Abstract
As the traditional Chinese medicine, the fresh fruits of Amorpha fruticosa L. were applied for the treatment of carbuncle, eczema and burn (Das et al., 2007). However, little is known about the functional roles of the fruits of Amorpha fruticosa L. during wound healing progress. In the present study, we evaluated both antimicrobial potential against a wide range of microorganisms and wound healing activity of the seven compounds isolated from the fruits of Amorpha fruticosa L in vitro and in vivo. Our results showed that compounds I (6a,12a-dehydroamorphin), V (dehydrosermundone) and VI (tephrosin) isolated from the fruits of Amorpha fruticosa L. performed dominant antimicrobial potential against microorganisms. Moreover, these compounds significantly enhanced fibroblasts proliferation and migration, leading to promotion of wound healing. Thus, it could be possible for the therapeutic utilization of Amorpha fruticosa L. for wound healing in the future.
Keywords: Amorpha fruticosa L., anti-bacterial, wound healing
Introduction
The use of plant makes it possible to double our life span in the 20th century and the natural products from plants have been an overwhelming success in modern society. Amorpha fruticosa L. is a shrub plant belonging to the genus Amorpha in Leguminosae family (Lee et al., 2006). There are about twenty-five species plants in genus Amorpha in the whole world. Only the species fruticosa had been introduced to be planted in China and had a wide distribution in Liaoning Province of China. The therapeutic utilization of Amorpha fruticosa L. for wound healing could date back to the beginning of civilization (Das et al., 2007). As the Traditional Chinese Medicine theories described, the fresh fruits of Amorpha fruticosa L. were applied for the treatment of carbuncle, eczema and burn (Das et al.,2007). In the China, Record of Natural Resources of Chinese Materia Medica, the effectiveness of dried fruits of Amorpha fruticosa L. was applied as paste externally twice or thrice a day for wound-healing purpose (Liu et al.,2005).
Wound healing is a complex, integrated series of biochemical, cellular and physiological processes (Martin, 1997). The purpose of repairing events is to resist pathogens invasion, establish the integrity of the damaged tissue and reconstruct physiological function of skin. The healing process in full-thickness skin wounds includes inflammation, wound contraction, development of granulation tissue, scar formation and tissue remodeling (Singer and Clark, 1999) which are concurrent but independent to each other. During wound reparation progress, a critical step is the proliferation and migration of the resident epidermal keratinocytes and dermal cells from the wound edge and surrounding epidermis to the wound bed (Babu and Wells, 2001; Eming et al., 2007). The dermal cells, including dermal fibroblasts and dermal microvascular endothelial cells, perform important function in this reparation process. Topical application of compounds with enhancement of dermal cells proliferation and migration is to improve significantly wound healing (Pan et al., 2010). In previous study, we reported the isolation from the PE, AcOEt and n-BuOH of the fruits of Amorpha fruticosa L. Seven compounds were obtained and their structures were elucidated (Wang et al., 2010), and we evaluated both antimicrobial potential against a wide range of microorganisms and wound healing activity of the seven compounds isolated from the fruits of Amorpha fruticosa L. in vitro and in vivo.
Experimental Section
Plant
The fruits of Amorpha fruticosa L. were collected from Dalian City of Liaoning province, China in July. It was authenticated by vice Prof. Yun-peng Diao, college of pharmacy, Dalian Medical University and a voucher specimen has been deposited in pharmacognosy laboratory under specimen number AF0017.
Cells, animals and microorganisms
The mouse fibroblast cell line (L929) was obtained from Chinese Academy of Sciences and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100µg/ml streptomycin. All cells were cultured in a humidified atmosphere of 5% CO2 at 37°. Healthy, male, Sprague-Dawley rats, weighing 200–220g, were supplied by the Animal Experimental Center of Dalian Medical University and housed one per cage for a week before the study in a room with controlled environment (12-h light/dark cycle, 23±2° and relative humidity 70%). They were also allowed free access to standard laboratory diet and water. All experimental procedures were approved by the Animal Research Ethics Committee of Dalian Medical University, Dalian, China. The Bacterial (Bacillus subtilis, Bacillus cerculences, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Klebsiella aeruginosa) were got from China Center for Type Culture Collection.
Wound healing test in vitro
MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) viability assay was performed as previously described with the following modification (Do et al.,2008). Briefly, cells were seeded into 96-well plate with 0.6×104 cells/well. After 24 h attachment, various concentrations (1–1000µg/ml) of seven compounds were applied to the cells in serum-free RPMI 1640, and incubation was extended for another 48 h. Then the cells were washed with PBS and 200 µl MTT (0.5 mg/ml) was added to each well and further incubated for 4 h. The MTT solution was carefully removed by aspirating, and the formazan product was dissolved in 150 ml DMSO. Absorbance was measured at 570nm on a microplate reader (Bio-TEK Instrument, Winooski, VT).
Transwell assay
The migration activity of fibroblasts was performed using 24-well transwell chamber with 8.0um pore polycarbonate filter inserts (Costar, Cambridge, MA, USA). Cells (3×104 cells/well) suspended in serum-free RPMI 1640 containing 0.1% BSA were overlaid in the upper chamber of each transwell. In each lower chamber, 500ul of serum-free RPMI 1640 containing 0.1% BSA in the presence or absence of compounds was added. Then the inserts were incubated at 37° in a humidified atmosphere containing 5% CO2 for 24h. The cells that had not penetrated the filters were removed using cotton swabs. The migrated cells attached to the bottom side were fixed in 100% methanol for 10 min and stained with Wright-Giemsa. The cells migrating the lower surface of the filter in five microscopic fields of 400× magnification were counted in each filter. Triplicate samples were acquired and the data were expressed as the average cell number of 15 fields.
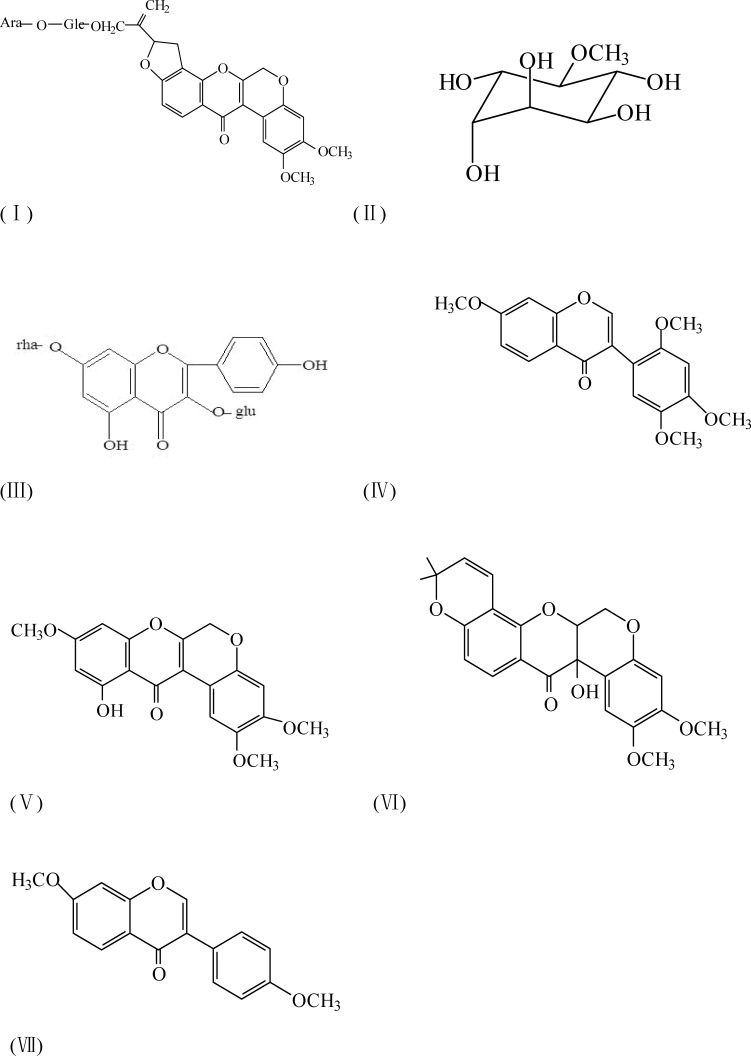
Structures of seven compounds
The compounds were named as 6a,12a-dehydroamorphin(I), D-3-O-methyl-chiro-inositol(II), Kaempferol-3-gluco-7-rhamnoside(III),7,2′,4′,5′-tetrom-ethoxy-isoflavone(IV), dehydrosermundone(V), tephrosin(VI),7,4′-dimethoxyisoflav one(VII). Their structures are showed as followed:
Wound healing test in vivo
Drug formulations
Based on preliminary screening, dose-response study of seven compounds isolated from the fruits of Amorpha fruticosa L. were performed to determine the optimal concentration range. For the present study of wound healing evaluation in vivo, seven 10%(w/w) ointment were prepared, with 5 mg of every compound formulated with 50 mg of Vaseline.
Excision wounds model
A total of 54 rats were randomly divided into nine groups of six animals each. The animals were anesthetized by an intra-peritoneal injection of 10% chloral hydrate (0.3ml/100g), and the dorsal surface of the rat was shaved and the underlying skin was cleaned with povidone iodine. An excision wound was created on the dorsal aspect of the rats by cutting away a 500mm2 full thickness of skin. The seven experimental groups received isolated compounds' ointment. The control group received the vehicle alone. The positive group received recombinant human epidermal growth factor (rhEGF) ointment. All drugs were applied topically once a day until the wounds completely healed. The wounds were measured every other day by placing a transparent paper over the wound and tracing it out. The parameters studied were percentage wound contraction calculated using the following formula: percent wound contraction = (original wound area -unhealed area)/original wound area ×100%.
Incision wounds model
A total of 54 rats were randomly divided into nine groups of six animals each. After anesthesia, disinfection and skin preparation, a paravertebral long incision of 4 cm length was made through full thickness of the skin at a distance about 1.5 cm from the middle on right side of the back. The wounds were closed with interrupted sutures of 0.5cm apart using using sterile surgical thread (No. 000) and a curved needle (No. 11) (Udupa et al.,1995). The nine groups were treated with the same manner as the excision model for 9 days. On day 9, sutures were removed and the tensile strength of healed wounds was measured with a tensiometer and calculated using the following formula: tensile strength = breaking strength/cross sectional area of skin.
Determination of the hydroxyproline content
On the day 22 after excision wounds creation, the healed wound tissues were excised and analyzed for hydroxyproline content, which is a basic constituent of collagen. Tissues were dried in a hot air oven at 60°C to constant weight and were hydrolysed in 6N HCl at 130 °C for 4 h in sealed tubes. The hydrolysate was neutralised to pH 7.0 and was subjected to Chloramine-T oxidation for 20 min. The reaction was terminated by addition of 0.4M perchloric acid and colour was developed with the help of Ehrlich reagent at 60°C (Woessner, 1961) and measured at 560 nm using a spectrophotometer.
Histological examination
The excised tissue of excision wounds was fixed in 10% neutral buffered formalin, dehydrated in graded ethanol, cleared in xylene, and embedded in paraffin. Five-micron-thick sections, including the epidermis, the dermis, and the subcutaneous panniculus carnosus muscle, were mounted on glass slides, dewaxed, rehydrated in distilled water, and stained with hematoxylin and eosin(HE). All subsequent analyses were performed by an experienced pathologist without knowledge of the previous treatment to evaluate the degree of re-epithelization, granulation tissue formation and collagen organization in the wound
Antimicrobial activity
Minimal Inhibitory concentration (MIC) determination
Minimum inhibition concentration assay was performed in nutrient broth containing 0.05% phenol red and supplemented with 10% glucose (NBGP). All the test compounds were initially dissolved in DMSO and the solution obtained was added to NBGP to a final concentration of 5000 µg/ml for each crude extract. This was serially diluted by twofold, to obtain concentration ranging from 5000 µg/ml to 4.9 µg/ml. One hundred microlitres of each concentration was added to a 96-well microplate containing 95µl of NBGP and 5 µl of standard inoculum, the appropriate inoculums size for standard MIC was 105 CFU/ml. The final concentration of DMSO in the well was less than 1%. The negative control well was composed of 95 µl of NBGP and 5 ul of the standard inoculum. Chlormycetin was taken as the positive control and the concentration was diluted from 64 µg/ml to 0.063 µg/ml. The plates were covered with a sterile plate sealer, then agitated to mix the content of the wells using a plate shaker and incubated at 37 °C for 24 h. The assay was repeated twice and microbial growth was determined by observing the chromatic change in the wells (red when there is no growth and yellow when there is growth). The lowest concentration demonstrating no color change in the well was considered as the MIC (Dash et al., 2001).
Minimum bactericidal concentrations (MBC)
For the determination of MBC, a portion of liquid (5ul) from each well that showed no change in color was plated on agar plate and incubated at 37°C for 24 h. The lowest concentrations that yielded no growth after this sub-culture were taken as the MBC (Srinivas et al.,2008).
Results
Wound healing activity in vitro
Cell viability of fibroblast
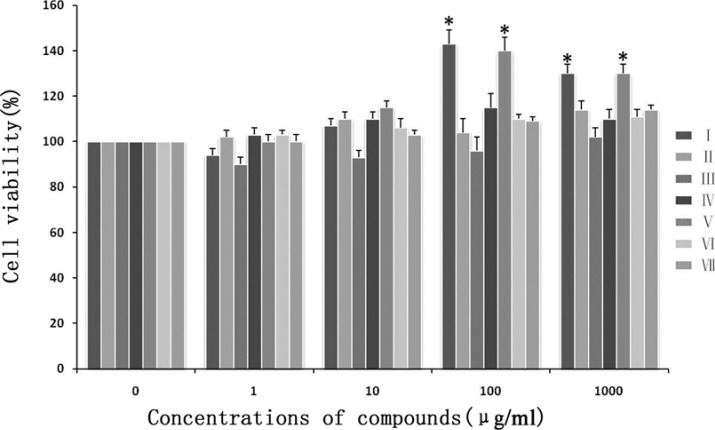
MTT cell viability assay was performed in response to different concentrations of the seven compounds. The result demonstrated that at lower concentrations (1 and 10µg/ml), no clear effect was seen with all compounds. At higher concentrations (100 and 1000 µg/ml), only compound I and V increased cell viability (p<0.05), but the cell viability was no significant difference with the concentration of 100 µg/ml and 1000 µg/ml (p<0.05)(Figure 1). So 100ug/ml of seven compounds was used in the following experiments.
Figure 1.
Viability of fibroblasts after different compounds treatment measured with MTT assay. Cells were exposed to gradually increased concentration of different compounds. The results are represented as a percentage of absorbance relative to control cells (100%). * Differ (P<0.05) from the control
Cell migration of fibroblast
Transwell assay is a commonly used model for analyzing the molecular mechanism underlying cell migration. With this migration assay, the fibroblasts were cultured in serum-free media with 1% BSA for 24 h in the presence or absence of seven different compounds. The results showed that compound I and V significantly induced cell migration at 100 µg/ml compared with control group and other five compounds (p<0.05), and there is no difference within the above two compounds (p<0.05) (Figure 2).
Figure 2.
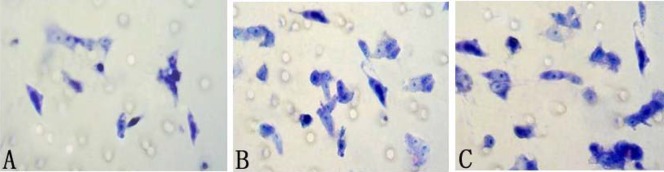
Effect of different compounds on fibroblast migration activity in transwell assay. After treated with compound I(B) and V(C), the fibroblasts demonstrated significant capability of migration, compared with absence of any compounds(A).
Wound healing activity in vivo
The percent wound contraction of excision wounds
Starting from day 4 to day 16 after wound creation, the wound contraction of compound I, V and positive control group was found to be significant compared with negative control group and other compounds (p<0.05). On day 18, wounds in compounds I, V and positive control treated group healed completely. Wounds in other five compounds and negative control treated group did not heal until day 22 after wound creation (Table 1).
Table 1.
The percent wound contraction of excision wounds at different time point
| Group | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | Day 16 | Day 18 | Day 20 | Day 22 |
| Compound I |
17.9±1.0 | 36.2±1.8* | 43.5±2.0* | 62.6±2.1* | 77.3±1.8* | 86.1±2.4* | 90.7±2.0* | 96.8±1.9* | 100* | 100* | 100 |
| Compound II |
16.0±1.1 | 30.2±1.3 | 37.6±2.2 | 57.4±1.6 | 64.8±1.9 | 76.3±1.8 | 82.4±1.4 | 87.5±1.9 | 92.5±2.1 | 96.3±2.1 | 100 |
| Compound III |
16.5±1.3 | 31.0±1.1 | 39.3±2.9 | 56.8±2.0 | 63.3±1.6 | 76.1±2.6 | 83.7±1.5 | 88.5±1.9 | 91.7±2.0 | 96.9±1.1 | 100 |
| Compound IV |
15.7±1.5 | 32.1±2.0 | 39.5±1.9 | 56.1±2.2 | 64.1±1.4 | 77.0±1.6 | 84.3±3.1 | 87.9±1.6 | 92.8±2.1 | 96.3±1.4 | 100 |
| Compound V |
16.8±1.0 | 35.7±1.6* | 42.4±2.4* | 60.7±3.1* | 76.9±3.1* | 85.2±2.3* | 90.5±2.0* | 96.1±1.1* | 100* | 100* | 100 |
| Compound VI |
16.2±0.9 | 31.9±1.0 | 38.0±2.1 | 58.3±2.5 | 65.8±2.3 | 75.2±2.1 | 84.9±2.3 | 89.1±2.7 | 93.5±3.1 | 97.0±1.4 | 100 |
| Compound VII |
17.3±1.0 | 31.1±1.3 | 38.3±3.0 | 54.9±2.5 | 63.5±1.5 | 75.9±1.6 | 82.9±2.6 | 87.0±2.6 | 93.0±1.2 | 96.5±1.3 | 100 |
| Positive control |
18.3±1.4 | 39.3±1.5* | 45.2±2.3* | 63.7±1.4* | 77.4±2.6* | 86.6±2.7* | 92.1±1.9* | 97.3±1.1* | 100* | 100* | 100 |
| Negative control |
16.2±1.1 | 31.5±2.1 | 38.2±1.7 | 55.5±2.0 | 63.6±1.9 | 75.7±2.1 | 83.4±1.5 | 87.2±3.1 | 92.2±2.3 | 95.4±1.8 | 100 |
Values are mean ± S.D.
P<0.05 as compared to negative control group.
Tensile strength of incision wounds and hydroxyproline content of excision wounds
The tensile strength and hydroxyproline content in compound I and positive control treated group was the highest, and significantly higher than negative control treated group (p<0.01). The above two healing parameters in compound V was also higher than negative control treated group (p<0.05). In contrast, the difference between other four compounds and negative control treated group was not found to be statistically significant (Table 2).
Table 2.
Tensile strength of incision wounds and hydroxyproline content of excision wounds
| Tensile strength(g/cm2) | Hydroxyproline(mg/g tissue) | |
| Compound IT | 547.3±7.9# | 80.3±4.4# |
| Compound II | 431.5±8.3 | 63.1±5.1 |
| Compound III | 440.3±7.4 | 58.4±3.7 |
| Compound IV | 451.8±6.0 | 63.6±4.9 |
| Compound V | 500.2±8.9* | 70.7±4.2* |
| Compound VI | 442.0±8.1 | 63.2±5.9 |
| Compound VII | 449.8±6.4 | 59.3±4.7 |
| positive control | 568.1±8.3# | 84.6±5.3# |
| Negative control | 436.5±7.6 | 60.2±4.1 |
Values are mean ± S.D.
P<0.01 as compared to negative control group
P<0.05 as compared to negative control group
Histological examination
When the excision wound healed, the tissue was excised to evaluate the healing quality under light microscope. The progression of new epithelium, granulation tissue formation and collagen organization related to wound healing in compound I, V and positive control group were better compared to compound II, III, IV, VI, VII and negative group. Wounds in compound I, V and positive control group demonstrated that the collagen fiber was more matured with few capillaries, robust fusiform fibrocytes and regenerative folliculus and sebaceous glands. In contrast, in compound II, III, IV, VI, VII and negative control group, there was more granulation tissue within the wound bed showing poorly-organized capillary, many fibroblast, moderate collagen and no regenerative folliculus or sebaceous glands. In addition, compared with compound II, III, IV, VI, VII and negative group, a thicker continuous epithelial line covering the whole wound bed was observed in compound I, V and positive control group (Figure 3).
Figure 3.
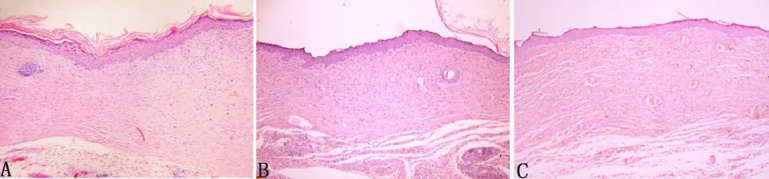
Histological compare in the character of excision wound healing (100× magnification). Wounds of all groups demonstrated a continuous epithelial line covering the whole wound bed. However, the epithelium was thicker in compared with negative control group. Moreover, in compound I(A) and V(B) group, the granulation was matured showing capillary vertically oriented, robust fusiform fibrocytes and moderate well-arranged collagen. In contrast, in negative control group(C), the granulation tissue was un-matured with capillary poorly-organized, many fibroblast and slight collagen formation.
Antimicrobial activity
Among the seven compounds (I – VII) from the fruits of Amorpha fruticosa L, compound I, V and VI exhibited higher inhibitory effect towards all the pathogens than other four compounds. The MIC and MBC of these compounds against pathogens was summarized in Table 1. The results showed that Bacillus subtilis, Staphylococcus aureus and Pseudomonas aeruginosa were most sensitive to compound VI. Bacillus cerculences was most sensitive to compound I and VI equally. Escherichia coli was most sensitive to compound V, and Klebsiella aeruginosa was most sensitive to compound I respectively (Table 3).
Table 3.
MIC and MBC of compounds from Amorpha fruticosa L against different bacteria (µg/ml)
| compounds | Bacillus subtilis | Bacillus cerculences | Staphylococcus aureus | Escherichia coli |
Pseudomonas aeruginosa |
Klebsiella aeruginosa | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| I | 156.3 | 312.5 | 156.3 | 312.5 | 156.3 | 156.3 | 312.5 | 312.5 | 312.5 | 312.5 | 156.3 | 312.5 |
| II | — | — | — | — | — | — | — | — | — | — | — | — |
| III | 2500 | 2500 | 1250 | 2500 | — | — | — | — | — | — | — | — |
| IV | 1250 | 1250 | 1250 | 2500 | 5000 | 5000 | 2500 | 2500 | 5000 | 5000 | 2500 | 2500 |
| V | 156.3 | 312.5 | 312.5 | 312.5 | 78.2 | 156.3 | 156.3 | 156.3 | 312.5 | 312.5 | 312.5 | 312.5 |
| VI | 156.3 | 156.3 | 156.3 | 312.5 | 78.2 | 78.2 | 156.3 | 312.5 | 156.3 | 156.3 | 312.5 | 312.5 |
| VII | 2500 | 2500 | 5000 | 5000 | 2500 | 2500 | 1250 | 2500 | — | — | 2500 | 2500 |
| Chlormycetin | 1 | 2 | 1 | 2 | 2 | 4 | 0.5 | 1 | 1 | 2 | 1 | 2 |
Discussion
Wound healing involving interactions of multiple cell types, various growth factors, their mediators, and the extracellular matrix proteins is a complicated biological process. Physiological healing of wound takes place by itself and does not require much other intervention, but some risk factors such as the defect of local blood supply and infection could delay the healing process (Keeling et al., 2010; Leaper,2010). Many natural products based on plant have been found to have therapeutic effects as promoters of wound healing (Woollard et al., 2007; Skórkowska-Telichowska et al., 2010). Amorpha fruticosa L. leaves and fruits have been used for treating wounds in traditional medicine. In previous study of our laboratory, the compounds isolated from Amorpha fruticosa L. were mainly rotenoids, which have demonstrated many pharmacological activities, such as antitumor, antivirus and antibacterial action, inhibition of phosphodiesterase, hepatoprotection, fish poison and insecticide activity (Petkov et al., 1983; Li et al., 1993). The results of present study using wound healing experiments in animal models, biochemical analysis of wound biopsy, and in vitro studies showed that three different compounds isolated from the fruits of Amorpha fruticosa L. have antibacterial activity and promote the healing of wounds.
Fibroblast is a major cell type participating in wound healing process which is responsible for the synthesis, deposition, and remodeling of the extracellular matrix (Hinz,2010). During wound repair, fibroblasts initially proliferate in situ, then migrate from the wound edges to the wound site, proliferate again, and subsequently produce collagen (Werner et al.,2007). Collagen is a crucial constituent of extracellular matrix and the amount of hydroxyproline is a reflection of concentration of collagen (Bhattacharjee and Bansal, 2005). Therefore, the more hydroxyproline represents the faster rate of collagen synthesis corresponding to enhanced wound healing. In present study, according to the results of MTT assay and transwell assay which is widely used for detection of cell proliferation and migration activity in vitro, compound I, V and VI isolated from the fruits of Amorpha fruticosa L. demonstrated significantly enhanced effect on fibroblasts in proliferation and migration, thus promoting wound healing. And this finding was further confirmed by biochemical assay for hydroxyproline content in vivo, which means collagen synthesis was improved after topical application of related compounds, so that the amount of collagen increasing was one of the reasons for the wound healing promotion.
When wound healing starts, it is prone to be attacked by bacteria. It is well known that infections may delay wound healing and cause failure of healing, even wound deterioration (Guo and Dipietro,2010). Microbial pathogens delay wound healing through several mechanisms, including persistent production of inflammatory mediators, metabolic wastes and toxins; causation of tissue hypoxia, the granulation tissue hemorrhagic and fragile; reduction of fibroblast number and collagen production; continuous activation of neutrophils, which produce cytolytic enzymes and free oxygen radicals, and competition with host cells for nutrients and oxygen necessary for wound healing (Macri and Clark,2009). Thus, keeping the wound axenic or maintaining a low bio-burden is a crucial aim in wound healing. In the present study, the inhibitory effects of seven compounds isolated from the fruits of Amorpha fruticosa L on six strains referring to Gram positive and Gram negative bacteria growth were evaluated. As a result of rotenoids structure, the compounds I, V and VI demonstrated a more significant antibacterial effect comparing with other four compounds. To our knowledge, in aerobic organisms the rotenone-insensitive enzymes provide at least two vitally important functions. First, they can reduce NADH produced by multiple dehydrogenases, thus not maintaining and controlling general oxidative metabolism. Second, they also never serve as the energy-transducing devices accumulating free energy from the redox gap between NADH/NAD+ and the quinone pair as the proton-motive force (PMF) across the coupling membranes. By observing the chemical structures of compound I, V and VI, the slight difference of antibacterial activity may mainly come from the 12a-OH which connected with 12a-C (Fang and Beattie, 2002; Grivennikova et al., 2007). Therefore, the compounds I, V and VI may account for the commendable activity of Amorpha fruticosa L against the test pathogens, and the topical application of the three compounds on wounds would prevent microbes from invading the susceptible state.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (No. 30901950, 81270052 and 81102791), from Dalian Scientific and Technological Foundation( No 2010J21DW022).
References
- 1.Babu M, Wells A. Dermal-epidermal communication in wounds. Wounds. 2001;13:183–189. [Google Scholar]
- 2.Bhattacharjee A, Bansal M. Collagen structure: the Madras triple helix and the current scenario. IUBMB Life. 2005;57(3):161–172. doi: 10.1080/15216540500090710. [DOI] [PubMed] [Google Scholar]
- 3.Das PK, Goswami S, Chinniah A, Panda N, Banerjee S, Sahu NP, Achari B. Woodfordia fruticosa: traditional uses and recent findings. J Ethnopharmacol. 2007;110(2):189–199. doi: 10.1016/j.jep.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Dash GK, Suresh P, Ganapaty S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. Journal of Natural Remedies. 2001;1:105–110. [Google Scholar]
- 5.Do GM, Choi MS, Kim HJ, Woo MN, Lee MK, Jeon SM. Soy pinitol acts partly as an insulin sensitizer or insulin mediator in 3T3-L1 preadipocytes. Genes Nutr. 2008;2(4):359–364. doi: 10.1007/s12263-007-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 7.Fang J, Beattie DS. Novel FMN-Containing Rotenone-Insensitive NADH Dehydrogenase from Trypanosoma brucei Mitochondria: Isolation and Characterization. Biochemistry. 2002;41(9):3065–3072. doi: 10.1021/bi015989w. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikova VG, Kotlyar AB, Karliner JS, Cecchini G, Vinogradov AD. Redox-dependent change of nucleotide affinity to the active site of the mammalian complex I. Biochemistry. 2007;46(38):10971–10978. doi: 10.1021/bi7009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Keeling JJ, Hsu JR, Shawen SB, Andersen RC. Strategies for managing massive defects of the foot in high-energy combat injuries of the lower extremity. Foot Ankle Clin. 2010;15(1):139–149. doi: 10.1016/j.fcl.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Leaper DJ. Risk factors for and epidemiology of surgical site infections. Surg Infect (Larchmt) 2010;11(3):283–287. doi: 10.1089/sur.2010.022. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Kang HY, Kim CH, Kim HS, Kwon MC, Kim SM, Shin IS, Lee HY. Effect of new rotenoid glycoside from the fruits of Amorpha fruticosa LINNE on the growth of human immune cells. Cytotechnology. 2006;52:219–226. doi: 10.1007/s10616-006-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wang H, Chang J, McPhail A, McPhail D, Terada H, Konoshima T, Kokumai M, Kozuka M, Estes J, Lee K. Antitumor Agents, 138. Rotenoids and Isoflavones as Cytotoxic Constituents from Amorpha fruticosa. Journal of Natural Product. 1993;56(5):690–698. doi: 10.1021/np50095a005. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Wang ET, Chen WX. Diverse rhizobia associated with woody legumes Wisteria sinensis, Cercis racemosa and Amorpha fruticosa grown in the temperate zone of China. Syst Appl Microbiol. 2005;28(5):465–477. doi: 10.1016/j.syapm.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Macri L, Clark RA. Tissue engineering for cutaneous wounds: selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol Physiol. 2009;22(2):83–93. doi: 10.1159/000178867. [DOI] [PubMed] [Google Scholar]
- 17.Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 18.Pan TL, Wang PW, Al-Suwayeh SA, Chen CC, Fang JY. Skin toxicology of lead species evaluated by their permeability and proteomic profiles: a comparison of organic and inorganic lead. Toxicol Lett. 2010;197(1):19–28. doi: 10.1016/j.toxlet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Petkov E, Uzunov P, Kostova I, Somleva T, Ognyanov I. Inhibition of rat heart phosphodiesterase by some rotenoids and isoflavonoids. Planta Med. 1983;47(4):237–239. doi: 10.1055/s-2007-969996. [DOI] [PubMed] [Google Scholar]
- 20.Singer AJ, Clark RA. Cutaneous wound healing. New Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 21.Skórkowska-Telichowska K, Zuk M, Kulma A, Bugajska-Prusak A, Ratajczak K, Gasiorowski K, Kostyn K, Szopa J. New dressing materials derived from transgenic flax products to treat long-standing venous ulcers-a pilot study. Wound Repair Regen. 2010;18(2):168–179. doi: 10.1111/j.1524-475x.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas Reddy B, Kiran Kumar Reddy R, Naidu VG, Madhusudhana K, Agwane SB, Ramakrishna S, Diwan PV. Evaluation of antimicrobial, antioxidant and wound-healing potentials of Holoptelea integrifolia. J Ethnopharmacol. 2008;115(2):249–256. doi: 10.1016/j.jep.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Udupa D, Kulkarni R, Udupa SL. Effect of Tridax procumbens extracts on wound healing. International Journal of Pharmacology. 1995;33:37–40. [Google Scholar]
- 24.Wang SY, Wang K, Xin Y, Lv DC. Maggot excretions/secretions induces human microvascular endothelial cell migration through AKT1. Mol Biol Rep. 2010;37(6):2719–2725. doi: 10.1007/s11033-009-9806-x. [DOI] [PubMed] [Google Scholar]
- 25.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small portion of this amino acid. Archives of Biochemistry and Biophysics. 1961;193:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 26.Woollard AC, Tatham KC, Barker S. The influence of essential oils on the process of wound healing: a review of the current evidence. J Wound Care. 2007;16(6):255–257. doi: 10.12968/jowc.2007.16.6.27064. [DOI] [PubMed] [Google Scholar]
- 27.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]