Abstract
Literature is scanty on the interaction potential of Hibiscus sabdariffa L., plant extract with other drugs and the affected targets. This study was conducted to investigate the cytochrome P450 (CYP) isoforms that are inhibited by the extract of Hibiscus sabdariffa L. in vitro. The inhibition towards the major drug metabolizing CYP isoforms by the plant extract were estimated in human liver microsomal incubations, by monitoring the CYP-specific model reactions through previously validated N-in-one assay method. The ethanolic extract of Hibiscus sabdariffa showed inhibitory activities against nine selected CYP isoforms: CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. The concentrations of the extract which produced 50% inhibition of the CYP isoforms ranged from 306 µg/ml to 1660 µg/ml, and the degree of inhibition based on the IC50 values for each CYP isoform was in the following order: CYP1A2 > CYP2C8 > CYP2D6 > CYP2B6 > CYP2E1 > CYP2C19 > CYP3A4 >> CYP2C9 >> CYP2A6. Ethanolic extract of Hibiscus sabdariffa caused inhibition of CYP isoforms in vitro. These observed inhibitions may not cause clinically significant herb-drug interactions; however, caution may need to be taken in co-administering the water extract of Hibiscus sabdariffa with other drugs until clinical studies are available to further clarify these findings.
Keywords: Cytochrome P450, Hibiscus sabdariffa, Herb-drug interaction, Anthocyanin, N-in-one assay
Introduction
Herbal remedies have been in use over the ages until synthetic drugs became available, however, there has been resurgence in the use of herbal remedies. This resurgence has led to increase in the concomitant use of herbs with conventional medicines with the belief that the potency of either the herb or the conventional medicine may be enhanced. But the concomitant use of herbs with conventional medicines has given rise to various potential interactions which may not be beneficial to the user. For example Ginkgo biloba causes spontaneous bleeding when co-administered with aspirin, ibuprofen or warfarin (Bressler, 2005). Panax ginseng induces mania when used with phenelzine and St. John's Wort reduces the plasma concentrations of midazolam, digoxin and indinavir (Hu et al., 2005).
Some of these potential herb-drug interactions occur when the pharmacokinetic profile of either product is altered significantly as a result of their co-administration. However most interactions occur during metabolism especially phase 1 metabolism which is mediated by cytochrome P450 (CYP) isoforms. CYP isoforms are responsible for the metabolism of about 70% of prescription drugs (Karyekar et al., 2002). The induction or inhibition of CYP isoforms by any of the product of herb and drug combination may lead to increased side effect, toxicity or therapeutic failure. Many CYP isoforms such as CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 have been implicated in pharmacokinetic interactions in humans. Some herbs have been shown to inhibit or induce some CYP isoforms for example acute usage of St. John's Wort (SJW) has little effect on major CYPs isoforms (1A2, 2D6, 2C9, 3A) but chronic usage induces CYP3A (Wang et al., 2001). Echinaceae on the other hand alters the action of CYP3A (Gorski et al., 2004), and Ginkgo biloba extract induces CYP2C19 (Yin et al., 2004).
Herbs are taken as powdered products, standardized extracts, tinctures or as beverages but herbal beverages are quite popular worldwide due to perceived beneficial effects. An herbal drink that is very popular in Nigeria and some other parts of the world is the water beverage of Hibiscus sabdariffa which is used for entertainment at social gatherings. Known as “Rosselle” in Mexico, “Zobo” in Nigeria and “Karkade” in Germany, it is also used in folk medicine as a laxative and diuretic. Studies have shown that it can be used in the treatment of hypertension and it has actually been shown to have antihypertensive effect comparable to captopril, in man (Odigie et al., 2003; Herrera-Arellano et al., 2004; Olaleye & Akindahunsi, 2005). The anthocyanin pigments of Hibiscus sabdariffa possess antioxidative, hypocholesterolemic and hepatoprotective activities (Wang et al., 2000; Tsai and Huang, 2004).
A common practice in some part of the world is the use of juices, sodas and sometimes herbal drinks such as the water beverage of Hibiscus sabdariffa (Zobo) to administer drugs (Huang & Lawrence 2004; Fakeye et al., 2007). The water extract of Hibiscus sabdariffa caused a reduction in the elimination of acetaminophen and diclofenac and also a reduction in the bioavailability of chloroquine (Mahmoud et al., 1994; Fakeye et al., 2007; Kolawole and Maduenyi, 2004). The mechanism responsible for this pharmacokinetic herb-drug interaction has not been fully elucidated.
Cytochrome P450 interactions are often considered to be a first step evaluation for possible herb-drug interactions in man. In vitro CYP inhibitory studies showed that alcoholic extracts of herbs tend to have more inhibitory effect than water extract on the same CYP isoforms where the herb demonstrated CYP inhibition (Strandell et al., 2004 Gwaza et al., 2009; Sevior et al., 2010). This study approached the in vitro inhibition of selected CYP isoforms by an ethanolic extract of Hibiscus sabdariffa L.
Materials and methods
Extraction of plant material
Dried calyxes of Hibiscus sabdariffa were purchased from Gbagi market, in Ibadan, Oyo state, Nigeria and was identified and authenticated at the Forestry Research Institute of Nigeria (FRIN), Ibadan with herbarium number FHI 106934. Extraneous matters were handpicked and the calyxes dried in Gallenkamp size three oven, at 40°oC until a constant weight was obtained. The dried calyxes were pulverised with blender (Kenwood, model number: OWBL436003, China,) for 3 minutes and 100 g of the dried powdered calyxes of the plant was infused with 1L of absolute ethanol at room temperature for 4 hours. The solution was decanted and the residue further extracted with another 1 L absolute ethanol for 4 hours. The solutions were pooled, sieved and concentrated with rotary evaporator (Buchi Rotavapor R-210, model number: 0800014803, Switzerland) at 40°C and freeze dried (Lyotrap plus® freeze drier, model number: 912350, Great Britain).
Anthocyanin determination
The total monomeric anthocyanin (TMA) content of the ethanolic extract of Hibiscus sabdariffa was determined as Cyanidin-3-glucoside using the pH differential method described by Wrolstad et al., (2005).
An aliquot portion of the ethanolic extract of Hibiscus sabdariffa was taken and appropriate dilution factor was determined by diluting the test portion of the extract solution with pH 1.0 buffer (potassium chloride, 0.025 M) until absorbance at 520 nm was within the linear range of the spectrometer used (PerkinElmer Lambda 25UV/Vis spectrometer, model number: 501508080511, Singapore). The dilution factor obtained was used to prepare two dilutions of the extract solution, one with pH 1.0 buffer and the other with pH 4.5 buffer (sodium acetate, 0.4 M). The absorbance of these two dilutions were determined at 520 nm and 700 nm and read against a blank cell filled with distilled water. The difference in the absorbance was used in calculating the TMA present in 100 g of the powdered plant material. This was done in triplicate.
CYP-inhibition experiments
Pooled microsomes for metabolite profiling and CYP-inhibition studies were obtained from BD Biosciences Discovery Labware (Bedford, MA). The human liver microsomes (HLM) pool containing 20 mg protein/ml (Lot#99268) consisted of liver samples from 25 donors of both genders. The cocktail-approach (N-in-one assay) for elucidating inhibition towards CYP-specific model reactions was conducted as described earlier in detail (Turpeinen et al., 2005; Tolonen et al., 2007). This method of assay had been employed by several other studies (Turpeinen et al., 2006; Abass et al., 2009; Turpeinen et al., 2009; Sevior et al., 2010). Briefly, each incubation mixture contained 0.5 mg microsomal protein/ml, 0.1 M phosphate buffer (pH 7.4), 1mM NADPH (Nicotinamide Adenosine Dinucleotide Phosphate), and ten probe substrates for major drug-metabolizing CYPs. Substrates, (the target CYPs and final concentrations) in the incubations were: melatonin (CYP1A2, 5 µM); coumarin (CYP2A6, 2 µM); bupropion (CYP2B6, 2 µM); amodiaquine (CYP2C8, 5 µM); tolbutamide (CYP2C9, 8 µM); omeprazole (CYP2C19 and CYP3A4, 5 µM); dextromethorphan (CYP2D6, 1 µM); chlorzoxazone (CYP2E1, 10 µM); midazolam (CYP3A4, 1 µM) and testosterone (CYP3A4, 5 µM). The dried extract was dissolved with methanol and added to the incubation mixture to obtain final concentrations of 0 (solvent control), 0.001, 0.01, 0.1, 1, 10, 100 and 1000 µg/ml. The final amount of methanol in the incubation mixtures was 1% (v/v). The reaction mixture, in a final volume of 200 µl, was preincubated for 2 minutes at 37°C in a shaking incubator block (Eppendorf Thermomixer 5436, Hamburg, Germany) before the reaction was initiated by the addition of NADPH. Each reaction was terminated after 20 minutes by adding 100 µl of ice-cold acetonitrile containing 1 µM phenacetin as an internal standard (IS). Samples were subsequently cooled in an ice bath to precipitate the proteins and stored at −20°C until analyzed. This was done in duplicates. The IC50 values were determined graphically from the logarithmic plot of inhibitor concentration against percentage of activity remaining after inhibition using GraphPad Prism 5.40 software (GraphPad Software Inc., San Diego, CA). All data points represent the average of duplicate incubations. The enzyme activities in the presence of inhibitors (ethanolic extract of Hibiscus sabdariffa) were compared with the vehicle incubations. This assay method was validated using positive controls (Turpeinen et al., 2005; Tolonen et al., 2007)
To evaluate the possible matrix effects to LC/MS/MS analysis of probe metabolites by the complex plant extract, extra control incubations where the extract was spiked after termination of metabolic reactions were performed and no matrix effects were observed at concentration at or below 100 µg/ml for any of the probe metabolites. Peak area of 7-hydroxycoumarin was 74% and peak area of hydroxychlorzoxazone was about 52%, when 1000 µg/ml of extract was added to the control incubation, suggesting some matrix suppression for these two probe metabolites. The peak areas for 7-hydroxycoumarin and hydroxychlorzoxazone at 1000 µg/ml were corrected based on the observed relative matrix effect in comparison to control incubations by multiplying the observed peak areas with the observed matrix suppression factor.
Liquid chromatography-mass spectrometry in monitoring the CYP specific model reactions
The analysis of probe metabolites from CYP-specific marker reactions were conducted with a liquid chromatography-tandem mass spectrometry (LC/MS/MS) method modified from an earlier paper where the inter and intraday variations of method and precision for all metabolites and the limit of quantification (LOQ) had been validated (Tolonen et al., 2007). Briefly, a Waters Alliance 2695 HPLC system (Waters Corp., Milford, MA) was used together with a Phenomenex Kinetex C18 column (2.1 × 50 mm column with 2.6 µm particle size) and an on-line filter at 40°C. The injection volume was 6µl. The HPLC eluents were aqueous 0.1% acetic acid (pH 3.2, A) and methanol (B). The gradient elution from 2% – 50% – 80% B was applied in 0 – 0.5 – 3.0 min, followed by column equilibration, giving a total time of 7 min/injection. The eluent flow rate was 0.4 ml/min. The data was acquired using a Waters Quattro Ultima triple quadrupole mass spectrometer equipped with a Z-spray ionization source. Multiple Reaction Monitoring (MRM) mode using both positive and negative polarity (separate injections for each polarity). For all compounds the capillary voltage used was 4200 V in positive ion mode and 3500 V in negative ion mode. The desolvation temperature was 400°C and the source temperature 150°C. Nitrogen was used as a drying gas and as a nebulizer gas. The collision cell argon pressure was set to 3.5 × 10−3 mbar. The MRM transitions and the compound dependent parameters were as previously described (Tolonen et al. 2007). The instruments were controlled using MassLynx 4.1 software.
Data analysis
The total monomeric anthocyanin (TMA) content of the extract was determined using the formula
Where A = [(A520nm − A700nm)pH 1.0 − (A520nm − A700nm)pH 4.5]
MW = molecular weight in gmol−1; ɛ = molar extinction coefficient in Lmol−1cm−1
DF = dilution factor; I = pathlength in cm.
(Where MW = 449.2 g/mol; ɛ = 26 900 L/cm/mol for Cyanidin-3-glucoside)
The percentage enzyme activity remaining was plotted against the concentrations of ethanolic extract of Hibiscus sabdariffa (EEHS) for each CYP isoforms using GraphPad Prism 5.40. The model equation for the plot was:
% EAR = 100/(1 + 10(EEHS − Log IC50)).
Where EEHS = log of EEHS concentration in the incubations; IC50 = 50% inhibitory concentration, % EAR = percentage enzyme activity remaining.
Results
The percentage yield of the ethanolic extract of Hibiscus sabdariffa was 25.0% (approximately 7 ml of concentrated extract weighing 25.04 g was recovered from 100 g of powdered plant calyxes) and the total monomeric anthocyanin content calculated as Cyanidin-3-glucoside was 0.583 g ± 0.13 [mean ± standard deviation] per 100 g of the dried powder.
A logarithmic plot of the percentage enzyme activity remaining (%EAR) against the different concentrations of the EEHS shows inhibitory activities of the extract on all the nine CYP isoforms screened (Table 1). The %EAR for the solvent control was 100%.
Table I.
IC50 values for the in vitro inhibition of nine CYP isoforms by ethanolic extract of the calyxes of Hibiscus sabdariffa
| Metabolic reactions | OH-MEL | OH-COU | OH-BUP | desEt-AMO | OH-TOL | deM-OME | 5OH-OME | O-deM-DEX | OH-CLZ | 3OH-OME | SO2-OME | 6β-OH-TES | 1α-MID |
| CYP isoforms | 1A2 | 2A6 | 2B6 | 2C8 | 2C9 | 2C19 | 2C19 | 2D6 | 2E1 | 3A4 | 3A4 | 3A4 | 3A4 |
| IC50 (µg/ml) | 306 | 1660 | 481 | 424 | 744 | 621 | 546 | 446 | 506 | 633 | 600 | 1307 | 589 |
| Std Error (LogIC50) | 0.084 | 0.072 | 0.089 | 0.086 | 0.136 | 0.088 | 0.076 | 0.118 | 0.092 | 0.073 | 0.075 | 0.223 | 0.041 |
| 95%C.I of IC50 | 202 | 1159 | 310 | 276 | 378 | 401 | 375 | 248 | 320 | 441 | 412 | 432 | 480 |
| to | to 2379 | to | to | to 1464 | to | to | to | to | to | to | to | to | |
| 463 | 748 | 650 | 960 | 796 | 800 | 800 | 910 | 872 | 3958 | 722 | |||
| IC50 (Litre/dose unit)* |
7.1 | 1.2 | 4.3 | 4.8 | 2.8 | 3.3 | 3.8 | 4.6 | 4.1 | 3.2 | 3.4 | 1.6 | 3.5 |
Values are calculated using a non-linear regression analysis program (GraphPad Prism v5.40). Melatonin 6-hydroxylation (OH-MEL), Coumarin 7-hydroxylation (OH-COU), Bupropion hydroxylation (OH-BUP), Amodiaquine de-ethylatiion (desEt-AMO), Tolbutamide hydroxylation (OH-TOL), Omeprazole de-methylation (deM-OME), Omeprazole 5-hydroxylation (5OH-OME), Dextromethorphan O-demethylation (O-deM-DEX), Chlorzoxazone hydroxylation (OH-CLZ), Omeprazole 3-hydroxylation (3OH-OME), Omeprazole 2-sulphoxidation (SO2-OME), Testosterone 6β-hydroxylation (6β-OH-TES), Midazolam 1α-hydroxylation (1OH-MID).95% confidence interval (95%C.I).
IC50 values converted to reflect volume: One dose unit (=300 ml of herbal drink = 8,18 grams of plant = 2,05 grams of dried extract) should be dissolved to this volume to give the IC50 concentration.
Ethanolic extract of Hibiscus sabdariffa inhibited the following reactions: 6-hydroxylation of melatonin catalysed by CYP1A2; 7-hydroxylation of coumarin catalysed by CYP2A6; hydroxylation of bupropion catalysed by CYP2B6; deethylation of amodiaquine catalysed by CYP2C8; hydroxylation of tolbutamide catalysed by CYP2C9; 5-hydroxylation of omeprazole and de-methylation of omeprazole both catalysed by CYP2C19; O-demethylation of dextromethorphan, catalysed by CYP2D6; 6-hydroxylation of chlorzoxazone catalysed by CYP2E1; and 3-hydroxylation of omeprazole, omeprazole sulphoxidation, 1α-hydroxylation of midazolam and 6β-hydroxylation of testosterone all catalysed by CYP3A4. The IC50 values in µg/ml ranged from 306 µg/ml for CYP1A2 to 1660 µg/ml for CYP2A6 while the IC50 values calculated in litre/unit dose ranged from 1.2 litre for CYP2A6 to 7.1 litre for CYP1A2 (Table 1). The order of inhibition of the investigated CYP isoforms by EEHS was 1A2 > 2C8 >2D6 >2B6 >2E1 > 2C19 > 3A4 >> 2C9 >>2A6.
The IC50 values of the EEHS compared with the IC50 values for selective CYP isoform inhibitors were significantly higher (Table 2), all the values were >100 µg/ml for all the CYP isoforms while those of the inhibitors as reported in a previous study (Turpinen et al., 2005) ranged from 0.011 µg/ml to 17.47 µg/ml.
Table 2.
Comparison of the IC50 values of EEHS with selective N-in-one assay CYP isoform inhibitors.
| CYP | Substrate | Inhibitor | Inhibitor | Inhibitor | Inhibitor IC50 | IC50 values of |
| isoform | MW | IC50 values | values | EEHS | ||
| (g/mol)g | (µM)*h | (µg/ml)** | (µg/ml) | |||
| 1A2 | Melatonin | fluvoxamine | 318.335 | 0.08 | 0.026 | 305.7 |
| 2A6 | Coumarin | tranylcypromine | 364.5 | 1 | 0.365 | 1661 |
| 2B6 | Bupropion | Ticlopidine | 263.79 | 0.1 | 0.026 | 481.2 |
| 2C8 | Amodiaquine | Quercetin | 302.236 | 57.8 | 17.47 | 423.8 |
| 2C9 | Tolbutamide | Sulphaphenazole | 314.362 | 0.2 | 0.063 | 743.8 |
| 2C19 | Omeprazole | Fluconazole | 306.271 | 5.7a | 1.746 | 546.2 |
| Omeprazole | 6.4b | 1.96 | 620.5 | |||
| 2D6 | Dextrmethorphan | Quinidine | 324.417 | 0.035 | 0.011 | 445.8 |
| 2E1 | Clozapine | Pyridine | 79.1 | 36.6 | 2.90 | 505.5 |
| 3A4 | Midazolam | Ketoconazole | 531.43 | 1.8c | 0.957 | 588.9 |
| Testosterone | 0.07d | 0.037 | 1307 | |||
| Omeprazole | 0.08e | 0.043 | 633.2 | |||
| Omeprazole | 0.13f | 0.069 | 599.6 |
*Data adapted from Turpeinen et al., 2005. aIC50 value for omeprazole demethylation. bIC50 value for omeprazole 5-hydroxylation. cIC50 value for midazolam hydroxylation. dIC50 value for testosterone 6-hydroxylation. eIC50 value for omeprazole 3-hydroxylation. fIC50 value for omeprazole sulphoxidation, **Values obtained by dividing the product of g and h with 1000. EEHS - ethanolic extract of Hibiscus sabdariffa.
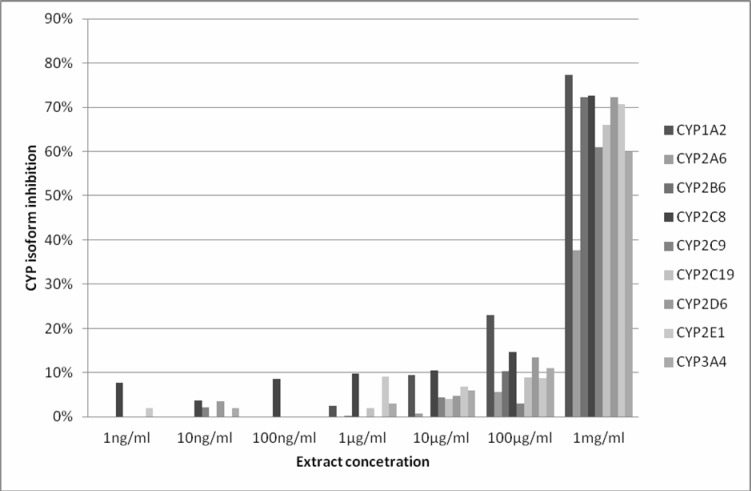
Figure 1 shows that the extract was only able to inhibit all the CYP isoforms significantly at 1 mg/ml (1000 µg/ml). The percentage CYP isoform inhibition at this concentration was between 60 and 80% for all the enzymes except for CYP2A6 which was below 40%. Little or no enzyme inhibition was noticed at extract concentrations of 1 ng/ml, 10 ng/ml and 100 ng/ml.
Figure 1.
Inhibition of CYP isoforms by the extract of Hibiscus sabdariffa. Data are shown as percentage inhibition of the control; each point represents an average of duplicate incubation. For CYP2C19 and CYP3A4 average percentage enzyme inhibition was used for all the substrate metabolites monitored.
Discussion
The extract of H. sabdariffa showed some inhibitory activities against all the nine CYP isoforms studied with a relatively high IC50 values when compared with the controls. Previous study by Prommetta et al., (2006) using aqueous extract of H. sabdariffa in rat reported that the extract had no effect on the total hepatic CYP contents and the activities of CYP isoforms: 1A1, 1A2, 2B1, 2B2, 2E1 and 3A. Ethanolic extract was used in our study instead of water extract because the concentration of the anthocyanin Cyanidin-3-glucoside maker in both extracts is not significantly different (Fakeye et al 2008). We also used Human Liver Microsomes which is more representative of human CYP complements. This may explain why we observed for the first time to the best of our knowledge, inhibitory activities of the extract of Hibiscus sabdariffa on cytochrome P450 enzymes. It was observed that the extract of H. sabdariffa inhibited CYP isoforms that are involved in the metabolism of drugs during phase I metabolic process. The inhibitory activities may become obvious and clinically significant when the plant extract is concomitantly administered with drugs with narrow therapeutic index or drugs that undergo capacity limited metabolism such as phenytoin, carbamazepine, theophylline and digoxin. However, comparing the IC50 values obtained in this study with other IC50 values obtained in similar studies using herbal extracts and also with the IC50 values of specific CYP isoform inhibitors; ethanolic extract of Hibiscus sabdariffa may be classified as a weak inhibitor of cytochrome P450 (Gwaza et al., 2009; Sevior et al., 2010) since the IC50 values were greater than 100 µg/ml. Thus its inhibitory effect on the metabolism of co-administered drugs may not be clinically significant.
Hibiscus sabdariffa contains anthocyanins (Delphinidin-3-sambubioside, Cyanidin-3-sambubioside, Delphinidin-3-glucoside and Cyanidin-3-glucoside), flavonoids such as gossypetine, sabdaretine & hibiscetine (Gautam, 2004), saponins and alkaloids (Mahadevan et al., 2009). There is a dearth of information on the pharmacokinetics properties of phytochemicals from herbs; especially the human oral bioavailability of these herbal components. Few studies available indicates oral bioavailability for anthocyanin, flavonoids and saponins, with the anthocyanins and flavonoids having short half lives (Wu et al., 2002; Yu et al., 2012)
In vitro studies have shown that some phytochemicals namely coumarins, saponins, flavonoids and anthocyanins have inhibitory activities on CYP isoforms (Kim et al., 1997; Kimura et al., 2010; Sand et al., 2010). These compounds which are also present in the calyx of Hibiscus sabdariffa might have been responsible for the observed inhibitory activities of the extract.
Prior clinical studies reported a decrease in the terminal half-life of acetaminophen without a significant corresponding change in AUC and clearance (Kolawole and Maduenyi, 2004); a reduction in the metabolic clearance of diclofenac (Fakeye et al., 2008) and a reduction in the bioavailability of chloroquine (Mahmoud et al., 1994) when these drugs were administered concomitantly with the extracts of H. sabdariffa. These drugs are metabolised by more than one CYP isoform: acetaminophen is metabolised by CYP isoforms 1A2, 2A6, 2D6, 2E1 and 3A4 (Patten et al., 1993); diclofenac by CYPs isoforms 2C9 and 3A4 (Tang et al., 1999) and chloroquine is metabolised by CYP2C8, 2D6 and 3A4/5 (Ducharme and Farinotti 1996; Kim et al., 2003). Drugs metabolised by more than one CYP isoforms or drugs that have other dominant metabolic pathways such as glucoronidation and sulphoxidation may not be significantly affected by inhibitors of only one of the CYP isoform since there is an alternate route of metabolism except if the inhibitor is able to inhibit all the metabolic pathways of the drug concerned. However, a potent inhibitor of most or all the CYP isoforms responsible for the metabolism of a drug may significantly affect the drug's metabolic clearance. Though, the isoforms involved in the metabolism of acetaminophen and diclofenac are inhibited by the extract of H. Sabdariffa; while this may partly explain the slight reduction observed in the metabolic clearance of diclofenac it cannot be proffered for the reduction in the elimination of acetaminophen (since its AUC was not affected) and the reduction in the bioavailabilty of chloroquine.
Usually the inhibitory effect of herbal alcoholic extracts as compared with those of water extracts on CYP isoforms for the same herb have been shown to be slightly different when such activity is demonstrated. The alcoholic extracts are slightly inhibitorier (Strandell et al., 2004; Gwaza et al., 2009; Sevior et al., 2010). Thus it may be expected that the water beverage of Hibiscus sabdariffa may show lesser inhibitory activities towards the nine CYP isoform.
Due to complex nature of the extract and because the individual compounds responsible for the inhibition are not known, molar IC50 values cannot be determined. In order to estimate the significance of the observed inhibitions, the IC50 values were converted to reflect the volume to which a unit dose of the extract must be diluted to give 50% inhibition, using the method described previously by Strandell et al, (2004). When representing the IC50 values in liters/dose units, the possibility of reaching in vivo concentrations that may cause significant inhibition, can be estimated. One dose unit (300ml of the herbal beverage) corresponding to 8.18 g of the plant powdered calyxes as reported by Fakeye et al, (2008) or 2.05 g of the freeze-dried extract used in this study need to be diluted to this volume to cause 50% inhibition. For example, an IC50 of 5 Liters/dose indicates that one dose unit diluted into a volume equivalent to the approximate blood volume of an average person would result in a concentration of inhibitory compounds equivalent to the IC50 value of CYP isoform inhibition. The likelihood of a notable inhibition is higher, when the IC50 values in Liters/dose units are higher. Strandell et al. (2004) concluded that an IC50 value < 0.8 Liters/dose would not be inhibitory. For the extract used in this study the IC50 values in litre/dose were almost equal to the blood volume of an average human for CYP 2B6, 2C8, 2C19, 2D6, 2E1 (approximately 4 L) and for CYP 1A2 it was approximately 7 L which is slightly higher than the average human blood volume. These results according to Strandell et al, (2004) suggest that the extract is likely to cause in vivo inhibition. However, there is need for further investigation in vivo to explore its potential for interactions when administered concomitantly with other drugs.
Conclusion
This study showed that the ethanolic extract of the calyxes of Hibiscus sabdariffa inhibited nine Cytochrome P450 isoforms though at high concentrations. Based on this study, Hibiscus sabdariffa may be classified as a weak inhibitor of Cytochrome P450 enzymes. Nonetheless, caution should be taken in co-administering the water beverage with other drugs until in vivo data are available to make informed decision regarding interactions between the beverage and other drugs metabolised by cytochrome P450 isoforms.
Acknowledgement
The authors wish to appreciate Mr Gbenga Adeyemi for assisting in the determination of the anthocyanin content of the extract and Dr. Dele Odeniyi for providing the software used in the analysis.
List of non-standard abbreviations
- TMA
Total Monomeric Anthocyanin
- EEHS
ethanolic extract of Hibiscus sabdariffa
- EAR
Enzyme activity remaining
References
- 1.Abass K, Turpeinen M, Pelkonen O. An evaluation of the cytochrome P450 inhibition potential of selected pesticides in human hepatic microsomes. J Environ Sci Health. 2009;44:553–563. doi: 10.1080/03601230902997766. [DOI] [PubMed] [Google Scholar]
- 2.Bressler R. Herb-drug interactions: Interactions between Ginkgo biloba and prescription medications. Geriatrics. 2005;60:30–33. [PubMed] [Google Scholar]
- 3.Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet. 1996;31(4):257–274. doi: 10.2165/00003088-199631040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fakeye T O, Adegoke A O, Omoyeni O C, Famakinde A A. Effect of water extract of Hibiscus sabdariffa, Linn (Malvaceae) - “Roselle” on excretion of a diclofenac formulation. Phytother Res. 2007;21(1):96–98. doi: 10.1002/ptr.2019. [DOI] [PubMed] [Google Scholar]
- 5.Fakeye T O, Anirban P, Bawankule D U, Khanuja S P S. Immunomodulatory effect of extracts of Hibiscus sabdariffa L. (Family Malvaceae) in a mouse model. Phytother Res. 2008;22:664–668. doi: 10.1002/ptr.2370. [DOI] [PubMed] [Google Scholar]
- 6.Gautam R D. Sorrel - A lesser-known source of medicinal soft drink and food in India. Nat Prod Rad. 2004;3(5):338–342. [Google Scholar]
- 7.Gorski J C, Huang S M, Pinto A, Hamman M A, Hilligoss J K, Zaheer N A, Desai M, Miller M, Hall S D. The effect of Echinacea on cytochrome p450 activity in vivo. Clin Pharmacol Ther. 2004;75(1):89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Gwaza IL, Wolfe A R, Benet L Z, Guglielmo B J, Chagwedera T E, Maponga C C, Masimirembwa C M. In vitro inhibitory effects of Hypoxis obtusa and Dicoma anomala on cyp450 enzymes and pglycoprotein. Afr J Pharm Pharmacol. 2009;3(11):539–546. [Google Scholar]
- 9.Herrera-Arellano A, Flores-Romero S, Chavez-Soto M A, Tortoriello J. Effectiveness and tolerability of a standardized extract from Hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11:375–382. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Hu Z, Yang X, Ho P C, Chan S Y, Heng P W, Chan E, Duan W, Koh H L, Zhou S. Herb-drug interactions: A literature review. Drugs. 2005;65(9):1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 11.Huang S M, Lawrence J L. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food Interactions: What have we learned? J Clin Pharmacol. 2004;44:559–569. doi: 10.1177/0091270004265367. [DOI] [PubMed] [Google Scholar]
- 12.Karyekar C S, Eddington N D, Dowling T C. Effect of St. John's wort extract on intestinal expression of cytochrome P4501A2: Studies in LS180 cells. J Postgrad Med. 2002;48(2):97–100. [PubMed] [Google Scholar]
- 13.Kim H J, Chun Y J, Park J D, Kim S J, Roh J K, Jeong T C. Protection of rat liver microsomes against carbon tetrachloride-induced lipid peroxidation by red ginseng saponins through cytochrome P450 inhibition. Planta Medica. 1997;63:415–418. doi: 10.1055/s-2006-957724. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Park J, Lee J, Lim S. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Archives Pharmacol Research. 2003;26:8631–8637. doi: 10.1007/BF02976712. [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Ito H, Ohnishi R, Hatano T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem Toxicol. 2010;48(1):429–435. doi: 10.1016/j.fct.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Kolawole J A, Maduenyi A. Effect of zobo drink (Hibiscus sabdariffa water extract) on the pharmacokinetics of acetaminophen in human volunteers. Eur J Drug Metab Pharmacokinet. 2004;29:25–29. doi: 10.1007/BF03190570. [DOI] [PubMed] [Google Scholar]
- 17.Mahadevan N, Shivali P, Kamboj Hibiscus sabdariffa Linn - An overview. Nat Prod Radiance. 2009;8:77–83. [Google Scholar]
- 18.Mahmoud B M, Ali H M, Homeida M M, Bennett J L. Significant reduction in chloroquine bioavailability following co-administration with the Sudanese beverages Aradaib, Karkadi and Lemon. J Antimicrob Chemother. 1994;33(5):1005–1009. doi: 10.1093/jac/33.5.1005. [DOI] [PubMed] [Google Scholar]
- 19.Odigie I P, Ettarh R R, Adigun S A. Chronic administration of aqueous extract of Hibscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. J Ethnopharmacol. 2003;86(2–3):181–185. doi: 10.1016/s0378-8741(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 20.Olaleye M T, Akindahunsi A A. Recent Progress in Medicinal plants Plant Bioactives in Traditional Medicine. 9. Vol. 12. U.S.A: Stadium press LLC; 2005. Hypotensive activity of methanolic extract of the calyces of Hibiscus sabdariffa L on Normotensive rats; pp. 283–288. [Google Scholar]
- 21.Patten C J, Thomas P E, Guy R L, Lee M, Gonzalez F J, Guengerish F P, Yang C S. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 22.Prommetta P, Phivthong-ngam L, Chalchantipyuth C, Niwattisaiwong N, Lawanprasert S. Aqueous extract of the calyces of Hibiscus sabdariffa Linn: effects on hepatic cytochrome P450 and subacute toxicity in rats. Thai J Pharm Sci. 2006;30:8–18. [Google Scholar]
- 23.Sand P G, Dreiseitel A, Stang M, Schreier P, Oehme A, Locher S, Hajak G. Cytochrome P450 2C19 inhibitory activity of common berry constituents. Phytother Res. 2010;24(2):304–307. doi: 10.1002/ptr.2910. [DOI] [PubMed] [Google Scholar]
- 24.Sevior D K, Hokkanen J, Tolonen A, Abass K, Tursas L, Pelkonen O, Ahokas J T. Rapid screening of commercially available herbal products for the inhibition of major human hepatic cytochrome P450 enzymes using the N-in-one cocktail. Xenobiotica. 2010;40:245–254. doi: 10.3109/00498251003592683. [DOI] [PubMed] [Google Scholar]
- 25.Strandell J, Neil A, Carlin G. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomedicine. 2004;11:98–104. doi: 10.1078/0944-7113-00379. [DOI] [PubMed] [Google Scholar]
- 26.Tang W, Stearns R A, Wang R W, Chiu S H, Baillie T A. Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol. 1999;12:192–199. doi: 10.1021/tx9802217. [DOI] [PubMed] [Google Scholar]
- 27.Tolonen A, Petsalo A, Turpeinen M, Uusitalo J, Pelkonen O. In vitro interaction cocktail assay for nine major cytochrome P450 enzymes with thirteen probe reactions and a single LC/MS/MS run; analytical validation and further testing with monoclonal P450 antibodies. J Mass Spectrom. 2007;42:960–966. doi: 10.1002/jms.1239. [DOI] [PubMed] [Google Scholar]
- 28.Tsai P J, Huang H P. Effect of polymerization on the antioxidant capacity of anthocyanins in Rosella. Food Res Inter. 2004;37:313–318. [Google Scholar]
- 29.Turpeinen M, Korhonen L, Tolonen A, Uusitalo J, Juvonen R, Raunio H, Pelkonen O. Cytochrome P450 inhibition screening: Comparison of three assays. Eur J Pharm Sci. 2006;29:130–138. doi: 10.1016/j.ejps.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Turpeinen M, Tolonen A, Chesne C, Guillouzo A, Uusitalo J, Pelkonen O. Functional expression, inhibition and induction of CYP enzymes in HepaRG cells. Toxicol In Vitro. 2009;23:748–753. doi: 10.1016/j.tiv.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Turpeinen M, Uusitalo J, Jalonen J, Pelkonen O. Multiple P450 substrates in a single run: rapid and comprehensive in vitro interaction assay. Eur J Pharm Sci. 2005;24:123–132. doi: 10.1016/j.ejps.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Wang C J, Wang J M, Lin W L, Chu C Y, Chou F P, Tseng T H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–416. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Gorski J C, Brater C, Huang S M, Lesko L J, Hall S D. The effects of St. John's Wort on human cytochrome P450 activity. Clin Pharmacol Ther. 2001;70(4):317–326. [PubMed] [Google Scholar]
- 34.Wrolstad R E, Durst R W, Lee J. Tracking colour pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. [Google Scholar]
- 35.Wu X, Cao G, Prior R L. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132(7):1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- 36.Yin O Q, Tomlinson B, Waye M M, Chow A H, Chow M S. Pharmacogenetics and herb-drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14(12):841–850. doi: 10.1097/00008571-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Yu K, Chen F, Li C. Absorption, disposition and pharmacokinetic of saponins from Chinese medicinal herbs; what do we know and what do we need to know more? Curr Drug Metab. 2012. [May 3rd]. (available at: http://www.ncbi.nlm.nih.gov/pubmed/22292787. [DOI] [PubMed]