Abstract
Background & Aims
Repair from biliary damages requires the biliary specification of hepatic progenitor cells and the remodeling of ductular reactive structures into branching biliary tubules. We hypothesized that the morphogenetic role of Notch signaling is maintained during the repair process and have addressed this hypothesis using pharmacologic and genetic models of defective Notch signaling.
Methods
Treatment with DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine) or ANIT (alpha-naphthyl-isothiocyanate) was used to induce biliary damage in wild type mice and in mice with a liver specific defect in the Notch-2 receptor (Notch-2-cKO) or in RPB-Jk. Hepatic progenitor cells, ductular reaction, and mature ductules were quantified using K19 and SOX-9.
Results
In DDC treated wild type mice, pharmacologic Notch inhibition with dibenzazepine decreased the number of both ductular reaction and hepatic progenitor cells. Notch-2-cKO mice treated with DDC or ANIT accumulated hepatic progenitor cells that failed to progress into mature ducts. In RBP-Jk-cKO mice, mature ducts and hepatic progenitor cells were both significantly reduced with respect to similarly treated wild type mice. The mouse progenitor cell line BMOL cultured on matrigel, formed a tubular network allowing the study of tubule formation in vitro; γ-secretase inhibitor treatment and siRNAs silencing of Notch-1, Notch-2 or Jagged-1 significantly reduced both the length and number of tubular branches.
Conclusions
These data demonstrate that Notch signaling plays an essential role in biliary repair. Lack of Notch-2 prevents biliary tubule formation, both in vivo and in vitro. Lack of RBP-Jk inhibits the generation of biliary-committed precursors and tubule formation.
Keywords: Cholangiocytes, Alagille syndrome, Ductular reaction, GSI, Liver repair, Notch signaling
Introduction
Liver disease is the result of the interaction between ongoing liver cell damage and reparative mechanisms. Liver repair in the context of biliary diseases requires the replacement of lost or damaged cells, generation of new branching tubules, and production of a fibrovascular stroma that sustains the new tissue. When these highly integrated processes fail, liver repair becomes “pathologic” and results in architectural distortion and deposition of fibrous tissue into the portal spaces [1].
The hallmark of ongoing biliary repair is the presence of a “ductular reaction”. “Reactive cholangiocytes”, i.e., the epithelial component of the ductular reaction, are organized in clusters that do not encircle a lumen. However, they are in contiguity with the biliary tree and extensively participate in tissue remodeling, ultimately reorganizing into tubular structures [2]. The consequent increase in “ductal mass” is an important adaptive mechanism in cholestasis that prevents the development of extensive parenchymal necrosis [3].
Notch signaling, a strongly conserved developmental pathway involved in cell fate determination and stem cell biology [4], plays an important role in biliary development when Notch receptors expressed on ductal plate cells are stimulated by ligands expressed by the periportal mesenchyme [5]. Mutations in the Notch signaling factors Jagged-1 [6] or Notch-2 [7] cause Alagille syndrome (AGS), a cholangiopathy characterized by paucity of intrahepatic bile ducts, severe cholestasis, and extrahepatic manifestations [8]. We have documented that patients with AGS present a distinct pattern of liver repair responses [9]. Recent studies indicate that Notch signaling is activated in hepatic progenitors cells (HPCs) in human cholangiopathies [10,11]. Furthermore, by activating Numb, Wnt/β-catenin signaling inhibits the biliary specification of HPCs in favor of their hepatocytic specification [11]. In contrast to other morphogenetic pathways, like Wnt [12] and Hedgehog [13], Notch signals through direct cell-cell interaction [4].
These observations, suggest that Notch may be involved in several steps of biliary repair, in adult life. We have used genetic models of Notch loss of function (Notch-2 and RBP-JK liver-conditional knockout mice) to study the role of Notch signaling in the regulation of ductular morphogenesis during liver repair from biliary injuries.
Materials and methods
For reagents, immunohistochemistry and Western blotting see Supplementary Materials and methods.
Computer-assisted morphometry
K19 and SOX9 antibodies were used to quantify the ductular reaction. Reactive ductular cells are defined as K19 or SOX9 positive cells with a biliary phenotype arranged in irregularly shaped structures without a well-formed lumen, and HPCs are defined as small, oval or spindle-shaped cells positive for K19 or SOX9 with scant cytoplasm and oval nuclei, alone or in small clusters, localized in the parenchyma or at the portal interface [9,14] (Supplementary Materials and Methods).
Cell culture
Bipotential mouse oval cell line (BMOL) was kindly provided by Dr. Yeoh, University of Western Australia [15] (Supplementary Materials and Methods).
Silencing of Notch-1, Notch-2, and Jagged1
Gene silencing was performed using commercially available siRNAs against Notch-1, Notch-2, and Jagged-1 (Santa Cruz Biotechnology, Inc). Scramble RNAs were used to control for non-specific silencing effects. BMOL cells were transfected using the Lipofectamine 2000™ transfection reagent (Invitrogen) according to the manufacturer’s protocol (Supplementary Materials and Methods).
Animals and experimental protocol
All experiments were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. To produce a liver specific deletion of Notch-2, mice Notch-2flox/flox were crossed with mice doubly heterozygous for the Alb1-Cre transgene [16] and Notch-2del2 alleles on a C57BL/6J background (all from Dr. T. Gridley, Jackson Laboratory) [17]. Off-spring with the genotypes Alb1-Cre/+; Notch-2del2/ Notch-2flox are referred to as Notch-2-cKO.
Liver specific deletion of RBP-Jk, was obtained by crossing heterozygous Alb1-Cre; RBP-Jkflox/+ mice on a CD-1 outbred background (a kind gift from Dr. T. Honjo, Kyoto University and S. Huppert, Vanderbilt University) [18,19]. Offspring with the genotypes Alb1-Cre; RBP-Jkflox/flox are referred to as RBP-Jk-cKO.
Experimental and wild type mice were exposed to 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) at P30 or P75 (when indicated) and alpha-naphty-lisothiocyanate (ANIT) at P30 to induce biliary damage. DDC treatment, used to mimic intrahepatic obstructive cholestasis [20], was added to the diet at a concentration of 0.1% (BioServ, Inc., Frenchtown, NJ) for 10 days. ANIT treatment, used to induce biliary injury characterized by hyperplasia of the terminal branches of the biliary tree [21], was administered to the mice in a high dose (80 mg/kg) i.p., followed by a bi-weekly maintenance dose (50 mg/kg) for 4 weeks.
Dibenzazepine (DBZ) (Syncom BV, Netherlands), a γ-secretase inhibitor (GSI), was used to pharmacologic inhibit Notch signaling in vivo at the time of biliary damage induction [22]. DBZ was administered to wild type mice daily for 10 days via i.p. injection, at a concentration of 5 μmol/kg, in combination with or without DDC treatment.
At the end of each treatment, mice were sacrificed, liver tissues were explanted, and the two large lobes were fixed with formalin and embedded in paraffin. The small lobes were snap frozen in liquid nitrogen.
Tubule formation in vitro assay
BMOL cells were plated on a thick layer of matrigel at the density of 50,000/cm2 in growth medium. In this condition, 3 h after plating, cells begin to organize a network. Twenty-four hours later, this network forms visible interconnecting, mesh-like structures. A second layer of matrigel applied to the 24-h culture promotes the formation of complete tubular structures.
Confocal microscopy analysis was used to assess the 3D-tubular structure, after staining with Cellmask™ Orange. Images were obtained using a Zeiss LSM 510 confocal microscope using a 63x 1.4 NA-objective lens with excitation at 555 nm and emission at 567 nm. Serial optical sections were collected for 3D-reconstruction.
To inhibit Notch signaling, BMOL cells were treated with In Solution™ γ-Secretase Inhibitor IX (DAPT, 10 μM) (Calbiochem), at the time of plating on matrigel. The length of tubular structures and number of branches were measured using ImageJ software in 5 random, non-overlapping fields, in each condition. The images were captured with a contrast-phase microscope Olympus CK40 (Micro-Tech Optical (NE), Inc., Bloomfield, CT, USA) connected to a camera (Q-color 5 RTV, Qimaging, Canada).
Statistical analysis
Results are shown as mean ± SD. Statistical comparisons were made using one-way analysis of variance or the Wilcoxon-Mann-Whitney 2-sample rank sum test, where appropriate. In the latter, the p value was obtained from the exact permutation null distribution. The statistical analysis was performed using SAS software (SAS Institute Inc, Cary, NC). p values <0.05 were considered significant.
Results
Pharmacologic inhibition of Notch signaling reduces ductular reaction and HPCs in mice exposed to DDC
See Supplementary Results. To investigate whether Notch signaling participates in biliary repair, we induced biliary damage in WT mice by administering DDC [20] in the presence or absence of DBZ, to inhibit Notch signaling. As shown in Supplementary Fig. 1, DBZ significantly inhibited the increase in HPCs and ductular reaction (DR) induced by DDC. These are consistent with a role of Notch in biliary specification of HPCs, as suggested by Fabris [9] and Boulter [11]. However, because of the non-specific effects of DBZ [23], the demonstration of the role of Notch in biliary repair requires the use of genetic models.
Liver repair is altered in ANIT and DDC treated mice with conditional deletion of Notch-2 or RBP-Jk
Among the four Notch receptors, Notch-1 and 2 are expressed to significant extent in cholangiocytes and HPCs [24,25] (see also Supplementary Fig. 2). We studied two liver specific conditional knockout mice (Notch-2-cKO and RBP-Jk-cKO) in which deletion of Notch-2 or RBP-Jk, respectively, is under the control of the albumin promoter. Alb-Cre mediated recombination is specific to hepatoblasts, biliary cells, and hepatocytes [19,25]. RBP-Jk is an essential component of Notch signaling; thus, in RBP-Jk-cKO mice both Notch-1 and Notch-2 signaling is defective.
The phenotype of both mice has been previously described and results in paucity of bile ducts [19]. We characterized mouse phenotypes by staining with K19 and SOX-9 during the first 8 postnatal weeks. The SOX-9 transcription factor is the earliest marker expressed by biliary precursors [26,27]. At P30 and P60, the livers of Notch-2-cKO mice showed the same level of paucity of bile ducts at the periphery of the lobules and a significant increase in the number of K19, as well as SOX-9+ve cells (Supplementary Figs. 3–5) that extend out of the portal space; these cells are negative for Notch-2 (Supplementary Figs. 6 and 7). The livers of RBP-Jκ-cko mice showed ductopenia, and areas of parenchymal necrosis, and no increase in the number of K19/SOX-9+ve structures (Supplementary Figs. 3, 4 and 8). When analyzed at P30, we found an extensive expression of SOX-9 in hepatocellular-like cells. Interestingly, these SOX-9+ve cells were actually negative for the biliary/progenitor marker K19 and had a clear hepatocellular shape. These cells completely disappeared by P60, but ductopenia did not improve by P75, suggesting that these cells could be hepatocytes making an attempt to regenerate the biliary tree (Supplementary Figs. 8–10).
We exposed Notch-defective mice to two protocols of biliary damage (ANIT or DDC) starting at P30. After treatment with ANIT or DDC, the liver histology (Fig. 1 and Supplementary Fig. 11) and liver enzymes [28] (Supplementary Table 1) were different between Notch-2-cKO, RBP-Jκ-cKO mice and WT littermates. In WT mice, both damage protocols stimulated a DR, with increased HPCs, dysmorphic bile ducts and mature bile ducts [2]. In Notch-2-cKO mice, ANIT treatment caused an increase in dysmorphic K19+ve epithelial cells organized in clusters, but lacking a tubular structure. After DDC treatment, the K19+ve cells were arranged around irregular dilatations, lined by irregularly shaped epithelial cells with nuclear pleomorphism. Liver damage in RBP-Jκ-cKO mice was more severe: the near absence of ductular reaction was accompanied by the presence of areas of parenchymal necrosis. To exclude the possible interference of a postnatal liver injury, we repeated the DDC protocol in RBP-Jκ-cKO mice starting at P75, and we confirmed the absence of ductular reaction (Supplementary Fig. 8). Albeit more severe, the histological picture of RBP-Jκ-cKO mice was similar to the one seen in livers of WT mice after DDC + DBZ treatment (in which GSI treatment blocks signals from both Notch receptors). To exclude confounding effects due to possible alterations in xenobiotic metabolism, as previously described in other knockout models [12], we analyzed the expression of Cyp1A1, 1A2, 2E1, and 3A1 and we found them similar among the mouse lines (data not shown). These observations are consistent with the concept that Notch signaling plays a major role in liver repair.
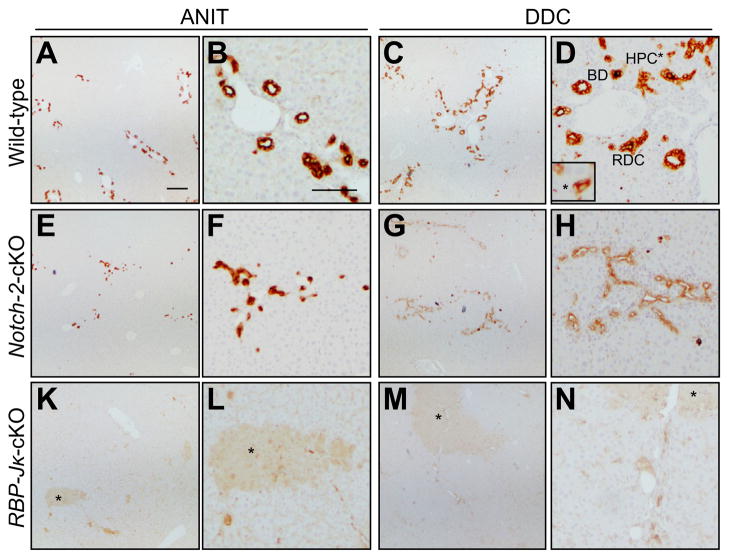
Fig. 1. K19 staining in Notch-2-cKO and RBP-Jk-cKO shows a defect in liver repair after ANIT and DDC-induced damage.
(A–D) WT, (E–H) Notch-2-cKO, and (K–N) RBP-Jk-cKO mice were treated with ANIT and DDC at P30 (see text for details). At the end of the treatment, the mice were sacrificed and liver tissues were stained with K19 antibody. After treatment with ANIT and DDC, WT mice developed the expected ductular reaction while Notch-2-cKO showed an increase in cholangiocyte-like structures with dysmorphic epithelial cells, faintly positive for K19 and missing a tubular structure. RBP-Jk-cKO showed no ductular reaction and presented parenchymal necrosis (*). The different components of ductular reaction are indicated in panel D: BD, bile ducts; RDC, reactive ductular cells; and HPC, hepatic progenitor cells. Scale bar is 200 μm in panels A, E, K, C, G, M and 100 μm in panels B, F, L, D, H, N.
RBP-Jk deletion, but not Notch-2 deletion affects biliary committment of HPC during biliary repair
An essential step during liver repair is the activation of HPCs. This bipotential compartment is able to differentiate into cells committed toward the hepatocellular or biliary lineage (RDCs), in order to increase the number of bile ducts at the periphery of portal spaces and rebuild the damaged biliary tree [3]. Thus, we quantified the number of biliary progenitor cells using SOX-9 immunostaining (Supplementary Materials and methods) after ANIT treatment or K19 immunostaining after DDC treatment.
The number of SOX9+ve and K19+ve HPCs at baseline was significantly higher in Notch-2-cKO but not in RBP-Jk;-cKO mice compared to their WT (Fig. 2A–D and Supplementary Table 2). After ANIT treatment, the number of HPCs significantly increased both in WT mice, and more so in Notch-2-cKO mice. On the contrary, in RBP-Jκ-cKO, the number of HPCs was significantly lower than in WT treated with ANIT (Fig. 2B and D and Supplementary Table 2). Similar results were confirmed after DDC treatment by quantification of K19+ve HPCs (Fig. 3A and B).
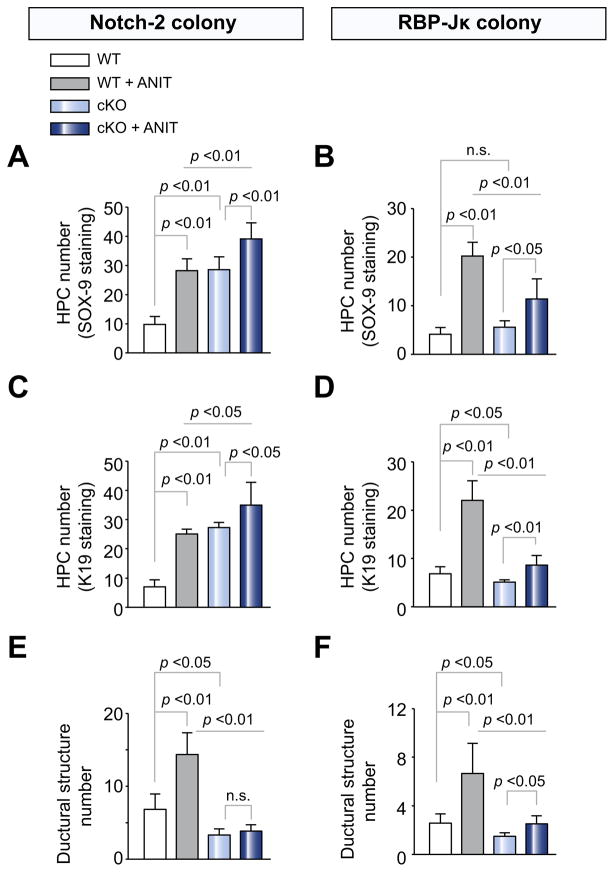
Fig. 2. Biliary progenitor cells and ductular structure quantification in WT, Notch-2-cKO, and RBP-Jk-cKO mice after treatment with ANIT.
Paraffin sections from Notch-2-cKO, RBP-Jk-cKO mice and their respective WT littermates, untreated or treated with ANIT, were stained for (A and B) SOX-9 or (C–F) K19. See Supplementary Materials and methods for quantification criteria. As shown in the bar graph, (A and C) Notch-2-cKO mice showed a significant increase in the number of HPCs (both SOX-9 or K19+ve) after ANIT damage. (B and D) Conversely, RBP-Jκ-cKO mice exhibited a significant reduction in the number of HPCs after treatment with ANIT. Variations in the baseline number of HPCs between (A) Notch-2-WT and (B) RBP-Jk-WT are due to differences of mouse strain. On the other hand, both (E) Notch-2-cKO mice and (F) RBP-Jκ-cKO mice showed a significant decrease in the number of K19+ve ductular structures after ANIT damage. Data represent average ± SD. (Data represent average ± SD, n = 4–8 mice per group; p values are reported). n.s., not significant.
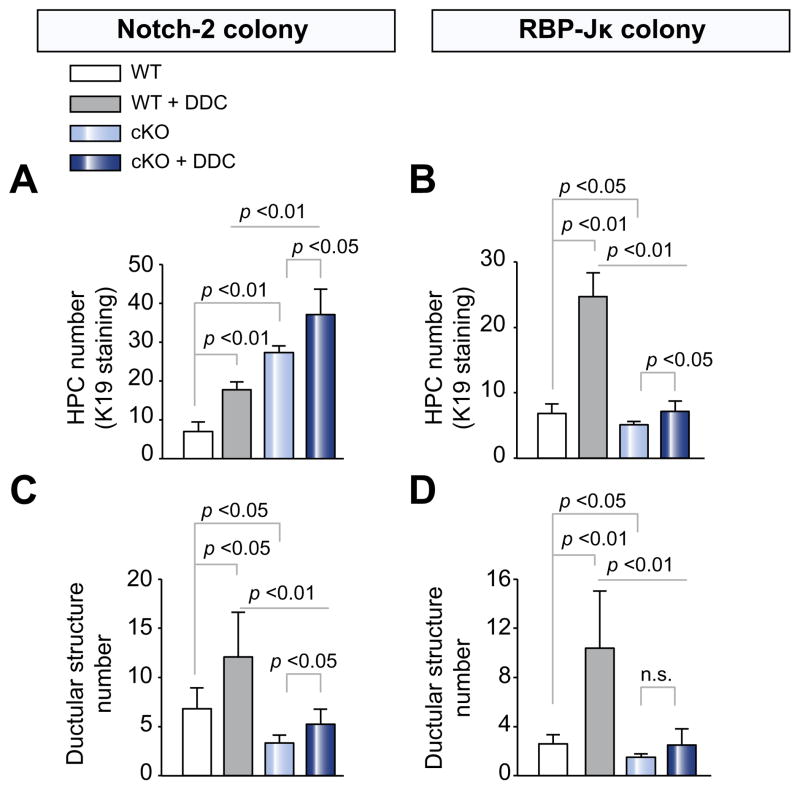
Fig. 3. Biliary progenitor cells and ductular structure quantification in WT, Notch-2-cKO, and RBP-JK-cKO mice after treatment with DDC.
Paraffin sections from Notch-2-cKO, RBP-Jk-cKO mice and their respective WT littermates, untreated or treated with DDC, were stained for K19. As shown in the bar graph, (A) Notch-2-cKO mice showed a significant increase in the number of HPCs after DDC damage. (B) Conversely, RBP-Jκ-cKO mice exhibited a significant reduction in the number of HPCs after treatment with DDC. On the other hand, both (C) Notch-2-cKO mice and (D) RBP-Jκ-cKO mice showed a significant decrease in the number of K19+ve ductular structures after DDC damage. (Data represent average ± SD, n = 4–8 mice per group; p values are reported). n.s., not significant.
Ductular structures are decreased in Notch-2 and RBP-Jk cKO mice during liver repair
The activation of HPCs and the subsequent increase in the number of RDCs during liver repair are aimed not only at replacing damaged cells, but also at restoring the tubular structures lost during liver injury [9]. Therefore, we quantified the number of ductular structures (Supplementary Materials and methods) in mice treated with ANIT and DDC.
As shown in Fig. 2E and F and Supplementary Table 3, after ANIT treatment, the number of ductular structures was significantly lower in Notch-2-cKO and RBP-Jκ-cKO mice, than in WT mice. Similar results were obtained after DDC treatment (Fig. 3C and D and Supplementary Table 3). The increased number of HPC and the decreased number of ductular structures observed in Notch-2-cKO mice again suggest that Notch-2 is not necessary for the generation of biliary-committed cells, but rather for the ability of biliary precursor cells to organize into tubular structures. Conversely, in RBP-Jκ-cKO mice, both HPCs and ductular structures are significantly reduced, resulting in more severe impairment of biliary repair.
GSI treatment and siRNA silencing of Notch-2 and Jagged1 inhibit tubule formation in vitro
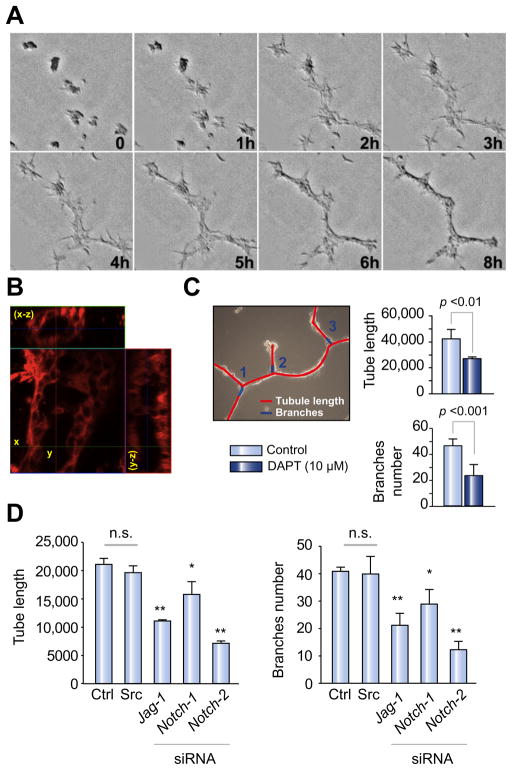
The process of tubulogenesis begins during embryonic life and continues after birth until the architecture of the biliary tree is completely developed [17]. Tubulogenesis also takes place in response to biliary injury and HPC activation. The mechanisms that regulate the initial phases of tubulogenesis during the reparative process are still unclear. To determine whether Notch plays a role in this process, we studied tubule formation in vitro using a well-characterized mouse liver progenitor cell line, BMOL [15]. When plated on a thick layer of matrigel matrix, BMOL cells migrated and organized into a mesh-like network of cells within 24 h from the time of plating (Fig. 4A). After a second layer of matrigel is overlayed, tubular structures are formed, as shown in Fig. 4B, in which the 3D-confocal imaging reconstruction reveals the presence of a luminal space inside the structure.
Fig. 4. Inhibition of Notch signaling affects tubule formation in BMOL cultured on matrigel, a model to study biliary tubulogenesis.
(A) BMOL plated on a thick layer of matrigel were imaged for 8 h and as, showed in the stack series (0–8 h), they migrated to organize a network of tubule structures (8 h). (B) Twenty-four h after plating in matrigel, the cell preparation was incubated with Cell mask TM Orange, a vital dye that stains cell plasma membranes, and processed by confocal microscopy analysis. Serial optical sections were collected for 3D-reconstruction. The confocal microscopy analysis (see x–z projection), revealed the presence of a lumen inside the structure. (C) BMOL cells cultured on matrigel were treated with DAPT at the time of plating. The length of tubules and number of branches were measured 24 h after plating in matrigel, using ImageJ software in 5 random, non-overlapping fields, as described. Treatment with DAPT significantly decreased the final length of the tubule network and the number of branches. (D) To confirm the involvement of Notch in tubulogenesis, tubule formation was quantified in BMOL cultured on matrigel 48 h after silencing Notch-1, Notch-2 or Jagged-1 genes. As shown in the bar graph, the tube length and number of branches were significantly reduced after silencing of Notch-2 and Jagged-1, and to a lesser extent Notch-1 (average ± SD, n = 3 independent experiments; *p <0.05, **p <0.01, n.s., not significant).
Using this in vitro tubulogenesis model, we studied the effects of pharmacologic and genetic inhibition of Notch pathway factors. BMOL cells were cultured in presence of the GSI compound DAPT (10 μM) [29]. The length of the tubules and number of branches were measured 24 h after plating, using the ImageJ software in 5 random, non-overlapping fields, captured with a contrast-phase microscope. As shown in Fig. 4C, treatment with DAPT significantly decreased the length of the tubular sections between the branches.
To confirm the specific involvement of Notch signaling, we repeated the experiments using BMOL cells after gene silencing of Notch-1, Notch-2, and Jagged1. As shown in Fig. 4D, tubule length and number of branches were significantly reduced 24 h after gene silencing, and this was particularly evident in the case of Notch-2 and Jagged-1 silencing. Gene expression analysis of Notch receptors (1–4) from the above experiments (Supplementary Fig. 2) showed no compensatory upregulation of Notch-3 and 4 in our silenced cells. These results confirm our hypothesis that inhibition of Notch signaling and, specifically Notch-2, impairs the ability of progenitor cells to arrange into a tubular-like structure.
Discussion
In response to liver damage, specialized portions of the biliary tree lining the terminal cholangioles and the canals of Hering give rise to hepatic progenitor cells (HPCs) [1]. These cells can eventually replace lost hepatocytes, and/or generate “ductular reactive” cells able to communicate with mesenchymal, endothelial, and inflammatory cells. To successfully repair the damage to the biliary epithelium, progenitor cells and reactive cholangiocytes must have the ability to proliferate and expand the ductular mass forming branching tubular structure [3]. Thus, “reactive” cholangiocytes, organized in tubeless structures, abandon their progenitor cell-like phenotype, characterized by motile properties and active production of growth factors and cytokines, to differentiate into cylindrical ion-secreting cells arranged around tubular structures [1]. If this process does not occur, bile may leak into the lobules, causing necrosis [1].
Liver repair is a highly integrated process in which a number of morphogenetic pathways, including Wnt/β-catenin and Hedgehog, are involved [12,13]. We had previously shown that in AGS, ductular reaction was altered with reduced ability to form biliary progenitor cells [9]. Increased expression of Notch-1, Notch-2, Jagged1/2, and Hes-1, in reactive ductules from patients with primary biliary cirrhosis [10] and primary sclerosing cholangitis [11], was recently reported.
In this study, we present strong evidence to support the idea that Notch signaling plays a fundamental role in the process of biliary repair. Using pharmacologic and genetic models of Notch loss of function [17,19], we showed that Notch-1 and 2 differentially regulate the biliary commitment of liver progenitor cells and the three-dimensional architecture of the biliary tree.
Consistent with Boulter et al. [11], pharmacologic inhibition of Notch in WT mice with GSI reduced the number of HPC and reactive ducts. GSIs, are broad inhibitors of Notch signaling that prevent the cleavage of NICD from Notch receptors [4]. Although widely used to study Notch signaling, GSI can also inhibit other proteolytic cleavage-dependent signaling pathways, thus reducing the specificity of the effects [23]. Thus, to gain further insight into the role of Notch signaling, we used two liver specific conditional KO mouse models (bearing a deletion of the Notch-2 receptor [17] or of RBP-Jκ [19], a transcription factor common to all Notch receptors). Only Notch-1 and Notch-2 are expressed to a significant extent by HPCs (Supplementary Fig. 2) and by cholangiocytes (data not shown). Thus, RBP-Jk-cKO cholangiocytes bear a defect of both Notch-1 and Notch-2, whereas in Notch-2-cKO mice, Notch-1 signaling remains functional.
Consistent with previous studies [19,30], the liver phenotype of untreated conditional KO mice shows a delay in bile duct maturation and a reduction in the number of bile ductules at the periphery of the biliary tree. Mice were viable and were not jaundiced. On the other hand, compared to wild type littermates, significant changes in biliary repair were found after treatment with DDC or with ANIT, particularly in the generation of HPC and the formation of biliary ducts.
SOX-9 is expressed by biliary-committed cells of the inner leaflet of the ductal plate cells at the duplication stage and subsequently by mature cholangiocytes [26,27] and biliary-committed HPCs [27]. In WT mice, the number of SOX-9+ve biliary progenitor cells increased by 3–4 fold after ANIT damage, along with the number of bile ducts. In Notch-2-cKO mice treated with ANIT, SOX-9+ve HPCs further increased. On the contrary, the number of tubular structures significantly decreased, suggesting that Notch-1 can substitute for Notch-2 in the biliary specification of HPCs but not in tubule formation. It has been reported that overexpression of Notch-1-specific or Notch-2 NICD, promotes ectopic biliary differentiation in the hepatic lobule [24,31,32] and hyperplasia of the terminal branches of the biliary system [19], whereas Notch-1 deletion does not alter liver development [25]. When both Notch-2 and Notch-1 signaling is blocked, as in RBP-Jκ-cKO mice, both the number of progenitor cells and the number of biliary ductules are significantly reduced. In RBP-Jκ-cKO mouse livers at postnatal day 30 (P30), we noticed an extensive expression of SOX-9 in cells with hepatocellular morphology that were negative for the biliary marker K19 and completely regressed by P60. We speculate that this phenomenon represents a transient expansion of hepatocytes making an attempt to become biliary cells, possibly under the control of other signaling pathways, such as Hedgehog [33]. We previously described a similar phenomenon in patients with Alagille syndrome, where we noticed an abundance of hepatobiliary cells positive for K7 (membranous staining), but negative for HNF-1β [9].
In Notch-2-cKO mice, ANIT treatment did not stimulate the expansion of ductal mass, while DDC treatment resulted in the development of irregular dilatations without a well-developed tubular structure. In RBP-Jκ-cKO mice, ductular reaction was nearly absent, wherein ANIT-induced liver damage was more severe; areas of parenchymal necrosis were present, instead of the irregular dilatations seen in Notch-2-cKO mice. Our data also show that the number of ductular structures was significantly decreased in both Notch-2-cKO and RBP-Jκ-cKO mice after damage, compared to WT injured mice. We speculate that Notch-2 is necessary to effectively form tubules. In fact, in the absence of Notch-2, the liver is still able to generate biliary progenitor cells in response to biliary injuries, but these cells cannot rebuild a tubular structure. On the other hand, in RBP-Jκ-cKO mice, lacking both Notch-1 and Notch-2 signaling, liver repair from a biliary damage is more profoundly affected and both the ability to generate HPCs and the process of tubulogenesis are impaired.
To repair biliary damage and prevent the development of extensive liver necrosis due to bile leakage into the parenchyma [1,3], progenitor cells and reactive cholangiocytes must have the ability to form new branching ductular structures. While the mechanisms of tubulogenesis taking place during development have been extensively studied [17,34], they have been neglected during liver repair. Using a bipotential mouse adult liver progenitor cell line that possesses an intermediate hepatobiliary/phenotype [15], we could model the initial phases of tubulogenesis in vitro (Fig. 4). We were able to show that pharmacologic Notch inhibition and genetic silencing of the Notch-2 receptor and Jagged1 ligand, significantly reduced tubule length and the number of lateral branches in our system, further reinforcing the role of Notch-2 signaling in promoting proper tubule formation. This in vitro model will be useful for future studies that will delve more deeply into different pathway components involved in biliary tubulogenesis.
In summary, our studies show that, after biliary damage, Notch-2 and RBP-Jk play a fundamental role in the activation of HPC and in ductal morphogenesis (See cartoon in Fig. 5). This is consistent with our earlier observation that in AGS patients, HPCs are unable to differentiate along the biliary lineage [9], and with recent reports showing stimulation/inhibition of Notch in liver progenitor cells undergoing biliary vs. hepatocytic differentiation [11]. Furthermore, our data show that proper biliary repair requires the coordinated and differential action of both Notch-1 and Notch-2. When Notch-2 is defective, biliary specification of HPC is not affected, however, tubular morphogenesis is impaired both in vivo and in vitro. The downstream mechanisms by which Notch-2 promotes tubulogenesis are at present unknown. A crosstalk between VEGF and Notch signaling plays a key role in vascular branching morphogenesis [35]; our lab is currently investigating its relevance in biliary repair [36].
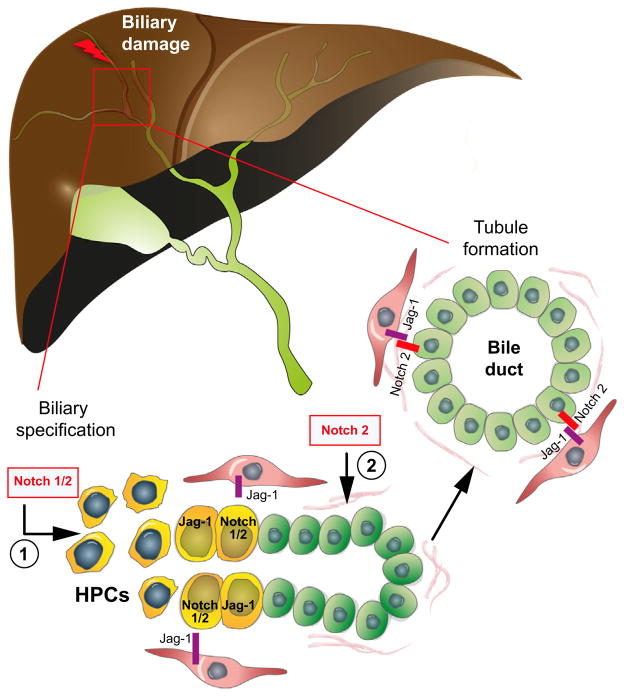
Fig. 5. Notch signaling is involved in biliary repair.
After biliary damage, HPCs and reactive cholangiocytes proliferate and expand the ductular mass forming branching tubular structures. Biliary specification of HPCs, requires a functional Notch signaling; (1) Notch-2 signaling (2) is essential for tubule formation, likely responding to Notch ligands expressed by the surrounding mesenchyma.
In conclusion, the demonstration of the role of Notch signaling in biliary specification of HPC and ductal morphogenesis adds a further important piece to the puzzle of biliary repair and paves the way for future investigations of the mechanism of bile duct formation; and indicates that it may be possible to modulate liver repair by targeting HPCs and Notch. Understanding the mechanisms of liver repair is a fundamental step for preserving organ function and prolonging survival of patients with liver disease.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. T. Gridley (The Jackson Laboratory, Bar Harbor, ME, USA) for the Notch-2flox/flox and Notch-2del2 mice, Dr. T. Honjo (Kyoto University, Japan) and Dr. S. Huppert, (Vanderbilt University, Nashville, TN, USA) for the Alb-Cre; RBP-JKflox/+ mice. We also thank Dr. G.C. Yeoh (The University of Western Australia, Australia) for the BMOL cell line.
Financial support
This work was supported by NIH grant DK079005, by NIH grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers, by CeLiveR, Fondazione S. Martino, Bergamo, by PRIN 2009ARYX4T005, by CARIPLO 2011-0470, by PSC Partners Seeking a Cure Foundation grant to M.S. and by a grant from Fondazione Telethon, GGP09-189 to L.F. R.F is a recipient of a Liver Scholar Award (American Liver Foundation).
Abbreviations
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- ANIT
alpha-na-phthyl-isothiocyanate
- RPB-Jk
recombination signal binding protein for immunoglobulin kappa J region
- K19
cytokeratin 19
- SOX-9
SRY (sex determining region Y)-box 9
- BMOL
bipotential mouse oval cell line
- AGS
Alagille syndrome
- HPC
hepatic progenitor cell
- RDC
reactive ductular cell
- DBZ
dibenzazepine
- GSI
γ-secretase inhibitor
- DR
ductular reaction
- Alb
albumin
- Cyp
cytochrome P450
- DAPT
LY-374973N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester
- P
postnatal day
- ALT
alanine transaminase
- NICD
Notch intracellular domain
- VEGF
vascular endothelial growth factor
- +ve
positive
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2013.02.025.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
The underlying research reported in the study was funded by the National Institute of Health (NIH).
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 3.Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol. 2012;56:1159–1170. doi: 10.1016/j.jhep.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2011;137:4061–4072. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 7.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, et al. NOTCH-2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003;12:R9–R13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- 9.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, et al. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- 11.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, et al. Hedgehog signaling regulates epithelial–mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 15.Tirnitz-Parker JE, Tonkin JN, Knight B, Olynyk JK, Yeoh GC. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int J Biochem Cell Biol. 2007;39:2226–2239. doi: 10.1016/j.biocel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One. 2008;3:e1851. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, et al. Inducible gene knocKOut of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- 19.Sparks EE, Huppert KA, Brown MA, Washington MK, Huppert SS. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology. 2010;51:1391–1400. doi: 10.1002/hep.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest. 1999;79:103–109. [PubMed] [Google Scholar]
- 21.Masyuk TV, Ritman EL, LaRusso NF. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. Am J Pathol. 2001;158:2079–2088. doi: 10.1016/S0002-9440(10)64679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe MS. Gamma-secretase as a target for Alzheimer’s disease. Adv Pharmacol. 2012;64:127–153. doi: 10.1016/B978-0-12-394816-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 24.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, et al. Liver-specific inactivation of Notch-2, but not Notch-1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 26.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golbar HM, Izawa T, Yano R, Ichikawa C, Sawamoto O, Kuwamura M, et al. Immunohistochemical characterization of macrophages and myofibroblasts in alpha-naphthylisothiocyanate (ANIT)-induced bile duct injury and subsequent fibrogenesis in rats. Toxicol Pathol. 2011;39:795–808. doi: 10.1177/0192623311413790. [DOI] [PubMed] [Google Scholar]
- 29.Teachey DT, Seif AE, Brown VI, Bruno M, Bunte RM, Chang YJ, et al. Targeting Notch signaling in autoimmune and lymphoproliferative disease. Blood. 2008;111:705–714. doi: 10.1182/blood-2007-05-087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, et al. Bile duct proliferation in liver-specific Jag1 conditional knocKOut mice: effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 31.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, et al. Constitutive Notch-2 signaling induces hepatic tumors in mice. Hepatology. 2012;57:1607–1619. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 32.Jeliazkova P, Jors S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, et al. Canonical Notch-2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology. 2013 doi: 10.1002/hep.26254. http://dx.doi.org/10.1002/hep.26254. [DOI] [PubMed]
- 33.Park J, Zhang JJ, Moro A, Kushida M, Wegner M, Kim PC. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev Dyn. 2010;239:514–526. doi: 10.1002/dvdy.22192. [DOI] [PubMed] [Google Scholar]
- 34.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch-1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 36.Fabris L, Strazzabosco M. Epithelial–mesenchymal interactions in biliary diseases. Semin Liver Dis. 2011;31:11–32. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.