google Scholar]
- 24.Rohman A, Riyanto S, Yuniarti N, Saputra WR, Utami R, Mulatsih W. Antioxidant activity, total phenolic, and total flavaonoid of extracts and fractions of red fruit (Pandanus conoideus Lam) Int Food Res J. 2010;17:97–106. [Google Scholar]
- 25.Sahnoun Z, Jamoussi K, Zeghal KM. Free radicals and antioxidants: human physiology, pathology and therapeutic aspects. Therapie. 1997;52:251–270. [PubMed] [Google Scholar]
- 26.Schmitt-Schillig S, Schaffer S, Weber CC, Eckert GP, Müller WE. Flavonoids and the aging brain. J Physiol Pharmacol. 2005;56:23–36. [PubMed] [Google Scholar]
- 27.Shahat AA, Nazif NM, Abousetta LM, Ibrahim NA, Cos P, Van Miert S, Pieters L, Vlietinck AJ. Phytochemical investigation and antioxidant activity of Duranta repens. Phytother Res. 2005;19:1071–1073. doi: 10.1002/ptr.1766. [DOI] [PubMed] [Google Scholar]
- 28.Sokołowska M, Oleszek A, Włodek L. Protective effect of alpha-keto acids on the oxidative hemolysis. Pol J Pharmacol. 1999;51:429–434. [PubMed] [Google Scholar]
- 29.Sun B, Fukuhara M. Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology. 1997;122:61–72. doi: 10.1016/s0300-483x(97)00078-4. [DOI] [PubMed] [Google Scholar]
- 30.Zima T, Fialová L, Mestek O, Janebová M, Crkovská J, Malbohan I, Stípek S, Mikulíková L, Popov P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci. 2001;8:59–70. doi: 10.1007/BF02255972. [DOI] [PubMed] [Google Scholar]