Abstract
Although, diabetes is reaching pandemic proportions, the exact etiology of either type 1 (T1D) or type 2 diabetes (T2D) remains to be determined. Mounting evidence, however, suggests that islet inflammation is a likely common denominator during early development of either type of the disease. In this review, we highlight some of the inflammatory mechanisms that appear to be shared between T1D and T2D, and we explore the utility of intravital imaging in the study of islet inflammation. Intravital imaging has emerged as an indispensable tool in biomedical research and a variety of in vivo imaging approaches have been developed to study pancreatic islet physiology and pathophysiology in the native environment in health and disease. However, given the scattered distribution of the islets of Langerhans within the “sea” of the exocrine pancreas located deep within the body and the fact that the islets only constitute 1 – 2% of the total volume of pancreatic tissue, studying the pancreatic islet in situ has been challenging. Here, we focus on a new experimental approach that enables studying local islet inflammation with single cell-resolution in the relevant context of the in vivo environment non-invasively and longitudinally and, thereby improving our understanding of diabetes pathogenesis.
Keywords: Pancreatic islet, inflammation, non-invasive, in vivo imaging, diabetes, T1D, T2D, insulin, ApoCIII, anterior chamber, intraocular transplantation
Introduction
Diabetes mellitus is a disease that is reaching pandemic proportions. It is a devastating condition characterized by loss of glucose homeostasis and onset of overt hyperglycemia. Chronic exposure to hyperglycemia typically precipitates in life-threatening complications which are the primary cause of diabetes-associated morbidities and mortalities. There are two types of diabetes mellitus. Insulin dependent or type 1 diabetes (T1D) is an autoimmune form of the disease typically diagnosed early in life and thought to be caused by a combination of genetic, environmental, and immunological factors that result in immune-mediated destruction of a critical mass of the insulin-producing pancreatic β-cells and onset of overt hyperglycemia. Type 2 diabetes (T2D), traditionally described as insulin independent, is a chronic disease characterized by slow and progressive deterioration of glucose homeostasis due to peripheral insulin resistance, as well as dysfunction of the pancreatic β-cells, which eventually leads to complete failure of the pancreatic islets to secrete insulin sufficiently and to adequately control glycemia. Like T1D, the exact etiology of T2D is not known but factors such as genetic predisposition, obesity, life style and eating habits are considered risk factors for the development of T2D and its associated complications. Insulin resistance has long been established as a hallmark of T2D, and studies have shown that obesity-induced inflammation within insulin target-tissues (i.e., liver, skeletal muscle, and adipose tissue) results in insensitivity/resistance to insulin and compromises its intracellular signaling and, thereby glucose uptake [1]. Deleterious effects of inflammation on insulin signaling are primarily mediated by pro-inflammatory cytokines and chemokines produced locally by the insulin target-tissues themselves and by activated tissue-resident inflammatory cells such as macrophages. Although β-cell dysfunction is classically thought to be the result of failure to cope with increased insulin demands due to chronic peripheral insulin resistance and hyperglycemia, recent genome-wide association studies have highlighted the role of pancreatic β-cell dysfunction in the early pathogenesis of T2D [2]. There is also a surprising scarcity of genetic variants related to insulin resistance, and most loci associated with T2D point to primary defects in the β-cells in the islets of Langerhans. Thus, in addition to disturbances in glucose metabolism in the periphery, dysfunction and/or apoptosis of the human β-cell in the endocrine pancreas contribute to the pathogenesis of T2D. Moreover, mounting evidence suggests that chronic, low-grade local islet inflammation is a common denominator contributing early to β-cell dysfunction and/or apoptosis during development of both T1D and T2D. Common inflammatory mechanisms have been suggested to play a key role during early pathogenesis of either type of the disease [3,4]. In this review, we highlight some of the major mechanisms that cause islet inflammation and are likely to contribute to the early pathogenesis of T1D and T2D. We also explore existing experimental approaches to study the immunobiology of pancreatic islets in the context of the living organism, and we focus on the utility of a recently established in vivo approach to simultaneously study non-invasively and longitudinally islet inflammation and β-cell dysfunction/apoptosis with single cell-resolution [5-7].
Islet inflammation and diabetes
Inflammation is a physiological response mounted by the organism to maintain homeostasis and to defend against harmful stimuli during infection or injury. Inflammatory responses are necessary for tissue remodeling or repair during growth and development or during wound healing. Inflammation is a complex biological process that involves the vascular and immune systems, as well as the involved target tissue. Inflammation is associated with significant active changes in the vascular wall that lead to increased blood flow, vessel diameter and permeability. Inflammatory changes in vascular endothelial cells are characterized by increased expression of surface receptors such as P-selectin, ICAMs (intercellular adhesion molecules), and VCAM-1 (vascular cell adhesion molecule-1) which facilitate leukocyte extravasation into the inflamed tissue [8]. These changes are initially induced by pro-inflammatory cytokines produced locally by tissue-resident innate immune cells (e.g., macrophages) and/or by the damaged cells of the tissue. Vascular changes in response to inflammation lead to fluid exudation into the interstitial space and increased presence of immune cells within the inflamed tissue.
Both T1D and T2D have been described as inflammatory diseases. Whereas the exact trigger(s) for either type of diabetes remains to be determined, emerging evidence suggests that the combination of genetic and environmental factors, as well as nutritional behavior leads to epigenetic changes that result in chronic low-grade islet inflammation during early pathogenesis of the disease [9,10]. Although, T1D is characterized by autoimmune attack against the β-cells by antigen-specific T effector cells of the adaptive immune system, studies have shown that chronic islet infiltration by inflammatory innate immune cells precedes direct β-cell damage by T effector cells and onset of overt hyperglycemia in T1D [11]. Moreover, T2D is classically considered the consequence of initial peripheral insulin resistance followed by dysregulation of the pancreatic islet function after failure of the β-cells to cope with chronic hyperglycemia, however, experimental findings in pre-diabetic subjects and in patients with established T2D indicate that islet inflammation is present several years prior to diagnosis of the disease [12,13]. These findings suggest that islet inflammation is a common denominator in the early pathogenesis of both T1D and T2D. While elimination of certain environmental risk factors and changes in life style and nutritional behavior can improve glycemic control in pre-diabetic and diabetic patients, other risk factors such as genetic predisposition and/or viral infections of β-cells, which can lead to chronic islet inflammation, may be more difficult to control or circumvent [14]. Therefore, improved understanding of the inflammatory mechanisms that lead to β-cell dysfunction and/or apoptosis might help develop therapeutic strategies to prevent or delay loss of glucose homeostasis and hyperglycemia onset in what might be considered “inevitable” in high risk diabetes-prone individuals. Delaying hyperglycemia impacts significantly on the quality-of-life of these individuals and reduces the propensity of life-threatening complications associated with chronic exposure to high levels of blood glucose.
Common inflammatory mechanisms in T1D and T2D
Mounting experimental evidence suggests that local islet inflammation contributes to the early pathogenesis of diabetes [10,11,15]. While islet inflammation may be in part a “byproduct” of the highly metabolic nature of the pancreatic islet and may serve a physiological role in the maintenance of the islet microenvironment, islet inflammation is likely to be exacerbated in the presence of diabetogenic risk factors (e.g., obesity, viral infection, and genetic background) in diabetes-prone individuals and may lead to “bystander” damage to islet cells. Numerous studies have independently investigated islet inflammation and the underlying mechanisms during development of T1D or T2D. Here, we highlight some of the inflammatory mechanisms that appear to be shared between the two types of the disease.
Cytokine-induced STAT1 and NF-kB signaling in β-cells
Maintenance of homeostasis and normal function of the pancreatic islet is a complex process that involves constant communication through paracrine signaling among the different endocrine cells of the islet, as well as through interactions with the vascular endothelial cells and resident innate immune cells (e.g. macrophages) present within the islets. Pancreatic islets from diabetic patients, however, have been shown to have increased immune cell infiltration and higher levels of pro-inflammatory cytokines and chemokines [10,16]. These are evident signs of increased islet inflammation in diabetes. While the exact triggers of increased islet inflammation remain to be determined, the chronic presence of activated innate immune cells leads to recruitment of adaptive immune cells into the islet. Moreover, local cross-talk between the innate and adaptive arms of the immune system is mediated in part through cytokine release. This in turn will lead to further islet inflammation and establishment of a chronic inflammatory microenvironment. Whereas all endocrine cells of the pancreatic islet may be exposed to this pro-inflammatory environment, the function and survival of the insulin-producing β-cells appear to be most affected. Studies clearly show that pro-inflammatory cytokines induce β-cell dysfunction and apoptosis [17,18]. Hence, detrimental effects of inflammation on islet function and overall glucose metabolism are likely to be mediated through cytokine-induced dysfunction and/or apoptosis of the insulin-producing β-cell during diabetes development.
Pro-inflammatory cytokines such as IL-1 (interleukin-1) beta, IL-6, IFN (interferon) gamma, and TNF (tumor necrosis factor) alpha have been shown to be upregulated in pancreatic islets in patients with T1D and T2D. It has been shown that cytokine injurious effects on the β-cell function and survival are primarily mediated through downstream signal transduction events involving the key transcription factors STAT1 (signal transducer and activator of transcription 1) and NF-kB (nuclear factor of kappa light polypeptide gene enhancer in B-cells) [19,20]. While cytokines are naturally produced during physiological immune responses to defend the host organism, the complex gene expression profiles induced by cytokine signaling can result in physiological or pathophysiological outcomes. In pancreatic β-cells, cytokine-induced STAT1 and NF-kB signaling pathways regulate complex gene networks associated with cell cycle and differentiation, endoplasmic reticulum and mitochondrial stress, inflammation, as well as cell survival and apoptosis [19,21,22]. It has been shown that selective down-regulation of the STAT1 or NF-kB pathways prevents cytokine-induced impairment in glucose-stimulated insulin secretion from pancreatic β-cells and reduces their apoptosis [23]. Moreover, in vitro and in vivo studies show that targeting the STAT1 pathway yields protective effects on β-cells in diabetic animal models and improves β-cell survival in isolated rodent and human islets [24,25]. These findings demonstrate that the cytokine-induced STAT1 and NF-kB signaling pathways play major roles in regulating the function and survival of β-cells and, are likely mechanisms in the pathogenesis of both T1D and T2D.
ApoCIII and β-cell dysfunction and apoptosis
Apolipoprotein CIII (ApoCIII) is naturally among the most abundant apolipoproteins in the circulation where it is found associated with chylomicrons and other lipoproteins. Circulating ApoCIII is largely produced by the liver where it inhibits activity of lipoprotein lipase, thereby decreasing metabolism of triglyceride-rich lipoproteins and reducing their uptake by hepatocytes [26,27]. Elevated serum levels of ApoCIII due to gene polymorphisms and other risk factors such as obesity are implicated in cardiovascular disease and atherosclerotic lesions. Negative effects of ApoCIII on the vascular system are thought to be mediated through reduced clearance of VLDL (very-low-density lipoproteins) and LDL (low-density lipoproteins) from the circulation and through other direct pro-inflammatory effects on the vascular endothelial cells [28,29]. Elevated serum levels of ApoCIII are also implicated in increased susceptibility to T1D and T2D, as well as in diabetes-associated life-threatening complications such as vascular pathologies, kidney failure, diabetic retinopathy and neuropathies [30,31].
Increased lipidemia and circulating free fatty acids during development of T2D in association with pathologically high levels of ApoCIII also contribute to peripheral insulin resistance, as well as pancreatic β-cell dysfunction/apoptosis through inflammatory mechanisms similar to those induced by cytokines involving the STAT1 and NF-kB signaling pathways as described above [32]. Other experimental evidence also indicates that high ApoCIII levels have direct negative effects on β-cell survival. Sera with high ApoCIII content from patients with established T1D were shown to induce β-cell dysfunction and apoptosis by disrupting the regulation of cytoplasmic free Ca2+ concentration ([Ca2+]i) [33]. Pathologically high concentrations of ApoCIII in diabetic serum were shown to increase the membrane density and open probability of voltage-gated Ca2+ channels leading to increased [Ca2+]i [34]. Chronic exposure to high [Ca2+]i due to high ApoCIII levels is likely to lead to β-cell dysfunction and apoptosis in diabetes-prone subjects. Consistently, studies show that lowering levels of circulating ApoCIII favors decreased incidence of diabetes and associated complications such as cardiovascular disease [35]. It was recently shown in diabetes-prone BB (DPBB) rats, which develop human-like T1D, that ApoCIII serum levels are elevated in pre-diabetic animals. It was also shown that high apoCIII levels in the serum derived from pre-diabetic DPBB rats could induce β-cell apoptosis in vitro. Importantly, treating pre-diabetic DPBB rats with antisense against ApoCIII delayed onset of T1D significantly [36]. These studies indicate that ApoCIII is a likely contributor to early pathogenesis of diabetes (i.e., before manifestation of hyperglycemia). It is of interest to note that a recent study in neonatal pancreatic islets from another strain of rats showed that ApoCIII could have protective effects on β-cells when exposed in vitro to cytokines to mimic the inflammatory stress in T1D [37]. This is likely the result of using neonatal islets as numerous studies show deleterious effects of high ApoCIII levels on islet function and glucose homeostasis [38,39].
Experimental evidence also indicates that the direct effects of ApoCIII on β-cells are likely mediated through distinct cell surface receptors including scavenger receptor class B type I, Toll-like receptor 2, and yet uncharacterized binding sites which signal through downstream effectors such as beta1 integrin, pertussis toxin-sensitive G proteins, NF-kB and protein kinases [40,41]. Studies in rodent islets indicate that the negative effects of ApoCIII on β-cells are likely to converge from multiple protein kinase signaling pathways, as only combined inhibition of PKA and Src kinase, with and without PKC co-inhibition, counteracted ApoCIII negative effects on the β-cells [42]. Moreover, knockdown of beta1 integrin prevented ApoCIII from hyperactivating Ca2+ channels in β-cells, indicating that ApoCIII effects on Ca2+ channels are mediated through integrin-dependent co-activation of PKA and Src kinase [42]. Furthermore, ApoCIII-triggered signaling through NF-kB can result in gene expression profiles consistent with those induced by cytokines leading to increased islet inflammation in early diabetes pathogenesis. Taken together, these findings suggest that high levels of circulating ApoCIII contribute to β-cell dysfunction and apoptosis through direct Ca2+-induced effects and inflammatory mechanisms and, thereby being involved in the pathogenesis of both types of diabetes.
Non-invasive study of islet inflammation in vivo
A great deal of research effort has been dedicated to studies investigating islet inflammation and its involvement in β-cell dysfunction and apoptosis during diabetes development. Many of the inflammatory mechanisms have been investigated in detail primarily in rodent islets in vitro, but translating such findings into the living organism can be very challenging. This is primarily due to the absence of critical components (i.e., vascular system, leukocyte extravasation, tissue infiltration, etc.) of inflammation in in vitro studies, which essentially offer a limited “snapshot” of a highly dynamic and progressive process. Another important challenge in biomedical research, in general, is the successful translation of intravital findings obtained in studies using in vivo animal models into human applications. Anti-inflammatory therapies for example successfully prevent or significantly delay onset of hyperglycemia in rodent models of T1D [43], but they have been less effective in human clinical trials [44]. This highlights the need to address the “discrepancy” between these seemingly inconsistent outcomes of studies performed in animals versus humans.
Part of this apparent discrepancy is due to the lack of a comprehensive understanding of the human disease process in the temporal and spatial context of the natural in vivo environment of the endocrine pancreas. This gap in knowledge is mainly the result of lack of experimental approaches that enable studies of the pancreatic islets and β-cells with sufficient resolution in humans. To address this technical limitation, powerful experimental approaches using animal models have been developed to study diabetes pathogenesis in vivo, but most of these techniques inherently lack critical aspects, which limits their utility in the longitudinal study of chronic inflammation in pancreatic islets. For instance, a recently developed powerful but highly invasive approach was used to study the development of autoimmune diabetes in mice using “intravital” imaging of the exposed pancreas [45]. This approach clearly enables in vivo studies of diabetes autoimmunity in the native pancreas, but it does not allow longitudinal studies of chronic islet inflammation during progression of diabetes pathogenesis in the same animal over extended periods of time. This is critically important in the study of T1D and, especially, T2D which is a chronic and slow progressive disease. Other non-invasive intravital approaches enable longitudinal studies of pancreatic islet mass, but they lack the spatial resolution to allow the study of islets or islet cells individually [46]. Therefore, our current understanding of the role of chronic low-grade islet inflammation in the early pathogenesis of either T1D or T2D remains incomplete.
Another reason for the apparent discrepancy between outcomes of animal and human studies is due to the inherent structural and functional differences between rodent and human pancreatic islets [47-49]. This is likely to contribute to more challenges when translating experimental findings from animals (e.g., rodents) into human applications. While studies in animal models will endure and undoubtedly continue to enrich our knowledge of critical biological processes that will not be possible to derive in vitro or in human subjects, a concerted effort is being invested in developing new approaches that allow the study of human islets under in vivo conditions. One of these approaches is to study human islets in animal models. Humanized mouse models have been developed to study human disease after introduction of the target human tissue into immune compromised mice either by gene expression or transplantation [50,51]. However, re-emergence of mouse immune cells and other technical limitations (e.g., increased susceptibility to radiation, shorter life-span, etc.), as well as the high cost of acquiring and maintaining humanized mice have limited their wide-spread use in biomedical research. Alternatively, mouse models of human diseases have been identified and/or developed. Examples include the non-obese diabetic (NOD) mice and the leptin-deficient (ob/ob) mice which are commonly used to study T1D and T2D, respectively. These mouse models have enabled the study of diabetes in vivo. Being able to investigate the disease process under in vivo conditions has significantly improved our understanding of diabetes. This has also spurred a need for imaging approaches that enable longitudinal visualization of the target tissue (i.e., pancreas/pancreatic islets) non-invasively in the living animal. Whereas intravital imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) or bioluminescence enable imaging of target tissue deep within the body and have been successfully used in diabetes research, they lack the spatial resolution to enable studies at the single-cell level within the pancreatic islet [46,52,53]. Alternatively, studies using confocal and two-photon fluorescence microscopy with single cell-resolution capabilities have been instrumental in the study and characterization of key biological and cellular processes that could not be predicted outside the context of the natural in vivo environment (e.g., lymph nodes, brain) [54,55]. Two-photon in vivo imaging of pancreatic islets has also been performed in highly invasive approaches that either expose the pancreas [45] or use thick pancreatic slices [56] to study the physiology and pathophysiology of the pancreatic islet in situ. Given the highly invasive nature of these approaches, other studies have used implantable window chamber-devices in an effort to be less invasive while studying pancreatic islets [57,58]. Whereas these different in vivo approaches enable high-resolution imaging of the pancreatic islet, they offer limited capabilities to allow studies in the same target tissue over extended periods of time. On the one hand, invasive in vivo approaches inherently limit repetitive imaging in the exposed and exteriorized pancreas. On the other hand, less-invasive window chamber-devices offer limited utility in studies requiring long-term monitoring of islet survival over the span of several months due to the relatively quick deterioration of the device after several weeks of implantation. Being able to non-invasively monitor and study with high-resolution individual pancreatic islets over extended periods of time, however, is critical in studies of diabetes, especially T2D, where effects of chronic low-grade islet inflammation slowly impact on β-cell function and survival during the progression of the disease.
To address this critical technical limitation, we have recently reported on an in vivo experimental approach that simultaneously enables non-invasive and longitudinal studies of islet immunobiology with single cell-resolution [59]. By transplanting isolated pancreatic islets into the anterior chamber of the mouse eye (Fig. 1) [60], we were able to study the physiology and pathophysiology of individual islet cells [6,7]. Using the same approach, we also studied longitudinally the islet survival and structural integrity during immune responses mounted against allogeneic pancreatic islets after transplantation (Fig. 2) [5]. Analysis of the T cell priming and activation in allogeneic recipients of pancreatic islets showed typical immune responses against intraocular islet grafts. Intraocular islet transplantation also enabled us to simultaneous study the immune cell infiltration and their interaction with target islet cells during the progression of islet allograft rejection. Notably, we were able to track individual infiltrating T effector cells and quantify their infiltration kinetics and movement dynamics inside and outside the same islet grafts at different time points after transplantation (Fig. 3). This unprecedented in vivo access to the islets revealed a novel ruffled phenotype of the T effector cells. Cytotoxic ruffled T cells exhibited unique morphologic and dynamic features within the islet allografts, where they increased motility during killing of multiple target islet cells simultaneously. Importantly, our studies showed that local production of the chemokines CCL5 and CXCL9/10 induced further activation of infiltrating T effector cells and their consequent acquisition of the ruffled phenotype within islet allografts in situ.
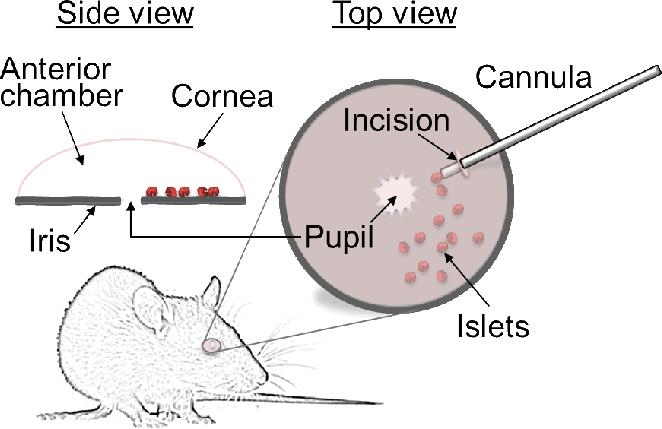
Fig. 1. Schematic depiction of pancreatic islet transplantation into the anterior chamber of the mouse eye.
Transplantation of pancreatic islets into the anterior chamber of the eye is a relatively simple and minimally invasive procedure. Isolated pancreatic islets are deposited on top of the iris through a small incision in the cornea of an anesthetized mouse. The islets are inserted into the anterior chamber using a small cannula. Once inside the anterior chamber, the islets adhere quickly to the iris where engraftment begins. Engrafted islets are revascularized and reinnervated similar to the native pancreas. Shown are top and side schematic views of the transplanted eye. For a detailed description of the transplantation procedure, see reference [60].
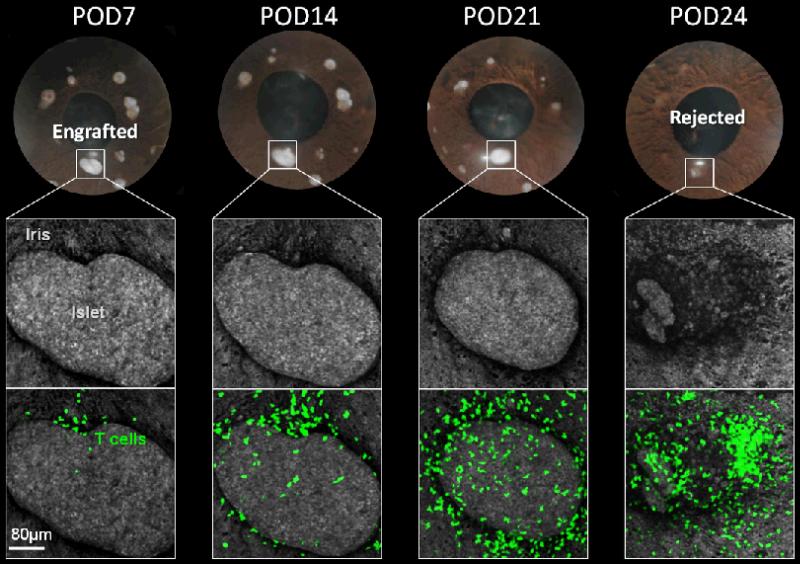
Fig. 2. Intraocular transplantation enables non-invasive and longitudinal in vivo imaging of individual islets with single cell-resolution.
A series of photos and confocal micrographs acquired in the same eye of a transplanted mouse highlighting the changes in the islet mass of an individual allogeneic pancreatic islet over an extended period of time after transplantation. The iris and the intraocular islet grafts were visualized by laser backscatter (reflection; grey). The transgenic recipient mouse expressed green fluorescent protein (GFP) in T effector cells; and GFP-labeled T cells (green) infiltration of the anterior chamber and into the islet graft is evident. Importantly, being able to monitor the same individual islet graft longitudinally revealed the destruction kinetics of the rejecting islet allografts in association with T cell infiltration.
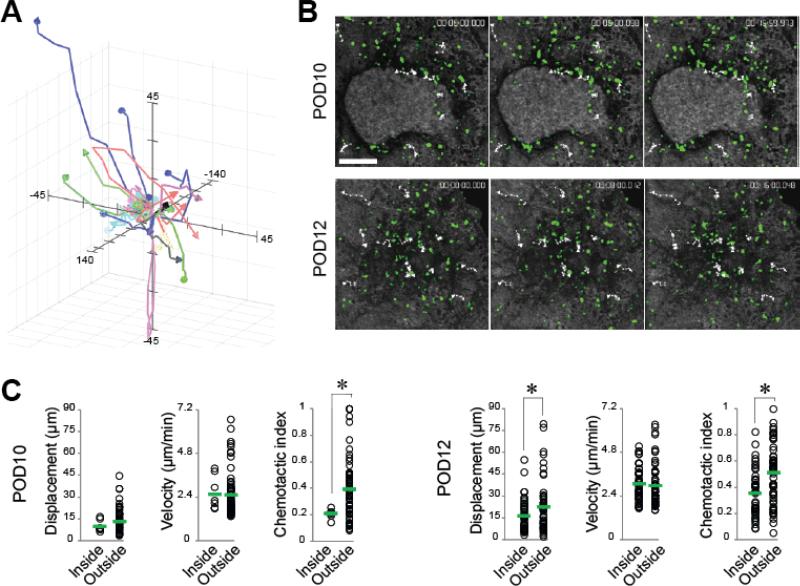
Fig. 3. Intraocular islet transplantation enables in vivo imaging and tracking of individual immune cells non-invasively and longitudinally the living animal.
(A) Representative flower plot of three-dimensional (3-D) trajectories of the movement of infiltrating T cells within pancreatic islets after transplantation. Each color-coded line represents a trajectory of an individual T cell. The T cells were systematically tracked inside and outside the islet allografts at different time points during progression of allorejection. (B) Selected snapshots of 3-D time-lapse recordings (shown as 2-D maximum projections) acquired on the same pancreatic islet allograft before rejection on postoperative day (POD)10 and after rejection at POD12. Shown in white dotted lines are representative movement trajectories of a few T cells (green) inside and outside the rejecting islet allograft. Notice the change in islet mass between POD10 and POD12 confirming that complete rejection of the islet occurred in ≤ 48 h. (C) Quantitative analysis of the dynamic behavior of infiltrating T cells within (i.e., inside) and outside the pancreatic islet allograft. The analysis was performed using specialized software and dynamic parameters were derived to quantify and characterize the cellular motility patterns before and after rejection. For detailed description of these parameters, see reference [5]. Each circle in the plots represents an individually tracked T cell. The means are shown as green bars and were compared using Student t-test. Notice the overall increase in the displacement and mean velocity of the T cells inside and outside of the islet on POD12 compared to POD10. Interestingly, despite their ability to move at similar mean velocities inside and outside the islet, the T cells constantly had significantly higher chemotactic index outside of the islet. This suggests that the movement of the T cells outside the islet is more directional due to an attractive chemotactic gradient emanating from the islet graft, Whereas the movement of T cells inside the graft is less directional despite their comparable velocity because the cells are already within the source of the chemokine gradient. These data further indicate that the T cells inside the graft are highly motile within short distances. This is likely due to active local mechanisms at play during ongoing rejection rather than physical constraints imposed by the dense islet parenchyma [5].
In addition to observing physiologically relevant immune responses against pancreatic islets transplanted into the anterior chamber of the mouse eye, our studies also show similar vascularization and innervation patterns of intraocular islet grafts compared to the native pancreas. We have shown that blood vessels within pancreatic islets transplanted into the anterior chamber of the mouse eye have structural and functional characteristics (e.g., capillary diameter and fenestration size) similar to those observed in the native pancreas [61]. We have also recently shown that the innervation patterns of pancreatic islet grafts are recapitulated in the anterior chamber of the eye to reflect that of the native pancreas of the original islet donor [62]. This was demonstrated by transplanting donor islets from different strains of mice with known differences in islet innervation patterns (e.g., C57BL6 versus 129X1 mice), or by transplanting human islets into the anterior chamber of the eye of immune compromised recipients (unpublished data). Collectively, our findings indicate that intraocular islets have similar structural and functional features as those in the native pancreas, and that they are subject to physiologically relevant immune responses consistent with other ectopic islet transplantation sites. Therefore, transplantation of pancreatic islets into the anterior chamber of the eye provides a versatile experimental tool to study longitudinally the immunobiology of pancreatic islets under normal conditions or during development of diabetes.
The anterior chamber of the eye as a natural “body window” for long-term imaging of pancreatic islet function and survival
Scientists have been transplanting different tissues into the anterior chamber of the eye for more than a century [63,64]. The anterior chamber appealed to scientists because of its immune privilege properties which may facilitate survival of foreign tissues with relatively little to no immune attack [65]. However, the history of eye disease and its natural clearance, as well as experimental studies in the eye demonstrate that the immune privilege of the anterior chamber of the eye is not absolute. The immune privileged status of the anterior chamber can be compromised by a variety of processes including, but not limited to, the initial “danger” signal generated by the transplantation procedure and the ensuing inflammation, and by reestablishment of lymphatic and blood vessels within the transplanted tissues (e.g., pancreatic islets) (Fig. 4) [66-68]. We have recently shown in our allotransplantation model that immune privilege of the anterior chamber of the eye is compromised after allogeneic islet transplantation as subsequent immune responses against intraocular islet allografts were similar to those observed during immune-mediated rejection of pancreatic islets transplanted under the kidney capsule [5]. Our studies also show similar immune cell infiltrates in intraocular NOD islet grafts compared to those observed in islet grafts under the kidney capsule or in islets of the native pancreas during T1D diabetes development [69]. Moreover, we consistently observe effective antigen-specific immune responses against intraocular islets bearing specific antigens ([70,71] and unpublished data), demonstrating the physiological relevance of immune responses observed in the anterior chamber of the eye once the immune privilege has been compromised.
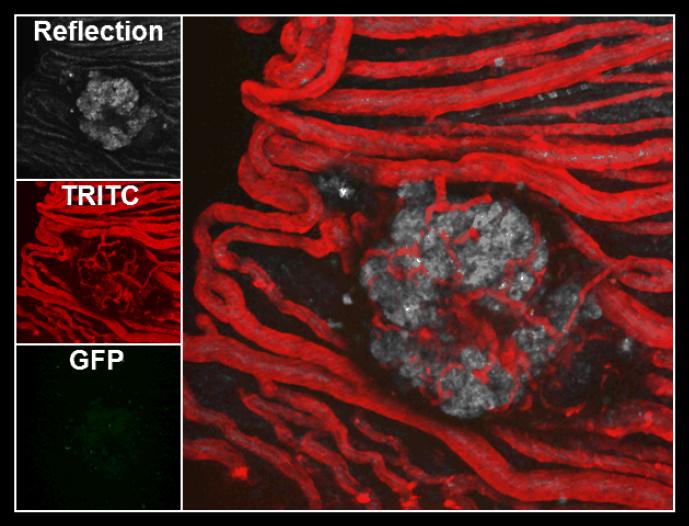
Fig. 4. Intraocular pancreatic islet grafts are revascularized by existing blood vessels of the iris.
Representative confocal micrograph a pancreatic islet transplanted in the anterior chamber of a mouse eye showing rich revascularization of the islet by existing iris blood vessels. The left panels show the islet graft visualized by laser backscatter (reflection), blood vessels visualized by injection of TRITC-labeled dextran, and infiltrating GFP-labeled T cells. The image on the right shows an overlay of the three images on the left. Revascularization of pancreatic islets in the anterior chamber of the eye contributes to breaking immune privilege and leads to precipitation of typical immune responses similar to those observed at other ectopic transplantation sites.
Another reason behind the appeal of the anterior chamber of the eye is its relative easy access and the simplicity and minimally invasive nature of the transplantation procedure into this site [60]. More importantly, the transparent nature of the cornea provides a natural “body window” through which transplanted tissue(s) can be monitored and studied non-invasively and longitudinally at the single cell level. Given these unique characteristics, we have recently explored the possibility of using the anterior chamber of the eye as an alternative site in clinical islet transplantation to treat T1D using a non-human primate (baboon) model [72]. Our studies in the baboon provided a proof-of-principle for the feasibility of using intraocular islet transplantation in larger animals. Importantly, similar to smaller animal models (e.g., mice) islet transplantation into the anterior chamber of the baboon eye enabled monitoring of islet survival and structural integrity long-term (≥ 350 days; Fig. 5). More importantly, there was a considerable improvement in glycemic control in the diabetic baboon transplanted in one eye. In this context, exogenous insulin requirements were also reduced significantly in spite of the relatively small transplanted islet mass compared to what is typically transplanted into the liver. While our studies in the baboon were performed under the cover of immunosuppression, learning how to harness the immune privilege properties of the anterior chamber of the eye offers a great potential for this approach in future clinical practice, where induction of immune tolerance for transplanted tissues (e.g., pancreatic islets) through ACAID (anterior chamber associated immune deviation) [73] may revolutionize the standard-of-care in transplantation therapies including that of pancreatic islets. This will reduce or eliminate the need for life-long immunosuppression and the life-threatening side-effects associated with chronic use of anti-rejection drugs.
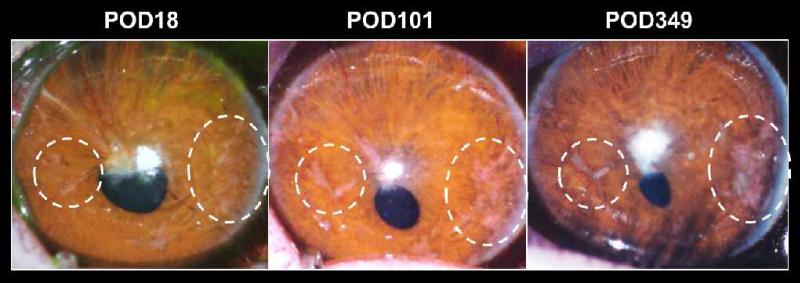
Fig. 5. The anterior chamber of the eye as a potential islet transplantation site in clinical application.
Serial photos of a baboon eye that was transplanted with pancreatic islets from an allogeneic donor highlighting the feasibility of intraocular islet transplantation in larger animals and its utility in monitoring the intraocular islet grafts over extended periods of time non-invasively. Notice in the dotted circles the longitudinal changes in the 2 selected clusters of islets. On POD18, the islets appear pale and less prominent due to absence of many blood vessels. On POD101 and POD349, the islets appear “rosy” and prominent due to the rich presence of blood vessels after complete revascularization of the islet grafts. These studies provided a proof-of-principle for a potential translation of intraocular islet transplantation into clinical practice.
In the meantime, intraocular transplantation continues to offer a valuable experimental tool to study different tissues under living conditions. In 1990, Adeghate and Donath transplanted embryonic pancreatic tissue into the anterior chamber of the eye and studied islet development over an extended period of time using light and electron microscopy [74]. In 2008, we combined transplantation into the anterior chamber of the mouse eye with high-resolution confocal and two-photon laser scanning microscopy to study the physiology of pancreatic islets in the living animal [6,7]. More recently, we extended the use of this approach to study the immune responses during allorejection or tolerance induction in vivo, as well as during development of autoimmune diabetes [5,69,70]. Together these studies demonstrate that intraocular islet transplantation is a versatile in vivo experimental tool to study the islet immunobiology in health and disease.
Studying the immunobiology of pancreatic islets during diabetes development in “plain sight”
We have established that transplantation into the anterior chamber of the eye represents a powerful experimental tool to study, in a physiologically relevant context, the immunobiology of pancreatic islets under living conditions. Our recent in vivo studies investigating the progression of autoimmunity during development of T1D in the NOD mouse revealed striking differences in the kinetics of islet destruction at onset of overt hyperglycemia due to the autoimmune attack against the insulin-producing β-cells in NOD islets, compared to allogeneic islets during immune-mediated rejection [69]. NOD islets appeared de-granulated but maintained their structural integrity during onset of overt hyperglycemia due to autoimmune diabetes. Interestingly, the NOD islets transiently increased in size before precipitous decline in their mass. These findings suggest that the NOD islets experience transient edema due to increased vascular leakage and islet inflammation in association with beta-cell dysfunction and failure to cope with hyperglycemia, before the eventual loss of islet integrity and complete destruction by autoimmune attack. By contrast, allogeneic islets were completely destroyed at onset of acute rejection and return to hyperglycemia. These results also showed that the rejection kinetics of allogeneic islets in the anterior chamber of the eye are similar, whether rejection is assessed either by direct in vivo imaging or glycemia measurement [5]. These findings highlight the important practical aspect of this approach which allowed non-invasive and longitudinal monitoring of the islets in the living animal, and helped reveal strikingly different features of the islet immunobiology during progression of allorejection and during development of autoimmune diabetes.
Moreover, we have recently used islet transplantation into the anterior chamber of the eye of mice that were adoptively transferred with T cells expressing different fluorescent proteins to study the effects of cellular interactions between antigen-specific effector T (Teff) cells and T regulatory (Treg) cells on the function and survival of β-cells ([70,71] and unpublished data). Our findings suggest that in situ Treg-Teff cell interactions within pancreatic islets through direct cell-cell contact play an important role in polarizing immune responses toward either cytotoxicity or tolerance. Our studies also show that killing of target β-cells by antigen-specific CD4+ Teff cells in our model is mediated through direct cell-cell contact, whereby shedding more light on a process that is not well established for these Teff cells in vivo [75]. We envision this approach, intraocular transplantation, enabling future studies to investigate the different killing mechanisms of CD4 and CD8 Teff cells in the context of the in vivo environment of a variety of tissues including pancreatic islets. Additionally, intraocular islet transplantation was recently used to investigate the role of macrophages in pancreatic islet graft rejection [76]. These studies showed that macrophage infiltration of allogeneic pancreatic islets after transplantation occurs in two phases. An early phase where recipient macrophages appear in the donor islets without apparent damage, and a later phase following massive graft infiltration by Teff cells in association with acute rejection. These data suggest that infiltrating macrophages within pancreatic islet grafts may fulfill dual roles: 1) an early role consistent with that of resident macrophages which contribute to setting up the inflammatory milieu that helps recruit effector cells into the grafts, and 2) a later active role in allograft destruction during acute rejection. We further anticipate that pancreatic islet transplantation into the anterior chamber of the eye will be particularly useful in illuminating our understanding of how M1 versus M2 macrophage polarization impacts on the survival of pancreatic islets after transplantation. Such studies may offer new intervention points to prevent or reduce loss of pancreatic islets after transplantation to treat T1D.
Furthermore, intraocular transplantation of different tissues (e.g., pancreatic islets, cancer cells, etc.) can serve as a drug screening platform to assess the longitudinal effects of different treatments on the function and survival of target tissue under in vivo conditions. We have taken advantage of this utility of intraocular transplantation to monitor the effects of different systemic treatments on T cell infiltration into pancreatic islets and to assess their impact on allogeneic islet survival after transplantation (Fig. 6). While these studies can be performed using other transplantation sites, such as under the kidney capsule, the anterior chamber of the eye offers the added advantages of being able to simultaneously monitor immune cell infiltration and assess survival of the same individual islets non-invasively and longitudinally. Intraocular transplantation also provides the ability to monitor and assess targeting of fluorescently-tagged reagents to the islets or other target tissues. Accurate tissue targeting can also be accomplished either by topical application or direct injection into the anterior chamber of the eye (Fig. 7). These features of the anterior chamber of the eye have valuable practical implications specifically in experiments where smaller quantities of reagents can be used in the anterior chamber of the eye, when availability or access to precious reagents is limited or when pharmacokinetic information (e.g., dose, solubility, etc.) of newly developed drugs is not available. In contrast to systemic application of investigational reagents, direct delivery into the anterior chamber of the eye also ensures accurate concentrations and minimizes/eliminates interference by other systemic tissues, thus, enabling better inquiry of the target tissue. These practical aspects are particularly valuable in the early screening and discovery process during development of new therapeutic agents.
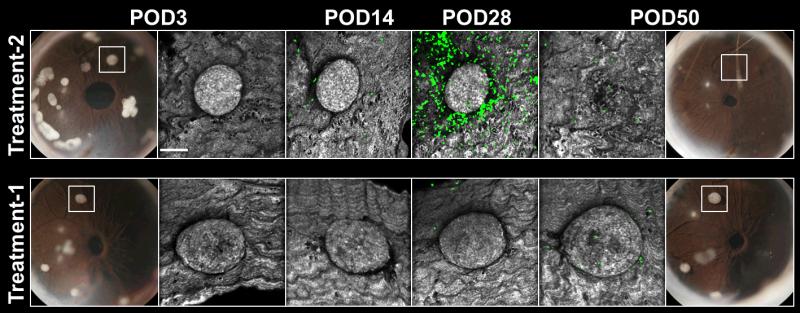
Fig. 6. Intraocular islet transplantation provides an experimental platform for screening new therapeutics longitudinally.
Serial photos and high-resolution confocal micrographs acquired on POD3, POD14, POD28, and POD50 of allogeneic pancreatic islets transplanted into the anterior chamber of the eye of mice expressing GFP in T cells. The mice were subjected to different experimental treatments administered systemically. Treatment-1 was with a proprietary lymphocyte-depleting agent and Treatment-2 was with an anti-chemokine agent. Digital images of the transplanted eyes on POD3 show individual islet grafts that were followed longitudinally using non-invasive in vivo imaging. Treatment efficacy was assessed based on islet infiltration by GFP-labeled T cells (green) and on islet structural integrity and survival in each treatment group. Islet grafts were visualized by laser backscatter (reflection; grey). These data demonstrate the utility of intraocular islet transplantation as a non-invasive drug screening tool during long-term in vivo studies.
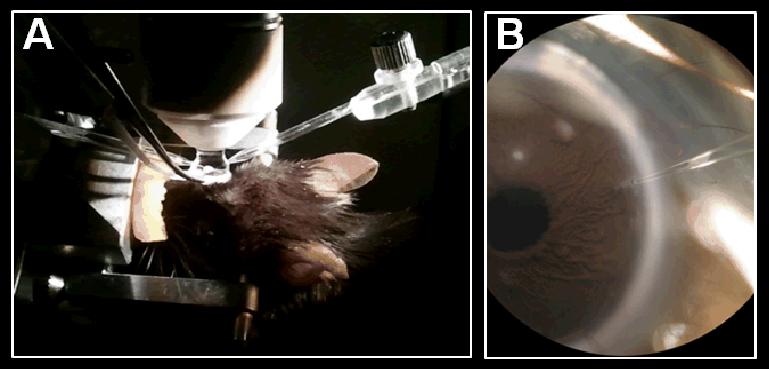
Fig. 7. The anterior chamber is a self-contained environment where local pharmacological manipulation of pancreatic islets can be performed.
(A) Photo of an anesthetized mouse mounted on a microscope where non-invasive in vivo imaging is performed on pancreatic islet transplanted into the anterior chamber of the eye. The image also shows a glass micropipette for direct injection into the anterior chamber. (B) Close-up view (under the microscope) of the micropipette inserted into the anterior chamber where direct injection of pharmaceutical agents and other reagents (e.g., cellular dyes, antibodies, etc.) can be performed. Furthermore, delivery of drugs and other compounds is also feasible with the proper formulation in the form of eye drops.
Conclusions
Diabetes mellitus poses a serious threat to public health worldwide. Both T1D and T2D are complex multifactorial diseases associated with life-threatening complications. While great strides in diabetes research have been accomplished, our current success in preventing this devastating disease remains limited. This is due in part to lack of a comprehensive understanding of the disease process and the complete etiology of both types of diabetes. Meanwhile, mounting evidence suggests that chronic low-grade islet inflammation is a common denominator in both T1D and T2D, where islet inflammation may play a critical role in the early pathogenesis of the disease. Studies have identified multiple inflammatory mechanisms that lead to β-cell dysfunction and/or apoptosis, and some of these mechanisms appear to be shared during development of both types of diabetes. It has been shown that cytokine-induced intracellular signaling involving the transcription factors STAT1 and NF-kB is a major inflammatory mechanism that leads to dysfunction of the pancreatic islet and contributes to diabetes pathogenesis. Other studies have also suggested that ApoCIII is an important etiological factor during early diabetes development. ApoCIII is thought to contribute to diabetes pathogenesis through its pro-inflammatory effects on the overall pancreatic islet environment and through its direct pro-apoptotic effects on β-cells by disturbing handling of [Ca2+]i. A better understanding of the inflammatory mechanisms underlying early dysfunction and/or apoptosis of the β-cells during diabetes development will undoubtedly impact on intervention approaches in the treatment of this devastating disease.
Inflammation, however, is a physiological process necessary for the maintenance of homeostasis within the pancreatic islet. Inflammation is a complex biological process that involves multiple body systems including the vascular and immune systems. Therefore, a better understanding of the role of islet inflammation in health and in disease is likely to be accomplished in the context of the natural environment of the living organism. Given the location and sparse distribution of the pancreatic islets within the “sea” of the exocrine pancreas, intravital studies of pancreatic islets have been traditionally hindered by technical limitations that restrict access to the islets with sufficient resolution. This has in turn motivated development of new in vivo approaches to study pancreatic islets under living conditions. While pioneering in vivo approaches have proved extremely valuable in many research and clinical applications, they offer limited capabilities to simultaneously study non-invasively and longitudinally the pancreatic islets at the single-cell level. This technical limitation was recently addressed by combining high-resolution confocal and two-photon microscopy with pancreatic islet transplantation into the anterior chamber of the eye [7]. This new approach enabled the study of the immunobiology of pancreatic islets with unprecedented detail for extended periods of time [5,6].
Whereas studies thus far using the anterior chamber of the eye have primarily focused on different aspects of islet physiology under normal conditions and in the context of immune responses during development of allorejection or autoimmune T1D, the unique features of intraocular islet transplantation are well-suited for the long-term study of islet inflammation and its chronic effects on β-cell function and survival in the context of T2D. For instance, this approach can be used in mice with a C57 genetic background to study the long-term effects of high fat diet-induced obesity on the local inflammatory environment of the pancreatic islet, where islet infiltration by immune cells (e.g., macrophages) and islet function and survival can be monitored non-invasively and longitudinally. Precise manipulation of the islet microenvironment in the anterior chamber of the eye can also be accomplished by local pharmacological intervention to better understand the mechanisms contributing to islet inflammation or β-cell dysfunction and/or apoptosis under different in vivo conditions. Thus, intraocular transplantation offers a valuable and versatile experimental tool to study a variety of tissues, including pancreatic islets, under different conditions in “plain sight”.
Ethics.
All aspects of the animal procedures including transplantation into the anterior chamber of the eye of mice or non-human primates were approved by the IACUC (Institutional Animal Care and Use Committee) of the University of Miami and the local animal ethics committees at Karolinska Institutet.
Acknowledgements
We thank Dr. Alejandro Caicedo for critical feedback on the manuscript. We acknowledge Drs. Anotnello Pileggi and Zhibin Chen and their research teams for fruitful collaborations on studies of autoimmunity and tolerance. Funding for the research studies behind this work was provided by grants from the National Institute of Health (NIH) through the National Institute of Digestive and Kidney Disease (NIDDK) (F32DK083226 and U01DK089538), the JDRFI (4-2004-361), and from the Diabetes Research Institute Foundation. Additional research support was provided through funds from Karolinska Institutet, the Swedish Research Council, the Swedish Diabetes Foundation, the Family Erling-Persson Foundation, the Family Knut and Alice Wallenberg Foundation, the Skandia Insurance Company Ltd., VIBRANT (FP7-228933-2), Strategic Research Program in Diabetes at Karolinska Institutet, the Novo Nordisk Foundation, the Stichting af Jochnick Foundation, and the Berth von Kantzow's Foundation.
Footnotes
Conflict of interest statement
P-OB is co-founder and CEO of BioCrine AB who is using the anterior chamber of the eye in vivo imaging platform for commercial purposes. MHA is consultant to BioCrine AB.
References
- 1.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timper K, Donath MY. Diabetes mellitus Type 2-the new face of an old lady. Swiss Med Wkly. 2012;142:w13635. doi: 10.4414/smw.2012.13635. [DOI] [PubMed] [Google Scholar]
- 3.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 4.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 5.Abdulreda MH, Faleo G, Molano RD, et al. High-resolution, noninvasive longitudinal live imaging of immune responses. Proc Natl Acad Sci U S A. 2011;108:12863–12868. doi: 10.1073/pnas.1105002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med. 2008;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speier S, Nyqvist D, Kohler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojcikiewicz EP, Abdulreda MH, Zhang X, Moy VT. Force spectroscopy of LFA-1 and its ligands, ICAM-1 and ICAM-2. Biomacromolecules. 2006;7:3188–3195. doi: 10.1021/bm060559c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert ER, Liu D. Epigenetics: the missing link to understanding beta-cell dysfunction in the pathogenesis of type 2 diabetes. Epigenetics. 2012;7:841–852. doi: 10.4161/epi.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56:234–241. doi: 10.1007/s00125-012-2762-3. [DOI] [PubMed] [Google Scholar]
- 12.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122:4667–4674. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol L, Grant WF, Comstock SM, et al. Pancreatic inflammation and increased islet macrophages in insulin resistant juvenile primates. J Endocrinol. doi: 10.1530/JOE-12-0424. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galleri L, Sebastiani G, Vendrame F, Grieco FA, Spagnuolo I, Dotta F. Viral infections and diabetes. Adv Exp Med Biol. 2012;771:252–271. doi: 10.1007/978-1-4614-5441-0_20. [DOI] [PubMed] [Google Scholar]
- 15.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 18.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 19.Moore F, Naamane N, Colli ML, et al. STAT1 is a master regulator of pancreatic {beta}-cell apoptosis and islet inflammation. J Biol Chem. 2011;286:929–941. doi: 10.1074/jbc.M110.162131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurzov EN, Ortis F, Cunha DA, et al. Signaling by IL-1beta+IFN-gamma and ER stress converge on DP5/Hrk activation: a novel mechanism for pancreatic beta-cell apoptosis. Cell Death Differ. 2009;16:1539–1550. doi: 10.1038/cdd.2009.99. [DOI] [PubMed] [Google Scholar]
- 21.Choi HJ, Hwang S, Lee SH, et al. Genome-wide identification of palmitate-regulated immediate early genes and target genes in pancreatic beta-cells reveals a central role of NF-κB. Mol Biol Rep. 2012;39:6781–6789. doi: 10.1007/s11033-012-1503-5. [DOI] [PubMed] [Google Scholar]
- 22.Barthson J, Germano CM, Moore F, et al. Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem. 2011;286:39632–39643. doi: 10.1074/jbc.M111.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldor R, Yeffet A, Baum K, et al. Conditional and specific NF-kappaB blockade protects pancreatic β-cells from diabetogenic agents. Proc Natl Acad Sci U S A. 2006;103:5072–5077. doi: 10.1073/pnas.0508166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menegazzi M, Novelli M, Beffy P, et al. Protective effects of St. John's wort extract and its component hyperforin against cytokine-induced cytotoxicity in a pancreatic beta-cell line. Int J Biochem Cell Biol. 2008;40:1509–1521. doi: 10.1016/j.biocel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 26.McConathy WJ, Gesquiere JC, Bass H, et al. Inhibition of lipoprotein lipase activity by synthetic peptides of apolipoprotein C-III. J Lipid Res. 1992;33:995–1003. [PubMed] [Google Scholar]
- 27.van der Ham RL, Alizadeh Dehnavi R, Berbée JF, et al. Plasma apolipoprotein CI and CIII levels are associated with increased plasma triglyceride levels and decreased fat mass in men with the metabolic syndrome. Diabetes Care. 2009;32:184–186. doi: 10.2337/dc08-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami A, Aikawa M, Alcaide P, et al. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation. 2006;114:681–687. doi: 10.1161/CIRCULATIONAHA.106.622514. [DOI] [PubMed] [Google Scholar]
- 30.Hokanson JE, Kinney GL, Cheng S, et al. Susceptibility to type 1 diabetes is associated with ApoCIII gene haplotypes. Diabetes. 2006;55:834–838. doi: 10.2337/diabetes.55.03.06.db05-1380. [DOI] [PubMed] [Google Scholar]
- 31.van Hoek M, van Herpt TW, Dehghan A, et al. Association of an APOC3 promoter variant with type 2 diabetes risk and need for insulin treatment in lean persons. Diabetologia. 2011;54:1360–1367. doi: 10.1007/s00125-011-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Juntti-Berggren L, Refai E, Appelskog I, et al. Apolipoprotein CIII promotes Ca2+-dependent β-cell death in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:10090–10094. doi: 10.1073/pnas.0403551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 35.Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmberg R, Refai E, Hoog A, et al. Lowering apolipoprotein CIII delays onset of type 1 diabetes. Proc Natl Acad Sci U S A. 2011;108:10685–10689. doi: 10.1073/pnas.1019553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storling J, Juntti-Berggren L, Olivecrona G, et al. Apolipoprotein CIII reduces proinflammatory cytokine-induced apoptosis in rat pancreatic islets via the Akt prosurvival pathway. Endocrinology. 2011;152:3040–3048. doi: 10.1210/en.2010-1422. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk KW, Rensen PC, Voshol PJ, Havekes LM. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr Opin Lipidol. 2004;15:239–246. doi: 10.1097/00041433-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Duivenvoorden I, Teusink B, Rensen PC, Romijn JA, Havekes LM, Voshol PJ. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 2005;54:664–671. doi: 10.2337/diabetes.54.3.664. [DOI] [PubMed] [Google Scholar]
- 40.Kawakami A, Aikawa M, Nitta N, et al. Apolipoprotein CIII-induced THP-1 cell adhesion to endothelial cells involves pertussis toxin-sensitive G protein- and protein kinase C alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2007;27:219–225. doi: 10.1161/01.ATV.0000249620.68705.0d. [DOI] [PubMed] [Google Scholar]
- 41.Xu S, Laccotripe M, Huang X, et al. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J Lipid Res. 1997;38:1289–1298. [PubMed] [Google Scholar]
- 42.Shi Y, Yang G, Yu J, et al. Apolipoprotein CIII hyperactivates β-cell Cav1 channels by coactivating PKA and Src kinase. 48th EASD Annual Meeting. Berlin, Germany. Diabetologia. 2012;55:S203. [Google Scholar]
- 43.Joventino IP, Alves HG, Neves LC, et al. The microalga Spirulina platensis presents anti-inflammatory action as well as hypoglycemic and hypolipidemic properties in diabetic rats. J Complement Integr Med. 2012;9 doi: 10.1515/1553-3840.1534. Article 17 (doi: 10.1515/1553-3840.1534) [DOI] [PubMed] [Google Scholar]
- 44.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppieters K, Martinic MM, Kiosses WB, Amirian N, von Herrath M. A novel technique for the in vivo imaging of autoimmune diabetes development in the pancreas by two-photon microscopy. PLoS One. 2010;5:e15732. doi: 10.1371/journal.pone.0015732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 47.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, et al. Alpha cells secrete acetylcholine as a nonneuronal paracrine signal priming β-cell function in humans. Nat Med. 2011;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda O, Tateishi H, Higuchi Y, et al. Novel genetically-humanized mouse model established to evaluate efficacy of therapeutic agents to human interleukin-6 receptor. Sci Rep. 2013;3:1196. doi: 10.1038/srep01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmstock N, Gonzalez FJ, Baes M, Annaert P, Augustijns P. PXR/CYP3A4-humanized mice for studying drug-drug interactions involving intestinal P-glycoprotein. Mol Pharm. 2013;10:1056–1062. doi: 10.1021/mp300512r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leblond F, Davis S, Valdés P, Pogue B. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J Photochem Photobiol B. 2010;98:77–94. doi: 10.1016/j.jphotobiol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toso C, Vallee JP, Morel P, et al. Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transpl. 2008;8:701–706. doi: 10.1111/j.1600-6143.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 54.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 55.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch. 2003;446:553–558. doi: 10.1007/s00424-003-1097-9. [DOI] [PubMed] [Google Scholar]
- 57.Sabek O, Gaber MW, Wilson CM, et al. Imaging of human islet vascularization using a dorsal window model. Transplant Proc. 2010;42:2112–2114. doi: 10.1016/j.transproceed.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 58.Bertera S, Geng X, Tawadrous Z, et al. Body window-enabled in vivo multicolor imaging of transplanted mouse islets expressing an insulin-Timer fusion protein. Biotechniques. 2003;35:718–722. doi: 10.2144/03354st01. [DOI] [PubMed] [Google Scholar]
- 59.Leibiger IB, Caicedo A, Berggren PO. Non-invasive in vivo imaging of pancreatic beta-cell function and survival - a perspective. Acta Physiol (Oxf) 2011;204:178–85. doi: 10.1111/j.1748-1716.2011.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdulreda MH, Caicedo A, Berggren P-O. Transplantation into the anterior chamber of the eye for longitudinal, non-invasive in vivo imaging with single-cell resolution in real-time. J Vis Exp. 2013;10:e50466. doi: 10.3791/50466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nyqvist D, Speier S, Rodriguez-Diaz R, et al. Donor islet endothelial cells in pancreatic islet revascularization. Diabetes. 2011;60:2571–2577. doi: 10.2337/db10-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez-Diaz R, Speier S, Molano RD, et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci U S A. 2012;109:21456–21461. doi: 10.1073/pnas.1211659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dooremaal JC. Die entwickelung der in fremden grund versetzten lebenden gewebe. Albrecht von Graefes Archiv für Ophthalmologie. 1873;19:359–373. [Google Scholar]
- 64.Falck B. Site of production of oestrogen in the ovary of the rat. Nature. 1959;184(Suppl 14):1082. doi: 10.1038/1841082a0. [DOI] [PubMed] [Google Scholar]
- 65.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18:162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 2010;10:493–497. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson TA, Apte RS. Angiogenesis in eye disease: immunity gained or immunity lost? Semin Immunopathol. 2008;30:111–119. doi: 10.1007/s00281-008-0113-8. [DOI] [PubMed] [Google Scholar]
- 68.Ksander B, Mammolenti M, Streilein J. Termination of immune privilege in the anterior chamber of the eye when tumor-infiltrating lymphocytes acquire cytolytic function. Transplantation. 1991;52:128–133. doi: 10.1097/00007890-199107000-00026. [DOI] [PubMed] [Google Scholar]
- 69.Faleo G, Abdulreda MH, Molano RD, et al. In vivo non-invasive imaging of the immunopathology of type 1 diabetes in NOD mice.. Keystone Symposium, Advances in Islet Biology; Monterey, California. 2012.p. 72. [Google Scholar]
- 70.Abdulreda MH, Miska J, Devarajan P, et al. Cell-cell contact in target tissues and immune damage versus protection. American Diabetes Association 73rd Scientific Sessions. Diabetes. 2013:A-2653. [Google Scholar]
- 71.Perez VL, Caicedo A, Berman DM, et al. The anterior chamber of the eye as a clinical transplantation site for the treatment of diabetes: a study in a baboon model of diabetes. Diabetologia. 2011;54:1121–1126. doi: 10.1007/s00125-011-2091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Streilein J. Anterior chamber associated immune deviation: the privilege of immunity in the eye. Surv Ophthalmol. 1990;35:67–73. doi: 10.1016/0039-6257(90)90048-z. [DOI] [PubMed] [Google Scholar]
- 73.Adeghate E, Donath T. Morphological findings in long-term pancreatic tissue transplants in the anterior eye chamber of rats. Pancreas. 1990;5:298–305. doi: 10.1097/00006676-199005000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Hahn S, Gehri R, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 75.Abdulreda MH, Molano RD, Molina J, et al. American Diabetes Association 70th Scientific Sessions. Diabetes: a Journal of the American Diabetes Association; Orlando, Florida: 2010. In vivo kinetics and dynamics of macrophages in pancreatic islet allografts. p. A360. [Google Scholar]