Abstract
The importance of endothelial dysfunction in the development and clinical expression of cardiovascular disease is well recognized. Impaired endothelial function has been associated with an increased risk of cardiovascular events. Endothelial function may be evaluated in humans by assessing vasodilation in response to stimuli known to induce the release of nitric oxide. A novel pulse amplitude tonometry device noninvasively measures vasodilator function in the microcirculation of the finger. This article reviews the recent studies that support the utility of digital pulse amplitude tonometry as a relevant test of peripheral endothelial function.
Introduction
The significance of endothelial dysfunction in cardiovascular disease is well established (Widlansky et al. 2003). In the healthy state, the endothelium produces a number of factors including nitric oxide that are essential for maintaining vascular homeostasis. Systemic risk factors damage endothelial cells lowering nitric oxide (NO) bioavailability. Endothelial dysfunction facilitates atherogenesis by promoting inflammation, thrombosis and cellular adhesion. Recent studies demonstrate higher risk of cardiovascular events in individuals with impaired endothelial function (Yeboah et al. 2007).
Since the initial identification of NO, a number of techniques have been employed to investigate endothelial vasomotor function in humans (McMackin et al. 2005). Endothelial function assessment has not yet been incorporated into routine risk stratification in part owing to technical and logistical limitations. The use of a novel digital pulse amplitude tonometry (PAT) device to measure endothelial function offers the possibility of an easily performed, rapid assessment of vascular function (Celermajer 2008). The current article reviews the use of digital PAT as an indicator of endothelial function.
Testing of Endothelial Function in Cardiovascular Disease
Endothelial health can be assessed by measuring vasodilator responses to interventions known to stimulate endothelial release of NO. Endothelial function testing has many potential applications in both research and clinical practice that are outlined in Table 1. A number of methodologies have been developed to measure endothelial vasomotor function in humans and these have been the subject of recent reviews (Barac et al. 2007, Deanfield et al. 2007, McMackin et al. 2005).
Table 1.
Noninvasive Endothelial Function Testing in Practice
| Potential Utility of Endothelial Function Testing |
| Discover novel cardiovascular risk factors |
| Risk stratify prior to onset of clinical cardiovascular disease |
| Risk stratify in patients with established cardiovascular disease |
| Predict clinical response to therapy |
| Investigate disease mechanisms in humans
|
| Criteria to Evaluate a Test of Endothelial Function |
| Relevant biological mechanism |
| Ease of use: transportable, limited training time, short test duration |
| Reproducible: across patient groups, locations, and time |
| Available population reference data on test distribution |
| Improves on current risk stratification tools: Acceptable discrimination, calibration, reclassification |
| Responds to therapeutic interventions |
| Improvement corresponds to reduced cardiovascular risk |
The most frequently employed noninvasive endothelial function test is flow-mediated vasodilation of the brachial artery assessed with ultrasound. Brachial artery flow-mediated vasodilation is lower in the presence of traditional risk factors and importantly predicts risk for cardiovascular events (Widlansky et al. 2003,Yeboah et al. 2007). A number of limitations have precluded the clinical adoption of brachial ultrasound based testing of endothelial function. In particular, the accurate measurement of brachial flow-mediated vasodilation requires extensive sonographer training, expensive equipment and labor intensive image analysis. In response to these limitations, investigators have developed new methodologies designed to be simple to perform. The ideal test for endothelial function would satisfy a number of important criteria as outlined in Table 1.
Measurement of Endothelial Function with the Use of Digital PAT
Emerging evidence supports the assessment of digital PAT as a measure of endothelial function. Assessment of vascular function with PAT involves measuring pulse amplitude in the fingertip at rest and following the induction of reactive hyperemia. The EndoPAT device (Itamar Medical, Israel) is a Federal Drug Administration approved, commercially available system, consisting of a fingertip plethysmograph capable of sensing volume changes in the digit with each arterial pulsation. The device used to measure digital vascular function is shown in Figure 1. The fingertip probe has a rigid external casing containing inflatable chambers. The uniform applied pressure field across the finger prevents venous pooling and partially unloads arterial wall tension. Volume changes in the fingertip are recorded digitally as pulse amplitude that can be tracked over time.
Figure 1.
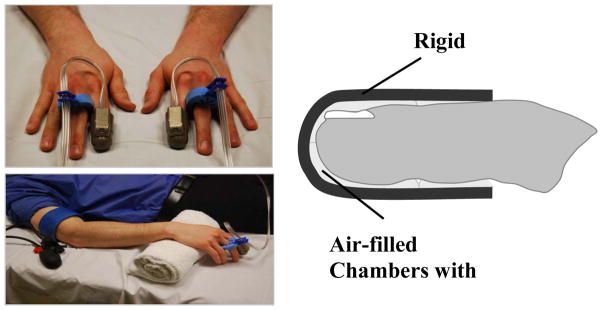
As shown at the top left, the thimble-like PAT device is placed on a finger from each hand. Tubing connects the device to a recording unit that transmits data directly to a computer. As shown in the diagram on the right, the device contains air-filled chambers that are inflated to approximate diastolic pressure throughout the study. Sensors allow detection of changes in finger volume with each arterial pulsation. A typical setup for a research study is shown in the bottom left panel. While the device is on the fingertip, the hand is elevated to allow the fingers to hang freely without touching any surfaces. A cuff is placed on one forearm that will be inflated to suprasystolic pressures to induce hyperemia.
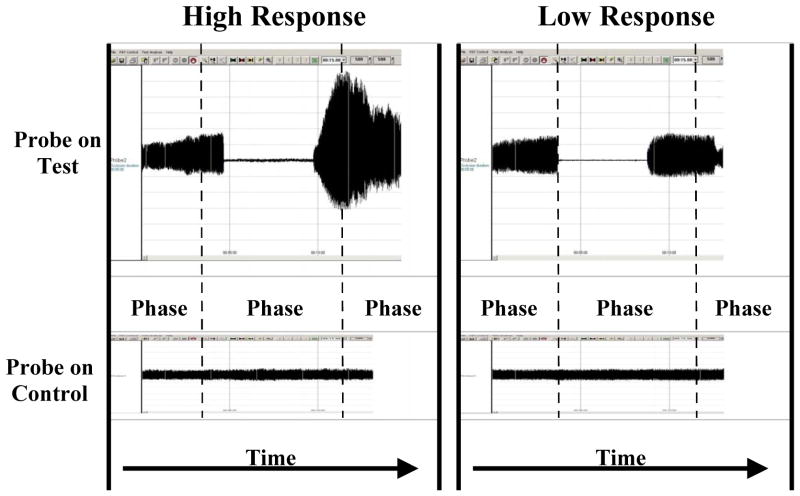
A complete digital PAT endothelial function test includes three phases: baseline, occlusion, and hyperemia. A PAT probe is positioned on one finger of each hand and set by the computer to inflate to 10 mmHg below diastolic pressure or 70 mmHg (the lower value is selected). Recordings are taken simultaneously from both fingers throughout the study. The response in the control finger not experiencing hyperemia can be used to adjust for systemic effects. After baseline data acquisition, a blood pressure cuff is inflated on one arm to suprasystolic pressures for 5 min. During the occlusion period, signals are absent from the hyperemic finger but continue from the control finger. Following cuff release, pulse amplitude increases in the hyperemic finger. The pulse amplitude recordings are digitized and analyzed by an automated, proprietary algorithm. Average pulse amplitude is calculated for each 30 sec intervals following cuff occlusion for up to 5 min. The baseline pulse amplitude also is measured and reported in standardized, arbitrary units. Figure 2 displays representative recordings from two individuals, one with high and one with low PAT hyperemic response.
Figure 2.
As shown, the tracings on the left are from an individual with a high PAT hyperemic response whereas the tracings on the right are from an individual with a low PAT hyperemic response. The study has three phases. Phase 1 records the baseline pulse amplitude from the probes on both fingers. Phase 2 is during the cuff inflation on one arm. In the test finger flow is occluded but continues in the control finger. Phase 3 is recorded after cuff release. In the test finger pulse amplitude rises rapidly in the healthy individual but not in the patient with coronary artery disease. In the control finger flow, there is minimal change in pulse amplitude after cuff release.
The automated assessment of vascular function with PAT produces reliable results. In over 2000 eligible participants in the Framingham Heart Study, the rate of technically interpretable studies was over 90% (Hamburg et al. 2008). The major reasons for technical inadequacy were incomplete cuff occlusion (3%), noisy signal quality (3%) and incomplete data acquisition (3%). A few patient-specific factors limit test performance, including Raynaud’s disease, a finger size that does not match the fingertip probe, and a very long fingernail. Owing to computerized analysis approach, interobserver measurement variability is minimized. The published data regarding the reproducibility of the digital vascular responses over time are limited. In two small studies of healthy adults, there was evidence supporting the reproducibility of the PAT hyperemic ratio measured on two days (Bonetti et al. 2003, Tomfohr et al. 2008). In 44 children with diabetes the mean coefficient of variation for the PAT hyperemic response measured 4 weeks apart was 14.8 % (Haller et al. 2007).
We recently reported the characteristics of the PAT hyperemic response in participants in the Third Generation cohort of the Framingham Heart Study (Hamburg et al. 2008). As shown in Figure 3, in the hyperemic finger, there was a time-dependent rise in pulse amplitude that on average peaked at 60–120 sec following cuff release. Notably, pulse amplitude increased modestly in the control finger, indicative of a systemic response to cuff occlusion. Owing to the presence of a systemic effect, a measure of the PAT hyperemic response was developed that incorporated the observed changes in the control finger. The PAT ratio was calculated in three steps. First, the ratio of the pulse amplitude in each 30 sec interval following cuff release to the baseline pulse amplitude was calculated for the hyperemic finger. Second, this ratio in the hyperemic finger was divided by the corresponding ratio in the control finger. Third, because the PAT ratio was not normally distributed and the error structure was heteroscedastic, we performed a natural logarithm transformation to arrive at the final PAT ratio.
The PAT ratio can be derived for each 30 sec interval following cuff release. The portion of the PAT hyperemic response shown to be dependent on endothelial NO release occurs from 60 to 120 sec following cuff deflation (Nohria et al. 2006). Notably, the relation between cardiovascular risk factors and the PAT hyperemic response was maximized in the 90 to 120 sec time interval (Hamburg et al. 2008). These findings support selecting the PAT hyperemic response recorded between one and two minutes after cuff deflation as the appropriate measure of digital vascular function.
The augmentation of pulse amplitude with hyperemia reflects a complex vascular response to ischemia in the digit (Mitchell et al. 2004). As the PAT device records pulse amplitude in the finger, changes in the PAT signal reflect, in part, vasodilator function of the digital microcirculation. Importantly, NO production by the endothelium has been shown to contribute to the PAT hyperemic response. In healthy volunteers, infusion of an endothelial NO synthase (eNOS) inhibitor blunted the rise in pulse amplitude measured in the minute following cuff release (Nohria et al. 2006). These findings suggest that the increase in digital pulse amplitude that occurs with hyperemia is dependent in part on flow-mediated release of NO and support the use of the PAT hyperemic response as a measure of endothelial function.
Clinical Studies Measuring Endothelial Function with Digital PAT
Investigators have developed evidence supporting the clinical relevance of the PAT hyperemic response. Selected studies measuring PAT hyperemic responses are summarized in Table 2. Multiple studies have examined the relation of PAT hyperemia to established measures of endothelial function. In addition, studies have examined the associations between traditional and novel cardiovascular risk factors and digital vascular function. Finally, investigators have measured the changes in the PAT hyperemic response after short-term interventions.
Table 2.
Selected studies of digital pulse amplitude tonometry hyperemic response
| Reference | Study population | Study details | Main findings |
|---|---|---|---|
| Observational | |||
| Community-based | |||
| Dhindsa et al 2008 | 40 healthy adults | PAT, FMD, brachial reactive hyperemia and brachial radial pulse wave velocity measured simultaneously | PAT hyperemic response correlated with FMD, brachial hyperemic flow but not pulse wave velocity |
| Hamburg et al 2008 | 1957 adults | PAT and cardiovascular risk factors | PAT hyperemic response lower with male sex, obesity, higher total/HDL cholesterol, diabetes, smoking, lipid-lowering therapy and higher with increasing age. |
| Referral or disease states | |||
| Kuvin et al 2003 | 89 Patients with chest pain referred for stress test | PAT and brachial measured simultaneously | PAT hyperemic response correlated with FMD and lower with risk factors and with positive stress test |
| Bonetti et al 2004 | 94 Patients referred for catheterization | PAT and coronary response to acetylcholine measured | PAT hyperemic response lower in patients with coronary endothelial dysfunction |
| Haller et al 2007 | 44 children | 24 with Type I DM, 20 healthy children | PAT hyperemic response lower in children with DM |
|
| |||
| Interventional | |||
| Bonetti et al 2003 | 23 Patients with refractory angina | 7 wk treatment Enhanced external counterpulsation | PAT hyperemic response increased after treatment |
| Nohria et al 2006 | 19 healthy subjects | Administration of inhibitor of eNOS inhibitor, L-NAME | PAT hyperemic response lower after L-NAME consistent with nitric oxide dependence |
| Schroeter et al 2006 | 12 healthy subjects | Ingestion of flavanol-rich cocoa or epicatechin | Flavanoid consumption increased PAT hyperemic response |
| Aversa et al 2008 | 20 men with diabetes | 4 week treatment with sildenafil or placebo | Sildenafil increased PAT hyperemic response and FMD |
A number of studies have investigated the relation between vascular function in the digit and other regions. In patients undergoing coronary angiography, lower PAT hyperemic response was associated with the presence of coronary endothelial dysfunction measured by acetylcholine response (Bonetti et al. 2004). Two studies reported a modest relation between PAT hyperemic response and brachial flow-mediated dilation (Dhindsa et al. 2008, Kuvin et al. 2003). Thus, in small studies, digital vascular function appears to be associated with endothelial function in the brachial artery. However, the PAT hyperemic response largely reflects vasodilator responses in digital microvessels whereas brachial artery flow-mediated dilation measures conduit artery vasodilation. Microvascular function as assessed by PAT has the potential to evaluate a distinct vascular response from conduit vessel flow-mediated dilation.
Several studies have examined the relation of clinical cardiovascular risk factors with the PAT hyperemic response. Kuvin and colleagues reported that the PAT hyperemic ratio was progressively lower with increasing burden of cardiovascular risk factors (Kuvin et al. 2003). In patients without obstructive coronary artery disease undergoing coronary angiography, increasing body mass index and decreasing HDL were associated with lower PAT hyperemic response in unadjusted analyses (Bonetti et al. 2004). Adolescents with type I diabetes also had lower hyperemic PAT responses compared to their healthy peers (Haller et al. 2007, Mahmud et al. 2008).
A number of emerging cardiovascular risk factors have been linked to impaired digital vascular responses in small studies. Polycystic ovarian syndrome, preeclampsia, obstructive sleep apnea, acute malaria infection, depression, mental stress, and postprandial hyperglycemia have all been associated with decreased PAT hyperemic response (Itzhaki et al. 2005, Lowenstein et al. 2007, Tomfohr et al. 2008, Yeo et al. 2007, Yinon et al. 2006, Martin et al. 2008, Crandall et al. 2009). These studies provide evidence that systemic states associated with cardiovascular risk impair microvessel vasodilator function in the fingertip reflected in lower PAT hyperemic responses.
We examined how cardiovascular risk factors relate to PAT measures in the Framingham Heart Study Third Generation cohort. In this large, community-based cohort, we reported that cardiovascular risk factors explained close to 16% of the variability in the PAT hyperemic response (Hamburg et al. 2008). A number of cardiovascular risk factors, including male sex, body mass index, total to high-density lipoprotein cholesterol ratio, diabetes mellitus, smoking, and lipid-lowering treatment were all associated with lower PAT hyperemic response. Surprisingly, increasing age was associated with higher PAT hyperemic response. The unanticipated positive association between advancing age and PAT requires further investigation in cohorts with a wider age range and potentially reflects differential aging responses in the digital vessels. Systolic blood pressure and C-reactive protein were not associated with PAT hyperemic response in multivariable models.
We also reported significant relations between cardiovascular risk factors and higher baseline pulse amplitude. Baseline pulse amplitude reflects blood flow, tone and compliance and may be an important indicator of microvessel structure and function. Overall, these findings suggest that early vascular abnormalities associated with systemic cardiovascular risk factors are reflected in digital vascular responses.
The presence of clinical cardiovascular disease is associated with impaired digital vascular function. Individuals with a stress test with evidence of ischemia had lower PAT hyperemic responses than those individuals without ischemia (Kuvin et al. 2003). In an ambulatory setting, patients with coronary artery disease had lower PAT hyperemic responses than individuals without established coronary disease (Kuvin et al 2007).
Although cross-sectional studies including large numbers of participants have excellent ability to adjust for potential confounding, they cannot establish the temporal relations between risk factors and PAT. Additional evidence for the utility of the PAT hyperemic response is provided by studies showing that short-term interventions of potential beneficial for vascular health improve the PAT hyperemic response. In patients with refractory angina, enhanced external counterpulsation therapy resulted in higher PAT hyperemic responses and a parallel reduction in anginal symptoms (Bonetti et al. 2003). Flavonoid-containing supplements improved the PAT hyperemic response (Barringer et al. 2008, Fisher et al. 2003, Schroeter et al. 2006). A number of additional therapies, including L-arginine infusion, sildenafil, air filtration to reduce particulate matter, treatment of malaria, therapy for obstructive sleep apnea, all similarly enhanced the PAT hyperemic response (Brauner et al. 2008, Aversa et al. 2008, Yeo et al. 2007, Itzhaki et al. 2007, Yeo et al. 2008). Overall, these studies in small groups of selected individuals indicate that the PAT hyperemic response is a dynamic measure of vascular function.
Limitations of Digital PAT
There are several limitations to digital vascular function testing as a measure of endothelial function. First, few studies have reported the PAT response to a direct vasodilator such as nitroglycerin; thus, the relative importance of endothelium-dependent and independent vasodilation in the PAT hyperemic response has not been defined in patients with risk factors or in disease states. Second, published studies reporting digital vascular responses have primarily included individuals of European descent; thus, it remains uncertain whether PAT hyperemic response varies by ethnicity or race. Third, digital vascular function is highly responsive to sympathetic tone and thus findings from a controlled research environment may not extend to routine practice settings. Finally, there is a cost associated with the probes and each probe can only be used one time.
Research Directions
A number of important questions require further investigation in order to define the clinical relevance and research utility of PAT for cardiovascular disease. The overall association of digital vascular function with cardiovascular risk factors in the Framingham Heart Study was modest at 16% and the contribution of individual risk factors was small. Thus, the determinants of digital vascular function remain incompletely defined. Future studies will define the heritability and relation of genetic variation to digital vascular function. The PAT hyperemic response, as a measure of microvessel function, may not yield equivalent information to measures of conduit artery vasodilation. Further studies in larger samples are required to define the relation between the PAT hyperemic response and alternate measures of endothelial function. More information regarding the variability in the PAT hyperemic response with time of day, ambient temperature, seasons, menstrual cycle, longitudinally and with disease status are necessary. The studies reporting a change in the PAT response with treatment have largely involved novel therapeutic strategies to improve cardiovascular health. Treatments with more established cardiovascular benefits such as statin therapy exist and it would be interesting to evaluate the effects of these drugs on PAT measures.
Individuals in the Framingham Heart Study and additional cohorts followed over time for the occurrence of cardiovascular events will provide further information regarding the prognostic relevance of the PAT hyperemic response.
Ongoing studies measuring endothelial function using PAT along with alternate methods will aid in determining the relative prognostic value of each vascular function test. Importantly for any endothelial function test to assist in clinical risk stratification, the results need to provide incremental risk prediction and reclassification of risk over standard risk factors (Vasan 2006, Pencina et al. 2008). Longitudinal studies will evaluate whether certain groups of individuals will derive particular benefit from digital vascular testing, such as those at intermediate risk of cardiovascular events. In addition, it will be important to establish whether improvements in the PAT hyperemic response are associated with lower cardiovascular risk.
Summary and Conclusions
There is abundant evidence linking endothelial dysfunction to atherosclerosis and to an increased risk of cardiovascular events. Thus, a simple and accurate endothelial function test is an attractive, noninvasive addition to cardiovascular risk stratification tools. There is considerable interest in developing the test of digital vascular function using PAT as a method for evaluating endothelial function. The advantages of this technique include ease of administration and an automated analysis program that facilitate acquisition of reliable data. Recent studies demonstrate a modest relation between cardiovascular risk factors and digital vasodilator responses. Treatments thought to confer cardiovascular benefit are able to reverse endothelial dysfunction in the fingertip. However, much additional work is needed to define the clinical relevance of the PAT hyperemic response. Further studies evaluating the clinical and genetic determinants and the predictive value of digital vascular function are required to establish the role of PAT in clinical and research practice.
Acknowledgments
Sources of Funding
Dr. Hamburg is supported by NIH grant HL083781. Dr. Benjamin receives support from RO1 HL076784, 1R01 AG028321, RO1 HL70100, and N01-HC 25195.
Footnotes
Disclosures
Dr. Benjamin has received an unrestricted research grant from Itamar Medical, the manufacturer of the PAT device.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aversa A, Vitale C, Volterrani M, et al. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet Med. 2008;25:37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- Barac A, Campia U, Panza JA. Methods for evaluating endothelial function in humans. Hypertension. 2007;49:748–760. doi: 10.1161/01.HYP.0000259601.38807.a6. [DOI] [PubMed] [Google Scholar]
- Barringer TA, Hatcher L, Sasser HC. Potential Benefits on Impairment of Endothelial Function after a High-fat Meal of 4 weeks of Flavonoid Supplementation. Evid Based Complement Alternat Med. 2008 doi: 10.1093/ecam/nen048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- Brauner EV, Forchhammer L, Moller P, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177:419–425. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- Celermajer DS. Reliable endothelial function testing: at our fingertips? Circulation. 2008;117:2428–2430. doi: 10.1161/CIRCULATIONAHA.108.775155. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Shamoon H, Cohen HW, et al. Post-challenge hyperglycemia in older adults is associated with increased cardiovascular risk profile. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-1829. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- Dhindsa M, Sommerlad SM, DeVan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol. 2008;105:427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Haller MJ, Stein J, Shuster J, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes. 2007;8:193–198. doi: 10.1111/j.1399-5448.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki S, Dorchin H, Clark G, et al. The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest. 2007;131:740–749. doi: 10.1378/chest.06-0965. [DOI] [PubMed] [Google Scholar]
- Itzhaki S, Lavie L, Pillar G, Tal G, Lavie P. Endothelial dysfunction in obstructive sleep apnea measured by peripheral arterial tone response in the finger to reactive hyperemia. Sleep. 2005;28:594–600. doi: 10.1093/sleep/28.5.594. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007;12:13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- Lowenstein L, Damti A, Pillar G, Shott S, Blumenfeld Z. Evaluation of endothelial function in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:208–212. doi: 10.1016/j.ejogrb.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Mahmud FH, Van Uum S, Kanji N, Thiessen-Philbrook H, Clarson CL. Impaired endothelial function in adolescents with type 1 diabetes mellitus. J Pediatr. 2008;152:557–562. doi: 10.1016/j.jpeds.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Martin EA, Tan SL, MacBride LR, et al. Sex differences in vascular and endothelial responses to acute mental stress. Clin Auton Res. 2008;18:339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Nohria A, Gerhard-Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Heiss C, Balzer J, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothelial function in healthy adolescent women. J Behav Med. 2008;31:137–143. doi: 10.1007/s10865-007-9141-4. [DOI] [PubMed] [Google Scholar]
- Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo TW, Lampah DA, Gitawati R, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci USA. 2008;105:17097–17102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006;27:328–333. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]