Abstract
Islet transplantation is the most exciting treatment option for individuals afflicted with Type 1 diabetes. However, the severe shortage of human pancreas and the need to use risky immunosuppressive drugs to prevent transplant rejection remain two major obstacles for the routine use of islet transplantation in diabetic patients. Successful development of a bioartificial pancreas using the approach of microencapsulation with perm-selective coating of islets with biopolymers for graft immunoisolation holds tremendous promise for diabetic patients because it has great potential to overcome these two barriers. In this chapter, we provide a detailed description of the microencapsulation process.
Keywords: Islets, Alginate, Microencapsulation, Immunoisolation, Diabetes, Transplantation
1 Introduction
The pancreas is a dual-function organ featuring both endocrine and exocrine tissue. Approximately one million cell clusters called the islets of Langerhans produce a variety of metabolic hormones including glucagon, pancreatic polypeptide, somatostatin, and insulin. Islets consist of four main cell types, namely; α cells: secrete glucagon (induces hepatic release of glucose); β cells: secrete insulin (promotes glucose uptake); δ cells: secrete somatostatin (regulates α and β cells); and PP cells: secrete pancreatic polypeptide. Thus, the islet plays a diverse and complex role in glucose metabolism and blood glucose homeostasis.
In the pancreas, insulin is released in proportional response to actual blood glucose levels. The insulin is released into the portal vein, where it predominately flows toward the liver, which is the major organ to store glycogen and about 50% of secreted insulin gets used in the liver. In addition, the insulin release is pulsatile which helps to maintain the insulin sensitivity of the hepatic tissue. Owing to severe shortage of human pancreas and the shortcomings of insulin therapy, a lot of effort has been made to develop an artificial pancreas.
The artificial pancreas is a technological development to enable Type 1 diabetic patients to automatically control their blood glucose, acting in essence like a healthy pancreas. The goals of the artificial pancreas are: (a) to improve presently popular but inefficient insulin therapy to attain a better glycemic control, thus avoiding the complication due to blood glucose fluctuations, and (b) to mimic normal stimulation of the liver by the pancreas and to normalize carbohydrate and lipid metabolism.
The bioengineering approach for designing a bioarti ficial pancreas has generally involved the development of either microcapsules, or macrocapsules, or other devices such as biocompatible sheet of encapsulated islets. When implanted, these constructs would substitute for the defective native endocrine pancreas (1). This chapter will focus on the microencapsulated islet construct, as it has advanced into the stage of clinical trials (2-5) and has significant promise to be a good alternative to pancreas transplantation.
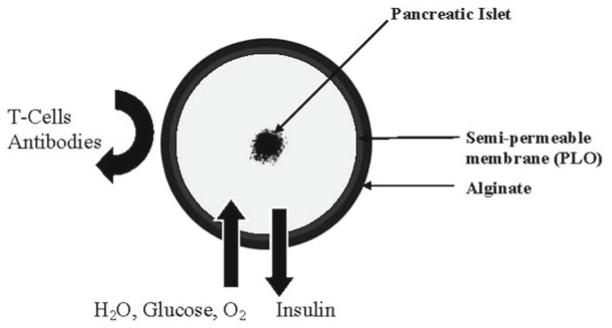
Alginate is attractive as a biomaterial for microencapsulation of cells because of its relative ease of gelling under mild conditions such as the presence of divalent cations as well as its biocompatibility (6) , hence this chapter will focus on the use of alginate for microencapsulation. Using alginate as the encapsulation polymer, the concept of islet immunoisolation is illustrated in Fig. 1 , which essentially incorporates a semipermeable membrane into the process because alginate does not have any appreciable permselectivity towards immune cells and other immunological factors such as antibodies that can potentially destroy the encapsulated cells.
Fig. 1.
Illustration of the principle of immunoisolation by microencapsulation
2 Materials
2.1 Chemicals
Alginate (Pronova UP LVM and UP LVG, Novamatrix, Sandvika, Norway).
Poly-l-Lysine (PLL) (P4957, Sigma-Aldrich, St. Louis, MO, USA).
Poly-l-Ornithine (PLO) (P5061, Sigma-Aldrich).
100 mM CaCl2 solution (C614-10, Fischer Scientific, Waltham, MA, USA).
55 mM sodium citrate solution (S467-3, Fischer Scientific).
0.9% sodium chloride solution (normal saline) (71376-5KG, Sigma-Aldrich).
10 mM HEPES solution (H3375-2KG, Sigma-Aldrich).
2.2 Equipment
Air-syringe pump droplet generator (see Note 1).
Electrostatic generator (see Note 2).
- Voltage generator (CZE1000R, Spellman High Voltage Electronic Corporation, Hauppauge, NY).
- Syringe pump.
- Stirring hotplate.
- 18 G Blunt tip needles.
Microfluidic devices (North Carolina State University, Raleigh, NC).
3 Methods
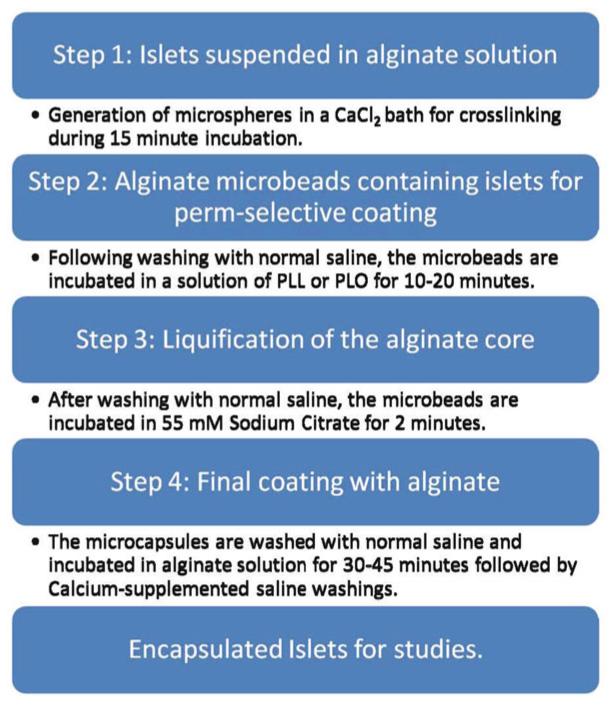
Our laboratory adopts a four-step process in islet microencapsulation, as illustrated in Fig. 2.
Fig. 2.
Schematic representation of the microencapsulation process
Islets are suspended in a solution of sodium alginate (usually from 1.2 to 1.8% w/v made up in normal saline with 5,000 islets suspended in 1 mL of alginate).
Microspheres of alginate containing one or two islets (depending upon the alginate–islet ratio in the suspension) are generated and allowed to gel into microbeads in a bath of 100 mM CaCl2 dissolved in 10 mM HEPES solution at 4°C, pH 7.4 (see Note 3).
Following two washes with normal saline, the microbeads are perm-selectively coated with variable concentrations of 0.1% PLL or 0.1% PLO for variable duration of time depending on the desired pore-size exclusion limit (12) (see Note 4).
Liquefaction of the alginate core of the microcapsules is achieved by a brief (2 min) incubation in 55 mM sodium citrate solution at 4°C.
Wash three times with normal saline, this is accomplished by allowing the capsules to settle on the bottom of a 100 mL beaker over the course of approximately 2 min.
External coating with a lower concentration (routinely about 10% of the concentration used in generating the initial microspheres), but our new procedure utilizes only a slightly lower concentration than the initial alginate concentration for microspheres (13, 14). This is accomplished by incubating the capsules with alginate for 5 min or 45 min if angiogenic protein is incorporated into the outer layer at 4°C (see Note 5).
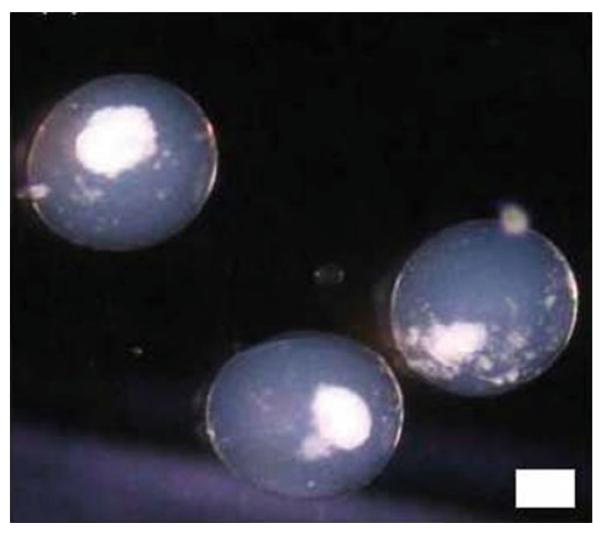
To cross-link the external alginate coat, a solution of normal saline with 22 mM calcium chloride has been recently described for the final washings of microcapsules (14) , albeit, normal saline is routinely used. Figure 3 shows some islets encapsulated in alginate–PLO–alginate (APA) microcapsules in our laboratory using the four-step process.
A critical component of the microencapsulation process is the device used to generate the initial alginate microbeads containing the islets. Within our lab we have used an eight-nozzle microfluidic device for the encapsulation of large numbers of islets (15) , using this device we are able to encapsulate islets using flow rates of 0.5–1.5 mL/min with air pressures of 4.0–5.0 psi. For smaller number of islets (>10,000) we have utilized an electro-spraying technique.
Fig. 3.
Encapsulated islets in an alginate microcapsule. Scale = 100 μm
Footnotes
The air-syringe droplet generator here is constructed with inhouse materials as previously described (7, 8). This two-nozzle device uses standard syringe needles (gauges 20–27) and generates capsules at air jacket pressures of 10 psi and alginate jacket pressures of 15 psi. An air-syringe droplet generator (CF-01) may also be purchased from Biorep technologies (Miami, FL, USA).
The electro-spraying device used here is similar to that previously described (9). Briefly a high voltage source was attached to an 18 G blunt tipped needle, which was positioned above an aqueous CaCl2 solution. Alginate was pumped through the 18-G needle using a syringe pump and droplets were allowed to fall into the CaCl2 solution.
One major advantage of using Ca2+ as the crosslinking cation is that an inner alginate core encapsulating islets can be liquefied in order to enhance the diffusion of permissible molecules to and from the microcapsules (10). The process of liquefaction is pretty delicate and has to be performed with utmost caution in order to avoid capsule breakage caused by high internal colloid-osmotic pressure after the “degelling” (11). The permselective coating with either PLL or PLO is achieved by incubating the alginate microbeads containing islets in 0.1% solution of the polymer for 20 min in order to obtain microcapsules with pore-size exclusion <100 kDa.
The preferred molecular weight range for both PLL and PLO for the purpose of perm-selective coating of alginate microbeads is 15–30 kDa.
Both PLL and PLO are polycationic polymers that require covering of their surface with a coat of the more biocompatible polyanionic alginate in order to prevent electrostatic interactions with cells and proteins after in vivo implantation.
References
- 1.Pareta RA, McQuilling JP, Farney A, Opara EC. Organ donation. INTECH Publishers; 2012. Bioartificial pancreas: evaluation of cru-cial barriers to clinical application; pp. 241–266. chapter 14. ISBN 979-953-307-081-9. [Google Scholar]
- 2.Soon-Shiong P, Feldman E, Nelson R, et al. Successful reversal of spontaneous diabetes in dogs by intraperitoneal microencapsulated islets. Transplantation. 1992;54:769–774. doi: 10.1097/00007890-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Calafiore R, Calabrese G, Basta G, et al. Microencapsulated pancreatic islet allograft into non-immunosuppressed patients with Type 1 diabetes. Diabetes Care. 2006;29(1):137–138. doi: 10.2337/diacare.29.1.137. [DOI] [PubMed] [Google Scholar]
- 4.Elliott RB, Escobar L, Tan PLJ, et al. Live encapsulated porcine islets from type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14:157–161. doi: 10.1111/j.1399-3089.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 5. http://www.newscientist.com/article/dn18730-pig-sushi-diabetes-trial-brings-xeno-transplant-hope.html.
- 6.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 7.Moya ML, Morley M, Khanna O, et al. Stability of alginate microbead properties in vitro. J Mater Sci Mater Med. 2012;23:903–912. doi: 10.1007/s10856-012-4575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolters GH, Fritschy WM, Gerrits D, et al. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 1991;3:281–286. doi: 10.1002/jab.770030407. [DOI] [PubMed] [Google Scholar]
- 9.Hongkwan P, Kim PH, Hwang T, et al. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. Int J Pharm. 2012;427:417–425. doi: 10.1016/j.ijpharm.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Garfinkel MR, Harland RC, Opara EC. Optimization of the microencapsulated islet for transplantation. J Surg Res. 1998;76:7–10. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann U, Mimietz S, Zimmermann H, et al. Hydrogel-based non-autologous cell and tissue therapy. Biotechniques. 2000;29:564–581. doi: 10.2144/00293rv01. [DOI] [PubMed] [Google Scholar]
- 12.Darrabie MD, Kendall WF, Opara EC. Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials. 2005;26(34):6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Opara EC, Mirmalek-Sani S-H, Khanna O, et al. Design of a bioartificial pancreas. J Investig Med. 2010;58(7):831–837. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna O, Moya ML, Opara EC, et al. Synthesis of multi-layered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95(2):632–640. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tendulkar S, Mirmalek-Sani S-H, Childers C, et al. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012;14(3):461–469. doi: 10.1007/s10544-011-9623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]