Abstract
This paper evaluated the antibacterial effect of medlar and hawthorn compound extract in vitro. Water extract method and ethanol extraction method was adopted to prepare the compound extracts, and disc diffusion method and improved test tube doubling dilution method were used to conduct the antibacterial test on the two common pathogenic bacteria, Staphylococcus aureus and Klebsiella pneumonia, in vitro. The results showed that medlar and hawthorn compound extract was moderately sensitive to Staphylococcus aureus, while its inhibiting effect on Klebsiella pneumoniae was particularly significant, moreover, the antibacterial effect of ethanol extract was better than water extract. Medlar and hawthorn compounds had good antibacterial effect on the two pathogenic bacteria.
Keywords: Medlar and hawthorn compound, content measuring, Antibacterial activity, Killing curve
Introduction
Medlar can nourish liver and kidney and regulate blood and dryness, and has the effects of liver protection, blood pressure decreasing, cholesterol decreasing and cosmetology. Hawthorn has the effects of appetite and digestion promoting, blood activating and stasis dissolving, anti-bacteria and inflammation diminishing, as well as body immunity improving, anti-aging, and blood sugar and blood lipid regulating. The compound composed of the two has the effects of liver and kidney nourishing, blood enriching, intellect improving and cardiovascular and cerebrovascular disease curing, as well as antibacterial and analgesic efficacies (Leung et al., 2001; Toyoda-Ono et al., 2004; Kim et al., 2000). To study its antibacterial effect, take advantage of its rich natural resources and expand the scope of application, this paper studies the antibacterial effect of the medlar and hawthorn compound extract in vitro.
Materials and Methods
Drugs
Medlar and hawthorn (YX-12-05) were bought from Nepstar Drugstore in Yinchuan City, Ningxia Province. They were identified by Doctor Nan Yi in our college as the dry and ripe fruit of Lycium barbarum L. and dry and ripe fruit of Crataegus Pinnatifida Bge., and the specimens are stored in the Specimen Room in the Pharmaceutical College.
Experimental bacteria
S.aureus, ATCC25923 and K. Pneumonia, ATCC700603 were provided by the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China.
Instruments, culture medium and reagents
Liquid chromatograph P3000 (Beijing Chuangxin Tongheng Science and Technology Co., Ltd); ZHJH-CH09B clean bench (Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd); DHG-101-3A heating and drying oven (Gongyi Yingyu Yuhua Co., Ltd); Difunctional vapour-bathing constant temperature vibrator (Jintan Jieruier Electric Co., Ltd);M302078 bacteria turbimeter (Beijing Zhongxi Yuanda Science & Technology Co., Ltd); Gallic acid control (110831-200803) (provided by Ningxia Institute for Drug Control)
Experimental methods
Preparation of medlar and hawthorn compound extract
Water extract: hawthorn (10 g) and medlar (5 g), were crushed respectively and passed through 40 mesh screen, added with 10-fold amount of distilled water, and extracted by heating and backflow three times, each time lasted 1 h, the extract solutions were combined, cooled, and filtered, and the filtrate was concentrated into extractum, and weighed to be 9.7 g, and the extractum rate is 64.7%.
50% ethanol extract: hawthorn (10 g) and medlar (5 g) were weighed out, crushed and passed through 40 mesh screen respectively, added with 10-fold amount of 50% ethanol, and extracted by heating and backflow three time, each time lasted 1 h, the extract solutions were combined, cooled, and filtered, and the filtrate was concentrated into extractum, and weighed to be 8.2g, and the extractum rate is 54.7%.
Chromatographic conditions
50% ethanol extract is prepared at the concentration of 1 mg/ml, standard substance is gallic acid, and chromatographic conditions are: Dima C18 column (150nm * 4.6mm, 5µm), mobile phase is methanol-0.5% phosphoric acid (5:95), detection wavelength is 276 nm, column temperature is 30., the sampling volume is 5 µl, and the flow rate is 0.8 mL/min.
Drug sensitive test (Tao et al., 2009)
0.2 mL of test bacterial solution, which was activated and diluted to 1/2 Mcfar turbidity, was evenly coated and inoculated in sterile nutrient broth agar, the drug disks were pasted on the inoculated agar plate, with six equidistant disks per plate, and each test had 3 parallels. They were inverted in culture incubator at 37°C and taken out after 24 h, the diameter of each inhibition zone was read with vernier caliper, each inhibition zone was read 3 times, and the three readings were averaged. All the parallel test data for all the inhibition zones were processed with SPSS16.0 software, and the data for each group were expressed with (X ±SD).
Minimal inhibitory concentration (MIC) determination (Mo et al., 2009)
Improved test tube doubling dilution method was adopted. Water extract and 50% ethanol extract were used respectively to carry out two groups of parallel tests. The bacterial solution diluted to 1/2 Mcfar turbidity was diluted with nutrient broth by 1:1000, and the diluted bacterial solution was obtained. 1.8 mL of diluted bacterial solution was added into the first tube and 1.0 mL into other tubes, then 0.2 mL of drug solution (crude drug 1 g/mL) was added into the first tube, after mixing uniformly, 1.0 mL was taken and added into the second tube. Doubly diluted to the 10th tube, abandon 1.0 mL, and the 11th tube was for the growth control, i.e. no drug, so as to observe whether the culture medium fits for bacterial growth. 2 mL of drug solution diluted with sterile nutrient broth was added into the 12th tube, in order to observe whether the test drug solution was contaminated. Three times of repeated tests were made to each test bacterium. The above test tubes were placed in 37°C incubator for 2 h and taken out for observation, the bacterium in the 11th tube presented turbid growth, while the 12th tube had no bacterial growth and presented transparent, the minimal drug concentration for sterile growth was determined as MIC.
Minimal bactericidal concentration (MBC) determination (Lin et al., 2003)
The culture solutions with the drug concentration over MIC were streak inoculated in the nutrient broth agar medium using 4 mm inoculating loop, each tube was inoculated to 2 plates, they were inverted in incubator at 37°C for 18 h and then taken out, the minimal drug concentration with no bacterial growth or less than five colonies was determined as MBC of the drug to this bacterium.
Killing curve (Okoli et al., 2005)
Drug concentrations of 1/2MIC, MIC, 2MIC, 4MIC, 8MIC and 16MIC were selected in the test tubes, bacterial solution was added to the tubes to make its concentration in tube as 105CFU/ml, at 0, 2, 4, 6, 8, 12, 18 and 24 h, tubes were fully mixed, sampled and diluted, the viable bacteria was counted with plate method, and the bacterial count-time curve was drawn.
Results and Discussion
Result of measuring Gallic acid content in the different extracts by HPLC
HPLC is adopted to make chromatographic analysis on Gallic acid in water and 50 ethanol extracts, there is Gallic acid in these two extracts, and its content is measured, see Figure 1.
Figure 1.
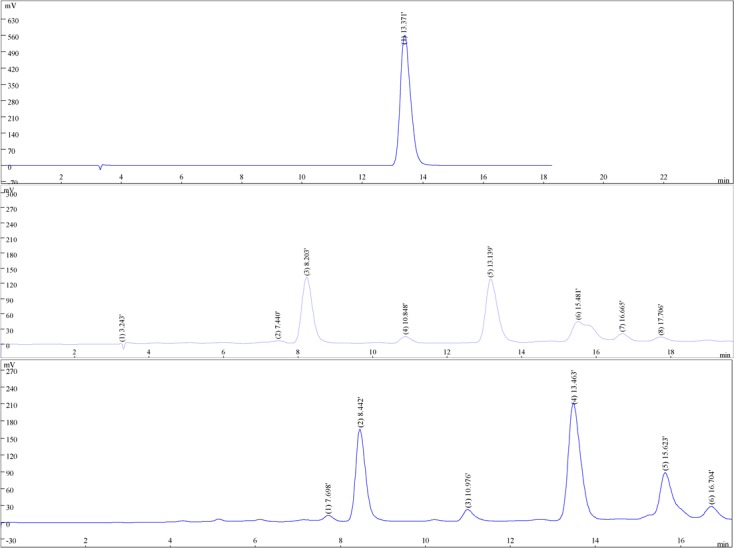
(a) the HPLC chromatograms of gallc acid, (b) the HPLC chromatograms of water extracts, (c) the HPLC chromatograms of 50% ethanol extracts
It can be seen from the result that Gallic acid content in water and 50% ethanol extracts is different, respectively 2.94% and 4.61%. Gallic acid content in 50% ethanol extract is significantly higher than that in water extract. Meanwhile, we find that the antibacterial activity of 50% ethanol extract is also higher than that of water extract.
Drug sensitivity measuring result
According to the determination standard for the qualitative result of anti-bacteria in vitro, the medlar and hawthorn compound extract is highly sensitive to Staphylococcus aureus and Klebsiella pneumoniae, and the antibacterial force to Klebsiella pneumoniae is the strongest. The results of the drug sensitive test of medlar and hawthorn compound to the two pathogenic bacteria are shown in Table 1.
Table 1.
Diameter of inhibiting bacteria circle of extracts (mm)
| Sample name | Diameter of inhibiting bacteria circle | |
| S.aureus | K. Pneumonia | |
| water extracts | 15.28±0.13 | 17.21±0.24 |
| 50% ethanol extracts | 19.33±1.12 | 23.87±0.18 |
MIC and MBC values of the different extracts
MIC and MBC values of the different extracts of medlar and hawthorn compound to Staphylococcus aureus and Klebsiella pneumoniae are low, which is in accordance with its inhibition zone result. MIC and MBC values of the 50% ethanol extract of medlar and hawthorn compound to the two pathogenic bacteria are lower than its water extract, as shown in Table 2.
Table 2.
MIC and MBC of inhibiting bacteria of extracts (mg/ml)
| Sample name | water extracts | 50% ethanol extracts | ||
| MIC | MBC | MIC | MBC | |
| S.aureus | 5 | 100 | 2.5 | 50 |
| K. Pneumonia | 2.5 | 50 | 0.625 | 2.5 |
Killing curve of 50% ethanol extract
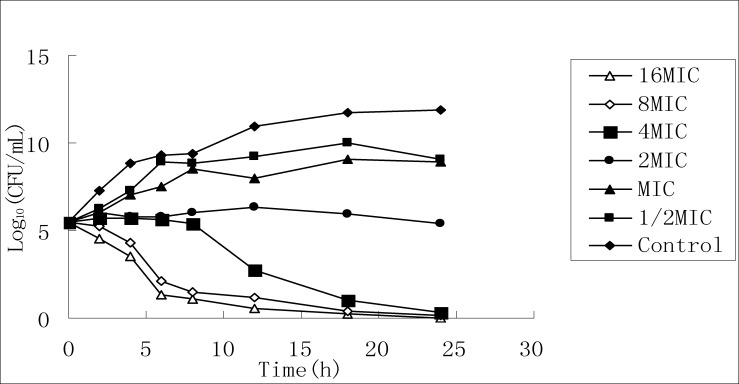
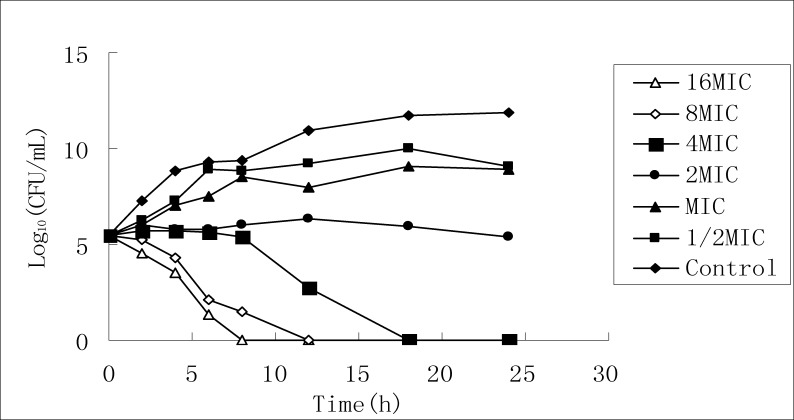
According to MIC test result, the stock solution of 50% ethanol extract is diluted respectively to six concentrations, and the killing curves for the two bacteria are drawn by taking different time points as the horizontal axis and taking the logarithms of the culture colony counts at the different time points as the vertical axis, as shown in Figure 2 and Figure 3.
Figure 2.
Killing S.aureus curve of 50% ethanol extracts
Figure 3.
Killing K. Pneumonia curve of 50% ethanol extract
It can be seen from FIG2 that for the effect on Staphylococcus aureus, as the extract concentration increases, bacterial growth at 24 h is completely inhibited, but it cannot completely kill the bacteria; it can be seen from FIG3 that the time for killing Klebsiella pneumoniae turns shorter, the killing effect increases and presents concentration dependence, and when the concentration increases above MIC value, it can completely kill the bacteria.
Hawthorn, mainly produced in Northern China, is a commonly used traditional Chinese medicine. There are more than 150 substances found and separated from hawthorn at present, and the main components are mostly phenolic compounds, such as apigenin, luteolin, quercetin, (+)-catechin and (−)-epicatechin, or dimer and polymer forms, such as procyanidin B2, B4 and B5, ursolic acid, and corosolic acid; cycloartane form, such as cycloartenol, oleanolic acid and crataegolic acid; also including a large quantity of organic acids, such as benzoic acid, Gallic acid, protocatechuic acid, chlorogenic acid, β-coumaric acid, caffeic acid, ferulic acid, anisic acid, vanillic acid, syringic acid, gentisic acid, etc (Melikouglu et al., 1999; Melikoglu et al., 2000; Mousallamy et al., 1998; Ulla et al., 2002; Woo et al., 1994; Carmen et al., 2001; Sun et al., 2000; Garcia et al., 1997). Hawthorn phenolic compound has many pharmacological activities, such as lowering blood lipid, lowering blood pressure, helping digestion, anti-oxidation, anti-bacteria, anti-tumor, etc. (Essaady et al., 1996; Choi et al., 2000; Hsu et al., 1997; Lee et al., 1994). Medlar mainly contains betaine, LBP, scopoletin, free amino acids, vitamins, etc, and it has sweet taste and moderate nature. Medlar has the effects of immune function strengthening, anti-bacteria, anti-aging, liver protection, blood sugar lowering, anti-tumor, blood pressure lowering and anti-oxidation (Kim et al., 1997; Peng et al., 2001; Leung et al., 2001; Toyoda-Ono et al., 2004; Kim et al., 2000).
Experimental results show that medlar and hawthorn compound extract has strong inhibitory effect on Staphylococcus aureus and Klebsiella pneumoniae. In general, the ethanol extract of medlar and hawthorn compound has better antibacterial effect than the water extract, and in coincidence, the main component gallic acid content in the ethanol extract is significantly higher than that in the water extract, and the reason may be related to that the extraction with ethanol as the solvent and coordinated with hot reflux can greatly increase the extraction rate of phenols and other active components in the drugs, so proper processing technology can be selected according to the difference in the nature of the active components in traditional Chinese medicine, to make it maximize the efficacy (Liu et al., 2009).
This experiment can focus on the antibacterial effect of medlar and hawthorn compound and makes HPLC to the main components of the extract, but its mechanism of antibacterial effect needs more in-depth study in the future. In recent years, along variety increase and wide application of antibiotics, the problem of bacterial resistance is growing, and adverse drug reaction is increasing. As chemosynthetic drugs are hard to develop and have significant toxic and side effects, while Chinese medicines and Chinese herbal compounds have less resistance to the majority of bacteria (Yu et al., 2007). Therefore, the research and development of antibacterial Chinese medicines has important significance on solving the problems of resistant strain production and antibiotics abuse (Wang et al., 2007).
Acknowledgments
This project was supported by 2009 National Supporting Project of Sciences and Technology (No. 2009BAI72B03) and 2011 National Natural Science Foundation of China (No. 81102893) and 2011 Ningxia science and technology key projects (No.2011-25).
References
- 1.Essaady D, Simon A, Ollier M, Maurizis JC, Chulia AJ, Delage C. Inhibitory effect of ursolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996;106:193. doi: 10.1016/0304-3835(96)04312-1. [DOI] [PubMed] [Google Scholar]
- 2.Choi BM, Park R, Pae HO, Yoo JC, Kim YC, Jun CD, Jung BH, Oh GS, So HS, Kim YM, Chung HT. Cyclic adenosine monophosphate inhibits ursolic acid-induced apoptosis via activation of protein kinase A in human leukaemic HL-60 cells. Pharmacol Toxxicol. 2000;86:53. doi: 10.1034/j.1600-0773.2000.d01-10.x. [DOI] [PubMed] [Google Scholar]
- 3.Hsu HY, Yang JJ, Lin CC. Effect of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111:7. doi: 10.1016/s0304-3835(96)04481-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee HY, Chung HY, Kim KH, Lee JJ, Kim WM. Induction of differentiation in the cultured F9 teratocarcinoma stem sells by triterpene acids. Cancer Research Clinical Oneology. 1994;120:513. doi: 10.1007/BF01221027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S Y, Choi Y H, Kim J, Kim Y C, Lee H S. New antihepatoxic cerebroside from Lycium chinese. Journal of Natural Product. 1997;60:274–276. doi: 10.1021/np960670b. [DOI] [PubMed] [Google Scholar]
- 6.Peng X, Tian G. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohy drate Research. 2001 doi: 10.1016/s0008-6215(00)00321-9. [DOI] [PubMed] [Google Scholar]
- 7.Leung I, Li W, Tso M, Lam T. Absorption and tissue distribution of zeaxanthin and lutein in rhesus monkeys after taking Fructus lycii (Gou Qi Zi) extract. Investigative Ophthalmology and Visual Science. 2001;42:466–471. [PubMed] [Google Scholar]
- 8.Toyoda-Ono T, Maeda M, Nakao M, Yoshimura M, Sugiura-Tomimori N, Fukami H. 2-O(a-D-glucopyranosyl) ascorbic acid, a novel ascorbic acid analogue isolated from Lycium Friut. Journal of Agricultural and Food Chemistry. 2004;52:2092–2096. doi: 10.1021/jf035445w. [DOI] [PubMed] [Google Scholar]
- 9.Kim S Y, Lee J E, Kim P H, Lee HS, Kim YC. LCC, a cerebroside from Lycium chinese, protects primary cultured rat hepatocytes exposed to galactoseamine. Phytotherapy Research. 2000;14:448–451. doi: 10.1002/1099-1573(200009)14:6<448::aid-ptr635>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Melikoglu G, Mericli F, Mericli A H. Flavonoids of Crataegus orientalis. Bollettino Chimico Farmaceutico. 1999;138:351–352. [Google Scholar]
- 11.Melikoglu G, Mericli A H. Flavonoids of Crataegus stevenii. Pharmazie. 2000;55:326–327. [PubMed] [Google Scholar]
- 12.Mousallamy E, Amani M D. Chemical investgation of the constitutive flavonoid glycosides of the leaves of Crataegus sinaica. Nat Prod Sci. 1998;4:53–57. [Google Scholar]
- 13.Ulla S, Heikki V, Risto K, Jari T, Juha K, Jussi-Pekka R, Into L, Raimo H. Isolation and identification of oligomeric procyanidins from Crataegus leaves and flowers. Phytochemistry. 2002;60:821–825. doi: 10.1016/s0031-9422(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 14.Woo P S, Soo Y C, Kyu L H. Chemical components from the fruits of Crataegus pinnatifida var. psilosa. Saengyak Hakhoechi. 1994;25:328–335. [Google Scholar]
- 15.Ahumada C, García D, Saenz T, Gómez A, Cert A. Influence of the parasite Viscum cruciatum Sieber on the chemical constituents of Crataegus monogyna Jacq. J Biosci. 2001;56:1091–1094. doi: 10.1515/znc-2001-11-1228. [DOI] [PubMed] [Google Scholar]
- 16.Sun M B, Ho K Y, Lee S M, Na MK, Lee CO, Lee JP, Bae K. Cytotoxic triterpenes from Crataegus pinnatifida. Arch Pharm Res. 2000;23:155–158. doi: 10.1007/BF02975505. [DOI] [PubMed] [Google Scholar]
- 17.Garcia MD, Saenz MT, Ahumada MC, Cert A. Isolation of three triterpenes and several aliphatic alcohols from Crataegus monogyna Jacq. J Chromatog, A. 1997;767:340–342. [Google Scholar]
- 18.Liu Y, Cui L, Zhao ZH, Xu G, Wang M, Li YH. Antibacterial activity on different extracts of 50 Chinese herbal medicines against Streptococcus suis in vitro. Journal of Northeast Agricultural University. 2009;40:90–93. [Google Scholar]
- 19.Yu Y, Yi ZB, Liang YZ. Validate antibacterial mode and find mainbioactive components of traditional chinese medicine aquilegia oxysepala. Bioorganic & Medicinal Chemistry Letters. 2007;17:1855–1859. doi: 10.1016/j.bmcl.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Wang YP, Guo SM. Research thinking on antibacterial effects of traditional Chinese medicine. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2007;28:5–7. [Google Scholar]
- 21.Tao WQ, Lei XY, Mai XF, Huang LY, Miao SY. In vitro antibacterial activities extracts from four plants used as traditional chinese medicine guanzhong. Journal of Wuhan Botanical Research. 2009;27:412–416. [Google Scholar]
- 22.Mo WG, Liu HG, Zheng CG. Antibacterial and acute toxic research on mango stone extract in vitro. Lishizhen Medicine and Materia Medica Research. 2009;20:1932–1933. [Google Scholar]
- 23.Lin D, Zhao GL, Liu JJ. Extraction of active components from flos lonicerae and their bacteriostatic test. Natural Product Research and Development. 2003;15:436–437. [Google Scholar]
- 24.Okoli S, Iroegbu CU. In vitro antibacterial activity of Synclisa scabrida whole root extract. Afr J Biotechnol. 2005;4:946–952. [Google Scholar]