Abstract
Although the pathological role of the immune system in several metabolic disorders, including type 1 diabetes mellitus (T1DM) and Addison’s disease, has long been recognized and studied, only in the last decade has it become apparent that the immune system plays a broad and more subtle role in local and systemic metabolism. It is now apparent that the immune system monitors and responds to specific metabolic cues in both pathologic and non-pathologic settings through a set of processes dubbed immunometabolism. Expansion of adipose tissue mass, activation of lipolysis, eating a high fat diet and even non-shivering thermogenesis all lead to the recruitment and activation of immune cells in key metabolic tissues. The responses are complex and not completely defined, and indeed, as is typical of rapidly evolving research areas, there are some conflicting reports, especially related to the metabolic consequences of manipulation of immune function. However, what is clear is the consensus that metabolic processes, especially obesity and obesity-related complications, activate both the innate and adaptive arms of the immune system. Canonical immune processes consist of discrete steps: surveillance, recognition, effector action and resolution. Over the last decade evidence for each part of the immune response has been found at the intersection of the immune system with metabolism. Although evidence for immune surveillance and modulation of metabolism has been found in the liver, muscle, hypothalamus and pancreas, immune cell function has been most intensively studied and best understood in adipose tissue where studies continue to provide insights into the intersection of the metabolic and immune systems. Here we review the modulation of immune cell populations in adipose tissue and discuss regulatory processes implicated in controlling the interface between metabolism and immunologic function.
Keywords: Adipose tissue, obesity, macrophages, T cells, B cells, iNKT cells, eosinophils, mast cells, neutrophils, immunometabolism
Innate Immune Cell Populations in Adipose Tissue
The first direct evidence for a coherent immunometabolic system came from studies of adipose tissue in mice that varied in adiposity [1,2]. Expression profiling revealed that variations in adiposity were tightly associated with a macrophage transcription signature in adipose tissue. When examined by both histology and fluorescence activated cell sorting (FACS), it became apparent that with increasing adiposity macrophages accumulate in adipose tissue depots. In lean mice and humans, macrophages constitute ~ 5% of cells in adipose tissue depots, but in the most obese rodents and humans macrophages make up as much as 50% of the cells in a depot [1,3]. Not only do they increase in numbers with increasing mass but they are also reduced with long-term weight loss. With long-term weight loss the macrophage content of adipose tissue falls commensurate with the adiposity of the individual [4–6].
Adipose tissue macrophages (ATMs), originally identified in murine fat depots by the expression of the macrophage protein, F4/80 (EMR1), are the most abundant immune cell in adipose tissue, representing more than half of leukocytes in depots from lean and obese animals [1]. However, ATMs are not a uniform population of cells. Antigenically distinct ATMs can be distinguished based on expression of immune markers and anatomic location. The first antigenically distinct subpopulation identified was the ATMs that express the integrin CD11c/ITGAX [7,8]. In adipose tissue from lean individuals macrophages that do not express CD11c (CD11c-) predominate. With increasing adiposity both ATM populations increase but CD11c expressing ATMs (CD11c+) predominate. Thus, both CD11c+ and CD11c- ATMs increase with obesity but CD11c+ expansion is much greater than CD11c- cells [9]. Originally characterized as classically activated or M1 polarized, the CD11c+ are thought to contribute disproportionately to the inflammatory phenotype and metabolic dysfunction of adipose tissue from obese individuals. Consistent with M1 polarization of CD11c+ ATMs several groups have reported that these cells are negative for the markers of alternatively activated macrophages including CD206 (MRC1), Arginase 1 (ARG1) and CD301 (MGL1). However, this has not been consistently found in rodents and humans [9,10]. Indeed, the dichotomous classification of ATMs as either classically or alternatively activated probably is simplistic and does not fully capture both the diversity of ATMs and their function. ATMs have been further subdivided based on their expression of antigens, including Ly6C, CD34, CCR2, CX3CR1. The functional role and ontological relationships of most subpopulations have not been well established but this antigenic diversity of ATMs hints at diverse and varied functions for ATMs within adipose tissue.
In addition to quantitative changes and alterations in antigenically distinct populations, obesity alters the morphology and location of ATMs. In lean individuals, ATMs are small and interspersed among adipocytes, but with the onset of obesity they aggregate and accumulate lipid, and some form multinucleated cells [1,11,12]. The aggregates formed what have been termed crown-like structures and are seen surrounding large lipid droplets that have been postulated to represent the vestigial lipids droplet of dead adipocytes [13]. Another subset of ATMs has been found in intimate association with vasculature structures specifically in expanding adipose tissue and have been implicated in vascular remodeling associated with expansion of adipose tissue [14]. These three anatomically distinct subsets imply functional niches that have yet to be fully characterized.
Although increases in the macrophage population were first to be recognized, the metabolic state of adipose tissue modulates the size of nearly all immune cell populations studied to date. Among myeloid cells, macrophages represent the largest and best studied population in adipose tissue but other myeloid cell populations also respond to changes in the metabolic state of fat. Neutrophils are myeloid cells that typically have short half-lives and are the most numerous in and critical for the initial response to bacterial infections and injury [15,16]. As part of the first wave of cells to migrate to sites of acute infection and injury, they facilitate the recruitment of macrophages, dendritic cells and lymphocytes and thereby participate in the evolution of the immune response. In adipose tissue of lean animals, neutrophils represent a small fraction of the total immune cells (<1%). Nonetheless, when mice are challenged with a high fat diet there is a rapid increase in adipose tissue neutrophils so that they represent ~2 % of the non-adipocyte cells. Some reports suggest that the increase in response to high fat feeding is largely transient [16] while other provide evidence of a more chronic increase in resident ATNs [17]. Dendritic cells are the closest ontological cogeners of macrophages, yet are perhaps the least well characterized of adipose tissue myeloid cells. A recent study found that a CD11c+, F4/80dim population of murine adipose tissue cells, largely absent from lean animals, can effectively present antigen and induce proliferation in a mixed lymphocyte population, arguing that authentic dendritic cells also accumulate in adipose tissue of obese individuals [18].
Mast cells are classic mediators of allergic reactions [19] but recent studies have implicated them in autoimmune disorders and atherosclerosis [20–22]. In adipose tissue, mast cells are increased several fold in obese compared to lean individuals and like some ATMs are found in close association with vasculature in both rodents and humans [23]. Eosinophils are primary effectors of classic anti-helminth responses, and through production of Il-4 can alternatively activate macrophages [24]. In contrast to ATMs, dendritic cells, mast cells and neutrophils, obesity appears to reduce Il-4 producing eosinophils, and thereby skew the phenotype of macrophages and other Il-4 regulated cells in adipose tissue [24]. More recent studies suggest that adipose tissue eosinophils themselves are dependent upon innate lymphoid type 2 cells in adipose tissue, which produce of Il-5 and Il-13 [25].
While the anatomical and morphologic differences identify distinct populations of ATMs, very little is known about the anatomy and morphology of other myeloid cells. Indeed, studies that have commented on the location of myeloid cells have noted their clustering in the crown-like structures of obese adipose tissue, with little mention of their location in lean individuals. A more detailed anatomical catalogue has not yet been produced but might provide insights into the relationship among immune cells in adipose tissue.
Adaptive Immune Cell Populations in Adipose Tissue
While studies dating to the 1990s found that NKT cell populations are reduced in the livers of obese mice [26], only more recent work has focused on the dynamic changes that occur in lymphocyte populations in adipose tissue. Similar to the effects of obesity on most myeloid populations, obesity increases the total T cells [27] and B cells [28,29] in adipose tissue but reduces small subpopulations that have been implicated in suppressing adipose tissue inflammation.
After macrophages, CD3+ T cells are the most abundant immune cells in adipose tissue and in toto increase in response to increases in adiposity [27]. Both CD4+ and CD8+ T cells increase in adipose tissue obese mice [30–32]. However, in contrast to the increase conventional CD4+ Th1 effector T cells there is a marked reduction in Foxp+ regulatory CD4+ T cells (Tregs), cells critical for maintaining self tolerance and dampening Th1 and Th17 responses. Consistent with their role as a primary regulator of adipose tissue inflammation, their reduction during the development of obesity-induced inflammation precedes the accumulation of macrophage populations [33]. An unusual feature of T cell populations in adipose tissue is their limited T-cell receptor repertoire [32–34], arguing that a specific set of antigens drives T cell function in fat depots, especially in obesity. Consistent with this hypothesis, high fat feeding rapidly induces replication of Th1 CD4+ T cells in adipose tissue.
Invariant NKT cells are lymphocytes that express a semi-invariant T-cell receptor and proteins typical of NK cells. iNKT are distinguished from conventional T cells by their ability to recognize lipid and glycolipids presented in the MHC-like molecule CD1d and thus would seem to be ideal candidates to recognize and respond to changes in lipid homeostasis in adipose tissue. Obesity reduces iNKT cells in the liver but leads to a small increase in adipose tissue depots [35]. The expression of CD1d on ATMs and other antigen presenting cells may provide a signal for iNKT activation and accumulation in fat of obese rodents.
Compared to iNKT and T cells, B cells constitute a far smaller population of lymphocytes in adipose tissue, and they have been less well characterized. However, as with most immune cells, B cells accumulate in adipose tissue of obese mice [29]. Further subpopulations of B cells have not been further characterized, however a provocative finding that IgG transferred from diabetic mice would confer insulin resistance suggests that a subset of B cells produce antibodies that are pathologic in the development of type 2 diabetes [29]. These provocative studies await replication and detailed analysis.
Activators of Immune Recruitment and Expansion in Adipose Tissue
The intersection between the immune and metabolic systems has classically been studied in the context of autoimmune disorders, including type 1 diabetes, Addison’s disease and Hoshimoto’s thyroiditis. In each of these diseases a breakdown in immune tolerance leads to a T-cell driven process that largely targets a specific cell or tissue [36]. The loss of tolerance in these autoimmune disorders appears to be unrelated to the underlying metabolic function of the cells, i.e the immune destruction is due to the immunologic identity of the cells and not their endocrine function or metabolic state per se [37].
In contrast, the interface of systems in immunometabolism is driven by the metabolic state of cells and tissue, not a primary defect in immune function [38]. These dynamic responses of immune cell content to adipose tissue mass argue that the immune system both monitors and recognizes the size of adipose tissue depots. The molecular signals of adiposity that are recognized by the immune system, however, remain incompletely defined but lipids, hypoxia, adipocyte death/stress have each been propose to help regulate immune cell recruitment [12,13,30,39,40]. The regulation of the most intensively studied population, ATMs in both chronic and acute metabolic states have provided insights as to the signals regulating immune cell recruitment.
A clue as to the nature of at least one signal, may come from studies of weight loss. Adiposity is not the only metabolic phenotype that modulates immune cell content of adipose tissue. Lipolysis and the release of free fatty acids by adipose tissue, whether induced by fasting or pharmacologic adrenergic activation, rapidly increases the immune cell content of adipose tissue [12,41]. Following an overnight fast the macrophage content of adipose tissue increases by ~ 20–30%. Activation of lipolysis by a β3-adrenergic agonist can increase by 3–4 fold the macrophage content of an adipose tissue depot within 24 hours. Genetic deletion of the key neutral lipase (ATGL/PNPLA2), which regulates triglyceride hydrolysis in adipocytes, prevents acute recruitment of macrophages to adipose tissue and argues that lipolysis or byproduct of lipolysis is being monitored by the immune system [12].
Obesity, to the degree that it increases insulin resistance, also increases the rate of basal lipolysis and the flux of non-esterified fatty acids out of adipose tissue. Indeed, a better predictor of ATM content than adiposity in is insulin resistance [3]. Hence, increases in the local concentration of NEFA or another product of lipolysis may be recognized by the immune system through innate immune system’s “danger signal” surveillance mechanisms. Indeed, toll-like receptors can bind saturated fatty acids and activate macrophage cell lines and bone marrow derived cells [42]. Genetic and pharmacologic manipulations of adipocyte lipolysis in obesity will test this hypothesis directly.
Non-shivering thermogenesis depends in part on uncoupled oxidative metabolism of fatty acids in brown adipose tissue. During a cold challenge the release of fatty acids from white adipose tissue depots depends upon adrenergic stimulation of lipolysis. Recent studies have found that cold challenges increase the number of alternatively activated adipose tissue macrophages and in an Il-4 receptor dependent manner these ATMs produce catecholamines necessary for sustaining thermogenesis. Similar to fasting and weight loss induced CD11c- ATM accumulation the acute increase in alternatively activated ATMs during a cold challenge may be driven by and amplify adrenergic activation of lipolysis [43].
Although obesity is the best studied regulator of immune cells in adipose tissue, several studies have paradoxically demonstrated that non-autoimmune lipodystrophies also induce recruitment of macrophages to adipose tissue. In rodent models of congenital and inducible lipodystrophies reduced adipocyte numbers are associated with large increases in adipose tissue macrophages [44,45]. Similarly, in congenital and HIV-associated lipodystrophies there is a higher content of macrophages in adipose tissue [46]. It has been proposed that obesity also increases the rate of adipocyte death, and thus perhaps through the release of lipids, activate macrophage recruitment and accumulation in adipose tissue. As discussed above two large populations of macrophages can be identified based on CD11c expression. In obesity, acute lipolysis and lipodystrophies both populations are increased but with lipolysis/weight loss and lipodystrophies the ratio of CD11c+ to CD11c- ATMs is lower than seen in obesity, arguing that there may be further differences in the processes, either qualitatively e.g. different signals, or quantitatively, e.g. acute vs chronic [44].
Tissue remodeling during development and vascularization employs macrophages in uptake of extracellular material and orchestrating aspects of endothelial and parenchymal development. Tissue hypoxia which in particular is a strong activator of neovascularization programs, and has been extensively studied in cancer biology, depends in part on macrophage function [47]. Consistent with a role for hypoxia in driving macrophage and immune cell recruitment, data from obese mice suggests that with the onset of obesity oxygen tension decreases in adipose tissue depots [39,40]. This decrease has been reported to activate in a HIF1a dependent manner chemokine expression in adipose tissue that can recruit macrophages and other immune cells.
MHC I and MCH II are the primary complexes through which adaptive immune system surveillance occurs. MHC I are expressed in nearly all nucleated cells and present to lymphocytes cytosolic proteins, providing a mechanism by which lymphocytes can monitor the presence of cytosolic proteins derived from intracellular pathogens. MCH II complexes are primarily expressed on professional antigen presenting cells, especially dendritic cells, macrophages and B-cells, and provide a mechanism for surveillance of the endocytic pathway for fragments of foreign proteins.
A recent study found that in mice obesity induces expression of MHC-II molecules by adipocytes [48]. These studies suggest that obesity induces adipocytes to express MHC II and through a leptin dependent mechanism leads to T-cell activation and subsequent recruitment of inflammatory ATMs. Consistent with MHC-II playing a role the surveillance of metabolic function animals deficient in MHC-II have a marked reduction in obesity-induced adipose tissue immune cell accumulation suggesting a model in which obesity turns adipocytes into antigen presenting cells that can then be monitored by lymphocytes [48].
Further buttressing a model in which T-cells and antigen driven processes control the immune response in adipose tissue is the observation of the limited T-cell receptor (TCR) repertoire of Tregs isolated from adipose tissue. Unlike Tregs in non-adipose tissue or conventional Tcells in adipose tissue, adipose tissue Treg TCR repertoire was remarkably redundant. These data suggest that a fat-specific antigen may be critical in maintaining a low inflammatory state in lean individuals [49]. This, of course, raises the question as to what protein or carbohydrate antigens are being recognized.
Conclusions
Over the last decade the field of immunometabolism has emerged as a dynamic study of two interwoven aspects of physiology. Adipose tissue has served as a key point of intersection with studies of obesity provided the primary context for exploring the interactions and interconnections. We now know how increases in adiposity alter each of the main immune cell populations. For adipose tissue macrophages and increasingly adipose tissue T-cells, subpopulations have been identified, and importantly we are beginning to describe the how the immune cell populations interact with each other (Figure 1). During this decade a large number of studies in rodents have suggest direct mechanistic links between specific immune cells and their phenotype and systemic metabolic function, and await validation in human studies. While it is likely that not all of the proposed models linking immune cells to systemic metabolism will prove correct or relevant to human physiology and disease, immunometabolism does offer new therapeutic targets and strategies to treat metabolic disease.
Figure 1. Immune Cell Populations Adipose Tissue.
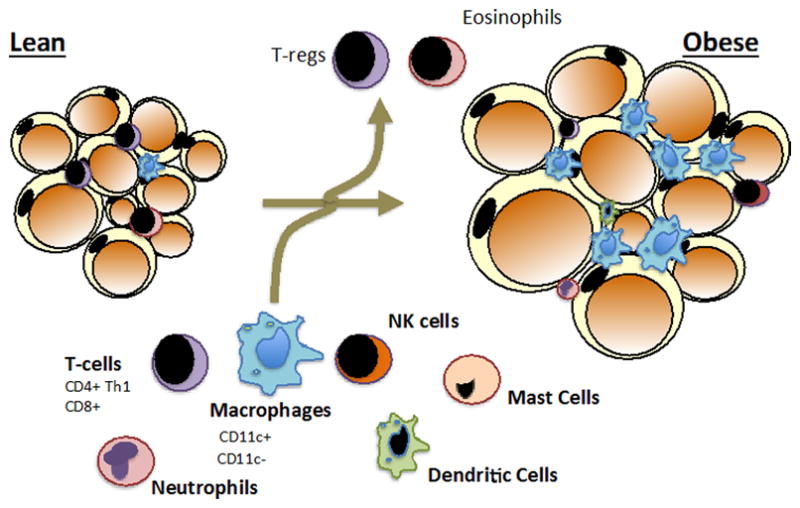
During the development of obesity adipocyte hypertrophy is accompanied by dynamic changes in immune cell populations. Nearly all immune cell study to date are quantitatively altered by obesity with most immune cells increasing in adipose tissue. The exceptions so far indentified include regulator T cells (Tregs) and eosinophils. Tregs and eosinophils have been implicated in attenuating inflammatory phenotypes of other adipose tissue immune cells, especially adipose tissue macrophages.
Acknowledgments
This work was supported by the NIDDK and a research grant from AstraZeneca.
Footnotes
The author declares no conflict of interest.
References
- 1.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortega Martinez de Victoria E, Xu X, Koska J, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 5.Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 6.Clement K, Viguerie N, Poitou C, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. Faseb J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 7.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 9.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosteli A, Sugaru E, Haemmerle G, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 15.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 20.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 22.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–822. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Divoux A, Sun J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 28.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Comm. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 31.Morris DL, Cho KW, Delproposto JL, et al. Adipose tissue macrophages function as antigen presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. doi: 10.2337/db12-1404. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 35.Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice lacking NKT cells but with a complete complement of CD8+ T-cells are not protected against the metabolic abnormalities of diet-induced obesity. PLoS One. 2011;6:e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy K. Janeway’s Immunobiology. New York: Garland Science; 2011. [Google Scholar]
- 37.La Torre D. Immunobiology of beta-cell destruction. Adv Exp Med Biol. 2012;771:194–218. doi: 10.1007/978-1-4614-5441-0_16. [DOI] [PubMed] [Google Scholar]
- 38.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333–342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 41.Capel F, Klimcakova E, Viguerie N, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58:1558–1567. doi: 10.2337/db09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci U S A. 2010;107:240–245. doi: 10.1073/pnas.0905310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 46.Sievers M, Walker UA, Sevastianova K, et al. Gene expression and immunohistochemistry in adipose tissue of HIV type 1-infected patients with nucleoside analogue reverse-transcriptase inhibitor-associated lipoatrophy. J Infect Dis. 2009;200:252–262. doi: 10.1086/599986. [DOI] [PubMed] [Google Scholar]
- 47.Squadrito ML, De Palma M. Macrophage regulation of tumor angiogenesis: implications for cancer therapy. Mol Aspects Med. 2011;32:123–145. doi: 10.1016/j.mam.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Deng T, Lyon CJ, Minze LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]


