Abstract
Calotropis Procera (CP) has been used in the management of toothache, fresh skin burns, gum bleeding as well as others to make it qualify as a medicinal plant. This study was designed to assess its wound-healing property in rabbits and its potentials for anti keloidal activity.Fresh latex of Calotropis were obtained and evaluated phytochemically. Fifteen male rabbits were used and four excisional wounds were created on each rabbit. The rabbits were divided into five groups of three each. Group 1 was the negative control and received no treatment. The wounds of group 2 animals were treated with 2mL of Calotropis latex; group 3 with 2mL honey; and group 4 with a mixture of 1ml honey and 1 mL of the latex. The animals in group 5 were given 2mg triamcinolone intramuscularly. All the groups had their wounds treated daily for 21 days. The wounds' diameters were measured on the day of wound creation, thereafter on days 7, 14 and 21 post wound creation. Biopsies of the wounds were taken on days 3 and 21 and viewed histologically. Phytochemical study of the latex revealed the presence of glycosides, tannins and alkaloids. The wounds were found to be significantly (p<0.05) reduced in groups treated with 50% latex in honey and triamcinolone, respectively, on day 7 post wound creation while there was a significant (p<0.05) reduction in wound surface area in all treated groups on days 14 and 21 post wound creation. Histological findings in untreated group showed thick bundle of collagen fibres some of which had broad based configurations, reminiscent of keloid. The group treated with 2mL of Calotropis latex revealed the presence of florid granulation tissues on day 3 while there was a marked reduction in quantity and size of collagen fibres on day 21 post wound creation which was comparable with what was seen for the triamcinolone-treated group.The general effect of Calotropis latex on wound-healing was noted. Likewise it's similarity to that of triamcinolone, an anti-keloidal agent; this makes it a probable candidate for future anti-keloidal study using a suitable model.
Keywords: Calotropis procera, latex, wound-healing, potential anti-keloidal property
Introduction
A wound is a breach in the normal tissue continuum resulting in a variety of cellular and molecular sequelae. Wound may be accidental or as a result of planned or deliberate surgical incision. Wound healing is a complex series of cellular events that brings about the restoration of the anatomic structure and function of damaged part of the body. The process of wound healing comprises of 3 overlapping phases, namely-inflammation, proliferation, and remodeling. At the time of wounding, the initial inflammatory phase occurs with the activation of the coagulation cascade that causes the release of cytokines which in turn stimulate chemotaxis of neutrophils and macrophages into the wound to begin early debridement [Wen-Hsiang et al.,2010]. Keloid, a type of scar, is a benign, proliferative, fibrous outgrowth having its origin in the sub papillary layer of the dermis, and which develops as a result of trauma in certain predisposed individuals.
Several medicinal agents of natural origin have been used for the treatment of wounds and keloids. Examples include Ocimum sanctum, O. gratissimum, alba, Eucalyptus globulus, Calotropis procera [Prasanna et al.,2007]. Calotropis procera Aiton (family: Asclepiadaceae), henceforth referred to as Calotropis, is native to South-west and South-east Asia and Africa and also occurs on the Caribbean Islands, in Central and South America [Lebrun et al.,1998].
In complementary and alternative medicine, the whole plant of Calotropis, leaves, barks as well as its latex have been employed in the treatment and management of many health conditions such as jaundice, joint pains, fever, asthma, snake bite, malaria, dysmenorrhoea, eczema and leprosy [Jan et al.1998, Pal et al.1998, Kirtikar et al.1975, Singh et al.1998, Parotta et al.,2001]. The latex is soaked in cotton for application into dental cavity to treat toothache [Suresh et al.,2005], gum bleeding [Kumar et al.,2007] and for dressing fresh skin burns [Kirtikar et al.,1975].
Rasik et al [Rasik et al.,1999] evaluated the latex of Calotropis for its wound healing potential in guinea pigs and found out that a 1.0% sterile solution effected a 20% reduction in wound area after a 7-day treatment regimen.
This study aims at evaluating the wound-healing property of Calotropis latex in rabbits with a view to relating the results of the histological findings to its probable anti-keloidal activity.
Materials and Methods
The plant was collected near the Obafemi Awolowo University campus, Ile-Ife, Nigeria. It was identified and authenticated by Mr. Gabriel Ibhanesebhor of the Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria and a voucher specimen (number UHI-16332) has been deposited at the Ife Herbarium, Obafemi Awolowo University, Ile-Ife, Nigeria. Fresh latex was collected from healthy plants by making small incisions near the youngest leaves, allowing the latex to flow into sterile Mac Cartney bottles. The latex was gently handled to maintain its integrity/potency during transport to the laboratory. The latex was then refrigerated until needed.
Phytochemical screening
Phytochemical screening was carried out on the latex using standard methods [Odebiyi et al.,1978, Trease et al.,1983]. for detecting the presence of secondary metabolites: alkaloids, carbohydrates, free reducing sugars, combined reducing sugars, tannins, saponins, glycosides, sterols, terpenes and flavonoids.
Animals
Fifteen male rabbits (1.4–2Kg) were obtained from the animal house, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria and maintained at standard conditions of 12 h light/dark cycle, relative humidity of 14 ± 1% and temperature of 22 ± 1°C. They were fed with standard rabbit feed (Guinea Feeds, Edo State, Nigeria) and water ad libitum. They were acclimatized for two weeks before the commencement of experiments. All experiments were performed according to the “Guide for the Care and Use of Laboratory Animals” [Janet et al., 1983].
Wound-healing study
The rabbits were gently shaved using an electric clipper to expose the skin after been anaesthetized with about 10mls of diethyl ether inhalation in a transparent bell jar. Four full thickness skin excisional wounds (two on each flank) were created on each rabbit with the use of a scalpel. The diametric widths of the wound were measured using a caliper. The rabbits were divided into 5 groups of three each. The three test groups were treated immediately after wound creation and daily by topical application of 2ml Calotropis latex, 1ml of 50% latex in honey and 1ml honey, respectively, for 21 days. Triamcinolone (2mg i.m.) was administered once to the positive control group, while the negative control group received no treatment for the 21-day test period. On days 3 and 21 post wound creation, biopsies of wounds were taken from the regenerated tissues and preserved in 10% buffered formalin at 25°C for histological study. The tissues were paraffin-embedded and prepared appropriately into Hematoxylin-and-Eosin (H & E) stained slides, at the Histopathology Laboratory of the Department of Morbid Anatomy and Forensic Medicine of Obafemi Awolowo University, Ile-Ife. All the slides were reviewed by the pathologists and photomicrographs were taken.
Statistical analysis
Numerical data were expressed as the mean ± standard error of mean (SEM). Statistical analysis of data was carried out using one-way analysis of variance (ANOVA) followed by student's t-test. Differences in mean were considered to be significant when P < 0.05
Results
Phytochemical screening
The results of the phytochemical tests carried out indicated the absence of saponins, phlobatanins, anthraquinones, and flavonoids in the Calotropis latex. Tests for glycosides indicated the presence of cardenolides with lactone ring, steroidal nucleus and desoxysugar. Tannins and alkaloids were also found to be present in the latex.
Wound-healing Study
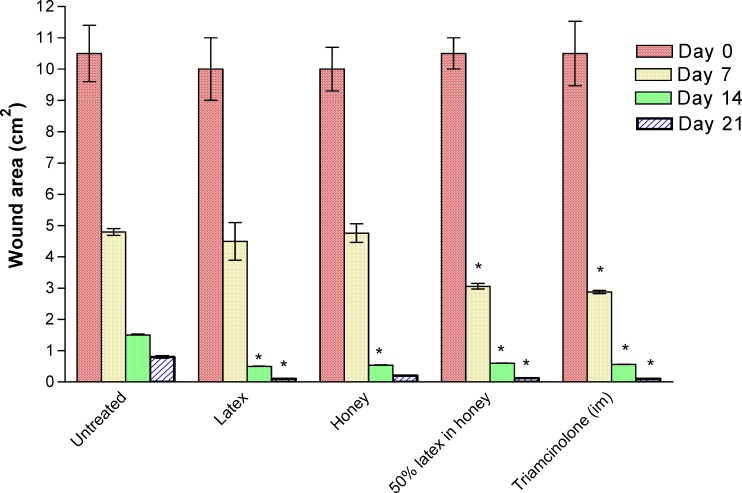
The effects of the test and control agents on the healing of the wounds created on the rabbits are indicated in the fig. 1 below:
Figure 1.
Wound-healing effect of Calotropis latex
n=12, where n= (number of rabbits per group X number of wounds created on each rabbit) [3X4]
* Significantly different from control at p<0.05
On day 7 of treatment, there was a significant (p<0.05) reduction in the wound surface area among the group administered with 50% of latex in honey and those treated with triamcinolone in comparison with the control, while there was no significant difference between the control group and those treated with only the latex and honey, respectively (Fig. 1).
On the 14th day of treatment, there was a significant (p<0.05) reduction in the wound surface area of all the treated groups when compared with the control, with the group receiving the latex alone exhibiting the highest wound contraction. On the 21st day of treatment, there was a significant (p<0.05) reduction in the surface area of the wounds for all the treated groups when compared with the control except the group of rabbits treated with honey alone. By this day, the groups treated with triamcinolone and the latex alone had the same extent of wound contraction.
Histopathology
The histological findings of the wounds treated with Calotropis on day 3 (Plate 4) showed florid granulation tissues when compared with the control (Plate 1) which showed some granulation tissues with necrotic areas. The wounds treated with triamcinolone had scanty granulation tissue (Plate 2) while wound treated with honey (plate 3) shows more granulation tissues when compared with the wound treated with triamcinolone.
Plate 4.
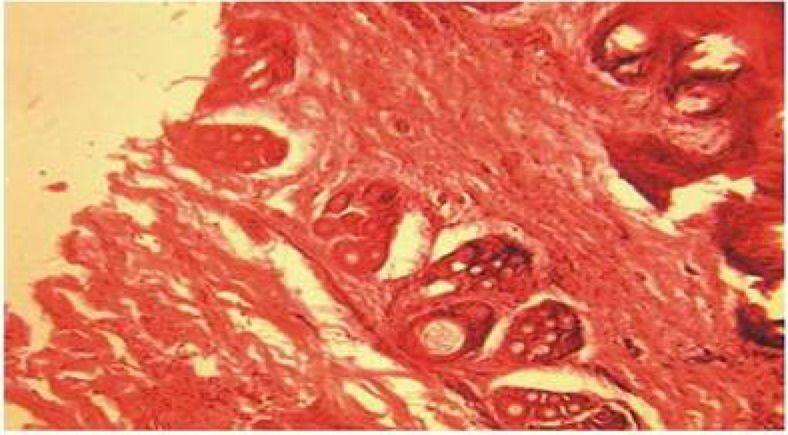
Section of the wound treated with Calotropis procera on day 3, showed the presence of florid granulation tissues. (H&E Stain. Medium power magnification).
Plate 1.
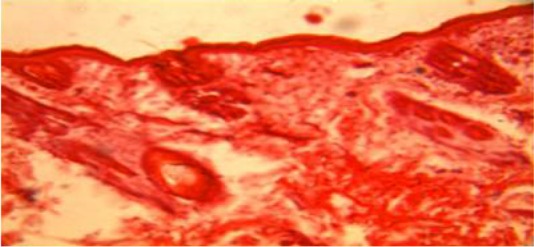
Section of the untreated wound on day 3, showed some granulation tissues with necrotic areas. (H&E Stain. Medium power magnification).
Plate 2.
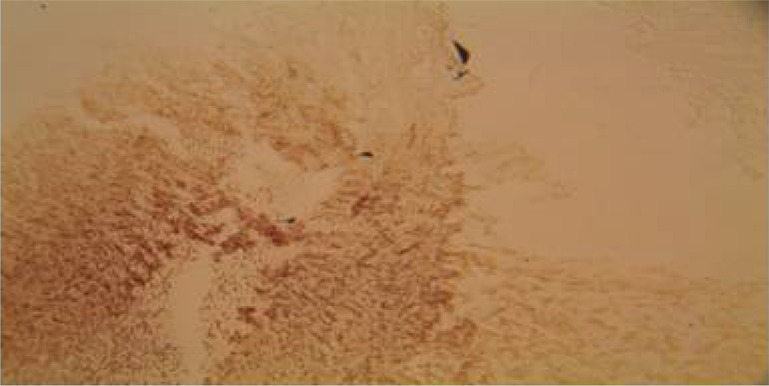
Section of the wound treated with triamcinolone on day 3. Note the scanty granulation tissue. (H&E Stain. Medium power magnification).
Plate 3.
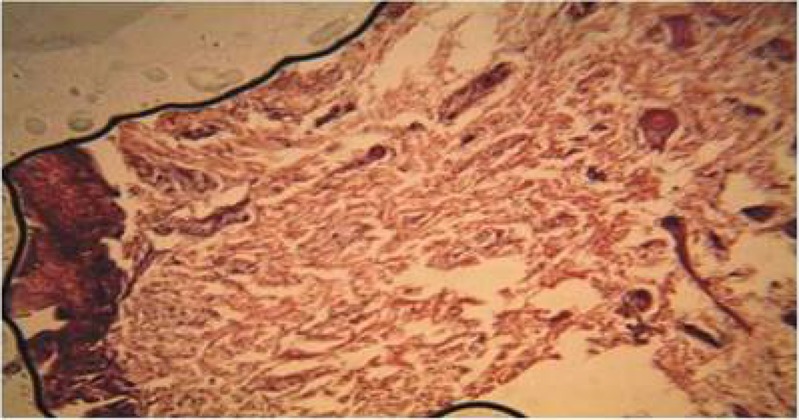
Section of the wound treated with honey on day 3, showed more granulation tissues but less than the quantity seen in wound treated with Calotropis procera. (H&E Stain. Medium power magnification).
The histological sections from the wounds treated with Calotropis on day 21 revealed wounds composed of pale looking collagen fibres with marked reduction in its thickness (Plate 7). The histology of the untreated wounds revealed homogenous eosinophilic, haphazardly arranged, thick bundle of collagen fibres some of which have broad based configurations (Plate 5) while those treated with triamcinolone also showed marked reduction in the thickness of the collagen fibres (Plate 9), which is comparable with the reduction noticed in group treated with Calotropis alone (Plate 7).
Plate 7.
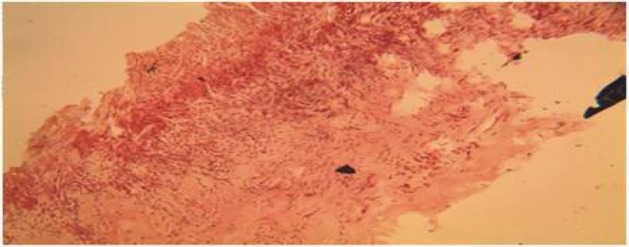
Section of the wound treated with Calotropis procera on day 21, showed marked reduction in the thickness of the collagen fibres. (H&E Stain. Medium power magnification).
Plate 5.
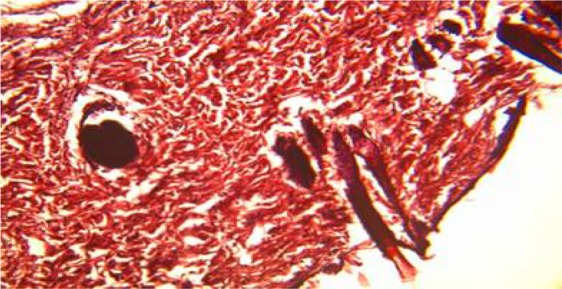
Section of the untreated wound on day 21, showed homogenous eosinophilic, haphazardly arranged thick bundle of collagen fibres. H&E Stain. Medium power magnification).
Plate 9.
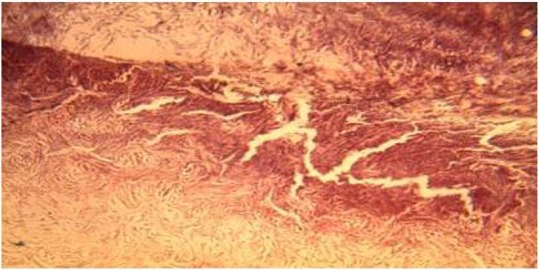
Section of the wound treated with triamcinolone on day 21, showed marked reduction in the thickness of the collagen fibres. (H&E Stain. Medium power magnification).
The histology of the wounds treated with 50% latex in honey showed moderate reductions in the thickness of the fibres (Plate 8) while the wound treated with honey alone showed some reductions in the thickness of the fibres (plate 6).
Plate 8.
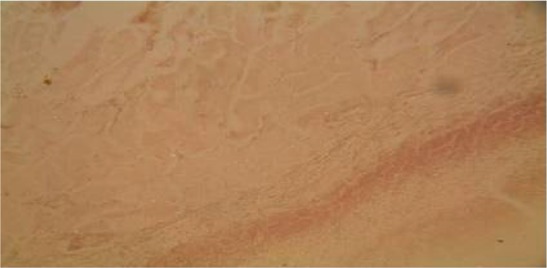
Section of the wound treated with combination of 50% honey and 50% Calotropis procera on day 21. Note also the marked reduction in the thickness of the collagen fibres but the reduction is less when compared with plate 7.
Plate 6.
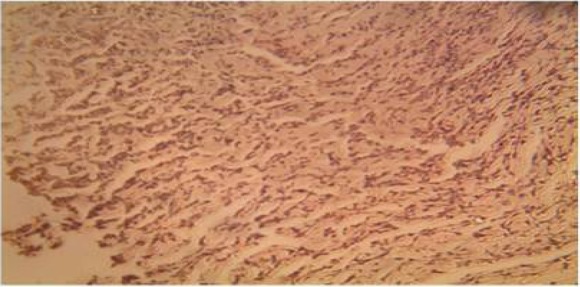
Sections of the wound treated with honey on day 21, showed some reductions in the thickness of the collagen fibres. (H&E Stain. Medium power magnification).
Discussion
Calotropis latex contains tannins which are known to promote wound healing mainly due to their astringent and antimicrobial properties that contribute to wound contraction and increased rate of epithelisation [Shivananda et al., 2007]. This could in part have contributed to the observed wound healing activity of the latex observed in this study.
During the inflammatory stage of wound healing, macrophages, neutrophils, and other cells get activated with the release of mediators of inflammation such as vascular endothelial growth factors and these exert a pleiotropic effect on different fibroblast activities, leading to the production of elevated quantities of extracellular matrix (ECM), resulting in excessive tissue fibrosis and unrestrained growth of keloid lesions [Robles et al., 2007]. Hypertrophic scars and keloids are aberrant forms of wound healing and are indications of exaggerated function of fibroblasts and excess accumulation of ECM during wound healing [Wen-Hsiang et al.,2010] in which case there is a dysregulated response to cutaneous injuries, which results in an excessive deposition of collagen [Shin et al.,2004]. Hypertrophic scars and keloids are not exactly same. Hypertrophic scar has raised and firm surface, red or pink in colour and usually confined to wound area. Keloid has raised firm and irregular surface usually dark red and pigmented in colour. It extends into the surrounding skin. Contrary to hypertrophic scars, keloids do not regress with time, are difficult to revise surgically, and do not provoke scar contractures [Ehrlich et al., 1998].
The pattern of histological appearance observed in the untreated wounds in this study is consistent with the histological appearance obtainable in hypertrophic scars and Keloids. The reduction in the number and width of the collagen fibres by Calotropis latex may be explained by its well reported anti-inflammatory properties [Alencar et al.,2004]. In addition, the phytochemical screening of the latex revealed the presence of steroidal nucleus in the test for glycosides. This may indicate that its anti inflammatory mechanism of action is similar to that of triamcinolone which is a steroid. Further studies will be needed to ascertain or complement this despite the generally known fact that lower animals do not form keloids
The wounds treated with triamcinolone also showed significant reduction in the thickness and quantity of collagen fibres. Triamcinolone is indicated in the treatment of keloids [Hochman et al.,2008]. While the exact mechanisms of action of steroids, such as triamcinolone, in the treatment of keloids are still unclear, their anti-keloidal activities most likely result from suppression of the inflammatory response and from diminished collagen synthesis, inhibition of fibroblast growth and enhanced collagen degeneration [Wu Ws et al.,2006].
In this study, sections taken from wounds treated with triamcinolone showed scanty granulation tissues admixed with pale looking well oriented collagen fibres. The implication of this is that although triamcinolone may not be excellent in wound healing, it displays some therapeutic usefulness in the control of high rate collagen synthesis that characterizes keloids.
The florid granulation tissue observed in wounds infiltrated with honey is supportive of its use in the management of wounds [Dunford et al.,2000]. Its inclusion in our experimental design was to see if its wound healing was comparable with that of Calotropis and to determine the possibility of synergism in their wound-healing activity. While this combination yielded a significant reduction in the area of wound in comparison with the control group, the group treated with honey alone did not show a significant wound contraction. This showed that 50% Calotropis latex in honey yielded a better wound contraction than honey alone. There was also a marked reduction in the thickness of the collagen fibres in this group as demonstrated histologically. It is noteworthy that honey alone produced a similar effect even though it was less than that of calotropis alone or 50% Calotropis in honey. This may indicate a possibility of exploration of honey as a potential candidate for anti-keloidal research in the near future.
Conclusion
Calotropis latex, in this study, demonstrated dual effects on wound healing. Its ability to induce florid granulation tissues favoured wound healing, while its ability to inhibit exaggerated response of fibroblasts and its subsequent accumulation of collagens in the matrix might be supportive of its potential anti-keloidal activity. The general apparent reduction in the quantity and width of the broad band collagens in the group treated with C. latex in comparison to the control suggest that the growth of the collagen is inhibited by C. latex. The result of this study suggests that C. latex can be a potential source of therapeutic agents that can be used in the treatment of keloid. Howard Jacob notes that rats and humans are 90% identical at the genetic level. This implies that the observations in this study might be of benefit in humans. However, despite these observations, the findings in this study can't be directly extrapolated in humans because majority of the drugs shown to be safe in animals end up failing in clinical trials [Shank et al.,2009].
We therefore advocate for further works to establish the probable anti-keloidal property of Calotropis latex.
Acknowledgements
We like to acknowledge the contributions of Prof E O Elujoba (department of Pharmacology OAU) Mr. Kamarudeen (Pharmacology department, LAUTECH) and Dr Eziyi (department of Surgery Lautech) that were part of an initial trial of this plant on Webster Rats (Yet to be published) and the laboratory assistants/technicians of the Faculty of Pharmacy of both LAUTECH and OAU.
References
- 1.Wen-Hsiang S, Ming-Huei C, Wen-Ling L, Tsung-Shan T, Wen-Hsun C, Chien-Sheng C, Peng-Hui WO. Nonsteroidal Anti-Inflammatory Drugs for Wounds: Pain Relief or Excessive Scar Formation? Mediators Inflamm. 2010 doi: 10.1155/2010/413238. Article ID 413238, 8 pages, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasanna V, Habbu, Hanumanthachar J, Patil BS. Potential Wound Healers from Plant Origin. Phcog Rev. 2007;1(2):271–282. [Google Scholar]
- 3.Lebrun JP. Catalogue des plantes vasculaires de la Mauretanie et du Sahara occidental. Genève. 322 S. Boissiera: 1998. p. 55. [Google Scholar]
- 4.Jan G, Khan MA, Gul F. Ethnomedicinal Plants Used against Jaundice in Dir Kohistan Valleys (NWFP) Pakistan Ethno Leaflets. 2009;13:1029–1041. [Google Scholar]
- 5.Pal DC, Jain SK. Tribal Medicine. 206 Bidan Sarani, Calcutte. SZ: Naya Prakesh; 1998. [Google Scholar]
- 6.Kirtikar KR, Basu BD. Indian Medicinal Plants. III. Delhi: Periodical Experts; 1975. pp. 1609–1611. [Google Scholar]
- 7.Singh V, Pandey RP. Ethnobotany of Rajastan, India. Jodhpur: Scientific Publishers; 1998. p. 367. [Google Scholar]
- 8.Parotta JA. Healing Plants of the Peninsular India. Winslow, Buck, Singapore: MRM Graphics Ltd.; 2001. p. 917. [Google Scholar]
- 9.Kumar Suresh, Goyal Sangeeta, Cauhan Aruna, Parveen Farzana. Some New Ethnomedicinal uses of Milkweed in the Indian Desert. Indian J Traditional Knowledge. 2005;4(4):448–455. [Google Scholar]
- 10.Kumar VL, Arya S. Medicinal uses and pharmacological properties of Calotropis procera. In: Govil JN, editor. Recent Progress in Medicinal Plants. Houston, Tex, USA: Studium Press; 2007. pp. 373–388. [Google Scholar]
- 11.Rasik M, Raghubir R, Gupta A, Shukla A, Dubey MP, Srivastava S, Jain HK, Kulshreshta DK. Healing potential of C. procera on dermal wounds in Guinea pigs. J Ethnopharmacol. 1999;68(1–3):261–266. doi: 10.1016/s0378-8741(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 12.Nayak Shivananda. Influence of Ethanol Extract of Vinca rosea on Wound Healing in Diabetic Rats. Online J Biol Sc. 2006;6(2):51–55. [Google Scholar]
- 13.Robles DT, Moore E, Draznin M. Keloids: pathophysiology and management. Dermatol Online J. 2007;13:9. [PubMed] [Google Scholar]
- 14.Shin D, Minn KW. The effect of myofibroblast on contracture of hypertrophic scar. Plast Reconstr Surg. 2004;113(2):633–640. doi: 10.1097/01.PRS.0000101530.33096.5B. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich HP, Desmouliere A, Diegelman RF, Cohen, Compton CC, Garner WL, Kapanci Y, Gabbiani G. Morphological and Immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1998;145(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- 16.Alencar NM, Figueiredo IS, Vale MR, Bitencurt FS, Oliveira JS, Ribeiro RA, Ramos MV. Anti-inflammatory effect of the latex from Calotropis procera in three different experimental models: peritonitis, paw edema and hemorrhagic cystitis. Planta Med. 2004;70(12):1144–1149. doi: 10.1055/s-2004-835842. [DOI] [PubMed] [Google Scholar]
- 17.Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional Triamcinolone Acetonide for Keloid Treatment: A Systematic Review. Aesth Plast Surg. 2008;32(4):705–708. doi: 10.1007/s00266-008-9152-8. [DOI] [PubMed] [Google Scholar]
- 18.Wu WS, Wang FS, Yang KD. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. 2006;126:1264–1271. doi: 10.1038/sj.jid.5700274. [DOI] [PubMed] [Google Scholar]
- 19.Dunford C, Cooper R, Molan P, White R. The use of honey in wound management. Nurs Stand. 2000;5; 15(11):63–68. doi: 10.7748/ns2000.11.15.11.63.c2952. [DOI] [PubMed] [Google Scholar]
- 20.Odebiyi A, Sofowora AE. Phytochemical Screening of Nigerian Medicinal Plants - Part III. Iloydia. 1978;41:243–246. [PubMed] [Google Scholar]
- 21.Trease GE, Evans WC. Pharmacognosy. 12th ed. Vol. 387. London: Baillere Tindall; 1983. pp. 475–476. [Google Scholar]
- 22.Guide for the Care and Use of Laboratory Animals. Eighth Edition. National Academies Press; p. 58.p. 59.p. 77. Downloaded from: http://www.nap.edu/catalog/12 910.html. [Google Scholar]
- 23.Shank N, Greek R, Greek J. Are animal models predictive for humans? Philosophy, Ethnics and Humanities in Medicine. 2009;4:2. doi: 10.1186/1747-5341-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]