Abstract
Background
Children with asthma have increased prevalence of food allergies. The relationship between food allergy and asthma morbidity is unclear.
Objective
We aimed to investigate the presence of food allergy as an independent risk factor for increased asthma morbidity using the School Inner-City Asthma (SICAS), a prospective study evaluating risk factors and asthma morbidity among urban children.
Methods
We prospectively surveyed 300 children from inner-city schools with physician-diagnosed asthma, followed by clinical evaluation. Food allergies were reported including symptoms experienced within one hour of food ingestion. Asthma morbidity, pulmonary function, and resource utilization were compared between children with food allergies and without.
Results
Seventy-three (24%) of 300 asthmatic children surveyed had physician- diagnosed food allergy, and 36 (12%) had multiple food allergies. Those with any food allergy independently had increased risk of hospitalization (OR: 2.35, 95% CI: 1.30–4.24, p=0.005), and use of controller medication (OR: 1.99, 95% CI: 1.06–3.74, p=0.03). Those with multiple food allergies also had an independently higher risk of hospitalization in the past year (OR: 4.10 95% CI: 1.47–11.45, p=0.007), asthma-related hospitalization (OR: 3.52, 95% CI: 1.12–11.03, p=0.03), controller medication use (OR: 2.38 95% CI: 1.00–5.66, p=0.05), and more provider visits (median 4.5 versus 3.0, p=0.008). Furthermore, lung function was significantly lower (% predicted FEV1 and FEV1/FVC ratios) in both food allergy category groups.
Conclusions
Food allergy is highly prevalent in inner-city school-aged children with asthma. Children with food allergies have increased asthma morbidity and health resource utilization with decreased lung function, and this association is stronger in those with multiple food allergies.
Keywords: asthma, food allergy, hospitalization, morbidity, prevalence, resource utilization, risk
INTRODUCTION
Asthma affects approximately 18.7 million adults and 7 million children in the United States.1, 2 Asthma prevalence is also higher among children than adults, and among racial minorities.3 In 2008, asthma accounted for an estimated 10.5 million lost school days in children.4 Food allergy prevalence in children is also increased; with an 18% increase in reported food allergy in children under 18 from 1997 to 2007.5 Studies in atopic children with food allergies have shown a higher risk for asthma development, and severe food allergy has been associated with greater odds of having a diagnosis of asthma.6–8 Children with food allergies and asthma are at increased risk of food- induced anaphylaxis and death.9, 10 The interrelationship between food allergy and asthma morbidity, however, is not well understood. Recently, a general US population study demonstrated an association between food allergy and severe asthma exacerbations.11 Less is known regarding the presence of food allergy as an independent risk factor for increased asthma morbidity in children, particularly among poor, urban environments. In our study, we aimed to investigate the relation between food allergy and asthma morbidity, in urban students with asthma. Our a priori hypothesis was that a current diagnosis of food allergy would be a significant predictor of increased asthma morbidity in students with asthma in our school-based inner-city asthma cohort.
METHODS
In this analysis, three hundred subjects with physician-diagnosed asthma were enrolled from 2008 to 2011 in the School Inner-City Asthma Study (SICAS), a prospective study of urban school-aged students in the northeast. The comprehensive study design and methods for SICAS are previously published.12 Briefly, students with asthma were screened and recruited from urban elementary schools. Eligibility requirements were modeled after other National Inner-City asthma studies13 including physician-diagnosed asthma along with the presence of wheezing, current use of controller medicines for asthma, or unscheduled physician or health care visits for asthma in the 12 months prior to screening. Exclusion criteria included significant pulmonary disease other than asthma, significant cardiovascular disease requiring daily medication, use of beta-blocker medication, and an inability to complete study procedures. After obtaining informed consent, trained interviewers administered an extensive baseline survey to the child’s caregiver that included questions regarding demographics, past medical history, and detailed sections regarding asthma morbidity and food allergy. The baseline survey was modeled after validated questionnaires from other inner-city asthma studies.13 Asthma morbidity outcomes included clinical symptoms, effect on activities of daily living, effect on parent or other caregiver, resource utilization, and lung function measurements. The food allergy section included comprehensive symptoms experienced within one hour of food ingestion for reported foods. Our definition of IgE-mediated food allergy was consistent with other studies14, 15 and included cutaneous (pruritis, urticaria, edema), gastrointestinal (mouth/throat itching, abdominal pain, vomiting), respiratory (throat tightness, cough, dyspnea, wheezing), and cardiovascular (hypotension, syncope) symptoms. Subjects were not considered to have food allergy if reported symptoms after food ingestion were not consistent with IgE-mediated food allergy. Pulmonary function testing (Koko spirometer, nSpire Health, Inc., Longmont, CO, USA) was performed according to ATS guidelines16. All lung function results were analyzed by a study physician to ensure fulfillment of acceptable criteria. The study was approved by the Boston Children’s Hospital and Brigham and Women’s Hospital Institutional Review Board.
Statistical Methods
The univariate analysis of the relation between food allergy variables and outcome measures employed chi-squared analysis for categorical variables and independent t test or Wilcoxon rank-sum test for continuous variables, depending on evidence for or against normal distributions, respectively. Stepwise logistic regression was used to select multivariate models, while adjusting for potential confounders and examining interactions. Variables included in the final models satisfied a change-in-estimate criterion [‡10% in the odds ratio (OR) or beta coefficient estimate] or were significant at the p <0.05 level. All analyses were conducted using SAS v9.2 (Cary, NC: SAS Institute Inc).
RESULTS
Characteristics of the SICAS participants
Descriptive characteristics of study participants are presented in table 1. Three hundred elementary school subjects were enrolled from June 2008 to November 2011. Fifty percent of subjects were male, with a mean age of 7.9 years and an age range of 5 to 13 years. Thirty-six percent of subjects were black, 34% hispanic, 18% mixed, and less than 5% were white. In this impoverished, primarily non-white cohort, 47% of subjects reporting income had an annual household income less than $25,000, and 71% reported income less than $45,000. There was a family history of asthma in 80% of subjects, and a diagnosis of eczema in 50%. Over 20% of subjects had frequent rescue medication use (≥2 times a week in past 4 weeks). One-quarter of subjects had parents/caregivers who rated their child’s asthma control as poor. Over one-third of subjects were exposed to at least one family member who smoked at home.
TABLE I.
Descriptive characteristics of study participants
| Characteristic | Count |
|---|---|
| Subjects | 300 |
| Male | 151 (50.3%) |
| Mean age (range) | 7.9 (5–13) |
| Race | |
| • White | 14 (4.7%) |
| • Black | 108 (36.0%) |
| • Hispanic | 103 (34.3%) |
| • Mixed | 53 (17.7%) |
| • Other a | 22 (7.4%) |
| Annual household income b | |
| • < $25,000 | 118 (47.0%) |
| • $25,000 – $44,999 | 60 (24.0%) |
| • > $45,000 | 73 (29.2%) |
| Family history of asthma | 239 (79.7%) |
| Diagnosis of eczema | 145 (48.5%) |
| Diagnosis of food allergy | 73 (24.3%) |
| Home tobacco smoke exposurec | 105 (35.0%) |
| Mean FEV1, % predictedd | 101.8% (SD 19.3) |
| Mean FEV1/FVCd | 0.87 (SD 0.07) |
| Dyspneae | 36 (12.3%) |
| Rescue medication usef | 64 (21.4%) |
| Parent-rated poor asthma controlg | 74 (25.3%) |
Includes Asian, Haitian, or Native American subjects
N=251, as 49 subjects chose not to report household income
Includes subjects with ≥1 family member who smokes at home
Spirometry data includes 258 subjects who met established criteria described in methods.
Shortness of breath ≥ 3 times a week in the past 4 weeks
Rescue inhaler use ≥2 times a week in the past 4 weeks
Parent-rated assessment of asthma control in the past 4 weeks
Food Allergy Characteristics
Seventy-three (24.3%) of 300 asthmatic subjects had physician-diagnosed food allergy. Figure 1 displays the percentage prevalence of individual food allergies. Of the 73 subjects with food allergy, peanut was the most prevalent (43.8%) followed by tree nuts (30.1%). Multiple food allergies were present in 49.3% of food-allergic subjects (n=36). We defined multiple food allergies as presence of food allergies from 2 distinct food groups, consistent with other studies.17 For example, a subject reporting allergy to milk and peanut would be classified as having multiple food allergies, whereas a subject reporting allergy to only several tree nuts would not meet criteria for multiple food allergies. Figure 2 displays the percentage prevalence of specific symptoms reported within one hour of food ingestion. Urticaria was most common (61%), followed by skin erythema (56.2%) and throat itching (45.2%). Respiratory symptoms were common, including dyspnea (34.2%), cough (28.8%), and wheezing (30.1%). Abdominal pain (24.7%) and vomiting (23.3%) were also commonly reported.
Fig. 1.
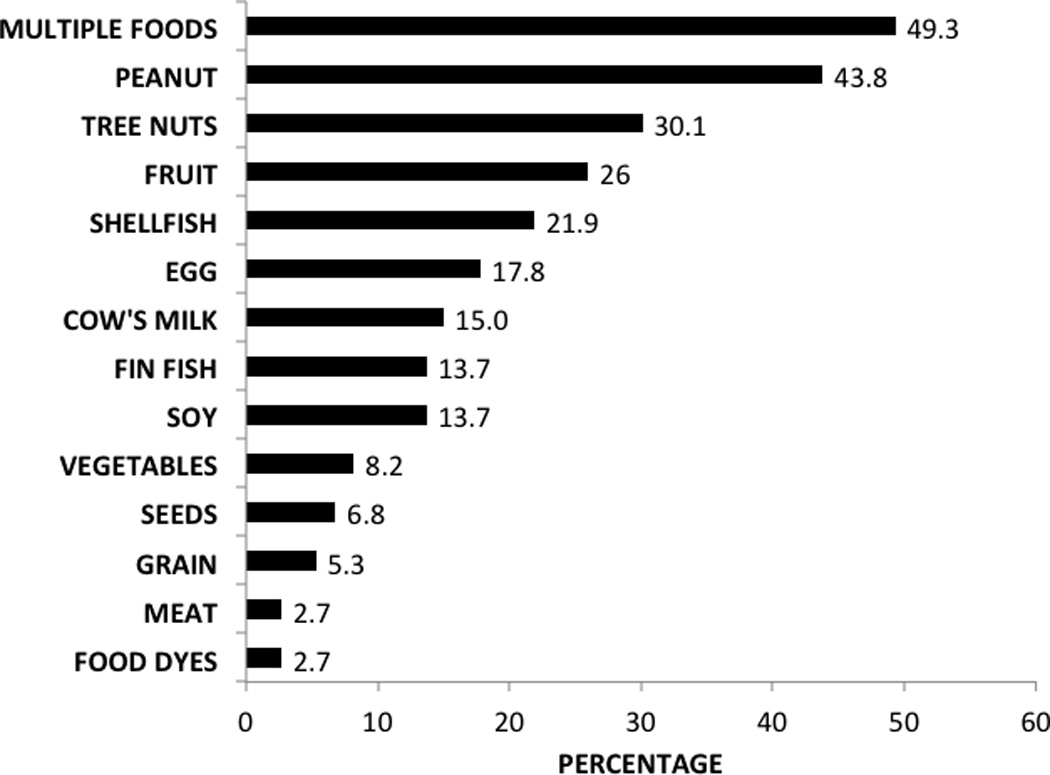
Percentage prevalence of food allergies (n=73)
* Includes subjects with food allergies from at least 2 above categories
Fig. 2.
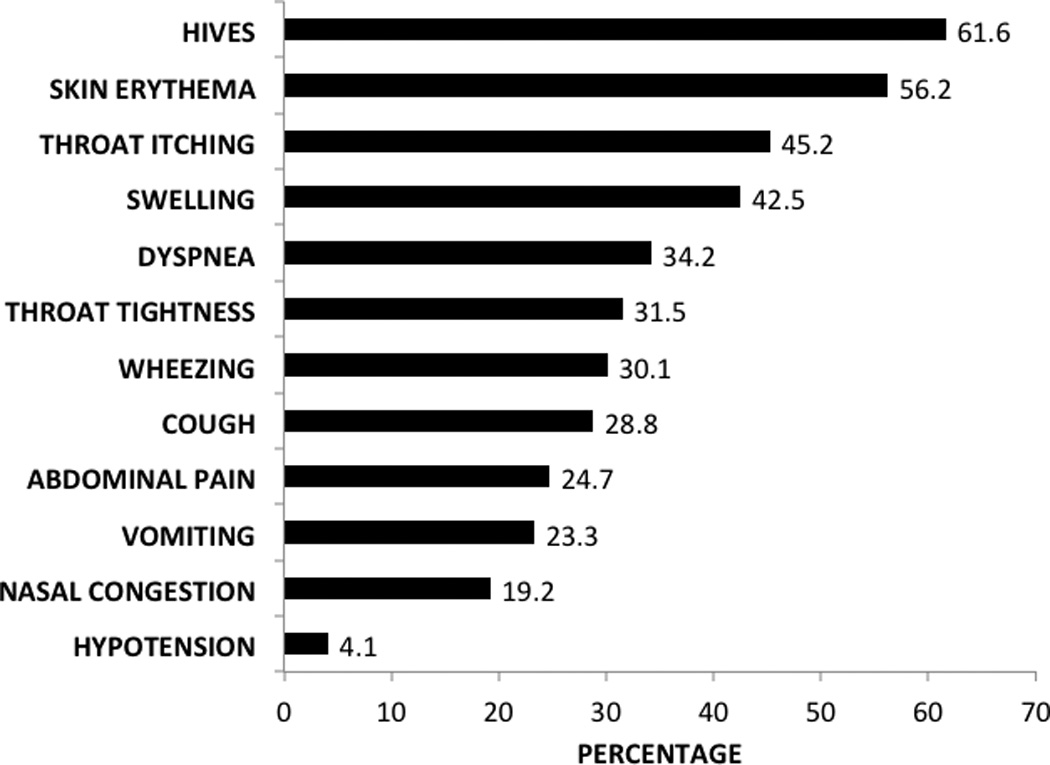
Symptoms experienced within 1 hour of food ingestion
Any Food Allergy and Asthma Morbidity
The association between food allergy and asthma morbidity outcomes is presented in tables 2 and 3. All results in the tables represent multivariable models, which included adjustments for age, gender, race, annual house hold income, tobacco smoke exposure, and physician diagnosis of eczema. Subjects with any food allergy had almost 2 times increased risk of daytime symptoms (≥3 days with wheeze and/or cough in the past 2 weeks) (p=0.047), over 2 times the risk of lifetime hospitalization (p=0.005), and weak associations with increased hospitalizations in the past 12 months (p=0.07). Asthmatic children with any food allergy had 2 times the risk of asthma controller use compared to those without food allergy (p=0.03). No significant association was seen with reported symptoms of dyspnea (shortness of breath ≥3 times a week in the past 4 weeks), parent-perceived asthma control, missed school days due to asthma, or unscheduled asthma visits to a provider. Percent-predicted FEV1 scores were lower in the food allergy group (mean 98.13% versus 102.99%, p=0.004). FEV1/FVC scores were also lower in the any food allergy group (mean 0.855 versus 0.870, p=0.01).
TABLE II.
Association between presence of food allergy and binary asthma morbidity outcomes
| Outcome | Any Food Allergy (n=73) |
No Food Allergy (n=227) |
Adjusted OR (CI) |
p Value | Multiple Food Allergy (N=36) |
No Food Allergy (N=227) |
Adjusted OR (CI) |
p Value |
|---|---|---|---|---|---|---|---|---|
| Increased daytime cough/wheezea | 32% | 21% | 1.95 (1.01–3.75) | 0.047 | 42% | 21% | 3.11 (1.38–7.00) | 0.006 |
| Dyspneab | 15% | 11% | 1.83 (0.76–4.41) | 0.18 | 14% | 11% | 1.44 (0.46–4.56) | 0.53 |
| Perceived poor asthma controlc | 27% | 25% | 1.38 (0.71–2.67) | 0.34 | 25% | 25% | 1.10 (0.46–2.64) | 0.84 |
| Any unscheduled asthma visits, past year | 42% | 44% | 0.77 (0.42–1.40) | 0.39 | 42% | 44% | 0.76 (0.35–1.64) | 0.48 |
| Hospitalization, lifetime | 62% | 40% | 2.35 (1.30–4.24) | 0.005 | 78% | 40% | 5.08 (2.13–12.18) | 0.0003 |
| Hospitalization past 12 months | 14% | 8% | 2.31 (0.94–5.72) | 0.07 | 22% | 8% | 4.10 (1.47–11.45) | 0.007 |
| Hospitalization for asthma, past 12 months | 10% | 7% | 1.91 (0.68–5.38) | 0.22 | 17% | 7% | 3.52 (1.12–11.03) | 0.03 |
| Asthma controller medication use | 74% | 58% | 1.99 (1.06–3.74) | 0.03 | 78% | 58% | 2.38 (1.00–5.66) | 0.050 |
Multivariate models were adjusted for age, race, gender, annual household income, tobacco smoke exposure, and history of eczema.
≥ 3 days with wheezing and/or cough in the past 2 weeks
Shortness of breath ≥ 3 times a week in the past 4 weeks
Parent-rated assessment of asthma control in the past 4 weeks
TABLE III.
Association between presence of food allergy and continuous asthma morbidity outcomes
| Outcome | Any Food Allergy (n=73) |
No Food Allergy (n=227) |
p Value | Multiple Food Allergy (n=36) |
No Food Allergy (n=227) |
p Value |
|---|---|---|---|---|---|---|
| No. of asthma-related missed school days in past year, median (IQR) | 4.0 (1.0–10.0) | 3.0 (0.0–7.0) | 0.21 | 5.0 (2.0–10.8) | 3.0 (0.0–7.0) | 0.10 |
| No. of visits to provider in past year, median (IQR) | 4.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.19 | 4.5 (3.0–6.0) | 3.0 (2.0–5.0) | 0.008 |
| FEV1, % predicted, mean (SD) | 98.13% (21.82) | 102.99% (18.36) | 0.004 | 97.13% (23.81) | 102.99% (18.36) | 0.0007 |
| FEV1/FVC, mean (SD) | 0.855 (0.0823) | 0.870 (0.0724) | 0.01 | 0.849 (0.103) | 0.870 (0.0724) | 0.01 |
| FEF25–75, % predicted, mean (SD) | 124.61% (168.36) | 112.99% (45.59) | 0.73 | 146.18% (235.36) | 112.99% (45.59) | 0.48 |
Multivariate models were adjusted for age, race, gender, annual household income, tobacco smoke exposure, and history of eczema.
IQR = interquartile range
SD = standard deviation
Multiple Food Allergies and Asthma Morbidity
Subjects with multiple food allergies had over 3 times increased risk of daytime symptoms (p=0.006). These subjects had over 5 times the risk of lifetime hospitalization (p=0.0003), over 4 times increased risk of hospitalization in the past 12 months (p=0.007) and over 3.5 times increased risk of asthma-related hospitalization in the past 12 months (p=0.03). Multiple food allergic subjects had over 2 times increased risk of asthma controller medication use (p=0.050). Number of unscheduled visits to a health care provider for asthma was significantly higher in the multiple food allergy group (median 4.5 versus 3.0, p=0.008). Lung function was lower in the multiple food allergy group (% predicted FEV1, mean, 97.13% vs. 102.99%, p=0.0007), as were FEV1/FVC scores (mean 0.849 vs. 0.870, p=0.01). There were no significant associations seen between subjects with multiple food allergies and reported symptoms of dyspnea, parent-perceived asthma control, unscheduled asthma visits to a provider in the past 12 months, or percent-predicted FEF25–75 values.
DISCUSSION
The goal of our study was to determine whether the presence of food allergy is associated with increased asthma morbidity as an independent risk factor in elementary students from a school-based, poor, urban, primarily non-white asthma cohort. This study provides evidence for increased asthma morbidity in children with any food allergy, and this risk was higher in students with multiple food allergies. We found that students with asthma and multiple food allergies have significantly increased daytime asthma symptoms, asthma controller medication use, asthma-related hospitalizations, unscheduled health visits to a provider, and decreased lung function. These independent associations remained when adjusting for other atopic disease including physician-diagnosed eczema, suggesting that concurrent food allergy diagnosis is an independent risk factor for increased asthma morbidity.
Peanut and tree nuts were the most prevalent food allergies in this school-aged cohort. The somewhat lower prevalence of cow’s milk and egg allergy may be indicative of our older, elementary school-aged cohort. The higher overall prevalence of food allergy seen in children with asthma in our study is consistent with other national studies. Data from the National Health and Nutrition Examination Survey (NHANES) demonstrated increased prevalence of food sensitization in children with asthma compared to those without.11 In 2005, Wang et al. investigated the effect of food allergen sensitization on asthma severity, using IgE measurements from serum samples obtained from the National Cooperative Inner City Asthma Study. Results showed 45% of patients sensitized to at least one food and 26% of asthmatic subjects with probable food allergy based on food allergen-specific IgE cutoffs. There was no report of clinical symptoms in this study. Wang’s group found that children sensitized to at least one food had higher rates of asthma hospitalizations and required more steroid medications, concluding that food allergen sensitization may be a marker for increased asthma severity.18 Our study concurs with these results, and also adds the component of current clinical symptoms upon ingestion. Given that sensitization alone does not necessarily correlate with clinical allergy, our findings in this poor, urban elementary school cohort provides further evidence of food allergy as a marker for increased asthma morbidity in vulnerable, at risk populations. Recently, Krogulska et al. demonstrated increased bronchial hyper responsiveness in children with food allergy independent of whether or not respiratory symptoms were present.19 Malmberg et al. demonstrated that compared with controls, children with a history of positive cow’s milk specific IgE showed signs of airway inflammation, including increased exhaled nitric oxide levels and more pronounced bronchial responsiveness to histamine at school age.20
While we found an association between food allergy and multiple measures of asthma morbidity, this does not imply a causal relationship, and was not the focus of our study. There is evidence for food-triggered asthma exacerbations, and Liu’s study from the NHANES investigation suggests that food allergy-triggered asthma exacerbations may be under-recognized.11 A recent study by Tepper et al. found that infants sensitized to milk or egg allergens had increased airway responsiveness to methacholine when compared with controls without food sensitization.21 Previously, Bock studied respiratory reactions induced by food challenges in children with pulmonary disease, finding that out of 410 children with a history of asthma, 279 (68%) reported food-induced asthma. The study revealed positive blinded food challenges in 60% of the 279 patients, with 24% of those subjects developing wheezing during the challenge.22 Wheezing was usually present along with other non-respiratory symptoms, however. More studies are needed to investigate the direct effect of food allergy on asthma exacerbations.
Our study revealed lower lung function in subjects with any or multiple food allergies, with significant differences seen in mean % predicted FEV1 scores and mean FEV1/FVC ratios. Small airway differences were not seen. Small airway disease is a current area of interest, particularly in asthmatic children with normal FEV1 and FEV1/FVC values. The lack of significant differences seen in mean % predicted FEF25–75 values in our study may relate to the variability seen in other studies using FEF25–75 measurements23.
Our study has several strengths, including a large sample of inner-city children with asthma, detailed survey phenotypic data including presence of physician-diagnosed food allergy and specific current symptoms elicited upon food ingestion. We acknowledge that food allergy was not confirmed with double-blind, placebo-controlled food challenges, the gold standard in food allergy diagnosis, which was beyond the scope and focus of this study. However, subjects did fulfill clinical criteria including reported symptoms experienced within one hour that were consistent with IgE-mediated food allergy, and methods modeled from other studies that give reasonable diagnosis based on clinical algorithms24–26. Our study suggests that understanding comorbid symptoms of food allergy and asthma morbidity should be further evaluated, particularly in a school-based setting. As in all survey studies, recall bias is possible. However, the addition of current reported symptoms of food allergy in association with current asthma morbidity is a strength. Our asthma definitions and outcomes were modeled after other NIAID funded inner-city asthma studies which focus on physician diagnosis and symptoms27–29. We recognize that doctor’s diagnosis of asthma and symptoms may reflect reporting bias. However, both parental reports of wheeze and doctor’s diagnosis of asthma have consistently been associated with lower lung function, skin test reactivity and airways responsiveness in pediatric epidemiologic asthma literature30. A parental report of doctor’s diagnosis is a standard definition in respiratory epidemiology, is based on a standardized questionnaire, and is used by the National Center for Health Statistics and other government agencies in surveys of the U.S. population. Physicians tend to under diagnose asthma in early childhood, and our results are likely conservative31.
Additionally, our study involved an urban, predominantly non-white elementary school-aged student population, and results may not be as generalizable to other asthmatic populations. However, this vulnerable group has been established to have significantly higher risk and morbidity and relationships in this group are not well understood. Perhaps future school surveillance studies evaluating food allergy and asthma comorbidity may be useful in furthering our understanding of two highly prevalent and allergic conditions affecting many poor, urban, youth.
CONCLUSIONS
In summary, we found increased prevalence of food allergies in poor, primarily non-white, inner-city school-aged students with asthma. We found independent risk factors of higher asthma morbidity in food-allergic subjects including increased daytime asthma symptoms, hospitalizations, controller medication use, health care utilization, and decreased lung function. Based upon these findings, it is reasonable that children with asthma and food allergies may require increased surveillance by their physicians and caretakers in order to optimize asthma control.
Highlight Box.
What is already known about this topic? Food allergy prevalence is increased in people with asthma. The relationship between food allergy and asthma morbidity is not well described.
What does this article add to our knowledge? The presence of food allergy and multiple food allergies is associated with increased asthma morbidity, resource utilization, and decreased lung function.
How does this study impact current management guidelines? Impoverished, urban, non-white children with both food allergies and asthma may require greater asthma surveillance than those with asthma alone.
Acknowledgments
Funding: Dr. James Friedlander is supported by grant T32 AI007512-25. The School Inner-City Asthma Study is supported by grants R01 AI 073964, R01 AI 073964-02S1 and K24 AI 106822 (PI: Wanda Phipatanakul, MD, MS). This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Institutes of Health Award UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
We thank Lincoln Diagnostics for the gracious support in providing allergy skin testing supplies, and Greer Inc, for the gracious support in allergen skin testing reagents.
Abbreviations
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- FEF25–75
Forced expiratory flow 25%–75%
REFERENCES
- 1.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat. 2010;10(249):1–207. [PubMed] [Google Scholar]
- 2.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat. 2010;10(247):1–82. [PubMed] [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 5.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 6.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder A, Kumar R, Pongracic JA, et al. Food allergy is associated with an increased risk of asthma. Clin Exp Allergy. 2009;39(2):261–70. doi: 10.1111/j.1365-2222.2008.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr Opin Allergy Clin Immunol. 2009;9(2):128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 10.Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003;112(1):168–174. doi: 10.1067/mai.2003.1569. [DOI] [PubMed] [Google Scholar]
- 11.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipatanakul W, Bailey A, Hoffman EB, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma. 2011;48(10):1007–1014. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell H, Senturia Y, Gergen P, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24(4):237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Wang J. Management of the patient with multiple food allergies. Curr Allergy Asthma Rep. 2010;10(4):271–277. doi: 10.1007/s11882-010-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Sampson HA. Food allergy. J Clin Invest. 2011;121(3):827–835. doi: 10.1172/JCI45434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Ahn SS, Sicherer SH. Prevalence of allergy to multiple versus single foods in a pediatric food allergy referral practice. J Allergy Clin Immunol. 2010;125:AB216. [Google Scholar]
- 18.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005;115(5):1076–1080. doi: 10.1016/j.jaci.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Krogulska A, Dynowski J, Wasowska-Krolikowska K. Bronchial reactivity in schoolchildren allergic to food. Ann Allergy Asthma Immunol. 2010;105(1):31–38. doi: 10.1016/j.anai.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Malmberg LP, Saarinen KM, Pelkonen AS, Savilahti E, Makela MJ. Cow's milk allergy as a predictor of bronchial hyperresponsiveness and airway inflammation at school age. Clin Exp Allergy. 2010;40(10):1491–1497. doi: 10.1111/j.1365-2222.2010.03567.x. [DOI] [PubMed] [Google Scholar]
- 21.Tepper RS, Llapur CJ, Jones MH, et al. Expired nitric oxide and airway reactivity in infants at risk for asthma. J Allergy Clin Immunol. 2008;122(4):760–765. doi: 10.1016/j.jaci.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bock SA. The incidence of severe adverse reactions to food in Colorado. J Allergy Clin Immunol. 1992;90(4 Pt 1):683–685. doi: 10.1016/0091-6749(92)90143-p. [DOI] [PubMed] [Google Scholar]
- 23.Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Sorkness CA. Classifying asthma severity in children mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170(4):426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 24.Gaffin JM, Sheehan WJ, Morrill J, et al. Tree nut allergy, egg allergy, and asthma in children. Clin Pediatr (Phila) 2011;50(2):133–139. doi: 10.1177/0009922810384720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stutius LM, Sheehan WJ, Rangsithienchai P, et al. Characterizing the relationship between sesame, coconut, and nut allergy in children. Pediatr Allergy Immunol. 2010;21(8):1114–1118. doi: 10.1111/j.1399-3038.2010.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta RS, Springston EE, Smith B, Pongracic J, Holl JL, Warrier MR. Parent report of physician diagnosis in pediatric food allergy. J Allergy Clin Immunol. 2013;131(1):150–156. doi: 10.1016/j.jaci.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell H, Senturia Y, Gergen P, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997;24(4):237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 29.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold DR, Wypij D, Wang X, et al. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med. 2004;149(5):1198–1208. doi: 10.1164/ajrccm.149.5.8173760. [DOI] [PubMed] [Google Scholar]
- 31.Weiss ST. Epidemiology and heterogeneity of asthma. Ann Allergy Asthma Immunol. 2001;87(1 Suppl 1):5–8. doi: 10.1016/s1081-1206(10)62188-6. [DOI] [PubMed] [Google Scholar]


