Abstract
The PET radioligand 11C-PBR28 targets translocator protein (18 kDa) (TSPO) and is a potential marker of neuroimmune activation in vivo. Although several patient populations have been studied using 11C-PBR28, no investigators have studied cognitively impaired patients who would require anesthesia for the PET procedure, nor have any reports investigated the effects that anesthesia may have on radioligand uptake. The purpose of this study was to determine whether the anesthetic propofol alters brain uptake of 11C-PBR28 in healthy subjects.
Methods
Ten healthy subjects (5 men; 5 women) each underwent 2 dynamic brain PET scans on the same day, first at baseline and then with intravenous propofol anesthesia. The subjects were injected with 680 ± 14 MBq (mean ± SD) of 11C-PBR28 for each PET scan. Brain uptake was measured as total distribution volume (VT) using the Logan plot and metabolite-corrected arterial input function.
Results
Propofol decreased VT, which corrects for any alteration of metabolism of the radioligand, by about 26% (P = 0.011). In line with the decrease in VT, brain time–activity curves showed decreases of about 20% despite a 13% increase in plasma area under the curve with propofol. Reduction of VT with propofol was observed across all brain regions, with no significant region X condition interaction (P = 0.40).
Conclusion
Propofol anesthesia reduces the VT of 11C-PBR28 by about 26% in the brains of healthy human subjects. Given this finding, future studies will measure neuroimmune activation in the brains of autistic volunteers and their age and sex-matched healthy controls using propofol anesthesia. We recommend that future PET studies using 11C-PBR28 and concomitant propofol anesthesia, as would be required in impaired populations, include a control arm to account for the effects of propofol on brain measurements of TSPO.
Keywords: anesthesia, cognitive impairment, neuroinflammation
Neuroimmune activation is measured in vivo using PET radioligands that target translocator protein (18 kDa) (TSPO) (1). Several groups have studied neuroimmune activation in this way in healthy subjects and in patients with various diseases, including epilepsy, multiple sclerosis, small strokes, and mild to moderate Alzheimer dementia (2–5). No published reports exist on patient populations with more profound cognitive or communicative impairments, as these patients are unable to tolerate the technical aspects of PET studies, which may include placement of an arterial line and intravenous lines, as well as remaining motionless in the scanner for about 2 h.
Deep sedation or anesthesia is necessary for patients who are unable to tolerate the requirements of clinical and research imaging studies, such as MRI or PET (6–8). The effects of anesthetic agents on the binding of various PET radioligands to their target receptors are highly variable (9). Binding of the PET radioligand may be decreased (10), increased (11), or unchanged (12) with the addition of anesthesia. For example, in healthy human volunteers, sevoflurane was found to increase γ-aminobutyric acid type A (GABAA) receptor binding of 11C-flumazenil (13). In contrast, ketamine does not affect GABAA receptor binding (12), suggesting that different anesthetics will produce different outcomes that do not necessarily correlate with what is known about the preferential affinities for various anesthetics and receptors in the brain. Ouchi et al. demonstrated that in monkeys, the effects on receptor binding can vary among brain regions and with the concentration of anesthetic (14). The finding that there is a dose effect of anesthetic has also been shown for isoflurane in GABAA receptor density measurements (15). In summary, the literature indicates that anesthetics can increase, decrease, or not affect radioligand binding to a target receptor, and these findings may vary across brain regions and with anesthetic dose.
To our knowledge, no published reports exist on the effects of anesthesia on radioligands that target TSPO. In this first human study on the effects of anesthesia on TSPO binding in vivo, we selected the anesthetic propofol because of widespread use in clinical settings, a quick onset of action, and a favorable safety profile when used in a well-controlled setting (16). Of the radioligands that target TSPO, we selected 11C-PBR28 (17), because it has high sensitivity to specificity (18), because low-affinity binders of this radioligand (which occur in about 10% of the population) can be readily excluded using a peripheral blood assay, and because the 20-min half-life of 11C makes it feasible to perform PET on each subject twice in a day without concern for residual radioactivity. Although heterozygous and homozygous variants of a single nucleotide polymorphism confer mixed-affinity and high-affinity binding states (19), we planned an intrasubject comparison to look at the effects of propofol, thus making this polymorphic variant irrelevant for the main outcome measure of this study.
In sum, the specific purpose of this study was to determine whether intravenous propofol affects brain uptake of 11C-PBR28 in healthy subjects. The results of this study can be used to inform the design of future studies in patients who would require anesthesia, including those with more profound impairment of cognition, communication, and anxiety, as is seen in autism.
MATERIALS AND METHODS
Radiopharmaceutical Preparation
We synthesized 11C-PBR28 as described in our Investigational New Drug Application 76,441 (http://pdsp.med.unc.edu/snidd/) (20). In brief, we methylated the O-desmethyl analog of 11C-PBR28 using 11C-iodomethane. Using reverse-phase high-performance liquid chromatography, we then isolated 11C-PBR28. Radiochemical purity was greater than 97%. Specific activity at the time of injection was 92.9 ± 50.2 GBq/μmol (mean ± SD, n = 20).
Human Subjects
Approval for this study was obtained from the Combined Neurosciences Institutional Review Board of the National Institute of Mental Health and the Radiation Safety Committee of the National Institutes of Health. Verbal and written informed consent was obtained from all subjects in this study. We included subjects who were considered medically and psychiatrically healthy on the basis of history, Structured Clinical Interview for DSM Disorders–Nonpatient, Wechsler Abbreviated Scale of Intelligence (21,22), Social Responsiveness Scale, physical examination, electrocardiography, urine toxicology, urinalysis, and blood testing (complete metabolic profile; complete blood count with differential; thyroid stimulating hormone level; rapid plasma Reagin test for syphilis; HIV; hepatitis A, B, and C antibody testing; and vitamin B12 level). Before the PET scans, we confirmed that none of the participants were low-affinity binders via in vitro competitive binding assays using peripheral leukocytes (19). Full eligibility criteria are available at www.clinicaltrials.gov (NCT01322555). The participants in this study were 10 healthy subjects: 5 men and 5 women; age, 29.8 ± 7.2 y; weight, 83.0 ± 19.2 kg; and body mass index, 26.5 ± 4.2.
MRI Procedures
Each subject underwent 1.5- or 3-T clinical brain MRI to exclude anatomic abnormalities before study enrollment. The scan series included T1-weighted, T2-weighted, and fluid attenuation inversion recovery sequences in the axial and coronal orientations. MRI data were also used for normalization of PET data into Montreal Neurologic Institute space.
PET Scan Procedures
We followed the general procedure for PET image acquisition and reconstruction described previously by our group (23). An arterial catheter was placed in the radial artery after good collateral circulation had been confirmed. We monitored for safety by checking blood (complete blood count with differential, complete metabolic profile, and thyroid-stimulating hormone) and urine (urinalysis) within 24 h before and after the PET scans. For the baseline (awake) scan, blood pressure was obtained before tracer injection and at 15, 30, and 60 min after injection. The electrocardiogram was continuously monitored during the scan. Data were acquired on an Advance Nxi tomograph (GE Healthcare), which has a reconstructed resolution of 5.3 mm in full width at half maximum at the center of field of view in all directions in 3-dimensional mode. We acquired an 8-min transmission scan of the brain using rotating 68Ge rods for subsequent attenuation correction. We then administered 680 ± 15 MBq (range, 662–714 MBq; n = 20; 688 ± 13 baseline scans; 673 ± 14 scans with propofol; within-subject difference, 2.6% ± 3.1%) of 11C-PBR28 intravenously over 60 s using a PHD 2000 syringe pump. We acquired 33 frames of dynamic emission scans, with acquisition cycles increasing in duration from 30 s to 5 min. In total, the emission scan was 120 min. PET images were reconstructed with ordered-subset expectation maximization in 33 subsets with 4 iterations and were corrected for attenuation.
Intravenous Propofol Sedation and Anesthesia
All subjects were evaluated by an anesthesiologist before enrolling in this study. Intravenous propofol sedation and anesthesia were performed as described by Bishu et al. (24). In brief, the subjects fasted for the 8 h preceding propofol administration. After completing the baseline PET scan while awake, they were provided the opportunity to empty their bladders. They then returned to the scanner and were reconnected to monitors including continuous electrocardiography, arterial blood pressure, capnography, oxygen saturation, and skin temperature. A continuous infusion of propofol was initiated intravenously about 15 min before radioligand injection. A nasopharyngeal airway was inserted after loss of the corneal blink reflex to ensure airway patency. Propofol was continuously administered over the subsequent 120-min emission scan to ensure motionlessness and lack of verbal response. On completion of the scan, all subjects were monitored for about 1 h in the postanesthesia care unit until the discharge criteria of that unit were met. The subjects were discharged from the hospital the following morning.
Analysis of PET Images
PET images were analyzed as previously described (23), with minor modifications. Data were reconstructed on a 128 × 128 matrix with a pixel size of 2.0 × 2.0 × 4.25 mm in the x-, y-, and z-axes, respectively. All data were corrected for attenuation and scatter. Frames were realigned relative to frame 18 and then were co-registered to each subject's MRI scan using Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology). PET and MRI data were normalized to Montreal Neurologic Institute space and then imported into pixelwise modeling software PMOD 3.2 (PMOD Technologies), where the anatomic automatic labeling template was applied to obtain radioactivity counts across the following 10 brain regions: frontal cortex, parietal cortex, occipital cortex, striatum, thalamus, cingulum, hippocampus–amygdala, insula, thalamus, and cerebellum. Data from bilateral regions were the mean of both the left and the right regions. Radiometabolite-corrected arterial input function and the Logan plot (25) were used for all subjects to calculate total distribution volume (VT) because, in 1 subject, an unconstrained 2-compartment model applied in a previous report (23) gave unrealistic VT values that were 5–10 times greater than the VT of the other subjects because of unusually slow washout from the brain and poor identifiability of k4.
Statistical Analysis of Outcome Measures
Outcome measures (VT, free fraction of radioligand [fp], and area under the curve [AUC] of parent radioligand in plasma) (26) in baseline versus propofol-sedated groups were analyzed using SPSS 15.0 software (release 19.0.0; SPSS Inc.). The Wilcoxon signed-rank test was applied for comparisons between fp and AUC, because the Shapiro–Wilk test indicated significant deviations from a normal distribution. VT values in the 10 regions were compared between awake and anesthetized conditions using within-subject, 2-way repeated-measures ANOVA. Statistically significant values were defined as those having a P value of less than 0.05.
RESULTS
Clinical Characteristics of Participants
The demographics of the study participants reflect a cohort balanced by sex and race and demonstrating average intelligence without evidence of impaired social reciprocity (Table 1). We selected younger adults for this study (mean age, 26.5 ± 7.3; n = 10) to assess a more homogeneous population unlikely to be influenced by any age-related changes in TSPO (4). The cohort was slightly overweight (body mass index, 26.5 ± 4.2, n = 10), reflecting the population of adults in the local area (27). Although we excluded low-affinity binders of 11C-PBR28, 7 of 10 subjects were heterozygous for the Ala147Thr polymorphism of TSPO, conferring mixed-affinity binding (19,28). Because this study had an intrasubject design, the affinity state of each individual did not affect statistical comparisons between baseline and propofol PET scans.
TABLE 1.
Clinical Demographics
| Parameter | Value |
|---|---|
| Sex (n) | |
| Male | 5 |
| Female | 5 |
| Race (n) | |
| Caucasian | 6 |
| African-American | 2 |
| Asian | 1 |
| Hispanic | 1 |
| Age (y) | |
| Mean ± SD | 28.5 ± 7.3 |
| Range | 21–41 |
| Body mass index | |
| Mean ± SD | 26.5 ± 4.2 |
| Range | 19.8–33.8 |
| TSPO polymorphism (n) | |
| Mixed-affinity binders | 7 |
| High-affinity binders | 3 (19) |
| Social Responsiveness Scale (39)* | |
| Mean ± SD | 19.0 ± 10.0 |
| Range | 11–35 |
| Verbal IQ† | |
| Mean ± SD | 107.2 ± 11.2 |
| Range | 90–125 |
| Performance IQ | |
| Mean ± SD | 114.0 ± 9.1 |
| Range | 103–133 |
| Total IQ | |
| Mean ± SD | 111.7 ± 9.3 |
| Range | 97–125 |
Adverse Events
There were no adverse events related to propofol in this study, consistent with other reports that propofol is safe when administered in a well-controlled setting by an anesthesiologist (16). The only adverse event in this study was a presyncopal vasovagal episode during insertion of the first intravenous line in a subject hours before administration of propofol or radioligand. Each subject in this protocol demonstrated a clear sensorium before discharge from the hospital.
Decreased Binding of TSPO
Propofol anesthesia decreased binding of TSPO by about 26% (F1,9 = 10.10, P = 0.011), as measured by the decreased VT of 11C-PBR28 (Fig. 1). This reduction in VT was observed globally across brain regions, with no significant region X condition interaction (P = 0.40). Given that we had no a priori hypotheses regarding specific brain regions that might be affected more than others, data were analyzed using Logan plotting of large regions of interest. For our primary outcome measure, we applied the Logan plot rather than an unconstrained 2-compartment model (23) because the latter inaccurately measured VT in 1 subject because of poor identifiability of k4. By excluding the subject with poor k4 identifiability, we obtained a similar reduction in VT by both the unconstrained 2-compartment model and the Logan plot, which supported application of the Logan plot for all subjects in the current study. After exclusion of 1 subject, the Logan plot showed a decrease of 26% in VT (F1,8 = 7.53, P = 0.025), and an unconstrained 2-compartment model obtained a similar decrease of 32% in VT (F1,8 = 9.32, P = 0.016).
FIGURE 1.
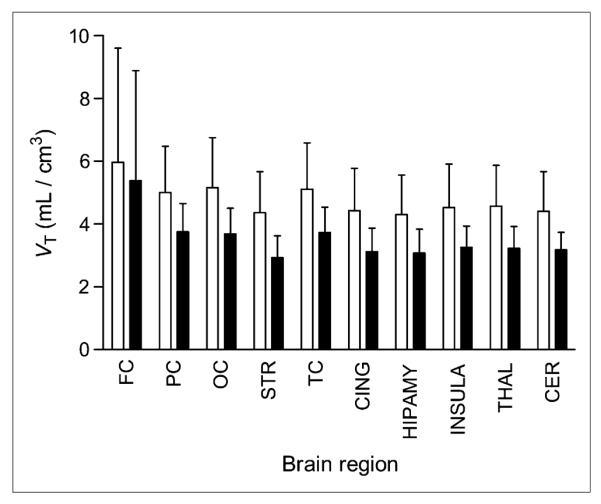
Decreased binding of TSPO as measured by VT of 11C-PBR28 across brain regions. Relative to baseline scans (white bars), VT is about 26% (P = 0.011) lower in scans with propofol anesthesia (black bars). Error bars denote SD. FC = frontal cortex; PC = parietal cortex; OC = occipital cortex; STR = striatum; TC = temporal cortex; CING = cingulum; HIPAMY = hippocampus-amygdala; INSULA = insula; THAL = thalamus; CER = cerebellum.
Insignificant Differences in Plasma Free Fraction Between Baseline and Propofol Scans
To control for any effects that propofol or its solvents might have had on concentrations of free radioligand, we calculated fp for each subject before each scan (baseline and propofol). These data were collected because it is unknown whether propofol changes concentrations of free 11C-PBR28 (as may happen indirectly by displacing 11C-PBR28 from plasma proteins, for example) and only free 11C-PBR28 is available to enter the brain. Blood for measurement of fp was obtained from each subject's arterial line about 5 min before radioligand injection. In parallel with obtaining plasma data from each subject at each scan, we measured fp from a control blood sample obtained from a composite of healthy volunteers from other studies. We then normalized fp from all scans using measurements from the pooled control blood sample to account for fluctuations in day-to-day measurements of fp (29). Using this approach, we found insignificant differences (P = 0.83) in fp between baseline and propofol scans (fp was 3.91% ± 0.96% and 3.82% ± 0.58% in baseline and propofol scans, respectively).
Although only insignificant differences in fp between the baseline and propofol studies were noted, the variability of measurements was moderate to high (30). For baseline studies, fp was 3.9 ± 1.0 (coefficient of variance, 25%), and for propofol studies, fp was 3.8 ± 0.6 (coefficient of variance, 15%). The source of the high variability in fp measurements was unclear but could have resulted from technical differences in blood processing, such as time between obtaining and processing the sample and operator differences. Given that insignificant differences in fp between the baseline and propofol scans were observed in conjunction with a moderate to high degree of variability in fp, we decided to report VT (as opposed to VT/fp) as the outcome measure for this study.
Lower Brain Activity but Higher Plasma Concentrations of Parent Radioligand in Scans Obtained with Propofol
We compared both brain activity and plasma concentrations of parent radioligand in the baseline and propofol scans to determine whether the decreased VT observed in the propofol scans might be an artifact resulting from decreased levels of available parent radioligand. Propofol significantly decreased brain activity calculated as AUC by 20% (baseline, 126.8 ± 15.4 standardized uptake value [SUV]·min; with propofol, 105.4 ± 11.4 SUV·min; P < 0.001; Fig. 2). If plasma discrepancies could explain this change in brain activity, we would expect to see lower plasma concentrations of parent radioligand in propofol studies. However, we observed the opposite—plasma concentrations of parent radioligand were higher in scans obtained with propofol (Fig. 3). For plasma curves, the peak was higher for propofol scans, consistent with a 13% higher calculated AUC for propofol scans (5,418 ± 637 SUV·min) versus baseline scans (4,803 ± 727 SUV·min) (P = 0.02) (Fig. 3). Furthermore, the greater decrease in VT (26%) than in brain activity (20%) is well explained by the 13% higher levels of plasma 11C-PBR28 with propofol because VT is equivalent to the AUC of brain divided by that of plasma 11C-PBR28 from time zero to infinity. Therefore, our main outcome of decreased TSPO binding (VT) in the presence of propofol was unlikely to have been only an artifactual outcome secondary to changes in metabolism or blood profiles.
FIGURE 2.
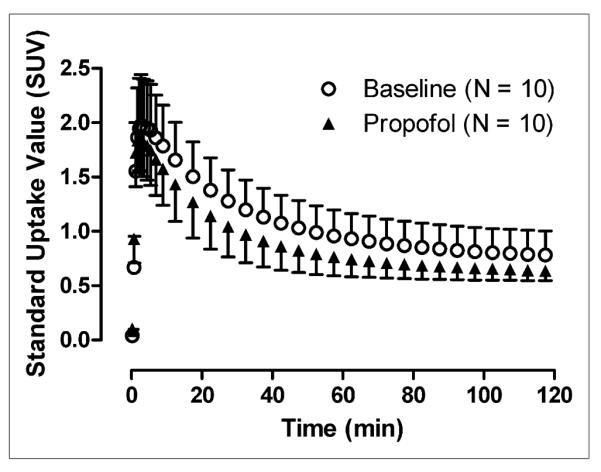
Mean brain time–activity curves for 10 subjects over entire brain show that, compared with baseline scans, scans acquired with propofol demonstrate SUV reduced by about 20% (baseline, 126.8 ± 15.4 SUV·min; with propofol, 105.4 ± 11.4 SUV·min). Time–activity curves for other 10 brain regions showed similar pattern of reduction in SUV with addition of propofol. Peak SUV in baseline and propofol scans was about 2.0.
FIGURE 3.

Plasma concentrations of parent radioligand are higher in sedated scans. Time–activity curves show percentage SUV vs. time (min) for baseline (◯) vs. propofol (▲) scans. Peak is higher for sedated scans, consistent with our higher calculation of AUC for propofol (5,418 SUV·min) vs. baseline (4,803 SUV·min) scans. Inset shows data from 0 to 10 min, and larger graph shows data from entire scan. Of note, there appears to be little variation in tails of blood curves.
DISCUSSION
The main outcome of this study was that TSPO binding was decreased in the presence of propofol anesthesia by about 26% (P = 0.011). Reduction of TSPO binding was observed globally across all brain regions. Furthermore, no clear confounders were noted when considering possible artifactual causes of this main outcome, including altered arterial input function (Fig. 3) and differences in technical procedures (Table 2). It should also be noted that VT is in theory independent of cerebral blood flow (31), and we acquired both brain and blood data far beyond the peak time, ensuring no artifacts from cerebral blood flow (Figs. 2 and 3).
TABLE 2.
Comparison of Parameters in Baseline (n = 10) Versus Propofol (n = 10) Scans
| Parameter | Baseline | Propofol | % change | P |
|---|---|---|---|---|
| Injected mass associated with radioactive dose (nmol/kg) | 0.092 ± 0.04 | 0.098 ± 0.03 | +6.5% | 0.35 |
| Radiochemical purity (%) | 97.6 ± 1.8 | 97.9 ± 0.7 | +0.3% | 0.33 |
| Plasma50% (min) | 9.66 ± 2.3 | 10.84 ± 2.7 | +12.2% | 0.09 |
| Tmax (min) | 1.36 ± 0.14 | 1.13 ± 0.12 | −16.9% | 0.00 |
| Parent plasma AUC0→120 | 4,803.2 ± 726.8 | 5,418.2 ± 637.2 | +12.8% | 0.02 |
% change = (propofol − baseline)/baseline; plasma50% (min) = time from peak to 50% decrease from peak of 11C-PBR28 concentration in arterial plasma; AUC0 → 120 = area under curve of 11C-PBR28 concentration in arterial plasma calculated from time 0 to 120 min.
Thus, propofol altered 11C-PBR28 binding to the TSPO receptor and this finding cannot be attributed to an artifactual effect. We do not know how or why receptor binding may have changed. For some neurotransmitter receptors, decreased binding was hypothesized to be caused by release of the native neurotransmitter in the presence of anesthetic (13,15). Such a condition would not apply to TSPO, which has no endogenous neurotransmitter and which is protected intracellularly as protein in the outer membrane of mitochondria (32). We can only speculate.
Propofol has several mechanisms of action that might affect TSPO binding of a radioligand. These include reduction of GABA in the cerebrospinal fluid of the spinal cord (33), retraction of neurites in glial cells (34), retrograde movement of synaptic vesicles in cortical neurons (35), reduced phosphorylation of mitogen-activated protein kinases involved in GABAA signal transduction (36), reduction in neurotransmitter release via SNARES (soluble N-ethylmaleimide-sensitive factor receptor attachment proteins) (37), and blockage of sodium channels (38).
No reports exist implicating these mechanisms as directly involved in TSPO stability, processing, localization, or affinity state. A host of indirect interactions could also account for our observation that TSPO binding of the radioligand is reduced with propofol. For example, it is possible that reducing GABAA signal transduction decreases a terminal signaling molecule that serves as a cofactor for TSPO stability. Without this cofactor, TSPO may not be in the appropriate conformation to bind radioligand. Lastly, the literature lacks negative results on any interactions between propofol and TSPO. Published molecular studies, whether positive or negative, are needed to address the mechanisms for reducing TSPO binding by 11C-PBR28 in the presence of propofol.
Regardless of the precise mechanisms, our data have demonstrated that healthy human subjects anesthetized with propofol have a 26% decrease in TSPO binding in vivo. Further, although safety evaluation of the anesthesia regimen used is beyond the scope of this study, others have demonstrated the safety of propofol sedation and anesthesia in both clinical (16) and research (6,7) settings. In keeping with the findings of others, our use of propofol sedation and anesthesia in healthy volunteers was not associated with any untoward events. We are now measuring neuroimmune activation in the brains of individuals with autism using 11C-PBR28 PET scans under propofol sedation and anesthesia. Future studies will compare data obtained from autistic individuals with data from healthy controls without autism, all of whom were scanned using propofol. We recommend that future PET studies assessing TSPO in the context of propofol deep sedation and anesthesia (as would be required in impaired populations) contain a control arm to account for the effects of propofol. Such a control arm may also prove useful for other study designs using anesthesia, which may affect radioligand uptake.
CONCLUSION
Propofol anesthesia reduces the VT of 11C-PBR28 by about 26% in the brains of healthy human subjects. Given this finding, future studies will measure neuroimmune activation in the brains of autistic volunteers and their age- and sex-matched healthy controls using propofol anesthesia. We recommend that future PET studies using 11C-PBR28 and concomitant propofol anesthesia, as would be required in impaired populations, include a control arm to account for the effects of propofol on brain measurements of TSPO.
ACKNOWLEDGMENTS
We thank Holly Giesen and Gerald Hodges for recruiting healthy volunteers; Audrey Thurm and Susan Swedo for supervising the IQ and Social Responsiveness Scale testing of healthy volunteers; the NIH PET Department, Kimberly Jenko, and David Clark for scanning and blood analysis; and Ioline Henter for editorial assistance.
Footnotes
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. PMOD Technologies (Zurich, Switzerland) provided its image analysis software. No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–2319. doi: 10.1007/s00259-008-0908-9. [DOI] [PubMed] [Google Scholar]
- 2.Hirvonen J, Kreisl WC, Fujita M, et al. Increased in vivo expression of an inflammatory marker in temporal lobe epilepsy. J Nucl Med. 2012;53:234–240. doi: 10.2967/jnumed.111.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh U, Fujita M, Ikonomidou VN, et al. Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol. 2011;6:354–361. doi: 10.1007/s11481-010-9243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulyás B, Vas A, Toth M, et al. Age and disease related changes in the translocator protein (TSPO) system in the human brain: positron emission tomography measurements with [11C]vinpocetine. Neuroimage. 2011;56:1111–1121. doi: 10.1016/j.neuroimage.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Gulyas B, Toth M, Vas A, et al. Visualising neuroinflammation in post-stroke patients: a comparative PET study with the TSPO molecular imaging biomarkers [11C]PK11195 and [11C]vinpocetine. Curr Radiopharm. 2012;5:19–28. doi: 10.2174/1874471011205010019. [DOI] [PubMed] [Google Scholar]
- 6.Kiringoda R, Thurm AE, Hirschtritt ME, et al. Risks of propofol sedation/anesthesia for imaging studies in pediatric research: eight years of experience in a clinical research center. Arch Pediatr Adolesc Med. 2010;164:554–560. doi: 10.1001/archpediatrics.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amundsen LB, Artru AA, Dager SR, et al. Propofol sedation for longitudinal pediatric neuroimaging research. J Neurosurg Anesthesiol. 2005;17:180–192. doi: 10.1097/01.ana.0000171734.63879.fd. [DOI] [PubMed] [Google Scholar]
- 8.Borgwardt L, Larsen HJ, Pedersen K, Hojgaard L. Practical use and implementation of PET in children in a hospital PET centre. Eur J Nucl Med Mol Imaging. 2003;30:1389–1397. doi: 10.1007/s00259-003-1263-5. [DOI] [PubMed] [Google Scholar]
- 9.Elfving B, Bjornholm B, Knudsen GM. Interference of anaesthetics with radioligand binding in neuroreceptor studies. Eur J Nucl Med Mol Imaging. 2003;30:912–915. doi: 10.1007/s00259-003-1171-8. [DOI] [PubMed] [Google Scholar]
- 10.Xie G, Gunn RN, Dagher A, et al. PET quantifications of muscarininc cholinergic receptors with [N-11C-methyl]-benztropine and application to studies of propofol-induced unconsciousness in healthy human volunteers. Synapse. 2004;51:91–101. doi: 10.1002/syn.10292. [DOI] [PubMed] [Google Scholar]
- 11.Tokugawa J, Ravasi L, Nakayama T, et al. Distribution of the 5-HT1A receptor antagonist [18F]FPWAY in blood and brain of the rat with and without isoflurane anesthesia. Eur J Nucl Med Mol Imaging. 2007;34:259–266. doi: 10.1007/s00259-006-0228-x. [DOI] [PubMed] [Google Scholar]
- 12.Salmi E, Langsjo JW, Aalto S, et al. Subanesthetic ketamine does not affect 11C-flumazenil binding in humans. Anesth Analg. 2005;101:722–725. doi: 10.1213/01.ANE.0000156951.83242.8D. [DOI] [PubMed] [Google Scholar]
- 13.Salmi E, Kaisti KK, Metsahonkala L, et al. Sevoflurane and propofol increase 11C-flumazenil binding to gamma-aminobutyric acidA receptors in humans. Anesth Analg. 2004;99:1420–1426. doi: 10.1213/01.ANE.0000135409.81842.31. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi T, Ochiai R, Takeda J, Tsukada H, Kakiuchi T. Combined effects of propofol and mild hypothermia on cerebral metabolism and blood flow in rhesus monkey: a positron emission tomography study. J Anesth. 2006;20:208–214. doi: 10.1007/s00540-006-0411-z. [DOI] [PubMed] [Google Scholar]
- 15.Gyulai FE, Mintun MA, Firestone LL. Dose-dependent enhancement of in vivo GABA(A)-benzodiazepine receptor binding by isoflurane. Anesthesiology. 2001;95:585–593. doi: 10.1097/00000542-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH, Pediatric Sedation Research Consortium The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the pediatric sedation research consortium. Anesth Analg. 2009;108:795–804. doi: 10.1213/ane.0b013e31818fc334. [DOI] [PubMed] [Google Scholar]
- 17.Brown AK, Fujita M, Fujimura Y, et al. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48:2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- 18.Kreisl WC, Fujita M, Fujimura Y, et al. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage. 2010;49:2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briard E, Zoghbi SS, Imaizumi M, et al. Synthesis and evaluation in monkey of two sensitive 11C-labeled aryloxyanilide ligands for imaging brain peripheral benzodiazepine receptors in vivo. J Med Chem. 2008;51:17–30. doi: 10.1021/jm0707370. [DOI] [PubMed] [Google Scholar]
- 21.Wechlser D. The Measurement of Adult Intelligence. Williams and Wilkins; Baltimore, MD: 1939. [Google Scholar]
- 22.Wechlser D. The Measurement and Appraisal of Adult Intelligence. Williams and Wilkins; Baltimore, MD: 1958. [Google Scholar]
- 23.Fujita M, Imaizumi M, Zoghbi SS, et al. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishu S, Schmidt KC, Burlin TV, et al. Propofol anesthesia does not alter regional rates of cerebral protein synthesis measured with L-[1-11C]leucine and PET in healthy male subjects. J Cereb Blood Flow Metab. 2009;29:1035–1047. doi: 10.1038/jcbfm.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radio-ligand binding from time-activity measurements applied to [N-11C-methyl]-(-) cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 26.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 28.Guo Q, Owen DR, Rabiner EA, Turkheimer FE, Gunn RN. Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. Neuroimage. 2012;60:902–910. doi: 10.1016/j.neuroimage.2011.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickstein LP, Zoghbi SS, Fujimura Y, et al. Comparison of 18F- and 11C-labeled aryloxyanilide analogs to measure translocator protein in human brain using positron emission tomography. Eur J Nucl Med Mol Imaging. 2011;38:352–357. doi: 10.1007/s00259-010-1622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin SG. Understanding and using statistics in nuclear medicine. J Nucl Med. 1979;20:550–558. [PubMed] [Google Scholar]
- 31.Carson RE. Tracer kinetic modeling in PET. In: Bailey DL, Townsend DW, Valk PE, Maisey MN, editors. Positron Emission Tomography: Clinical Practice. Springer-Verlag; London, U.K.: 2003. pp. 127–160. [Google Scholar]
- 32.Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9:971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- 33.Mu X, Wu A, Wu J, et al. Effects of anesthetic propofol on release of amino acids from the spinal cord during visceral pain. Neurosci Lett. 2010;484:206–209. doi: 10.1016/j.neulet.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 34.Turina D, Loitto VM, Bjornstrom K, Sundqvist T, Eintrei C. Propofol causes neurite retraction in neurones. Br J Anaesth. 2008;101:374–379. doi: 10.1093/bja/aen185. [DOI] [PubMed] [Google Scholar]
- 35.Turina D, Bjornstrom K, Sundqvist T, Eintrei C. Propofol alters vesicular transport in rat cortical neuronal cultures. J Physiol Pharmacol. 2011;62:119–124. [PubMed] [Google Scholar]
- 36.Miao Y, Zhang Y, Wan H, Chen L, Wang F. GABA-receptor agonist, propofol inhibits invasion of colon carcinoma cells. Biomed Pharmacother. 2010;64:583–588. doi: 10.1016/j.biopha.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Herring BE, McMillan K, Pike CM, Marks J, Fox AP, Xie Z. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites. J Physiol. 2011;589:1103–1115. doi: 10.1113/jphysiol.2010.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haeseler G, Karst M, Foadi N, et al. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155:265–275. doi: 10.1038/bjp.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21:2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]


