Abstract
Understanding Nitric Oxide (NO) in innate anti-viral immunity and immune-mediated pathology is hampered by incomplete details of its transcriptional and signaling factors. We found in macrophages that IRF3, ERK MAP-kinases, and PKR are essential to NO production in response to RNA-virus mimic, poly I:C, a TLR3 agonist. ERK's role in NO induction may be through phosphorylation of serine-171 of IRF3 and expression of NO-inducing cytokines, IL-6 and IFN-β. However, these cytokines induced less NO in IRF3 knockout or knockdown macrophages. These findings show that ERK and IRF3 coordinate induction of NO by macrophages in response to stimulation of TLR3.
Keywords: Interferon Response Factor-3, poly I:C, ERK MAP-kinase, Interleukin-6, Macrophages, Nitric Oxide
Introduction
Nitric oxide (NO) production by macrophages plays an important role in innate immunity and disease progression. NO can contribute to host defense by limiting viral infections [1,2], but also contribute to lung pathology during influenza virus infection [3]. Additionally, NO is an important component of central nervous system disorders such as Alzheimer's disease, multiple sclerosis, and amyotropic lateral sclerosis [4,5]. Therefore, an understanding of signaling pathways that contribute to NO production is important for both the study and treatment of numerous human diseases.
Toll-like receptors (TLRs) recognize pathogen associate molecular patterns and induce signal transduction pathways that lead to activation of innate immune responses [6]. In particular, TLR3 recognizes double stranded RNA (dsRNA) and the dsRNA analog, poly I:C, and induces expression of antiviral genes and secretion of reactive oxygen species such as NO [1,7]. Recognition of poly I:C by TLR3 recruits the adapter Toll/IL-1R (TIR) domain-containing adaptor-inducing IFN-β (TRIF) and activates the kinases TBK1 and IKKε, which phosphorylate the C-terminus of interferon regulatory factor 3 (IRF3) [7,8], leading to its activation, and expression of cytokines such as IFN-β [9] or IL-6 [10]. However, poly I:C also leads to activation of non-canonical kinases such as mitogen activated protein kinases (MAPKs) [11,12]. Interestingly, early studies on IRF3 activation pointed to its phosphorylation by MAPKs such as JNK and p38 [13-15]. It remains unclear in which contexts, if any, MAPK phosphorylation could lead to IRF3 activation.
In this study, we found that extracellular signal related kinase (ERK) MAPK inhibitor, U0126, protein kinase R (PKR) inhibitor, 2-aminopurine, and phosphoinositide-3-kinase (PI3K) inhibitor, LY294002, all reduced NO induced by poly I:C in the mouse macrophage cell line, RAW264.7, and primary mouse macrophages. In addition, U0126 inhibited poly I:C-induced IFN-β, IL-6, and iNOS expression as well as induction of NO downstream of these cytokines. Furthermore, data showing ERK phosphorylation of IRF3 and decreased IL-6- or IFN-β-induced NO in IRF3 knockout or knockdown macrophages suggest that activation of IRF3 contributes to IL-6-induced NO. Therefore, both poly I:C-induced IFN-β and IL-6 expression and induction of NO downstream of IL-6 and IFN-β could depend on ERK phosphorylation of IRF3.
2. Materials and Methods
2.1 Mice, cell lines, and reagents
B10.s mice were offspring of breeder pairs generously provided by Dr. Michel Brahic (Stanford University). IRF3 deficient mice (IRF3KO) on the C57BL/6 (BL/6) background were offspring of breeder pairs obtained from Dr. Karen Mossman (McMaster University) [16]. OT-II mice on a BL/6 background were used as IRF3+/+ mice and were obtained from Jackson Labs, (Bar Harbor, ME). RAW264.7 cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in culture media (DMEM with 10% FBS with 50μg/ml gentamycin). For stable selection of shRNAs, RAW264.7 cells were transfected with 2μg psh-IRF3 or psh-Control (psiRNA vector, Invivogen) with the Amaxa nucleofector. Stable lines were selected with 100μg/ml Zeocin. Knockdown was confirmed by western blot and qRT-PCR (data not shown). For transient knockdown of ERK MAPKs, 2 μM phosphorothioate-oligonucleotides (ODNs) that target ERK 1 and 2 (5′-GCCGCCGCCGCCGCCAT-3′) or scrambled ODNs (5′-CGCGCGCTCGCGCACCC-3′) were transfected into RAW264.7 cells with the Amaxa nucleofector as previously described [17]. E. coli lipopolysaccharide (LPS)O127:B8 was obtained from Sigma Chemical Co. (St. Louis, MO), and poly I:C from InvivoGen (San Diego, CA). The p38 MAPK inhibitor SB203580 (10μM), Myristoylated PKC inhibitor (400μM), PI3K inhibitor LY294002 (50μM), and ERK MAPK inhibitor U0126 (40μM) were from Promega Corporation (Madison, WI). The JNK MAPK inhibitor SP600126 (44μM) was from Biosource (Camarillo, CA) and the PKR inhibitor 2-aminopurine (5mM) from InvivoGen.
2.2 Macrophage Preparations
Peritoneal macrophages were elicited by i.p. injection of 2 ml sterile thioglycollate broth into mice. Three days later, the peritoneal cavities were flushed with 2 ml DMEM and cells were incubated at 1 × 106 cells/2 ml culture media. After overnight incubation, non-adherent cells were removed and 1 ml of culture medium added. Adherent cells were greater than 90% Mac-1+ as determined by FACS analysis [18]. These macrophages were either untreated or treated as described in the figure legends.
2.3 ERK kinase assay
40μg of IRF3-S171 peptide (DEGSSDLAIVSDPSQQLPSPNVNNFLNPAP) or IRF3-S135 peptide (GASPDTNGKSSLPHSQENLPKLFDGLILGP), or no peptide (synthesized from Thermo scientific/Fisher) was added to kinase buffer (40mM Tris, 20mM MgCl2, 0.1mg/ml BSA) containing 770μM ATP, and 250 ng active ERK1 (Signal Chem, Richmond, BC, Canada) or no ERK. The kinase reaction was allowed to proceed at room temperature for 40 min and phosphorylation was detected using the kinase detection kit from Promega.
2.4 RNA preparations and qRT-PCR
RNA was extracted from cells using the Purelink kit from Ambion/Invitrogen (Carlsbad, CA), according to the manufacturer's specifications. One hundred ng to one μg of RNA was reverse transcribed in .5mM each of dATP, dGTP, dTTP, and dCTP, 20 U of RNAse inhibitor with Superscript II reverse transcriptase (Invitrogen) at 42°C for 1.5 h followed by 95°C for 5 min. The cDNA was diluted 1:2 and 1 μl was incubated with .4mM of the following primer pairs (Invitrogen):
IFN-β sense 5′ ATGAACAACAG GTGGATCCTCC 3′
and anti-sense 5′ AGGAGCTCCTGACATTTCCGAA 3′;
IL-6 sense 5′ ATGAAGTTCCT CTCTGCAAGAGACT 3′
and antisense 5′ CACTAGGTTTGCC GAGTAGATCTC 3′;
iNOS sense 5′ CCCTTCCGAAGTTTCTGGCAGCAGC
and anti-sense 5′ GGCTGCAGAGCCTCGTGCTTTGG 3′;
or GAPDH sense 5′-TTGTCAGCAA TGCATCCTGCAC-3′;
and antisense 5′-ACAGCTTTCCA GAGGGGCCATC-3′. Quantitative (q) PCR reactions were run on an ABI Prism 7000 thermal cycler at 50 °C for 2 min, 95 °C for 10 min, 45 cycles of 95 °C for 15 s/60 °C for 30 s. Relative levels of mRNA were normalized to GAPDH by using the Ct value and the formula: 2−ΔΔCt.
2.5 Statistical Analysis
Statistical analyses were performed using GraphPad Prism Software. Student's two-tailed unpaired t test was used to determine the significance of differences between means; *p<05, **p<.01, ***p<.001, ****p<.0001.
3. Results
3.1 ERK is involved in the induction of NO in response to poly I:C and LPS in macrophages
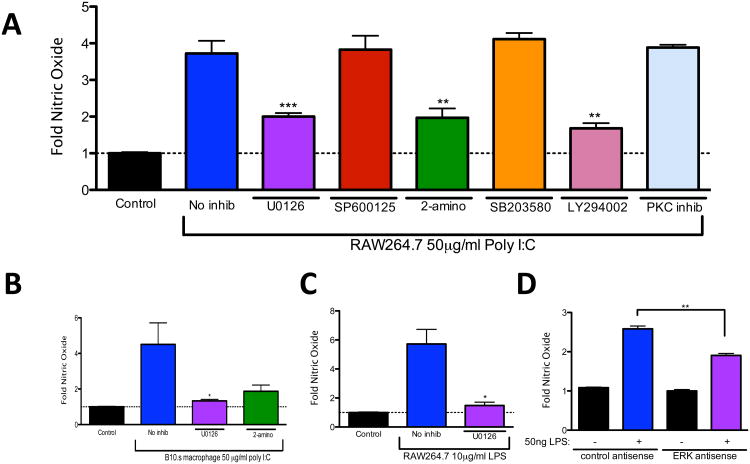
Viral RNA, and the dsRNA analog, poly I:C, signal through TLR3 and adapter TRIF to induce NO [1,19]. Although the TLR3/TRIF signaling cascade has been well studied, pathways that lead to poly I:C-induced NO production are not well understood. In order to reveal kinases that contribute to NO production during stimulation with dsRNA, RAW264.7 macrophages were pretreated with well-characterized specific inhibitors of ERK MAPK (U0126), JNK MAPK (SP600125), PKR (2-aminopurine), p38 MAPK (SB203580), PI3K (LY294002), or PKC for 30 minutes at previously optimized concentrations and then stimulated with 50μg/ml poly I:C for 24 h. Supernatants were then analyzed for NO secretion by way of Greiss assay. As shown in Figure 1A, poly I:C stimulated NO production in RAW264.7 macrophages. NO production was inhibited by U0126, 2-aminopurine, and LY294002, which indicate that ERK MAPK, PKR, and PI3K, respectively, are involved in poly I:C-induced NO. Because previous reports showed that PKR is involved in poly I:C-induced NO [19] and LY294002 blocks poly I:C-induced IRF3 activation [20], we decided to focus our subsequent studies on ERK MAPK. Consistent with our observations in RAW264.7 macrophages, the induction of NO in poly I:C-treated primary macrophages from B10.s mice was also ERK dependent (Fig. 1B). In order to further investigate a role for ERK MAPK in NO induction, these experiments were repeated using LPS, which signals through TLR4 in either a TRIF dependent or TRIF-independent manor [21], activates MAPKs [13], and is also a potent inducer of NO [22]. LPS- stimulated NO production from RAW264.7 cells was significantly inhibited by U0126 (Fig. 1C) or by antisense ODN transient knockdown of ERK MAPKs (Fig. 1D). Taken together, these results show that ERK MAPK is a critical signaling molecule for the induction of NO in macrophages responding to either poly I:C or LPS.
Fig. 1.
ERK is involved in poly I:C-induced nitric oxide production in macrophages. (A) RAW264.7 cells were untreated (no inhib) or pretreated with U0126, SP600125, 2- aminopurine (2-amino), SB203580, LY294002, or PKC inhibitor for 30 minutes and then treated with 50μg/ml poly I:C for 24 hours or left untreated (control). (B, C) B10.s peritoneal macrophages (B) or RAW264.7 cells (C) were pre-treated with U0126 and then treated with 50μg/ml poly I:C (B) or 10μg/ml LPS (C), or transfected with ERK MAPK specific ODNs or scrambled control ODNs and then stimulated with 50 ng/ml LPS (D) for 24 hours or left untreated (control). The supernatant was removed and nitric oxide was analyzed by Greiss assay. Data are means +/− SEM of 4 to 6 samples per group.
3.2 ERK is required for maximum expression of IFN-β and IL-6 in poly I:C-treated macrophages
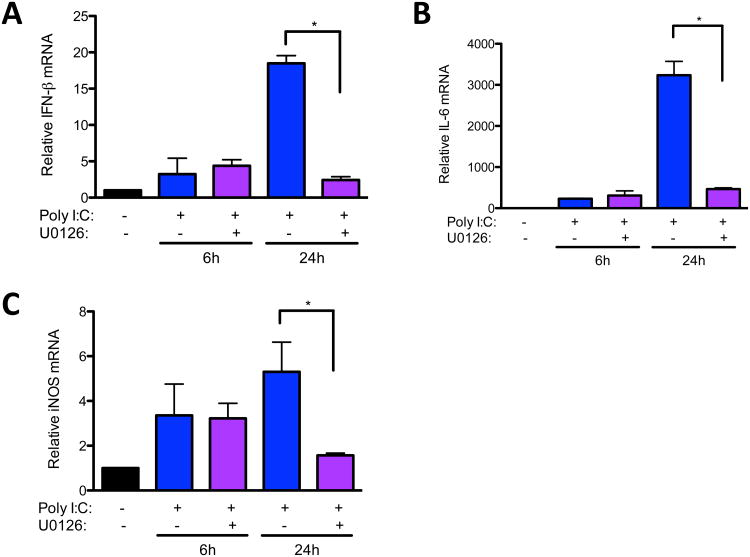
Previously, we found that ERK MAPK was required for maximum expression of IL-6 by macrophages infected with Theiler's virus, which is recognized, in part, by TLR3 [23,24]. Moreover, we showed that IL-6 was able to synergistically enhance NO in Theiler's virus infected macrophages [2]. Because of these data and the fact that poly I:C induces IFN-β, a potent inducer of NO [25] , we determined if reduced NO in U0126-pretreated macrophages could be due to decreased expression of IL-6 or IFN-β. Indeed, U0126 pretreatment significantly inhibited induction of both IFN-β (Fig. 2A) and IL-6 (Fig. 2B) in response to poly I:C. As expected, the reduction of poly I:C-induced IFN-β and IL-6 expression by U0126 pretreatment correlated with decreased expression of inducible nitric oxide synthase (iNOS) at 24 h. Therefore, the role of ERK in poly I:C-induced NO is partly at the level of transcription of cytokines, such as IFN-β and IL-6, and the induction of iNOS expression.
Fig. 2.
ERK is involved in poly I:C-induced IFN-β and IL-6 expression. (A, B) RAW264.7 cells were untreated or pretreated with U0126 for 30 minutes and then treated with 50μg/ml poly I:C for 6 or 24 hours. RNA was collected from cell lysates, reverse transcribed, and IFN-β (A), IL-6 (B) and iNOS (C) expression was analyzed by qRT-PCR. Data are means +/− SEM of 3 to 4 samples per group.
3.3 ERK is involved induction of NO downstream of IFN-β and IL-6
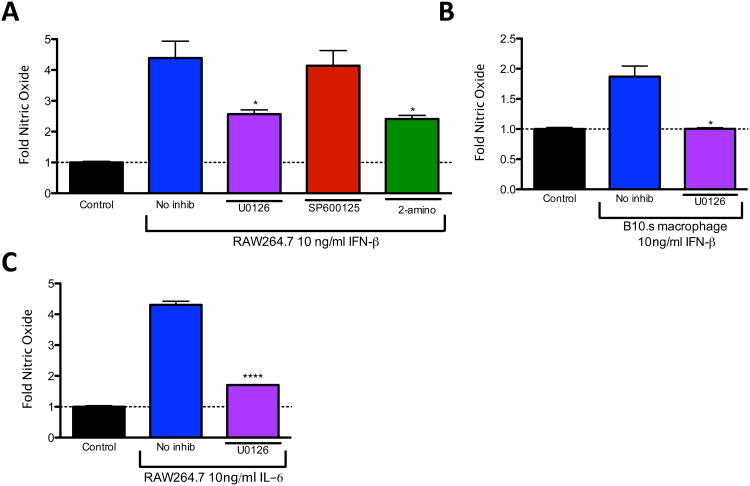
Although inhibiting ERK activity significantly reduced expression of IFN-β and IL-6 in response to poly I:C, ERK could also mediate NO production downstream of IFN-β and IL-6. In order to test whether ERK is involved in production of NO downstream of IFN-β and IL-6, RAW264.7 macrophages were pretreated with U0126, SP600125, or 2-aminopurine and then treated with IFN-β for 24 hours. Interestingly, both U0126 and 2-aminopurine significantly reduced induction of NO by IFN-β (Fig. 3A). U0126 also significantly reduced IFN-β-induced NO in primary macrophages from B10.s mice (Fig. 3B) and inhibited IL-6-induced NO production from RAW264.7 cells (Fig. 3C). These and the aforementioned results show that in addition to its involvement in poly I:C- induced IFN-β and IL-6 expression, ERK is involved in signaling downstream of IFN-β and IL-6 during NO induction.
Fig. 3.
ERK is involved in nitric oxide production downstream of IFN-β and IL-6. (A) RAW264.7 cells were pre-treated with no inhibitor (no inhib), U0126, SP600125 or 2-aminopurine (2-amino) for 30 minutes and then treated with 10 ng/ml IFN-β for 24 hours or left untreated (control). (B, C) B10.s peritoneal macrophages (B) or RAW264.7 cells (C) were pre-treated with U0126 and then treated with 10 ng/ml IFN-β (B) or 10ng/ml IL-6 (C) for 24 hours or left untreated (control). The supernatant was removed and nitric oxide was analyzed by Greiss assay. Data are means +/− SEM of 3 to 4 samples per group.
3.4 Role of IRF3 in ERK-mediated NO induction
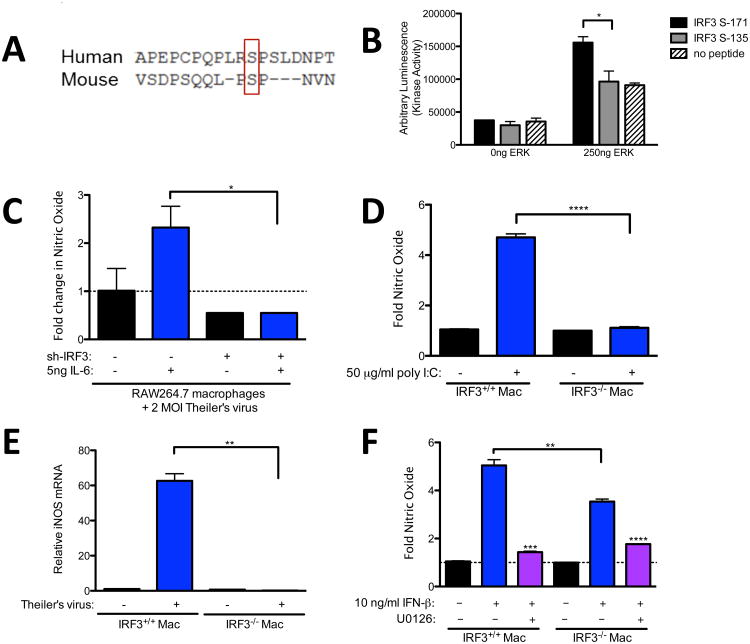
Recently, we found that IRF3 is involved in expression of both IFN-β and IL-6 from macrophages in response to Theiler's virus, as well as poly I:C (Moore et al., under review). Also, we found that IRF3 is critical for induction of interferon stimulated genes and IL-6-induced antiviral effects. Because both ERK and IRF3 are required for maximum IFN-β and IL-6 expression, as well as some of their downstream effects, we tested whether ERK could potentially activate IRF3 through phosphorylation. Using Scansite 3.0 software [26] to analyze the mouse IRF3 protein sequence, we identified a putative ERK kinase target at serine 171 (S171) of mouse IRF3. Importantly, alignment of human and mouse IRF3 with Clustal Omega from the European Bioinformatics Institute (EBI) revealed that this serine is conserved with human IRF3 (Fig. 4A) [27]. In order to test whether ERK could phosphorylate IRF3, we mixed purified activated ERK with 30-mer peptides that contain the putative ERK S171 site of IRF3 or with peptides that contain a confirmed DNA-protein kinase site of IRF3 at S135 [28]. In vitro kinase assays revealed a significant increase in kinase-dependent phosphorylation of the S171 peptide (Fig. 4B). In contrast, kinase dependent phosphorylation with the S135 peptides was equivalent to control, and can therefore be attributed to ERK autophosphorylation. Inactive ERK, used as a negative control, yielded no significant kinase dependent phosphorylation (data not shown). Therefore, IRF3 is a potential target of ERK dependent phosphorylation at IRF3 S171 but not IRF3 S135.
Fig. 4.
Role of IRF3 in poly I:C and IFN-β-induced NO. (A) Mouse and Human IRF3 were aligned using the Clustal Omega software from EBI. (B) ERK phosphorylation of S135, S172, or no peptide control solutions was performed as described in the materials and methods. (C) RAW264.7 stably expressing shIRF3 or control shRNA were infected with 2 MOI Theiler's virus with or without 10ng/ml IL-6. (D) Peritoneal macrophages from IRF3+/+ or IRF3−/−; mice were pre-treated with U0126 (D) or no inhibitor (D) and then treated with 50 μg/ml poly I:C (D), infected with Theiler's virus (E), or treated with 10ng/ml IFN-β (F) for 24 hours. The supernatant was removed and nitric oxide was analyzed by Greiss assay (D,F) or RNA was extracted for analysis of iNOS expression by qRT-PCR. Data are means +/− SEM of 3 to 4 samples per group.
To test if IRF3 is required for induction of NO in response to poly I:C, we compared poly-I:C-induced NO from RAW264.7 cells with shRNA plasmids that knocked down IRF3 expression. The IRF3 targeted shRNA prevented production of NO in response to IL-6 (Fig. 4C). We also compared poly-I:C-induced NO from IR F3+/+ macrophages on a Bl6 background with that from IRF3 deficient RF3−/−;) macrophages on a Bl6 background. Similar to RAW264.7 cells and B10.S macrophages, poly I:C induced significant NO production after 24 h treatment of IRF3+/+ macrophages (Fig. 4D). In contrast, IRF3 deficient (IRF3−/−;) macrophages did not produce NO in response to poly I:C (Fig. 4D). This lack of NO production in IRF3−/−; macrophages also correlated with a lack of iNOS induction (Fig 4E). These results show that IRF3 is critical to induction of NO in macrophages responding to poly I:C or IL6.
To test whether IRF3 and ERK are involved in NO production in macrophages downstream of IFN-β, IRF3+/+ or IRF3−/−; macrophages were pretreated with U0126 and then treated with 10 ng/ml IFN-β for 24 h. In contrast to poly I:C, IFN-β induced some NO from IRF3−/−; macrophages, albeit to a significantly lesser degree than from IRF3+/+ macrophages (Fig. 4F). Moreover, pretreatment with ERK inhibitor U0126 reduced NO in both IRF3+/+ and IRF3−/−; macrophages (Fig. 4F). Taken together, these results suggest that ERK phosphorylation of IRF3 contributes to NO production both through induction of IFN-β and IL-6 and also in signaling pathways downstream of these cytokines. However, other ERK targets in addition to IRF3 appear to be important inducers of NO downstream of IFN-β.
4. Discussion
Previously we showed that IL-6 is able to induce robust NO production from the RAW264.7 macrophage cell line [1,2]. The data in this report demonstrate that poly I:C, an agonist of the TLR3 pattern recognition receptor, also induces NO production from RAW264.7 cells. Furthermore, we show that ERK MAPK is involved in NO production by macrophages responding to poly I:C. ERK appears to contribute to NO production, in part, by promoting expression of NO-inducing cytokines such as IFN-β and IL-6, but is also involved in NO induction in response to these cytokines and in iNOS expression.
Herein the data also indicate that IRF3 is critical for poly I:C-induced NO production. This is consistent with our previous report which shows that IRF3 is involved in IL-6 and IFN-β expression during Theiler's virus infection of macrophages (Moore et al, under review). Surprisingly, in this report, we found that IRF3 is required for maximum induction of NO in response to exogenous IFN-β or IL-6. These data are similar to our previous findings in which IRF3 deficiency impaired IL-6 induction of interferon stimulated genes and impaired STAT1 phosphorylation in response to IL-6. Taken together, this suggests that IFN-β and IL-6 can signal, in part, in an IRF3-dependent manor. Herein, we suggest ERK as a possible link between the IFN-β or IL-6 receptors and IRF3. However, IFN-β-induced NO was still inhibited by U0126 in IRF3−/− macrophages, suggesting that IRF3 is only one of the targets for ERK downstream of IFN-β. Although these studies suggest that IRF3 is involved in amplifying NO induced by cytokines, further studies are required to define the mechanism of IRF3 involvement downstream of IFN-β and IL-6.
In addition to ERK, our results suggest that PKR and PI3K are other cell signaling factors associated with poly I:C-induced NO. The latter is consistent with a previous report showing that poly I:C-induced NO in astrocytes depends on PKR-mediated activation of nuclear factor κ-B (NF-κB). However, LY294002 has recently been shown to inhibit poly I:C-induced IRF3 activation by an unknown, PI3K- independent mechanism [29]. Therefore, it is possible that LY294002 reduces NO production by macrophages through inhibition of IRF3 activity directly and not through inhibition of PI3K activity.
Several early reports suggested that MAPKs are involved in IRF3 activation. In particular, both p38 MAPK and JNK MAPK have been shown to activate IRF3 [13,14]. However, our results indicate that lack of IRF3, but not inhibition of p38 or JNK, abolishes poly I:C-induced NO. Our results further implicate ERK in the activation of IRF3 that leads to production of NO by macrophages. A recent report showed that during cardiac fibrosis, angiotensin II promotes IRF3 activation by way of ERK phosphorylation [30]. In this system, they found that S339 of IRF3 to be the critical ERK phosphorylation site. Our results show that ERK phosphorylation of IRF3 at S171 may be an additional ERK phosphorylation target on IRF3.
In conclusion, these results show that poly I:C stimulation of macrophages induces NO in an ERK and IRF3-dependent manor that may involve ERK phosphorylation of IRF3. This is important because of the wide array of human diseases that are mitigated or exaggerated by NO production. This study, in conjunction with future studies, will provide insight into signaling pathways that promote NO production and may contribute to the development of treatments for NO-mediated diseases.
Highlights.
Maximum nitric oxide in response to poly I:C requires ERK
ERK is involved in poly I:C-induced IFN-β and IL-6 expression
ERK is involved in IFN-β and IL-6-induced nitric oxide
ERK phosphorylates IRF3 in vitro
IRF3 is required for maximum nitric oxide in response to poly I:C, IFN-β, and IL-6
Acknowledgments
This work was supported by funding from the University of Nebraska Medical Center College of Dentistry and University of Nebraska Lincoln, School of Biological Sciences, and supported by Award Number P30GM10350903 and P20GM103489 from the National Institute of General Medicine, a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- IRF3
Interferon Response Factor 3
- NO
Nitric Oxide
- TLR
Toll Like Receptor
- ERK
extracellular signal related kinase
- PKR
protein kinase R
- PI3K
phosphoinositide-3-kinase
- TIR
Toll/IL-1 Receptor
- TRIF
TIR domain-containing adaptor-inducing IFN-p
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κ-B
- IRF3KO
IRF3 deficient mice
- BL/6
C57BL/6 mice
- ODN
oligodeoxynucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta DR, Ashkar AA, Mossman KL. The nitric oxide pathway provides innate antiviral protection in conjunction with the type I interferon pathway in fibroblasts. PLoS One. 2012;7:e31688. doi: 10.1371/journal.pone.0031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore TC, Bush KL, Cody L, Brown DM, Petro TM. Interleukin-6 control of early Theiler's Murine Encephalomyelitis Virus replication in macrophages occurs in conjunction with STAT1 activation and nitric oxide production. J Virol. 2012 doi: 10.1128/JVI.01402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrone LA, Belser JA, Wadford DA, Katz JM, Tumpey TM. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013;207:1576–84. doi: 10.1093/infdis/jit062. [DOI] [PubMed] [Google Scholar]
- 4.Dhib-Jalbut S. Pathogenesis of myelin/oligodendrocyte damage in multiple sclerosis. Neurology. 2007;68:S13–21. doi: 10.1212/01.wnl.0000275228.13012.7b. discussion S43-54. [DOI] [PubMed] [Google Scholar]
- 5.Drechsel DA, Estevez AG, Barbeito L, Beckman JS. Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotox Res. 2012;22:251–64. doi: 10.1007/s12640-012-9322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF- kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci U S A. 2004;101:3533–8. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–6. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–51. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney SE, Kimbler TB, Firestein GS. Synoviocyte innate immune responses: II. Pivotal role of IFN regulatory factor 3. J Immunol. 2010;184:7162–8. doi: 10.4049/jimmunol.0903944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsen IB, Nguyen TT, Bergstrom B, Lien E, Anthonsen MW. Toll-like receptor 3-elicited MAPK activation induces stabilization of interferon-beta mRNA. Cytokine. 2012;57:337–46. doi: 10.1016/j.cyto.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Pisegna S, Pirozzi G, Piccoli M, Frati L, Santoni A, Palmieri G. p38 MAPK activation controls the TLR3-mediated up-regulation of cytotoxicity and cytokine production in human NK cells. Blood. 2004;104:4157–64. doi: 10.1182/blood-2004-05-1860. [DOI] [PubMed] [Google Scholar]
- 13.Navarro L, David M. p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J Biol Chem. 1999;274:35535–8. doi: 10.1074/jbc.274.50.35535. [DOI] [PubMed] [Google Scholar]
- 14.Nociari M, Ocheretina O, Murphy M, Falck-Pedersen E. Adenovirus induction of IRF3 occurs through a binary trigger targeting Jun N-terminal kinase and TBK1 kinase cascades and type I interferon autocrine signaling. J Virol. 2009;83:4081–91. doi: 10.1128/JVI.02591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, et al. The TAK1-JNK cascade is required for IRF3 function in the innate immune response. Cell Res. 2009;19:412–28. doi: 10.1038/cr.2009.8. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–48. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 17.Robinson CJ, Scott PH, Allan AB, Jess T, Gould GW, Plevin R. Treatment of vascular smooth muscle cells with antisense phosphorothioate oligodeoxynucleotides directed against p42 and p44 mitogen-activated protein kinases abolishes DNA synthesis in response to platelet-derived growth factor. Biochem J. 1996;320(Pt 1):123–7. doi: 10.1042/bj3200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petro TM. Disparate expression of IL-12 by SJL/J and B10.S macrophages during Theiler's virus infection is associated with activity of TLR7 and mitogen-activated protein kinases. Microbes Infect. 2005;7:224–32. doi: 10.1016/j.micinf.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Auch CJ, Saha RN, Sheikh FG, Liu X, Jacobs BL, Pahan K. Role of protein kinase R in double-stranded RNA-induced expression of nitric oxide synthase in human astroglia. FEBS Lett. 2004;563:223–8. doi: 10.1016/S0014-5793(04)00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X, Masic A, Liu Q, Zhou Y. Regulation of influenza A virus induced CXCL-10 gene expression requires PI3K/Akt pathway and IRF3 transcription factor. Mol Immunol. 2011;48:1417–23. doi: 10.1016/j.molimm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem. 2003;278:49751–62. doi: 10.1074/jbc.M305820200. [DOI] [PubMed] [Google Scholar]
- 22.Salvemini D, Korbut R, Anggard E, Vane J. Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1990;87:2593–7. doi: 10.1073/pnas.87.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Salleeh F, Petro TM. TLR3 and TLR7 are involved in expression of IL-23 subunits while TLR3 but not TLR7 is involved in expression of IFN-beta by Theiler's virus-infected RAW264.7 cells. Microbes Infect. 2007;9:1384–92. doi: 10.1016/j.micinf.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Hause L, Al-Salleeh FM, Petro TM. Expression of IL-27 p28 by Theiler's virus-infected macrophages depends on TLR3 and TLR7 activation of JNK-MAP-kinases. Antiviral Res. 2007;76:159–67. doi: 10.1016/j.antiviral.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Fujihara M, Ito N, Pace JL, Watanabe Y, Russell SW, Suzuki T. Role of endogenous interferon-beta in lipopolysaccharide-triggered activation of the inducible nitric-oxide synthase gene in a mouse macrophage cell line, J774. J Biol Chem. 1994;269:12773–8. [PubMed] [Google Scholar]
- 26.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–41. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpova AY, Trost M, Murray JM, Cantley LC, Howley PM. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc Natl Acad Sci U S A. 2002;99:2818–23. doi: 10.1073/pnas.052713899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao W, Qi J, Wang L, Zhang M, Wang P, Gao C. LY294002 inhibits TLR3/4-mediated IFN-beta production via inhibition of IRF3 activation with a PI3K-independent mechanism. FEBS Lett. 2012;586:705–10. doi: 10.1016/j.febslet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Tsushima K, et al. IRF3 regulates cardiac fibrosis but not hypertrophy in mice during angiotensin II-induced hypertension. FASEB J. 2011;25:1531–43. doi: 10.1096/fj.10-174615. [DOI] [PubMed] [Google Scholar]