Abstract
Background
Older patients with atrial fibrillation (AF) and coronary artery disease (CAD) face high risk of stroke and bleeding with antithrombotic therapy. Balancing safe and effective use of aspirin, clopidogrel, and warfarin in this population is important.
Methods
From the Duke Databank for Cardiovascular Disease, we identified patients with AF ≥65 years old with angiographically confirmed CAD from 2000 to 2010. Antithrombotic use was described across age and Congestive heart failure, Hypertension, Age >75 years, Diabetes, prior Stroke/transient ischemic attack (CHADS2) stroke risk and Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) bleeding scores. Death and the composite of death, myocardial infarction, and stroke by antithrombotic strategy were reported.
Results
Of 2,122 patients ≥65 years old with AF and CAD, 477 (22.5%) were ≥80 years old; 1,133 (53.4%) had acute coronary syndromes. Overall rates of aspirin, clopidogrel, and warfarin use were 83.4%, 34.6%, and 38.9%, respectively. Compared with patients 65 to 79 years old, more patients ≥80 years old were at high stroke risk (CHADS2 ≥2, 84.7% vs 57.8%) and high bleeding risk (ATRIA 5-10, 55.8% vs 23.3%). Warfarin use in both age groups increased with higher CHADS2 scores and decreased with higher ATRIA scores. Of patients ≥80 years old with CHADS2 ≥2, 150 (38.2%) received warfarin. Antithrombotic strategy was not associated with improved 1-year adjusted outcomes.
Conclusions
Among older patients with AF and CAD, overall warfarin use was low. Patients ≥80 years old at highest stroke risk received warfarin in similar proportions to the overall cohort. Further investigation into optimizing antithrombotic strategies in this population is warranted.
Atrial fibrillation (AF) and coronary artery disease (CAD) are prevalent with increasing age, and antithrombotic therapy is indicated for the treatment of both diseases. According to American Heart Association/American College of Cardiology/European Society of Cardiology guidelines, oral anticoagulation is a class IA recommendation in patients with AF at moderate to high risk of stroke.1 However, recommendations for antithrombotic treatment of patients with combined AF and CAD are less clear and differ between North America and Europe, for example, with respect to duration of oral anticoagulation plus dual antiplatelet therapy after drug-eluting stent implantation in AF patients with low/moderate bleeding but increased stent thrombosis/stroke risk.1-4
Clinicians are therefore faced with the dilemma of choosing the most appropriate antithrombotic regimen for patients with both AF and CAD. This is particularly challenging among older patients in whom the risks of stroke and bleeding are greater. Oral anticoagulation is more effective than antiplatelet therapy in reducing the risk of thromboembolic events associated with AF,5,6 but antiplatelet agents are preferred in patients with CAD as well as those receiving percutaneous coronary intervention (PCI).7 Unfortunately, combinations of aspirin, clopidogrel, and warfarin all increase the risk of bleeding.8
To facilitate selection of anticoagulation in patients with AF, risk scores have been developed to predict ischemic and bleeding risk.9-11 Previous work in patients with AF and acute coronary syndromes (ACS) has shown that use of warfarin is unrelated to or inversely related to risk of stroke or bleeding determined from these risk scores.12,13 Although older age has been postulated to explain part of this observed risk-treatment paradox, the role of risk assessment in selection of treatment for coexisting AF and CAD in older patients and the effect of antithrombotic therapy on outcomes in this population have not been well studied. We therefore examined antithrombotic strategies stratified by age and according to Congestive heart failure, Hypertension, Age >75 years, Diabetes, prior Stroke/transient ischemic attack (CHADS2) stroke risk and Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) bleeding scores and their association with clinical outcomes in older patients with AF and CAD.
Methods
Data were from the Duke Databank for Cardiovascular Disease (DDCD), a database of patients who have undergone cardiac catheterization and/or cardiac surgery at Duke University Medical Center. Patients are considered to have obstructive CAD and are included in the DDCD if they have ≥1 coronary lesion ≥50%. These patients have routine follow-up for myocardial infarction (MI), stroke, coronary revascularization procedures, rehospitalization, mortality, and medication use at 6 months, 1 year, and annually thereafter. Patients included in our analysis met the following criteria: age ≥65 years, diagnosis of obstructive CAD during cardiac catheterization between 2000 and 2010, diagnosis of AF within 6 months before index catheterization, and survival to hospital discharge. Aspirin, clopidogrel, and warfarin use was considered from day of catheterization through 30 days postprocedure. Actual hospital discharge medications were not available; medication use the day of discharge was considered a proxy for discharge medication and was obtained through query of the Duke Decision Support Repository, an electronic medical records system. This study was approved by the Duke Institutional Review Board.
Outcomes
Primary outcomes identified from the DDCD were death and the composite end point of death, MI, or stroke at 1 year. Bleeding outcomes were not available.
Risk stratification and scoring
The CHADS2 scores were calculated by assigning 1 point each for history of congestive heart failure, hypertension, age >75 years, or diabetes mellitus, and 2 points for history of stroke or transient ischemic attack.9 The ATRIA scores were determined by assigning 3 points each for anemia (hemoglobin level <13 g/dL in men, <12 g/dL in women) or severe renal disease (glomerular filtration rate <30 mL/min or dialysis-dependent), 2 points for age ≥75 years, and 1 point each for hypertension or prior bleeding.10 Patients were grouped into low-risk (0-3 points), intermediate-risk (4 points), and high-risk (5-10 points) categories. Calculation of the Outpatient Bleeding Risk Index (OBRI) was based on risk factors of age ≥65 years, history of stroke, history of gastrointestinal bleed, and the presence of one or more of the following comorbid conditions: serum creatinine >1.5 mg/dL, recent hematocrit <30%, diabetes mellitus, or history of MI.11
Statistical analyses
Age was analyzed as a continuous variable, but for presentation purposes, the cohort was divided into patients aged 65 to 79 years versus ≥80 years old. Patient and treatment characteristics were described for the overall population and for each subgroup. Baseline demographic and clinical characteristics are presented as medians with 25th and 75th percentiles for continuous variables and frequencies and percentages for categorical variables. Aspirin, clopidogrel, and warfarin use was described according to age, ACS at presentation and PCI status, and algorithm-predicted risks of ischemic and bleeding events using CHADS2 and ATRIA, respectively. A sensitivity analysis was performed using OBRI instead of ATRIA. Adjusted event rates according to antithrombotic treatment were calculated; these rates were based on the average risk of the cohort through estimation of event rates from all patients using multivariate Cox regression models developed for each end point and adjusted for baseline demographic and clinical characteristics listed in Table I. Ejection fraction was excluded from models due to >15% missing data. In-hospital events were excluded, and hospital discharge was considered “time 0.” Treatment and outcome relationships were evaluated with Wald χ2 tests. Before modeling, linearity assumptions for relationships between variables and outcomes were assessed using cubic polynomial spline plots of log hazard ratios for variable versus outcome. Linearity assumptions were satisfied with variable transformations. Violations of proportionality assumptions were evaluated with adjusted time-to-event plots with a treatment stratum. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). P < .05 was used to determine statistical significance.
Table I.
Patient characteristics and treatment by age
| Variable | Overall (N = 2122) | 65-79 y (n = 1645) | ≥80 y (n = 477) |
|---|---|---|---|
| Demographics | |||
| Median age (y) (25th, 75th) | 74.0 (69.0, 79.0) | 72.0 (68.0, 76.0) | 83.0 (81.0, 85.0) |
| Female sex, n (%) | 918 (43.3) | 667 (40.5) | 251 (52.6) |
| Race, n (%)* | |||
| White | 1760 (83.9) | 1368 (84.0) | 394 (83.7) |
| Black | 265 (12.6) | 200 (12.3) | 65 (13.8) |
| Native American | 55 (2.6) | 44 (2.7) | 11 (2.3) |
| Other | 18 (0.9) | 17 (1.0) | 1 (0.2) |
| Medical history, n (%) | |||
| Hypertension | 1531 (72.1) | 1183 (71.9) | 348 (73.0) |
| Hyperlipidemia | 1109 (52.3) | 871 (52.9) | 238 (49.9) |
| Smoking | 806 (38.0) | 658 (40.0) | 148 (31.0) |
| Diabetes | 529 (24.9) | 433 (26.3) | 96 (20.1) |
| CHF* | 912 (44.0) | 707 (43.9) | 205 (44.2) |
| NYHA class | |||
| I | 39 (1.9) | 28 (1.7) | 11 (2.4) |
| II | 212 (10.2) | 180 (11.2) | 32 (6.9) |
| III | 454 (21.9) | 346 (21.5) | 108 (23.3) |
| IV | 207 (10.0) | 153 (9.5) | 54 (11.6) |
| Median ejection fraction (%) (25th, 75th) | 55.1 (41.7, 64.5) | 56.0 (42.0, 64.7) | 52.9 (40.7, 63.4) |
| MI | 646 (30.4) | 477 (29.0) | 169 (35.4) |
| PCI | 252 (11.9) | 211 (12.8) | 41 (8.6) |
| CABG | 494 (23.3) | 379 (23.0) | 115 (24.1) |
| Cerebrovascular disease | 319 (15.0) | 235 (14.3) | 84 (17.6) |
| Stroke | 85 (4.0) | 64 (3.9) | 21 (4.4) |
| Peripheral vascular disease | 200 (9.4) | 154 (9.4) | 46 (9.6) |
| GI bleed | 85 (4.0) | 61 (3.7) | 24 (5.0) |
| Thyroid disease | 63 (3.0) | 47 (2.9) | 16 (3.4) |
| Presenting clinical characteristics | |||
| Median heart rate (beats/min) (25th, 75th) | 74.0 (63.0, 87.0) | 74.0 (63.0, 87.0) | 73.0 (64.0, 86.0) |
| Median BP (mm Hg) (25th, 75th) | |||
| Systolic | 142.0 (126.0, 160.0) | 141.0 (126.0, 159.0) | 143.0 (129.0, 164.5) |
| Diastolic | 77.0 (67.0, 87.0) | 78.0 (67.0, 88.0) | 77.0 (67.0, 85.0) |
| Median baseline GFR (mL/min) (25th, 75th) | 62.8 (49.7, 76.6) | 63.9 (51.3, 78.2) | 56.5 (46.0, 72.1) |
| Dialysis, n (%) | 38 (1.8) | 34 (2.1) | 4 (0.8) |
| Median hematocrit (%) (25th, 75th) | 38.0 (34.0, 42.0) | 39.0 (35.0, 42.0) | 37.0 (33.0, 41.0) |
| Stable CAD, n (%) | 989 (46.6) | 808 (49.1) | 181 (37.9) |
| Presenting with ACS, n (%) | 1133 (53.4) | 837 (50.9) | 296 (62.1) |
| MI | 383 (18.0) | 263 (16.0) | 121 (25.4) |
| Unstable angina | 750 (35.3) | 574 (34.9) | 175 (36.7) |
| No. of diseased vessels at catheterization, n (%) | |||
| 1 | 466 (22.0) | 363 (22.1) | 103 (21.6) |
| 2 | 350 (16.5) | 254 (15.4) | 96 (20.1) |
| 3 | 573 (27.0) | 425 (25.8) | 148 (31.0) |
| Median CHADS2 score (25th, 75th)* | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | 2.0 (2.0, 3.0) |
| 0, n (%) | 172 (8.3) | 172 (10.7) | 0 (0.0) |
| 1, n (%) | 578 (27.9) | 507 (31.5) | 71 (15.3) |
| ≥2, n (%) | 1324 (63.8) | 931 (57.8) | 393 (84.7) |
| Median ATRIA score (25th, 75th) | 3.0 (1.0, 5.0) | 3.0 (1.0, 4.0) | 5.0 (3.0, 6.0) |
| Low (0-3), n (%) | 1137 (53.6) | 948 (57.6) | 189 (39.6) |
| Intermediate (4), n (%) | 336 (15.8) | 314 (19.1) | 22 (4.6) |
| High (5-10), n (%) | 649 (30.6) | 383 (23.3) | 266 (55.8) |
| Treatment | |||
| PCI, n (%) | 551 (26.0) | 406 (24.7) | 145 (30.4) |
| BMS | 325 (15.3) | 228 (13.9) | 97 (20.3) |
| DES | 189 (8.9) | 146 (8.9) | 43 (9.0) |
| Other | 37 (1.7) | 32 (1.9) | 5 (1.0) |
| CABG, n (%) | 209 (9.8) | 176 (10.7) | 33 (6.9) |
25th, 75th indicates 25th and 75th percentiles, respectively. Abbreviations: CHF, Congestive heart failure; NYHA, New York Heart Association; GI, gastrointestinal; BP, blood pressure; GFR, glomerular filtration rate; BMS, bare-metal stent; DES, drug-eluting stent.
n ≠ 2,122 due to missing data.
No extramural funding was used to support this work, and the authors are solely responsible for the design and conduct of this study, study analyses, manuscript drafting and editing, and final manuscript contents.
Results
Patient characteristics and revascularization procedures
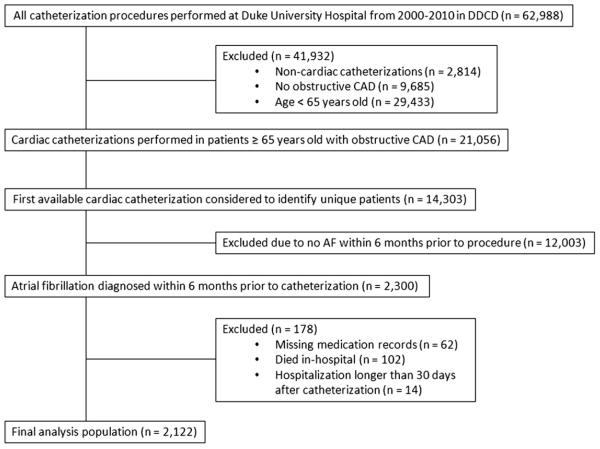
A total of 2,300 patients ≥65 years old with AF and CAD were identified. After we excluded patients with missing medication records (n = 62), hospitalization length of stay >30 days (n = 14), or in-hospital death (n = 102), 2,122 patients remained in the analysis population (Figure 1). Baseline patient characteristics and revascularization status by age are shown in Table I. Of the patients included in our analysis, 477 (22.5%) were ≥80 years old. Compared with patients 65 to 79 years old, patients ≥80 years old were more often female; more often had prior MI, cerebrovascular disease, stroke, and prior gastrointestinal bleed; and had a lower median left ventricular ejection fraction and glomerular filtration rate. Of the overall cohort, 1,133 patients (53.4%) presented with ACS; of these, MI at presentation was documented in 383 cases (33.8%). Patients ≥80 years old more frequently presented with ACS and MI and more often had multivessel CAD at cardiac catheterization than their younger counterparts. Based on the distribution of CHADS2 and ATRIA risk scores, patients ≥80 years old were at higher risk for both ischemic and bleeding events than patients 65 to 79 years old.
Figure 1.
Flow diagram of patient selection.
Coronary revascularization was performed in 35.7% of the overall study population; 26.0% received PCI, and 9.8% underwent coronary artery bypass graft surgery (CABG). Of all coronary revascularization procedures, 23.4% were performed in patients ≥80 years old. Patients 65 to 79 years old were more frequently referred for CABG, whereas patients ≥80 years old more frequently received PCI. Within the PCI cohort, the rate of bare-metal stent use was higher in patients ≥80 years old, whereas drug-eluting stent use was more frequent in younger patients.
Antithrombotic use
Antithrombotic medication use by age is shown in Table II. Overall rates of aspirin, clopidogrel, and warfarin use were 83.4%, 34.6%, and 38.9%, respectively. Combination therapy included use of dual antiplatelet therapy (aspirin and clopidogrel) in 26.7% of patients; warfarin plus antiplatelet therapy (aspirin, clopidogrel, or both) in 35.0% of patients; and combined warfarin, aspirin, and clopidogrel (“triple therapy”) in 7.2% of patients. Compared with patients 65 to 79 years old, those ≥80 years old were more commonly treated with antiplatelet therapy alone and received warfarin less frequently. Triple therapy use was higher in patients ≥80 years old than younger patients.
Table II.
Antithrombotic use by age
| Antithrombotic medication | Overall (N = 2122), n (%) | 65-79 y (n = 1645), n (%) | ≥80 y (n = 477), n (%) |
|---|---|---|---|
| No aspirin, clopidogrel, or warfarin | 254 (12.0) | 209 (12.7) | 45 (9.4) |
| Aspirin or clopidogrel alone* | 477 (22.5) | 372 (22.6) | 105 (22.0) |
| Aspirin and clopidogrel | 566 (26.7) | 415 (25.2) | 151 (31.7) |
| Warfarin alone | 83 (3.9) | 62 (3.8) | 21 (4.4) |
| Warfarin + (aspirin or clopidogrel)† | 590 (27.8) | 478 (29.1) | 112 (23.5) |
| Triple therapy | 152 (7.2) | 109 (6.6) | 43 (9.0) |
| Antiplatelet therapy alone‡,§ | 1043 (49.2) | 787 (47.8) | 256 (53.7) |
| Warfarin and antiplatelet therapy‡,§ | 742 (35.0) | 587 (35.7) | 155 (32.5) |
| Any aspirin§ | 1769 (83.4) | 1359 (82.6) | 410 (86.0) |
| Any clopidogrel§ | 734 (34.6) | 539 (32.8) | 195 (40.9) |
| Any warfarin§ | 825 (38.9) | 649 (39.5) | 176 (36.9) |
Clopidogrel alone used in 15 (0.7%) patients overall, 14 (0.9%) patients 65 to 79 years old, and 1 (0.2%) patient ≥80 years old.
Warfarin + clopidogrel used in 1 (0.0%) patient overall and 1 (0.1%) patient 65 to 79 years old.
Antiplatelet therapy defined as aspirin, clopidogrel, or both.
Categories within each division are not mutually exclusive.
Table III shows antithrombotic treatment stratified by ACS and PCI status. In contrast to the 12% of the overall population not discharged on aspirin, clopidogrel, or warfarin, 3.4% of patients presenting with ACS and none undergoing PCI were discharged without any of these medications. Almost all patients (97.6%) undergoing PCI received dual antiplatelet therapy, whereas 16.7% were treated with warfarin plus antiplatelet therapy.
Table III.
Antithrombotic use stratified by ACS and PCI status.
| Medications | ACS (n = 1133), n (%) |
PCI (n = 551), n (%) |
|---|---|---|
| None | 39 (3.4) | 0 (0.0) |
| Antiplatelet therapy alone* | 703 (62.1) | 459 (83.3) |
| Aspirin and clopidogrel | 519 (45.8) | 538 (97.6) |
| Warfarin only | 22 (1.9) | 0 (0.0) |
| Warfarin and antiplatelet therapy* | 369 (32.6) | 92 (16.7) |
Antiplatelet therapy defined as aspirin, clopidogrel, or both.
Antithrombotic use according to stroke and bleeding risk
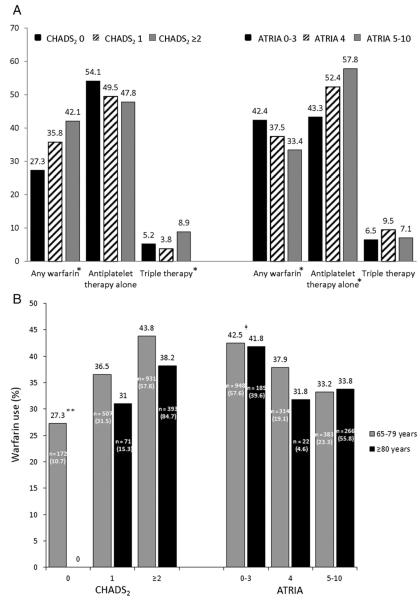
Distributions of CHADS2 and ATRIA scores are shown in Figure 2. As shown in Figure 3A, overall use of warfarin increased with higher CHADS2 scores and decreased with higher ATRIA scores. Treatment with antiplatelet therapy alone decreased with higher CHADS2 scores and increased with higher ATRIA scores. Use of triple therapy increased with higher CHADS2 scores but was unrelated to bleeding risk. Sensitivity analyses using the OBRI bleeding score produced similar results.
Figure 2.
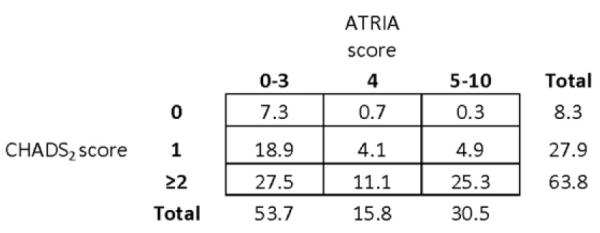
Overall distributions of CHADS2 and ATRIA scores. Shown are the distributions of stroke risk according to CHADS2 and bleeding risk according to ATRIA scores. The numbers in each box represent the percentage of patients in the total study population with the given CHADS2 and ATRIA scores.
Figure 3.
Antithrombotic use according to stroke and bleeding risk. A, The percentage of patients treated with warfarin, antiplatelet therapy alone (aspirin, clopidogrel, or both), and triple therapy (warfarin, aspirin, and clopidogrel) according to CHADS2 and ATRIA scores. B, Warfarin use according to CHADS2 and ATRIA scores in patients aged 65 to 79 years versus patients aged ≥80 years. Within each bar are the number of patients (n) in a given risk category and age group and the percentage of the overall age subgroup that this n represents.*P < .001; **P < .0001; ‡P < .01 for trends across corresponding categories.
Figure 3B shows warfarin use according to stroke and bleeding risk in patients 65 to 79 versus ≥80 years old. In both groups, warfarin use was concordant with risk of stroke and bleeding, increasing with higher CHADS2 scores and decreasing with higher ATRIA scores. Among patients with CHADS2 ≥2, warfarin was less frequently prescribed to patients ≥80 years old than those 65 to 79 years old (38.2% vs 43.8%). Warfarin use among patients with the highest ATRIA scores of 5 to 10 was similar in both groups (33.8% vs 33.2%).
Antithrombotic therapy and outcomes
There were 278 deaths at 1 year. Of these, 154 patients (14.8% of 1,043) were on antiplatelet therapy alone, 11 patients (13.3% of 83) were on warfarin only, and 96 patients (12.9% of 742) were on warfarin and antiplatelet therapy. There were 365 death/MI/stroke composite events at 1 year. Of these, 204 patients were on antiplatelet therapy alone (19.6% of 1,043), 13 patients were on warfarin only (15.7% of 83), and 125 patients were on warfarin and antiplatelet therapy (16.8% of 742). Comparisons of treatment with aspirin, clopidogrel, or both versus warfarin alone showed no difference in 1-year mortality (adjusted estimated event rate 14.5% vs 14.7%, adjusted hazard ratio [HR] 1.05, 95% CI 0.53-2.08, P = .89) or death/MI/stroke (18.2% vs 19.7%, HR 0.94, 95% CI 0.51-1.75, P = .85). For patients taking warfarin plus antiplatelet therapy compared with warfarin alone, no difference was observed for 1-year mortality (12.9% vs 14.7%, HR 0.90, 95% CI 0.46-1.76, P = .76) or death/MI/stroke (17.7% vs 19.7%, HR 0.92, 95% CI 0.50-1.70, P = .78). There were no significant interactions among treatment, age, and outcomes.
Discussion
The choice of antithrombotic therapy for patients with AF and CAD involves careful consideration of efficacy and safety, especially in older patients at greater risk for thromboembolic and bleeding events. We examined antithrombotic therapy use according to risk of stroke and bleeding in >2,000 patients ≥65 years old with both AF and CAD. Slightly over one-third of these patients received oral anticoagulation. Use of warfarin was concordant with patients’ risk, increasing with higher stroke risk and decreasing with higher bleeding risk; conversely, use of antiplatelet therapy decreased with increasing stroke risk and increased with higher bleeding risk. Among patients ≥80 years old with CHADS2 ≥2, the group at highest risk for stroke, the rate of warfarin use was <40% and was similar to the overall cohort of patients, suggesting that there seems to be no further selection of warfarin use in older patients at highest risk of stroke. There was no association between antithrombotic treatment strategy and improved outcomes.
Antithrombotic therapy in patients with AF and CAD
Although guidelines advocate use of oral anticoagulation for stroke prophylaxis in moderate to high risk patients with AF, this recommendation is not always followed. Despite a 64% reduction in stroke relative to placebo,5 warfarin is consistently underused in eligible populations, including older patients with AF and patients presenting with ACS complicated by AF.12-14 We demonstrated a similar trend in older patients with AF and CAD: 91.7% of patients were at moderate to high risk for stroke, yet only 38.9% were prescribed warfarin at discharge. Of note, the CHADS2 plus vascular disease, age 65-74, and female sex (CHA2DS2-VASc) score has been shown to better identify truly low stroke risk patients compared with CHADS2; however, it was of limited value in this analysis, as >96% of our population had CHA2DS2-VASc scores ≥2.15
Multiple reasons may have contributed to this low use of oral anticoagulation. The narrow therapeutic window of warfarin and associated bleeding risk may deter patients and physicians from using warfarin.16,17 Treatment decisions may have been deferred to the outpatient setting or delayed by in-hospital bleeding complications or surgical revascularization, and discharge medications may have been incompletely captured; this may explain the 12% of patients not discharged on antithrombotic therapy. Prior work has shown that physicians treating patients with multiple illnesses may focus on one and overlook others.18 This phenomenon could have contributed to the lower use of anticoagulation. Ultimately, during the period of our study, clear antithrombotic guidelines for patients with AF and CAD were lacking; uncertainty regarding the optimal therapeutic risk-benefit ratio and variations in treatment strategy are thus to be expected. Importantly, consensus recommendations have recently been published and advocate limited durations of triple therapy after stent implantation, regardless of bleeding risk,19 which may increase the low (16.7%) use of warfarin plus antiplatelet therapy in PCI patients observed in our analysis.
Atrial fibrillation patients with acute versus chronic CAD
Although the low rate of warfarin use in our population is consistent with prior studies, we found discrepant results regarding patterns of warfarin use according to stroke and bleeding risk. Previous studies of patients with AF and CAD have demonstrated conflicting trends in antithrombotic use: Data from the ST-segment elevation MI population showed a risk-treatment paradox, whereby warfarin use decreased as stroke risk increased,12 whereas warfarin use in the non–ST-segment elevation MI population has been unrelated to risk of stroke and has varied with respect to bleeding risk.13,20 Older age has been suggested as an explanation for these trends in warfarin use. We demonstrated that warfarin use in relation to stroke risk and bleeding risk was similar in patients 65 to 79 years old and those ≥80 years old.
Our findings may be accounted for by differences in study populations. Previous studies included patients with AF presenting with ACS and/or receiving PCI, populations who may have higher bleeding risk from more aggressive use of antithrombotic therapy and procedural complications. In contrast, we focused on antithrombotic treatment of AF and CAD in older patients and included a stable CAD population.
Antithrombotic use in patients ≥80 years old
Despite prior findings of an annual stroke risk of 23.5% in patients with AF 80 to 89 years old,21 >60% of patients ≥80 years old with CHADS2 ≥2 in our cohort did not receive warfarin. As risk factors for stroke and bleeding overlap, lack of warfarin use in this high stroke risk population may reflect concerns about bleeding. Bleeding with warfarin is more common in the first year of treatment in patients with AF ≥80 years compared with 65 to 79 years old,17 and older patients are at higher risk for bleeding after falls. However, the absolute benefit of warfarin in thromboembolic protection also increases with age,5,22 and fall risk may be overweighted in AF treatment decisions.16 Furthermore, physicians may favor platelet inhibitors over oral anticoagulation in older patients due to a perceived lower bleeding risk,23 although data from clinical trials show similar bleeding rates for aspirin plus clopidogrel versus warfarin alone.7 Our findings illustrate the need for increased awareness of potential benefits of anticoagulation in patients with AF with CAD, especially those at highest risk of stroke.
Clinical outcomes
Regarding outcomes, prior studies of patients with AF with ACS have found a clinical benefit of warfarin versus placebo and warfarin plus aspirin versus aspirin alone.12,24 In our cohort, no difference in end points was seen with the addition of antiplatelet therapy to warfarin, but our study was underpowered to detect incremental associations of antiplatelet therapy plus warfarin with clinical outcomes. Given the availability of new antithrombotic agents, future studies examining the effect of treatment on outcomes are warranted.
Study strengths and limitations
This is a large cohort of older patients with AF and angiographically confirmed CAD. However, relationships between antithrombotic treatment and outcomes in this observational study may be confounded, and minor violations of proportional hazards assumptions were found. Given low event rates in certain subgroups, our study was underpowered to detect modest differences in outcomes. Patients without medication records were excluded, and a proxy for discharge medications was used, which may have overestimated the 12% of patients not discharged on aspirin, clopidogrel, or warfarin. We also lacked data regarding both bleeding events, which precluded a net clinical benefit analysis, and AF and treatment duration; medication changes could have confounded our results. Despite data suggesting that Hypertension, Abnormal renal/liver function, Stroke, Bleeding history/predisposition, Labile international normalized ratio, Elderly (HAS-BLED) has greater predictive value for bleeding than ATRIA,25 limitations of data availability precluded full calculations and use of HAS-BLED. Finally, this study predates new AF and PCI treatment consensus guidelines, but our observations highlight both the overall low antithrombotic use in patients who might benefit and the influence of non–age-related factors on treatment selection that are still relevant for contemporary practice.
Conclusions
Treatment of coexisting AF and CAD in patients ≥65 years old is complicated due to competing risks of stroke and bleeding. Among these patients, overall use of warfarin was low (<40%) and was similar in patients ≥80 years old at highest stroke risk. Further study of antithrombotic therapy and outcomes for older patients with AF and CAD is needed.
Acknowledgement
The authors thank Peter J. Hoffman for his editorial contribution to this article. Connie N. Hess was funded by NIH grant 5T32HL069749-09.
Footnotes
William G. Stevenson, MD served as guest editor for this article.
Disclosures This work was supported internally by the Duke Clinical Research Institute. Renato D. Lopes, Christopher B. Granger, Eric D. Peterson, Jonathan P. Piccini, L. Kristin Newby, Linda K. Shaw, Kenneth W. Mahaffey, and John H. Alexander have posted their conflict-of-interest information online at https://www.dcri.org/about-us/conflict-of-interest/. Karen P. Alexander, Connie N. Hess, and Samuel Broderick have no conflicts to report.
References
- 1.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Huber K, Andreotti F, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary—a consensus document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2010;31:1311–8. doi: 10.1093/eurheartj/ehq117. [DOI] [PubMed] [Google Scholar]
- 4.Huber K, Airaksinen KJ, Cuisset T, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: similarities and dissimilarities between North America and Europe. Thromb Haemost. 2011;106:569–71. doi: 10.1160/TH11-08-0602. [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVEW): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 7.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–71. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 8.Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–41. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 9.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 10.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 12.Lopes RD, Elliott LE, White HD, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J. 2009;30:2019–28. doi: 10.1093/eurheartj/ehp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes RD, Starr A, Pieper CF, et al. Warfarin use and outcomes in patients with atrial fibrillation complicating acute coronary syndromes. Am J Med. 2010;123:134–40. doi: 10.1016/j.amjmed.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 15.Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–9. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- 16.Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161:241–6. doi: 10.1016/j.ahj.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 18.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–20. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 19.Faxon DP, Eikelboom JW, Berger PB, et al. Consensus document: antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting. Thromb Haemost. 2011;106:572–84. doi: 10.1160/TH11-04-0262. [DOI] [PubMed] [Google Scholar]
- 20.Wang TY, Robinson LA, Ou FS, et al. Discharge antithrombotic strategies among patients with acute coronary syndrome previously on warfarin anticoagulation: physician practice in the CRUSADE registry. Am Heart J. 2008;155:361–8. doi: 10.1016/j.ahj.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 22.van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40:1410–6. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 23.Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians’ fears often unfounded. Arch Intern Med. 2003;163:1580–6. doi: 10.1001/archinte.163.13.1580. [DOI] [PubMed] [Google Scholar]
- 24.Rothberg MB, Celestin C, Fiore LD, et al. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241–50. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 25.Roldán V, Marín F, Fernández H, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a ‘real world’ anticoagulated atrial fibrillation population. Chest. doi: 10.1378/chest.12-0608. [published online ahead of print; posted June 21, 2012] doi: 10.1378/chest.12-0608. [DOI] [PubMed] [Google Scholar]