Abstract
Extinction is diagnosed when patients respond to a single contralesional item but fail to detect this item when an ipsilesional item is present concurrently. Extinction has been studied mainly in the visual modality but it occurs also in other sensory modalities (touch, audition) and hence can be considered a multisensory phenomenon. The functional and neuroanatomical relations between extinction in different modalities are poorly understood. Here, we used voxel-based mophometry (VBM) to examine the neuronal substrates of visual versus tactile extinction in a large group of sub-acute patients (n = 454) with strokes affecting different vascular territories. We found that extinction deficits in tactile and visual modalities were significantly correlated (r = 0.341; p < 0.01). Several lesions within the right hemisphere were linked to extinction including the inferior parietal lobule, the superior parietal lobule, the middle frontal and occipital gyri, while lesions involving the superior temporal gyrus, inferior temporal gyrus and putamen were associated with tactile extinction. Damage within the middle temporal gyrus and superior temporal sulcus was linked to both deficits. We conclude that extinction in different modalities emerges after damage to both common (supra-modal) and distinct (modality specific) brain regions, and that contrasting sites emerge after damage to different vascular territories. We discuss the implications for understanding extinction as a multisensory disorder.
Keywords: Visual extinction, Tactile extinction, Stroke, Voxel-based morphometry
Highlights
► Damage to supra-modal vs. modality specific regions causes multisensory extinction. ► Visual compared to tactile extinction is caused by more posterior and dorsal damage. ► The study indicates the potential of using clinical CT scans to conduct VBM.
1. Introduction
Extinction is a common cognitive disorder following unilateral brain damage, diagnosed when patients who can respond to a single contralesional item fail to detect this item when an ipsilesional item is present concurrently (Bender and Teuber, 1946; Wortis et al., 1948; Critchley, 1953). It can be considered to be a disorder of attention characterized by a striking bias for the ipsilesional stimulus at the expense of a contralesional item, due to the brain lesion unbalancing the competition for selection from the stimuli on each side of space (Duncan et al., 1997; Driver and Vuilleumier, 2001). Similarly to other spatial attention disorders such as unilateral neglect, extinction has been mainly reported following right hemisphere strokes and is usually thought to be asymmetrically associated with the damage within the right hemisphere (e.g., Becker and Karnath, 2007; Stone et al., 1993; though see Ogden, 1985, for an opposite finding). The higher incidence of both extinction and neglect after damage to the right hemisphere suggests that the right hemisphere is dominant for the distribution of attention within extrapersonal space (Kinsbourne, 1977, 1987; Weintraub and Mesulam, 1988).
Extinction has been studied most extensively in the visual modality but it occurs also in other sensory modalities (touch, audition, and olfaction) and hence can be considered a multisensory phenomenon (e.g. Bellas et al., 1988a,b; De Renzi et al., 1984; Deouell and Soroker, 2000; Hillis et al., 2006; Ladavas et al., 2001; Maravita et al., 2000; Vaishnavi et al., 2001). Not surprisingly extinction has been also reported cross-modally, for example between vision and touch (e.g., Maravita et al., 2000; Sarri et al., 2006). Taking these different aspects together, the syndrome provides a unique opportunity to study the neural substrates of multisensory perception and spatial awareness. However, the functional and neuroanatomical relations between extinction in different modalities are poorly understood. It may be hypothesized that extinction emerges in different modalities after damage to both common (attention specific) and distinct (modality specific) brain regions, each of which may be modulated by competition between stimuli for selection (see Duncan et al., 1997). The overlapping spatial deficits across modalities could represent a number of factors. For example it could mean that: i) space is represented at the multi-modal level (see Driver and Spence, 1998a,b), ii) that there is a generalized cross-hemisphere competition and mutual inhibition (see Kinsbourne, 1977; Vallar et al., 1994) and/or iii) an important role of a central system that selects for subsequent action between contra- and ipsilateral stimuli irrespective of their original modality (see Bickerton et al., 2011; Chechlacz et al., in press). These hypotheses are not mutually exclusive. To date there have been relatively few studies that have attempted to systematically assess the neuroanatomical basis of extinction within and across modalities (though see Chechlacz et al., in press; Hillis et al., 2006; Vallar et al., 1994) and thus there is a lack of understanding about which neural regions support modality-specific and which modality-general cognitive processes. Furthermore, there are some discrepancies across the reported results. In cases of tactile extinction, for example, deficits have been linked to damage to posterior frontal cortex and the middle temporal gyrus (Kaplan et al., 1995), to the inferior parietal cortex (Hillis et al., 2006) and the post-central gyrus and putamen (Chechlacz et al., in press). Visual extinction has been linked to visual association cortex (BA19, Hillis et al., 2006), the angular gyrus (Vossel et al., 2011), the temporo-parietal junction (TPJ; Karnath et al., 2003; Ticini et al., 2010), and to the superior temporal sulcus, middle occipital gyrus and insula (Chechlacz et al., in press). Cross-modal deficits have been associated with damage to the inferior parietal cortex (Hillis et al., 2006; Kaplan et al., 1995) and the temporo-parietal junction (TPJ; Chechlacz et al., in press).
Some of the above discrepancies may arise because (i) relatively small numbers of patients have been studied (e.g., Kaplan et al., 1995; Ticini et al., 2010), (ii) some studies have assessed patients at an acute stage (e.g., Hillis et al., 2006) while others have tested chronic patients (e.g., Chechlacz et al., in press), (iii) some studies have used all-or-none (e.g., Karnath et al., 2003; Ticini et al., 2010) and others continuous measures of extinction (e.g., Chechlacz et al., in press; Vossel et al., 2011), and (iv) some studies fail to control for the neglect symptoms that frequently coincide with extinction (e.g., Hillis et al., 2006). An additional important factor contributing to these inconsistencies may be the difference in the general vascular territories affected by the stroke in the patients examined, as this determines the location of the lesion. This highlights a weakness of uni-mass voxel-based approaches, which assume a one-to-one mapping between function and lesion tested through correlations. A problem is that, if there is only a relatively small number of one type of patients in the sample (e.g., in a stroke sample patients with lesions around the posterior cerebral artery [PCA] will typically be lower in number than patients with a lesion in the territory of the middle cerebral artery [MCA]), then an overall VBM analysis will typically fail to reliably detect an association. Strikingly, Mort et al. (2003) reported differences in the critical lesions associated with neglect following MCA and PCA strokes (linked respectively to the inferior parietal lobe and the parahippocampal region), but this has not been attempted yet in patients with extinction (Mort et al., 2003). By separating out patients with damage to different stroke territories, we may gain a finer-grained analysis of lesion-symptom mapping. To address these points the current study examined extinction symptoms in an unselected group of patients with damage affecting different vascular territories. We first report results across the overall sample to give a general overview of lesion-symptom relations before examining variations in the relations according to the stroke territory.
The neuronal substrates of visual and tactile extinction were assessed on the data from a large group of sub-acute stroke patients (n = 454). Our analysis concentrated on the effects of grey matter lesions, which provided a clear delineation of the relations between extinction in the two modalities. We acknowledge, however, that visuospatial deficits may also arise as a consequence of white matter disconnections (Bartolomeo et al., 2007; Chechlacz et al., 2010, in press; Doricchi and Tomaiuolo, 2003; He et al., 2007; Karnath et al., 2009; Thiebaut de Schotten et al., 2008). Interestingly our previous work using MRI data has demonstrated that white matter disconnections arising from damage along the superior longitudinal fasciculus were associated with both visual and tactile extinction, while the cortical substrates varied with the stimulus modality (Chechlacz et al., in press). This last conclusion was tested further here.
To assess visual and tactile extinction we used matched procedures, minimizing the chances that non-specific task effects confounded the results. As the data were collected as a part of a large clinical trial, we used a relatively simple measure of extinction (with a relatively low number of event repetitions) and we assessed brain integrity from CT scans routinely used in clinical practice. In a previous analysis of visual neglect we have shown that VBM analyses of CT scans, based on large patient numbers, can yield highly reliable and interpretable results replicating previous findings observed using MRI scans (Chechlacz et al., 2012). We employed here whole brain statistical analyses using voxel-based morphometry (VBM; Ashburner and Friston, 2000) to evaluate common structure–function relationships. The analyses treated the behavioural measurements as continuous variables rather than as categorical scores, which increases both the ability to tease apart the neural substrates of extinction in different modalities (visual vs. tactile) and the overall sensitivity for detecting brain–behaviour associations. All analyses controlled for potential confounding factors including the aetiology (the type of stroke: ischaemia or haemorrhage), lesion size, age, gender, handedness, time from stroke to scan, patient overall orientation, anosognosia and presence of neglect symptoms. The analyses included the entire patient sample independently of lesion location or specific symptoms, thus providing an internal control for non-specific stroke effects and non-specific attentional deficits, not linked to extinction. This also enabled us to avoid biasing the results based on a-priori neuroanatomical or behavioural assumptions and therefore enabling us to generalize the observed findings to the entire sampled population. Finally, we evaluated how the lesion-symptom mapping may vary depending on the vascular territory affected by the stroke.
Our results highlight both common (attention specific) and distinct (modality specific) neural mechanisms of extinction and are discussed in relation to the organization of brain networks for spatial attention.
2. Methods
2.1. Participants
All patients were recruited as part of a large clinical study, the BUCS project (Birmingham University Cognitive Screen, http://www.bucs.bham.ac.uk), drawn from participating stroke units across the West Midlands of the United Kingdom. We used visual inspection of individual scans, blind to the category of patients, to exclude data from patients who either had enlarged ventricles or poor quality CT scans, to prevent artifacts in the neuroimaging analyses. A total of 454 sub-acute stroke patients (240 males and 214 females; average age of 69.9 years, range 30 to 93 years; see Table 1 for full demographic and clinical data) were included. Within this group 215 patients had middle cerebral artery (MCA) stroke and 47 posterior cerebral artery (PCA) stroke. The remaining 192 patients had other types of strokes including the anterior cerebral artery (ACA) area, basal ganglia and thalamus (LSA and AChA territories strokes) as well as the cerebellum. The study included both patients who suffered ischaemic stroke (417 patients) and haemorrhagic stroke (37 patients). Behavioural data were only collected from patients who were physically stable, willing to perform the task and had a concentration span of at least 60 min (judged clinically). Clinical and demographic data were obtained from the patients' clinical files. All participants provided written informed consent in agreement with ethics protocols approved by the National NHS ethic committee and local NHS trusts.
Table 1.
Patient's details: clinical and demographic data (n = 454, all recruited stroke patients).
| Mean (or number) | Std Deviation | Minimum value* | Maximum value* | |
|---|---|---|---|---|
| Age in years | 69.9 | 13.7 | 30 | 93 |
| Sex (male/female) | 240/214 | N/A | N/A | N/A |
| Aetiology (ISCH/BL) | 417/37 | N/A | N/A | N/A |
| Aetiology (MCA/PCA/other) | 215/47/192 | N/A | N/A | N/A |
| Handedness (right/left) | 404/50 | N/A | N/A | N/A |
| Stroke — CT scan in days** | 4.5 | 10.5 | 0 | 61 |
| Stroke — BCoS in days** | 23.0 | 20.7 | 1 | 90 |
| Orient1 | 7.5 | 1.3 | 0(0) | 8(8) |
| Orient2 | 5.5 | 0.9 | 0(0) | 6(6) |
| Anosognosia —Orient3 | 2.9 | 0.4 | 1(0) | 3(3) |
| Left TE uni asymmetry | 0.2 | 0.8 | 0(0) | 4(4) |
| Right TE uni asymmetry | 0.1 | 0.6 | 0(0) | 4(4) |
| Left TE bilat asymmetry | 0.8 | 2.0 | 0(0) | 8(8) |
| Right TE bilat asymmetry | 0.4 | 1.4 | 0(0) | 8(8) |
| Left VE uni asymmetry | 0.2 | 0.8 | 0(0) | 4(4) |
| Right VE uni asymmetry | 0.2 | 0.8 | 0(0) | 4(4) |
| Left VE bilat asymmetry | 0.8 | 2.2 | 0(0) | 8(8) |
| Right VE bilat asymmetry | 0.4 | 1.6 | 0(0) | 8(8) |
| AKCT accuracy | 39.9 | 14.4 | 4(0) | 50(50) |
| AKCT/TA left deficits | 1.3 | 3.6 | 0(0) | 20(25) |
| AKCT/TA right deficits | 0.5 | 2.1 | 0(0) | 20(25) |
*Minimum and maximum scores as measured in the studied group of stroke patients, numbers in brackets indicated minimum and maximum score for a given test; ** Interval between stroke onset and CT scan or cognitive assessment based on BCoS; A/KCT, Apples or Key Cancellation test; the maximum achievable score in these tests is 50 (AKCT accuracy). The cut-off for total numbers of target omissions i.e. accuracy score is 40/50. Spatial neglect is determined by whether patients miss targets (keys or full apples) on the left or right side of the page (asymmetry score calculated based on left- vs. right-side errors, AKCT/TA asymmetry score for targets, keys or full apples, indicating either left or right deficits); BL, bleed (haemorrhagic stroke); ISCH, ischaemic stroke; N/A, not applicable; Orient1, orientation measure assessing personal information; Orient2, orientation measure assessing time and space awareness; TE and VE; tactile and visual extinction tests, both tests consists of 4 unilateral left, 4 unilateral right and 8 bilateral trials, unilateral (uni) and bilateral (bilat) asymmetry scores are calculated based on left- vs. right-side misses.
2.2. Behavioural measures
2.2.1. Cognitive profile
The neuropsychological testing took place in the sub-acute phase following stroke onset (< 3 months) and the average stroke-to-test interval was 23 days (± 20.7; with 93% of patients being tested within two months and 76% of patients being tested within 1 month). The cognitive profile of each patient was derived using the BCoS, a test instrument developed to screen patients for a range of cognitive problems following stroke onset (Humphreys et al., 2012). The BCoS is aphasia and neglect-friendly and within 1 h provides assessment based on 23 tests within 5 broad cognitive domains: Attention and Executive functions, Memory, Language, Praxis/Control and planning of action, and Mathematical/Number abilities. The BCoS was administered in hospital settings. In this study we were interested in extinction deficits and based our analysis on 2 sub-tests: the Visual Extinction and Tactile Extinction tasks (see below for details). In addition we used the BCoS measure of spatial neglect as a covariate (Key or Apples Cancellation Tests; see below for details). The BCoS also provides assessment of a patient's awareness of his or her general setting and circumstance (the orientation questions that assess knowledge of personal information, awareness of time, place, medical condition and anosognosia). Three orientation measures derived from the BCoS were used in the current study: 1) eight questions regarding knowledge of personal information (e.g. ‘what is your first name?’); 2) six questions on the orientation of the patient in time and space (e.g.‘where are you right now?’); and 3) three questions measuring awareness of the medical condition and of the patient's own body (‘can you show me your left/right hand’; ‘why are you here?’; “do you have any problems moving your arms or legs”). The last orientation measure was used in the analyses to control for the presence of anosognosia.
2.2.2. Visual and tactile extinction
The examiner sat approximately one meter opposite at the participant's midline. For the visual extinction task, the examiner raised his/her left and right index fingers on either side of his/her head, approximately 20 cm from the nose. The testing was administered by experienced examiners who tried to maintain a consistent distance between their fingers and each patient's eyes. The instructions were as follows: “Look at my nose. Don't move your eyes. I will move my finger either on my left hand, on my right hand or on both hands simultaneously. Please tell me or show me which side moved. Always keep looking at my nose”. For each trial the examiner moved one or two finger(s) with two brief bending movements. For the tactile extinction test following instructions were given: “Put your hands on your knees (or on the table/bed cover). Now, close your eyes. I will touch your hand, either your left hand, your right hand or both your hands simultaneously. Please tell me or show me which hand I touched. Always keep your eyes closed”. The patient sat straight and symmetrically (no crossed arms or legs) and, using his/her own hand, the examiner touched the patient's hand(s) by gently tapping twice on the dorsal surface. In both tests there was a maximum time allowance of 15 s for each trial and the testing was abandoned if the patient showed no response on the first three trials. These patients were not entered in the current study due to the missing data. Both extinction tasks consisted of 4 unilateral left, 4 unilateral right and 8 bilateral trials. For each patient we calculated left and right asymmetry scores on two item trials and on unilateral trials. We also calculated the left and right extinction index.
2.2.2.1. Extinction index
The difference in the asymmetry scores on bilateral vs. unilateral trials was assessed, to index any spatially selective drop in response to two stimuli relative to the response to one stimulus. This was done separately for both left- and right-side items. To do this we calculated an extinction index i.e. the unilateral asymmetry score multiplied by two minus the bilateral asymmetry score, taking into account the difference in the number of trials. The extinction index and the asymmetry score for both left- and right-side unilateral items were entered into the statistical models.
For descriptive purposes, each patient's behavioural performance was classified based on cut-offs drawn from the BCoS and estimated from the dataset from healthy controls (Humphreys et al., 2012). Patients were classed as having a clinical deficit on measures of visual and/or tactile extinction if their scores on the task fell outside the control norms taken from 70 healthy controls without history of brain lesion or any neurological disorders. The cut-off scores for both visual and tactile extinction tasks are as follows: unilateral trials (both left and right) < 4 impaired; left bilateral trials < 7 impaired; right bilateral participants younger than 74 years old < 8 impaired and participants older than 75 years old < 7 impaired.
2.2.3. Neglect assessment
Spatial neglect was assessed using either the Key Cancellation or the Apples Cancellation task (Bickerton et al., 2011; Chechlacz et al., 2010).
2.3. Neuroimaging assessment
Computed Tomography (CT) scans were acquired for all patients as part of their routine clinical assessment following stroke and hospital admission. The average time between the stroke and the CT scan was 4.5 days (± 10.5, with 89% of cases within a week). Within the recruited group of patients (n = 454), 131 had no visible lesions. For 53 patients the failure to observe a lesion might be because the CT scans were acquired within 24 h of the stroke (a time frame when stroke impact on tissue density may not be evident). The neuroimaging data were acquired using the following scanners: Siemens Sensation 16, GE Medical System LightSpeed 16 and LightSpeed Plus. The images covered the whole brain with an in-plane resolution of 0.5 × 0.5 mm2 and a slice thickness varying between 4 and 5 mm. In comparison to a typical MR structural scan (T1-wieghted images) that have a resolution of 1 × 1 × 1 mm (e.g., data used in our previous studies, Chechlacz et al., 2010, 2012), the CT scans have higher double in-plane resolution (along the x, y axes) but much poorer ventral-dorsal resolution (along the Z-axis).
2.4. Neuroimaging analyses
2.4.1. Image preprocessing
Before the preprocessing stage, the quality of all CT scans was assessed by eye and all bad quality data sets (head movement or other image artefacts) were removed. Subsequently, the remaining CT images were pre-processed using SPM8 (Statistical Parametric Mapping, Welcome Department of Cognitive Neurology, London UK). The images were first re-aligned manually along the anterior–posterior-commissural (ac-pc) axes and then normalized (Ashburner and Friston, 2003) to an in-house CT template. The normalization was predominantly based on skull shape and was designed to transform the images into MNI space to optimize the next segmentation step. In the next step we used the unified segmentation algorithm as implemented in SPM8 (Ashburner and Friston, 2005). In this unified model, the tissue class priors are encoded by de-formable tissue probability maps through an iterative segmentation and normalization procedures. The a-priori tissue class maps indicate the probability of finding expected signal sources of grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), fat, bone and air (i.e. six different tissues classes), at each voxel of the image. As the CT scans were acquired following stroke, to account for the presence of an abnormal tissue associated with stroke, we adapted here a similar approach to (Seghier et al., 2008) and included an additional, seventh tissue class. Specifically, in the additional probability map we assumed that in each grey or white matter voxel there was a10% chance of it having a different intensity and thus representing an abnormal tissue class. The 10% probability for an abnormal voxel within the grey and white matter was estimated based on the ratio between average lesion size (computed for 160 patients, Chechlacz et al., 2012) and the grey plus white matter voxels.1 In addition, we constrained the classification of GM and WM to each being based on a single Gaussian (normal) distribution, whilst two Gaussian distributions were used to model the intensities in the abnormal tissue class. This last procedure was used to account for any possible in-homogeneity of the abnormal tissue. CT images as opposed to MRI do not suffer from field bias due to field strength inhomogeneity, therefore we did not correct for that during pre-processing. Supplementary Fig. 1 provides examples of output of the modified unified segmentation of the CT scans. In the final step of image pre-processing the segmented GM and WM images were smoothed with a 12-mm FWHM Gaussian filter to accommodate the assumption of random field theory used in the statistical analysis (Worsley, 2003). Finally, the quality of the segmentation and normalization procedures was assessed for each patient, and images where the segmentation failed were removed from the analyses. The pre-processed GM images were further used in the analyses to determine voxel-by-voxel relationships between brain damage and visuospatial deficits (see below).
Supplementary Fig. 1.
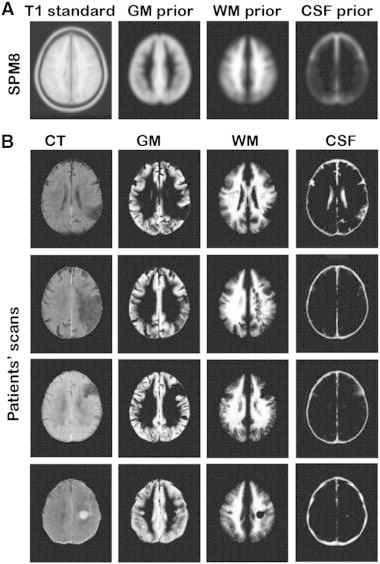
Modified unified segmentation. (A) T1 standard brain, GM (grey matter), WM (white matter) and CSF (cerebrospinal fluid) priors from SPM8. (B) Examples of output of the modified unified segmentation of patients' CT scans.
Before the preprocessing stage, the quality of all CT scans was assessed by eye and all bad quality data sets (head movement or other image artefacts) were removed. Subsequently, the remaining CT images were pre-processed using SPM8 (Statistical Parametric Mapping, Welcome Department of Cognitive Neurology, London UK). The images were first re-aligned manually along the anterior–posterior-commissural (ac-pc) axes and then normalized (Ashburner and Friston, 2003) to an in-house CT template. The normalization was predominantly based on skull shape and was designed to transform the images into MNI space to optimize the next segmentation step. In the next step we used the unified segmentation algorithm as implemented in SPM8 (Ashburner and Friston, 2005). In this unified model, the tissue class priors are encoded by de-formable tissue probability maps through an iterative segmentation and normalization procedures. The a-priori tissue class maps indicate the probability of finding expected signal sources of grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), fat, bone and air (i.e. six different tissues classes), at each voxel of the image. As the CT scans were acquired following stroke, to account for the presence of an abnormal tissue associated with stroke, we adapted here a similar approach to (Seghier et al., 2008) and included an additional, seventh tissue class. Specifically, in the additional probability map we assumed that in each grey or white matter voxel there was a10% chance of it having a different intensity and thus representing an abnormal tissue class. The 10% probability for an abnormal voxel within the grey and white matter was estimated based on the ratio between average lesion size (computed for 160 patients, Chechlacz et al., 2012) and the grey plus white matter voxels.1 In addition, we constrained the classification of GM and WM to each being based on a single Gaussian (normal) distribution, while two Gaussian distributions were used to model the intensities in the abnormal tissue class. This last procedure was used to account for any possible in-homogeneity of the abnormal tissue. CT images as opposed to MRI do not suffer from field bias due to field strength inhomogeneity, therefore we did not correct for that during pre-processing. Supplementary Fig. 1 provides examples of output of the modified unified segmentation of the CT scans. In the final step of image pre-processing the segmented GM and WM images were smoothed with a 12-mm FWHM Gaussian filter to accommodate the assumption of random field theory used in the statistical analysis (Worsley, 2003). Finally, the quality of the segmentation and normalization procedures was assessed for each patient, and images where the segmentation failed were removed from the analyses. The pre-processed GM images were further used in the analyses to determine voxel-by-voxel relationships between brain damage and visuospatial deficits (see below).
2.4.2. Voxel-based morphometry (VBM)
We used VBM (Ashburner and Friston, 2000) to assess the relationship between grey matter damage and visual as well as tactile extinction scores. (Please see the Supplementary material for analysis assessing the relationship between visual extinction and spatial neglect). The statistical analyses were carried out with SPM8 using smoothed GM maps and continuous behavioural scores. We used parametric statistics within the framework of the general linear model (Kiebel and Holmes, 2003).
All statistical models included both left and right extinction index scores as separate covariates. As the extinction deficits in the tactile and visual modalities were significantly correlated (left deficits, r = 0.34, p < 0.0001; right deficits, r = 0.20, p < 0.001), we examined common and distinct neuronal substrates of left and right visual versus tactile extinction in stepwise analyses. We used two models that included only either visual (Model 1) or tactile (Model 2) extinction measures as covariates of interest to report the individual effects in the absence of the other covariate of interest. To identify common regions involved in visual and tactile extinction, the statistical maps resulting from the above analyses were overlapped. Furthermore, we teased apart common and distinct neural substrates by examining how the individual effects of each covariate contributed to differences in the effect size based on a model including both visual and tactile extinction measures.
In all statistical models we included measures of other visuospatial problems: left and right asymmetry scores from the unilateral visual2 and tactile extinction trials and left and right spatial neglect. This enabled us to examine the neuronal substrates of extinction symptoms with effects of other visuospatial deficits, which may co-vary with extinction, eliminated. Additionally, to control for potentially confounding factors we included the following covariates in all analyses: age, gender, handedness, time from stroke to neuropsychological testing, time from stroke to scan, stroke etiology (ischaemia or haemorrhage) and 3 orientation measures (as described above). Finally, the size of the lesion visible on the CT scan (lesion volume) was rated for each patient from 0 to 5, where 0 means no visible lesion, 1 means small/precise lesion (up to 5 cm3), 2 means 1/4 of the hemisphere (up to 100 cm3), 3 means 1/2 of the hemisphere (up to 250 cm3), 4 means 2/3 to 3/4 of the hemisphere (up to 450 cm3) and 5 mean lesion encompassing entire hemisphere. As lesion size is a factor potentially contributing to the severity of any deficit, as well as a factor that varies with type of stroke, this information was then added as a covariate to all statistical models. To validate this rater-based categorical estimation of lesion size, we have computed the correlations between the assigned lesion-size category and the exact lesion volume computed for 160 stroke patients (see Chechlacz et al., 2012, for semi-automated lesion reconstruction method from CT scans). We found good reliability of the classification used here (r = 0.66 and p < 0.00001) based on the correlation between the scoring (0–5) and actual lesion volume from reconstructed lesion maps.
All analyses using the above models were carried out for the entire group of 454 patients (Analysis 1) and then separately for the following groups: (1) all patients with either MCA or PCA strokes (Analysis 2), (2) patients with only MCA strokes (Analysis 3), (3) patients with only PCA strokes (Analysis 4) and (4) all remaining patients after excluding MCA and PCA strokes (strokes other than affecting MCA and PCA vascular territories; Analysis 5).
We report only those results where there was a significant effect at p < 0.001 cluster-level corrected for multiple comparison with the amplitude of voxels surviving of p < 0.001 uncorrected across the whole brain and an extent threshold of 800 mm3 (> 100 voxels). The brain coordinates are presented in standardized MNI space. The anatomical localization of the lesion sites within the grey matter was based on the Anatomical Automatic Labeling toolbox (AAL toolbox, Tzourio-Mazoyer et al., 2002), the Duvernoy Human Brain Atlas (Duvernoy et al., 1991) and the Woolsey Brain Atlas (Woolsey et al., 2008).
3. Results
3.1. Behavioural findings
Table 1 presents a summary of the demographic and clinical data for all 454 patients, which corresponds to measures entered into statistical models used in VBM analyses, including performance on the Visual and Tactile Extinction tests. In this group, 161 (35.5%) had right hemisphere damage and 162 (35.7%) had left hemisphere damage clearly visible on CT scans, while 131 (28.8%) had no detectable lesion on CT scan.3 Out of 161 patients with right hemisphere damage, 45 patients showed left visual and 49 left tactile extinction, while out of the similar number (n = 162) of patients with left hemisphere damage, only 11 showed right visual and 26 right tactile extinction. Finally, among 131 patients with no detectable lesions, 12 showed left visual and 10 left tactile extinction, while 5 showed right visual and 9 right tactile extinction.
3.2. Neuroimaging findings
Our neuroimaging analyses focused on left extinction, in both the visual and tactile modalities. These symptoms were more prevalent than right side extinction (see above), and not surprisingly the results for right side extinction did not reach conventional levels of significance across our patients. We first carried out our analyses across the entire group of patients (n = 454), to give a general overview of the results, before contrasting the neural substrates of extinction for touch and vision following MCA (n = 215) and/or PCA (n = 47) strokes. We did not observe any brain regions significantly correlated with either visual or tactile extinction in the patients with strokes affecting vascular territories other than the MCA and PCA (these included ACA, LSA, AChA and cerebellum). No reliable neuroimaging findings in this group most likely resulted from large variability of lesion location and relatively small sample and symptom incidents.
3.2.1. Neural substrates of extinction across all patients
We found both common and dissociated neural substrates of visual and tactile extinction (Fig. 1A, B; Table 2). Across all the patients, lesions to the right IPL and the right middle frontal gyrus (MFG) were linked to left visual extinction, while lesions involving the right STG and the right inferior temporal gyrus (ITG) were associated with left tactile extinction. Damage within the right MTG and right STS was linked to both types of deficit (MNI coordinates 48–37 − 2; see Fig. 1A and B). In addition, lesions within the MTG extending posteriorly into the middle occipital gyrus (MOG) were related to visual but not tactile extinction.
Fig. 1.
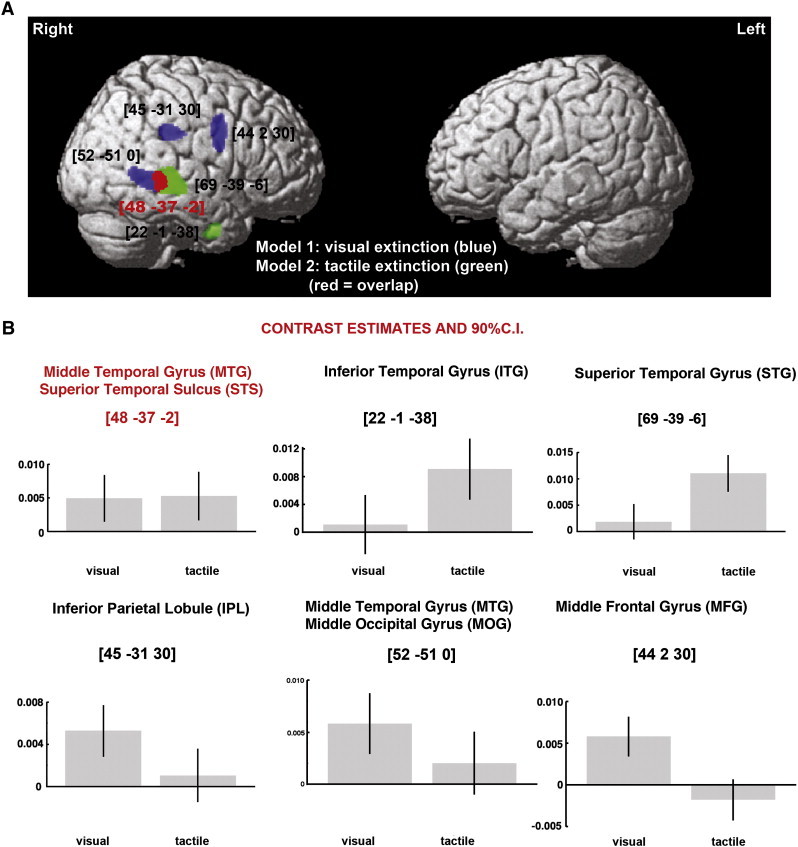
Grey matter substrates of left visual and left tactile extinction across the entire group of patients (VBM Analysis 1; n = 454). (A) The significant clusters identified in the VBM analyses, using either visual (blue) or tactile (green) extinction as covariates of interest, are plotted on a rendered brain. The red colour indicates the overlap between two statistical maps. (B) Individual effects of left visual and left tactile extinction corresponding to each peak voxel plotted to further illustrate common and dissociate neural substrates of extinction in the two examined modalities. The numbers in brackets indicate peak MNI coordinates.
Table 2.
Grey matter substrates of visual and tactile extinction (VBM Analysis 1: all patients).
| Model | Cluster level |
Voxel level |
Coordinates |
Brain structure (location) | |
|---|---|---|---|---|---|
| PFWE | Size | Z-score | X Y Z | ||
| Model 1: visual extinction | 0.000 | 341 | 4.00 | 45 − 31 30 | Right IPL (BA40) |
| 0.000 | 582 | 3.78 | 44 2 30 | Right MFG (BA6) | |
| 0.000 | 634 | 3.77 | 52 −51 0 | Right MTG (BA21) extending into STS and posteriorly to MOG (BA19/37) | |
| Model 2: tactile extinction | 0.000 | 1500 | 5.78 | 69 −39 −6 | Right STG (BA22) extending into STS |
| 0.000 | 163 | 3.85 | 22 −1 −38 | Right ITG (BA20) | |
Abbreviations: BA, Brodmann Area; IPL, inferior parietal lobule; ITG, inferior tempotal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus; STS, superior temporal sulcus; VBM, voxel-based morphometry.
3.2.2. Neural substrates of extinction following MCA and PCA strokes
We next repeated these analyses across all patients with lesions resulting only from MCA or PCA strokes and then separately for the patients with MCA and PCA strokes. Similarly, to previous analyses, across patients with both types of stroke, lesions to the right IPL and the right middle frontal gyrus (MFG) were linked to left visual extinction, while lesions involving the right STG and the right putamen were associated with left tactile extinction. Damage within the right MTG and right STS was linked to both types of deficit (MNI coordinates 50–44 -3; see Fig. 2A and B), but these lesions extended into the middle occipital gyrus (MOG) in patients with visual but not tactile extinction (Fig. 2A, B; Table 3).
Fig. 2.
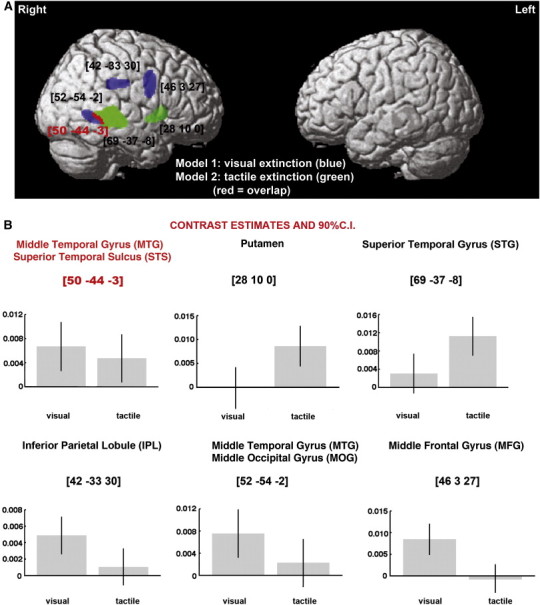
Grey matter substrates of left visual and left tactile extinction in strokes affecting only the MCA or PCA vascular territory (VBM Analysis 2; n = 262). (A) The significant clusters identified in VBM analyses using either visual (blue) or tactile (green) extinction as covariates of interest, are plotted on a rendered brain. The red colour indicates the overlap between two statistical maps. (B) Individual effects of left visual and left tactile extinction corresponding to each peak voxel plotted to further illustrate common and dissociate neural substrates of extinction in the two examined modalities. The numbers in brackets indicate peak MNI coordinates.
Table 3.
Grey matter substrates of visual and tactile extinction (VBM Analysis 2: MCA and PCA strokes).
| Model | Cluster level |
Voxel level |
Coordinates |
Brain structure (location) | |
|---|---|---|---|---|---|
| PFWE | Size | Z-scoret | X Y Zt | ||
| Model 1: visual extinction | 0.000 | 442 | 4.01 | 42 − 33 30 | Right IPL (BA40/39) |
| 0.000 | 903 | 3.90 | 46 3 27 | Right MFG (BA6) | |
| 0.000 | 277 | 3.60 | 52 − 54 − 2 | Right MTG (BA21) extending into STS and posteriorly to MOG (BA 19/37) | |
| Model 2: tactile extinction | 0.000 | 2309 | 5.09 | 69 − 37 − 8 | Right STG (BA22) extending into STS |
| 0.000 | 369 | 3.53 | 28 10 0 | Right putamen | |
Abbreviations: BA, Brodmann Area; IPL, inferior parietal lobule; ITG, inferior tempotal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; STG, superior temporal gyrus; STS, superior temporal sulcus; VBM, voxel-based morphometry.
The separate analysis of MCA and PCA strokes revealed that after an MCA stroke, visual extinction was linked to lesions within the IPL, MFG and MTG extending into the MOG (Fig. 3A; Table 4), while following a PCA stroke, visual extinction was linked to damage within the superior parietal lobule (Fig. 3B; Table 4). Finally, tactile extinction following an MCA stroke was linked to lesions within central regions of the STG and putamen (Fig. 3A; Table 4), while after a PCA stroke, tactile extinction was linked to ventral regions within the inferior temporal gyrus (Fig. 3B; Table 4). Damage within the right STS was linked to both types of deficit following MCA strokes (MNI coordinates 60–37 − 9; see Fig. 3A). These findings indicate that neural substrates of extinction were related to the vascular territories affected by stroke.
Fig. 3.
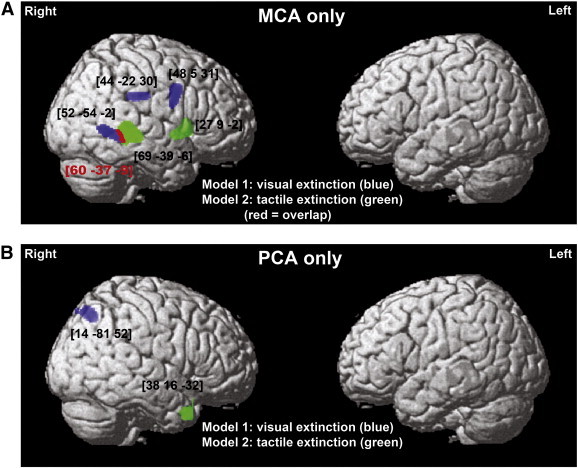
Grey matter substrates of left visual and left tactile extinction in strokes affecting only the MCA versus PCA vascular territory (VBM Analysis 3 and 4). The significant clusters identified in the VBM analyses in (A) the MCA group only (n = 215) or (B) the PCA group only (n = 47), using either visual (blue) or tactile (green) extinction as covariates of interest, are plotted on a rendered brain. The red colour indicates the overlap between two statistical maps. The numbers in brackets indicate peak MNI coordinates.
Table 4.
Grey matter substrates of visual and tactile extinction (VBM Analysis 3: MCA strokes only and Analysis 4: PCA strokes only).
| Model | Cluster level |
Voxel level |
Coordinates |
Brain structure (location) | |
|---|---|---|---|---|---|
| PFWE | Size | Z-score | X Y Z | ||
| Analysis 3: MCA strokes | |||||
| Model 1: visual extinction | 0.000 | 239 | 3.64 | 45 − 22 30 | Right IPL (BA40/39) |
| 0.000 | 454 | 3.61 | 48 5 31 | Right MFG (BA6) | |
| 0.000 | 388 | 3.61 | 52–54 -2 | Right MTG (BA21) extending into STS and posteriorly to MOG (BA19/37) | |
| Model 2: tactile extinction | 0.000 | 1284 | 5.71 | 69 − 39 − 6 | Right STG (BA22) extending into STS |
| 0.000 | 342 | 3.42 | 27 9 − 2 | Right putamen | |
| Analysis 4: PCA strokes | |||||
| Model 1: visual extinction | 0.001 | 247 | 3.67 | 14 -81 52 | Right SPL (BA7) |
| Model 2: tactile extinction | 0.000 | 198 | 4.69 | 38 16 − 32 | Right ITG (BA20) |
Abbreviations: BA, Brodmann Area; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; SPL, superior parietal lobule; STG, superior temporal gyrus; STS, superior temporal sulcus; VBM, voxel-based morphometry.
We note that regions observed in the MCA group match the findings from the entire sample, potentially because the MCA was the most prevalent stroke type. However the regions observed for the PCA group were not reliable when the entire sample is considered. This is probably because function-lesion relations within the PCA territories were only a characteristic of relative small number of patients, and hence these effects were diluted and did not reach reliability when the entire sample was considered.
While in the above analyses the number of patients in the PCA group was relatively small (n = 47) in relation to the number of covariates included in the statistical models, we replicated the results in additional analyses with reduced numbers of covariates (only 5 essential covariates i.e. left and right tactile or visual extinction, left and right spatial neglect and lesion volume; see Supplementary material). Thus the findings in the PCA group were not due to having an over-specified design.
4. Discussion
The current study examined the neuronal substrates of visual and tactile extinction in a large group of stroke patients. Specifically, we asked about anatomical dissociations as well as common neural substrates of visual and tactile extinction and whether extinction originating after strokes in different vascular territories may be associated with different anatomical–functional factors. The study demonstrated several important findings. In clear agreement with previous reports, both tactile and visual extinction were more frequent after right then left hemisphere lesion (Becker and Karnath, 2007; Chechlacz et al., in press; Stone et al., 1993). We also showed both common (modality independent/attention specific) and distinct (modality specific) neural correlates of extinction. Lesions to the MFG, IPL, SPL and MOG were linked to visual extinction, while lesions involving the STG and ITG were associated with tactile extinction. Damage within the MTG and STS was linked to both types of extinction. Secondly, we found that lesions associated with extinction differed according to the vascular territory of the stroke; specifically we found differences between MCA and PCA strokes. This suggests that extinction after strokes affecting different vascular territories may arise from different anatomical-functional factors.
4.1. Common and distinct neural substrates of visual and tactile extinction
The present results highlight both the overlap and the differentiation between the brain lesions linked to visual and tactile extinction in sub-acute stroke. The majority of previous studies either exclusively examined the neural substrates of visual extinction or they have provided evidence solely for modality specific extinction (Hillis et al., 2006; Karnath et al., 2003; Ticini et al., 2010). Thus our findings that damage within the MTG and STS is linked to both visual and tactile extinction provide important insights into understanding this multisensory disorder (Mattingley et al., 1997; Sarri et al., 2006). While lesion symptom-mapping studies rarely looked at the extinction as a multisensory phenomenon, there is a large body of evidence on neural correlates of multisensory processing including functional neuroimaging studies in humans as well as anatomical studies and single cell recordings in monkeys (Beauchamp et al., 2008; Calvert and Thesen, 2004; Hikosaka et al., 1988; Jones and Powell, 1970; Noesselt et al., 2007; Pandya and Seltzer, 1982; Sarri et al., 2006). The latter studies highlight a region within the superior temporal sulcus, receiving projections from visual, auditory and somatosensory cortices, as a multisensory convergence area (Bruce et al., 1981; Hikosaka et al., 1988; Jones and Powell, 1970; Pandya and Seltzer, 1982). In the macaque monkey brain this area is located in close proximity to the middle temporal area (MT), itself exclusively linked to visual perception and specifically motion-sensitive processing (Desimone and Ungerleider, 1986; Maunsell and van Essen, 1983a,b,c; Tanaka et al., 1986). Later neuroimaging studies in humans have demonstrated that, also in the human brain, the STS is a key area involved in multisensory integration. While earlier studies indicated that the STS multisensory region (STSms) underlies processing of auditory and visual information (Beauchamp et al., 2004a, 2004b; Calvert, 2001; Noesselt et al., 2007), later reports have confirmed that it is also involved in touch perception (Beauchamp et al., 2008). The present findings indicating that the damage to the area within MTG and STS underlies both visual and tactile deficits support the argument for the human STSms being multi-sensory in nature, whilst also suggesting that human multisensory regions are not restricted to the STS but extend to the MTG.
In contrast to results reported here, our previous study (Chechlacz et al., in press) of patients with chronic extinction (> 9 months post brain injury diagnosis) localized the temporo-parietal junction (TPJ) as the common site of lesions associated with extinction in both vision and touch. Although these discrepancies are somewhat puzzling, there are several important factors that may have contributed including: the different neuroimaging procedure (CT versus MRI), the time of testing (sub-acute versus chronic), the sample size (n = 50 versus n = 454) and most importantly the fact that, in the current study, visual and tactile extinction were measured using matched procedures (minimizing the chances that non-specific task effects confounded the results) but in Chechlacz et al. (in press) this was not the case. Specifically, tactile extinction was measured similarly to here but visual extinction was assessed using a more sensitive computer-based test with a large number of randomized trials. Interestingly, while the current work highlights the link between tactile and visual extinction within multisensory brain regions (STSms; Beauchamp et al., 2008; Hikosaka et al., 1988), our previous study highlighted the link within a neural region commonly associated with attention to and awareness of salient stimuli — factors that may have been stressed by the presentation conditions previously used (TPJ; Corbetta and Shulman, 2002; Downar et al., 2000, 2002). The current versus previous findings may therefore be seen as novel and interesting but also as complementary rather than contradictory.
Aside from this, we also generated clear evidence that the neural underpinnings of visual and tactile extinction differ, with several areas uniquely associated with each type of the disorder. Notably, lesions within the posterior MTG but extending into the MOG were correlated with visual but not tactile extinction. This suggests that the MTG/MOG region contains cells that weight the incoming stimuli on the basis of their locations in external space, with this being applied cross-modally in the MTG (see above) but with the weighting towards vision increasing gradually towards the MOG. This is in agreement with previous lesion-symptom mapping studies also linking the MOG (BA19) with visual extinction (e.g., Chechlacz et al., in press; Hillis et al., 2006) as well as with the anatomical location of visual area V5/MT (Desimone and Ungerleider, 1986; Huk et al., 2002; Tanaka et al., 1986). Along with the MOG, visual-specific extinction was observed after damage to neural areas associated with the ventral parts of the fronto-parietal attention network (Corbetta and Shulman, 2002) including the IPL and MFG, though when analysed selectively for PCA strokes the SPL was also implicated. These results are consistent with other reports linking visual extinction to lesions within the fronto-parietal network (Chechlacz et al., in press; Hillis et al., 2006; Ticini et al., 2010; Vallar et al., 1994), although it should be noted that a number of previous studies specifically linked visual extinction to lesions within TPJ (Karnath et al., 2003; Ticini et al., 2010). In the current study tactile extinction was linked to damage to the STG and putamen (after MCA stroke; also across the entire group of stroke patients) and, perhaps surprisingly, to the ITG (after PCA stroke; this region was also implicated in the analysis based on all patients; i.e. Analysis 1). While previous reports have also linked tactile extinction to damage within the basal ganglia (Chechlacz et al., in press; Vallar et al., 1994), the link between tactile extinction and damage within temporal cortex have not been previously reported – indeed tactile extinction has been more commonly associated with parietal damage (Chechlacz et al., in press; Hillis et al., 2006). Potentially, some of the discrepancies between the current data and the previous findings may be attributed to differences in the imaging modalities, i.e. CT scans versus anatomical and perfusion MRI, and/or the time of testing, i.e. sub-acute versus chronic patients. It should be also noted that while some previous lesion-symptom mapping classified patients as having or not having extinction (e.g., Vallar et al., 1994), our analyses here were based on continuous behavioural scores. Interestingly with accordance with current findings, previous single cell recordings in monkeys and fMRI studies in humans have linked some portions of the region lying between the superior temporal and inferior temporal gyri as responsive to unimodal tactile stimulation (Beauchamp et al., 2008; Hikosaka et al., 1988). Nevertheless, the findings linking the ITG with tactile extinction should be viewed with caution in relation to the evidence for this region being functionally associated with high-level visual processing (e.g., Allison et al., 1994; Gross, 1994; Tanaka, 1996; see below for further discussion).
In summary, our data provide compelling evidence that extinction in different modalities emerges after damage to both common (supra-modal) and distinct (modality specific) brain regions. This is in agreement with the ‘integrated competition’ account of attentional selection (Duncan, 1996; Duncan et al., 1997). Specifically, this account states that attentional competition depends on widespread neural networks serving different sensory modalities, with contrasting parts of the network (subsystems) having specialized functions. It follows that, while a unilateral lesion within particular brain region will produce an attentional imbalance affecting a wide range of stimulus properties and modalities, some lesions within specialized regions will affect directly one specific modality more than others (Duncan, 1996, see also Mattingley et al., 1997). Furthermore, our data extend previous findings with regards to the multisensory (visuo-tactile) integrated representation of peripersonal space (e.g. Ladavas and Farne, 2004; Ladavas et al., 1998). While previous studies have linked multisensory representation to bimodal neurons in premotor and parietal cortex based on evidence from the organization of receptive fields in the macaque brain, our findings demonstrate a role for integrated visuo-tactile representations in the temporal cortex of the human brain.
4.2. Neural substrates of extinction as a function of stroke vascular territory
One noteworthy aspect of our study is that the results varied as a function of the vascular territory affected by the stroke, particularly whether there was an MCA or PCA stroke. Visual extinction following MCA strokes was linked to lesions within the IPL, MFG and MTG extending into the MOG, while visual extinction following PCA strokes was linked to damage within the SPL. Furthermore, tactile extinction in the MCA group was associated with central regions of the STG and putamen, while tactile extinction in PCA group was linked to ventral regions within the ITG. We note that the SPL is at the border between two vascular territories (MCA and PCA). The fact that this region was highlighted only in the analysis of PCA strokes may reflect the increased signal-to-noise ratio when patients are sampled from only one stroke region. In the larger group analysis, effects of PCA stroke may have been watered-down because MCA strokes were more frequent — over two-thirds of all the patients with right hemisphere damage and left-side visual (36 out of 45) and tactile (34 out of 49) extinction here had MCA strokes.
Our findings strongly suggest that the territory of the stroke needs to be taken into consideration as a factor in lesion-symptom analyses when examining visuospatial disorders. Strokes affecting different vascular territories will result in specific patterns of lesion, with varied distribution across the brain (e.g., Phan et al., 2005; Stoeckel et al., 2007). Thus studies based largely on patients with strokes affecting a single vascular territory might fail to detect involvement of brain regions outside this vascular territory. For example, our study indicates the role of the SPL lesion in visual extinction and ITG lesions in tactile extinction following PCA strokes, demonstrating a link between lesions within these regions and extinction deficits. This was overlooked in the analysis based on combined MCA and PCA groups. The link between visual extinction symptoms and damage within the SPL in the PCA group and the IPL in the MCA group is in agreement with Gillebert et al. (2011). Based on data from patients with focal lesions within the intraparietal sulcus (IPS), Gillebert et al. (2011) propose that lesions within the superior parietal lobule have similar consequences to lesions within the inferior parietal lobule and that both can generate spatial attention deficits (for further review see also Vandenberghe et al., 2012). Futhermore, Ptak and Schnider (2011) provide compelling evidence that, in neglect patients, the IPS is a strong perdictor of spatial attention deficits. Importantly, in their lesion-symptom mapping analysis Ptak and Schnider defined IPS as a region of interest based on previous functional neuroimaging studies of spatial attention (Ptak and Schnider, 2011; for review of functional neuroimaging studies see Corbetta and Shulman, 2002). Taken together, these findings have important implications for understanding brain–behaviour relations, particularly in relation to the contrast between dorsal and more ventral regions of the attentional network (Corbetta et al., 2008). For example, prior fMRI and EEG/MEG data suggest that there may be early attentional orienting controlled by dorsal areas of the network along with slower registration of a stimulus within more ventral regions (for a review see Corbetta et al., 2008; see also DiQuattro and Geng, 2011; Doricchi et al., 2010). Our data would then suggest that visual extinction following SPL damage in PCA strokes reflects the loss of early attentional orienting whereas visual extinction following IPL-MFG lesions in MCA strokes reflects poor registration of contralesional stimuli for perceptual report. Furthermore, while the ITG has mainly been associated with high-level visual processing including visual object recognition (e.g., Allison et al., 1994; Gross, 1994; Tanaka, 1996), recent studies provide some evidence for the role of this area in the tactile processing. For example, functional neuroimaging studies have demonstrated that the ITG is bilaterally activated in tactile object recognition tasks as well as tests of tactile one-back repetition detection (e.g., Pietrini et al., 2004; Reed et al., 2004). These last results are in agreement with our findings linking ITG lesions to tactile extinction following PCA strokes. However, it might be the case that the activation of ITG in functional imaging studies of tactile recognition (i.e., Pietrini et al., 2004; Reed et al., 2004) may come about because a visual representation of the stimulus is evoked, fitting with the role of ITG in visual processing. Therefore, our finding that lesions within the ITG are linked with tactile extinction should be viewed with caution.
In summary, many cognitive functions are subserved by distributed neuronal networks, which can be revealed by testing patients where different parts of the networks are damaged. In particular our results suggest that extinction after strokes affecting different vascular territories may reflect contrasting relations between anatomy and brain function.
4.3. Methodological considerations
The present study demonstrates that it is possible to conduct lesion-symptom mapping using clinically-acquired CT scans and clinically obtained behavioural assessments (see also Chechlacz et al., 2012). However, there are several caveats that should be mentioned. One is that, although CT scans have some advantages (e.g., reduced field strength inhomogeneities, clear biological meaning of absolute signal, easier to compare across scanner cites), they also have obvious weakness, not least the reduced resolution relative to MRI scans.4 Whilst we acknowledge this, we also note that our image segmentation and normalization methods worked well with these scans (see the Supplementary material) and we were not beset by problems (e.g.) in image interpolation and re-sampling. The large scale of the present study enabled us to gain power even from the analysis of lower resolution images. Secondly, it should be noted that CT scans fail to detect cortical dysfunction within a region that is structurally intact but has inadequate cortical perfusion, and this dysfunction may contribute to cognitive deficits and affect our findings (see for example Hillis et al., 2005; Karnath et al., 2005; Ticini et al., 2010). Furthermore, it should be noted that, in the current study, our brain–behaviour relation analyses were limited to understanding the grey matter substrates of extinction syndrome, while extinction symptoms may result from white matter disconnections. For example we have previously demonstrated that white matter disconnections arising from damage along the superior longitudinal fasciculus are associated with both visual and tactile extinction (Chechlacz et al., in press). Nevertheless the present grey matter analyses usefully reveal both associative and dissociative aspects of visual and tactile extinction. A final caveat is that, as the behavioural results were derived from a large-scale clinical trial, there were limits on the amount of data that could be collected on the extinction tests. Certainly it would be useful to conduct more extensive tests, but this must be balanced against the gain of collecting data from a large sample of patients (Bickerton et al., 2011; Humphreys et al., 2012).
The following are the supplementary data related to this article.
Supplementary Fig. 2.
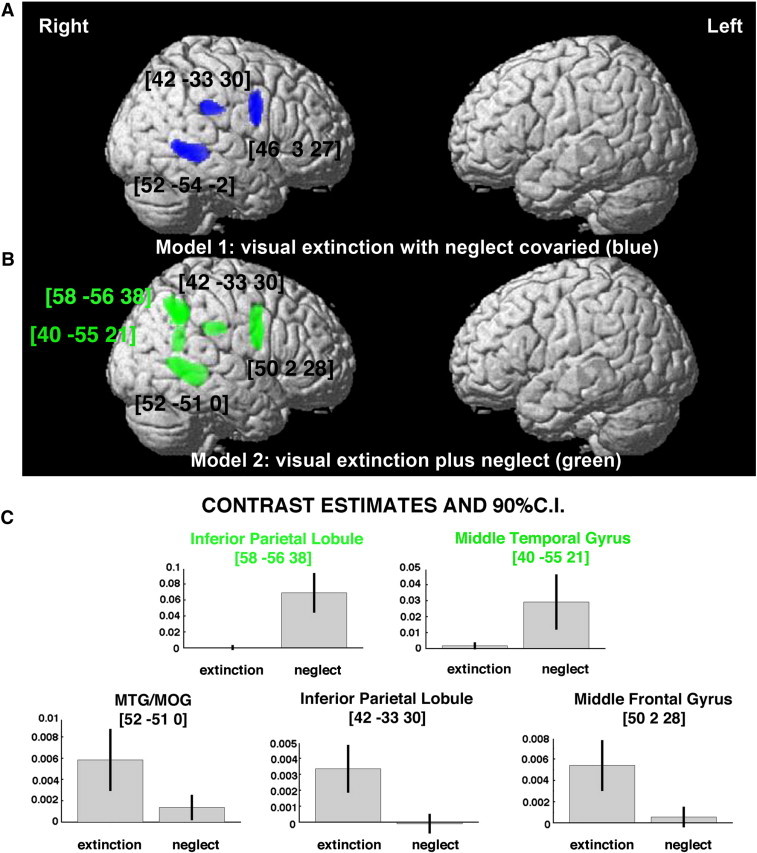
Grey matter substrates of left visual extinction and left neglect across the entire group of patients (n=454). (A) Significant clusters (blue; Model 1) identified in the VBM Analysis 1 (as described in the results section) using visual extinction as covariate of interest with neglect co-varied out plotted on rendered brain. (B) Significant clusters (green; Model 2) identified in additional analysis based on behavioural measures of both visual extinction and neglect in order to examine the main effect of both covariates. (C) Individual effects of left visual extinction and left spatial neglect corresponding to each peak voxel identified in analysis using statistical model looking at the effect of both covariates (Model 2 as shown in B) plotted to illustrate neural substrates of extinction versus neglect. The numbers in brackets indicate peak MNI coordinates. In both B and C the MNI coordinates labelled in green indicate clusters corresponding to dissociate neural substrates of neglect (58 -56 38/inferior parietal lobule and 40 -55 21/middle temporal gyrus). MTG/MOG, cluster within middle temporal gyrus extending into middle occipital gyrus.
Supplementary Fig. 3.
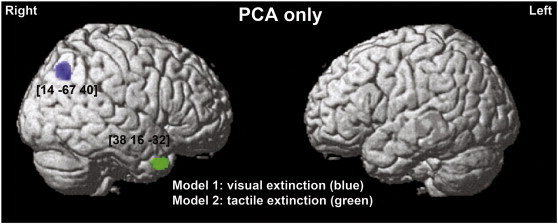
Grey matter substrates of left visual and left tactile extinction in strokes affecting the PCA vascular territory after reducing the number of covariates (only 5 essential covariates i.e. left and right tactile or visual extinction, left and right spatial neglect and lesion volume). The significant clusters identified in the VBM analyses, using an extinction measures within either the visual (blue) or tactile (green) modality are plotted on a rendered brain. The numbers in brackets indicate the MNI coordinates.
Supplementary material.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.01.013.
Acknowledgements
This work was supported by grants from the Stroke Association and the National Institute of Health Research (UK).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
The 10% probability for an abnormal voxel within the grey and white matter was estimated based on the ratio between the average lesion size and the grey plus white matter voxels. This was computed based on binary lesion maps semi-automatically reconstructed (from CT scans using a voxel-based analysis with SPM8) for 160 sub-acute stroke patients recruited a part of the same large clinical study i.e. BUCS project (as described in Chechlacz et al., 2012). Based on the reconstructed lesion maps, we then computed the average number of lesioned voxels across all patients in the sample (i.e. average lesion size). Finally we assumed that the probability for having a lesioned voxel equals the average number of lesioned voxels/the total number of voxels in the grey and white matter.
All patients in the current study were recruited from the BUCS cognitive screening trial using the BCoS instrument. The BCoS has rudimentary measures of visual and tactile sensory loss, based on trials where stimuli are presented unilaterally. Subsequently, we included in all statistical models left and right unilateral asymmetry scores (i.e. left vs. right unilateral misses) derived from the visual and tactile extinction tests as separate covariates to reduce the spurious effects of the presence of sensory impairments and visual field defects.
We note here that all 454 segmented CT scans were included in the first VBM analysis (including these with no visible lesions) and that it maybe the case that within the group of patients with no visible lesions some had sub-threshold changes in the grey matter density, which although no visible by naked eye on early acquired CT scans, these changes were detected by VBM analyses.
We note that typically CT will have relatively poor resolution on the Z-axis (inferior-superior) of 4–5mm, but high in plane resolution 0.5 × 0.5mm; this is in comparison to a typical research structural MRI that has a 1 mm axial slice thickness (Z-axis) but lower in plane resolution of 1 × 1mm. As is recommended for VBM analysis, the pre-processing of the data included a re-sampling of the data to 2 × 2 × 2 and smoothing of the data by 12 × 12 × 12mm FWHM. This meant increasing the resolution in the Z-axis, while down-sampling it in the X-axis and Y-axis.
References
- Allison T., McCarthy G., Nobre A., Puce A., Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Spatial normalization using basis functions. In: Frackowiak R.S.J., Friston K.J., Frith C., Dolan R., Price C.J., Zeki S., Ashburner J., Penny W.D., editors. Human Brain Function. 2nd edition. Academic Press; London: 2003. pp. 655–672. [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P., Thiebaut de Schotten M., Doricchi F. Left unilateral neglect as a disconnection syndrome. Cerebral Cortex. 2007;17:2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Argall B.D., Bodurka J., Duyn J.H., Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nature Neuroscience. 2004;7:1190–1192. doi: 10.1038/nn1333. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Lee K.E., Argall B.D., Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004;41:809–823. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Yasar N.E., Frye R.E., Ro T. Touch, sound and vision in human superior temporal sulcus. NeuroImage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E., Karnath H.O. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Bellas D.N., Novelly R.A., Eskenazi B., Wasserstein J. The nature of unilateral neglect in the olfactory sensory system. Neuropsychologia. 1988;26:45–52. doi: 10.1016/0028-3932(88)90029-2. [DOI] [PubMed] [Google Scholar]
- Bellas D.N., Novelly R.A., Eskenazi B., Wasserstein J. Unilateral displacement in the olfactory sense: a manifestation of the unilateral neglect syndrome. Cortex. 1988;24:267–275. doi: 10.1016/s0010-9452(88)80035-2. [DOI] [PubMed] [Google Scholar]
- Bender M.B., Teuber H.L. Phenomena of fluctuation, extinction and completion in visual perception. Archives of Neurology and Psychiatry. 1946;55:627–658. doi: 10.1001/archneurpsyc.1946.02300170075008. [DOI] [PubMed] [Google Scholar]
- Bickerton W.L., Samson D., Williamson J., Humphreys G.W. Separating Forms of Neglect Using the Apples Test: Validation and Functional Prediction in Chronic and Acute Stroke. Neuropsychology. 2011;25:567–580. doi: 10.1037/a0023501. [DOI] [PubMed] [Google Scholar]
- Bruce C., Desimone R., Gross C.G. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. Journal of Neurophysiology. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Calvert G.A. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cerebral Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Calvert G.A., Thesen T. Multisensory integration: methodological approaches and emerging principles in the human brain. Journal of Physiology, Paris. 2004;98:191–205. doi: 10.1016/j.jphysparis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Chechlacz M., Rotshtein P., Bickerton W.L., Hansen P.C., Deb S., Humphreys G.W. Separating neural correlates of allocentric and egocentric neglect: Distinct cortical sites and common white matter disconnections. Cognitive Neuropsychology. 2010;27:277–303. doi: 10.1080/02643294.2010.519699. [DOI] [PubMed] [Google Scholar]
- Chechlacz,M., Rotshtein, P., Hansen, P.C., Deb, S., Riddoch, M.J., Humphreys, G.W., in press. The central role of the temporo-parietal junction and the superior longitudinal fasciculus in supporting multi-item competition: Evidence from lesion-symptom mapping of extinction. Cortex. http://dx.doi.org/10.1016/j.cortex.2011.11.008 [DOI] [PubMed]
- Chechlacz M., Rotshtein P., Roberts K.L., Bickerton W.-L., Lau J.K.L., Humphreys G.W. Acute versus chronic prognosis of allocentric versus egocentric neglect symptoms: evidence from clinical scans. PLoS One. 2012;7(11):e47821. doi: 10.1371/journal.pone.0047821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M. Hafner; New York: 1953. The parietal lobes. [Google Scholar]
- De Renzi E., Gentilini M., Pattacini F. Auditory extinction following hemisphere damage. Neuropsychologia. 1984;22:733–744. doi: 10.1016/0028-3932(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Deouell L.Y., Soroker N. What is extinguished in auditory extinction? Neuroreport. 2000;11:3059–3062. doi: 10.1097/00001756-200009110-00046. [DOI] [PubMed] [Google Scholar]
- Desimone R., Ungerleider L.G. Multiple visual areas in the caudal superior temporal sulcus of the macaque. The Journal of Comparative Neurology. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiQuattro N.E., Geng J.J. Contextual knowledge configures attentional control networks. Journal of Neuroscience. 2011;31:18026–18035. doi: 10.1523/JNEUROSCI.4040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F., Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14:2239–2243. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Doricchi F., Macci E., Silvetti M., Macaluso E. Neural Correlates of the Spatial and Expectancy Components of Endogenous and Stimulus-Driven Orienting of Attention in the Posner Task. Cerebral Cortex. 2010;20:1574–1585. doi: 10.1093/cercor/bhp215. [DOI] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- Driver J., Spence C. Attention and the crossmodal construction of space. Trends in Cognitive Sciences. 1998;2:254–262. doi: 10.1016/S1364-6613(98)01188-7. [DOI] [PubMed] [Google Scholar]
- Driver J., Spence C. Crossmodal attention. Current Opinion in Neurobiology. 1998;8:245–253. doi: 10.1016/s0959-4388(98)80147-5. [DOI] [PubMed] [Google Scholar]
- Driver J., Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Duncan, J., 1996. Coordinated brain systems in selective perception and action, in: T Innui and J L McClelland, editors, Attention and Performance.Vol. XVI ed. MIT Press, Cambridge, MA, pp. 438–467.
- Duncan J., Humphreys G., Ward R. Competitive brain activity in visual attention. Current Opinion in Neurobiology. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Duvernoy H.M., Cabanis E.A., Vannson J.L. Springer-Verlag; Wien: 1991. The Human brain: surface, three-dimensional sectional anatomy and MRI. [Google Scholar]
- Gillebert C.R., Mantini D., Thijs V., Sunaert S., Dupont P., Vandenberghe R. Lesion evidence for the critical role of the intraparietal sulcus in spatial attention. Brain. 2011;134:1694–1709. doi: 10.1093/brain/awr085. [DOI] [PubMed] [Google Scholar]
- Gross C.G. How inferior temporal cortex became a visual area. Cerebral Cortex. 1994;4:455–469. doi: 10.1093/cercor/4.5.455. [DOI] [PubMed] [Google Scholar]
- He B.J., Snyder A.Z., Vincent J.L., Epstein A., Shulman G.L., Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Hikosaka K., Iwai E., Saito H., Tanaka K. Polysensory properties of neurons in the anterior bank of the caudal superior temporal sulcus of the macaque monkey. Journal of Neurophysiology. 1988;60:1615–1637. doi: 10.1152/jn.1988.60.5.1615. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Newhart M., Heidler J., Barker P.B., Herskovits E.H., Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. Journal of Neuroscience. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Chang S., Heidler-Gary J., Newhart M., Kleinman J.T., Davis C., Barker P.B., Aldrich E., Ken L. Neural correlates of modality-specific spatial extinction. Journal of Cognitive Neuroscience. 2006;18:1889–1898. doi: 10.1162/jocn.2006.18.11.1889. [DOI] [PubMed] [Google Scholar]
- Huk A.C., Dougherty R.F., Heeger D.J. Retinotopy and functional subdivision of human areas MT and MST. Journal of Neuroscience. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G.W., Bickerton W.L., Samson D., Riddoch M.J. Psychology Press; London: 2012. The Birmingham Cognitive Screen (BCoS) [DOI] [PubMed] [Google Scholar]
- Jones E.G., Powell T.P. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kaplan R.F., Cohen R.A., Rosengart A., Elsner A.E., Hedges T.R., III, Caplan L.R. Extinction during time controlled direct retinal stimulation after recovery from right hemispheric stroke. Journal of Neurology, Neurosurgery & Psychiatry. 1995;59:534–536. doi: 10.1136/jnnp.59.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H.O., Himmelbach M., Kuker W. The cortical substrate of visual extinction. Neuroreport. 2003;14:437–442. doi: 10.1097/01.wnr.0000059778.23521.88. [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Zopf R., Johannsen L., Fruhmann Berger M., Nagele T., Klose U. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain. 2005;128:2462–2469. doi: 10.1093/brain/awh629. [DOI] [PubMed] [Google Scholar]
- Karnath H.O., Rorden C., Ticini L.F. Damage to white matter fiber tracts in acute spatial neglect. Cerebral Cortex. 2009;19:2331–2337. doi: 10.1093/cercor/bhn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel S., Holmes A. The General Linear Model. In: Frackowiak R.S.J., Friston K.J., Frith C., Dolan R., Price C.J., Zeki S., Ashburner J., Penny W.D., editors. Human Brain Function. 2nd edition. Academic Press; London: 2003. pp. 725–760. [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Advances in Neurology. 1977;18:41–49. [PubMed] [Google Scholar]
- Kinsbourne M. Mechanisms of unilateral neglect. In: Jeannerod M., editor. Neurophysiological and neuropsychological aspects of spatial neglect. North-Holland; Amsterdam: 1987. pp. 69–86. [Google Scholar]
- Ladavas E., Farne A. Visuo-tactile representation of near-the-body space. Journal of Physiology, Paris. 2004;98:161–170. doi: 10.1016/j.jphysparis.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ladavas E., di Pellegrino G., Farne A., Zeloni G. Neuropsychological evidence of an integrated visuotactile representation of peripersonal space in humans. Journal of Cognitive Neuroscience. 1998;10:581–589. doi: 10.1162/089892998562988. [DOI] [PubMed] [Google Scholar]
- Ladavas E., Pavani F., Farne A. Auditory peripersonal space in humans: a case of auditory-tactile extinction. Neurocase. 2001;7:97–103. doi: 10.1093/neucas/7.2.97. [DOI] [PubMed] [Google Scholar]
- Maravita A., Spence C., Clarke K., Husain M., Driver J. Vision and touch through the looking glass in a case of crossmodal extinction. Neuroreport. 2000;11:3521–3526. doi: 10.1097/00001756-200011090-00024. [DOI] [PubMed] [Google Scholar]
- Mattingley J.B., Driver J., Beschin N., Robertson I.H. Attentional competition between modalities: extinction between touch and vision after right hemisphere damage. Neuropsychologia. 1997;35:867–880. doi: 10.1016/s0028-3932(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Maunsell J.H., van Essen D.C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. Journal of Neuroscience. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J.H., Van Essen D.C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. Journal of Neurophysiology. 1983;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- Maunsell J.H., Van Essen D.C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. Journal of Neurophysiology. 1983;49:1148–1167. doi: 10.1152/jn.1983.49.5.1148. [DOI] [PubMed] [Google Scholar]
- Mort D.J., Malhotra P., Mannan S.K., Rorden C., Pambakian A., Kennard C., Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Noesselt T., Rieger J.W., Schoenfeld M.A., Kanowski M., Hinrichs H., Heinze H.J., Driver J. Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. Journal of Neuroscience. 2007;27:11431–11441. doi: 10.1523/JNEUROSCI.2252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J.A. Anterior-posterior interhemispheric differences in the loci of lesions producing visual hemineglect. Brain and Cognition. 1985;4:59–75. doi: 10.1016/0278-2626(85)90054-5. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Phan T.G., Donnan G.A., Wright P.M., Reutens D.C. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. 2005;36:986–991. doi: 10.1161/01.STR.0000163087.66828.e9. [DOI] [PubMed] [Google Scholar]
- Pietrini P., Furey M.L., Ricciardi E., Gobbini M.I., Wu W.H., Cohen L., Guazzelli M., Haxby J.V. Beyond sensory images: Object-based representation in the human ventral pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R., Schnider A. The attention network of the human brain: relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologia. 2011;49:3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Reed C.L., Shoham S., Halgren E. Neural substrates of tactile object recognition: an fMRI study. Human Brain Mapping. 2004;21:236–246. doi: 10.1002/hbm.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri M., Blankenburg F., Driver J. Neural correlates of crossmodal visual-tactile extinction and of tactile awareness revealed by fMRI in a right-hemisphere stroke patient. Neuropsychologia. 2006;44:2398–2410. doi: 10.1016/j.neuropsychologia.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel M.C., Wittsack H.J., Meisel S., Seitz R.J. Pattern of cortex and white matter involvement in severe middle cerebral artery ischemia. Journal of Neuroimaging. 2007;17:131–140. doi: 10.1111/j.1552-6569.2007.00102.x. [DOI] [PubMed] [Google Scholar]
- Stone S.P., Halligan P.W., Greenwood R.J. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing. 1993;22:46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annual Review of Neuroscience. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hikosaka K., Saito H., Yukie M., Fukada Y., Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. Journal of Neuroscience. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Kinkingnehun S., Delmaire C., Lehericy S., Duffau H., Thivard L., Volle E., Levy R., Dubois B., Bartolomeo P. Visualization of disconnection syndromes in humans. Cortex. 2008;44:1097–1103. doi: 10.1016/j.cortex.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Ticini L.F., de Haan B., Klose U., Nagele T., Karnath H.O. The role of temporo-parietal cortex in subcortical visual extinction. Journal of Cognitive Neuroscience. 2010;22:2141–2150. doi: 10.1162/jocn.2009.21315. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaishnavi S., Calhoun J., Chatterjee A. Binding personal and peripersonal space: evidence from tactile extinction. Journal of Cognitive Neuroscience. 2001;13:181–189. doi: 10.1162/089892901564243. [DOI] [PubMed] [Google Scholar]
- Vallar G., Rusconi M.L., Bignamini L., Geminiani G., Perani D. Anatomical correlates of visual and tactile extinction in humans: a clinical CT scan study. Journal of Neurology, Neurosurgery & Psychiatry. 1994;57:464–470. doi: 10.1136/jnnp.57.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R., Molenberghs P., Gillebert C.R. Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia. 2012;50:1092–1103. doi: 10.1016/j.neuropsychologia.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Vossel S., Eschenbeck P., Weiss P.H., Weidner R., Saliger J., Karbe H., Fink G.R. Visual extinction in relation to visuospatial neglect after right-hemispheric stroke: quantitative assessment and statistical lesion-symptom mapping. Journal of Neurology, Neurosurgery & Psychiatry. 2011;82:862–868. doi: 10.1136/jnnp.2010.224261. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Mesulam M.M. Visual hemispatial inattention: stimulus parameters and exploratory strategies. Journal of Neurology, Neurosurgery & Psychiatry. 1988;51:1481–1488. doi: 10.1136/jnnp.51.12.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey T.A., Hanaway J., Gado M.H. 3rd ed. Wiley; Hoboken, N.J.: 2008. The brain atlas: a visual guide to the human central nervous system. [Google Scholar]
- Worsley K.J. Developments in random field theory. In: Frackowiak R.S.J., Friston K.J., Frith C., Dolan R., Price C.J., Zeki S., Ashburner J., Penny W.D., editors. Human Brain Function. 2nd edition. Academic Press; London: 2003. pp. 881–886. [Google Scholar]
- Wortis S.B., Bender M.B., Teuber H.L. The significance of the phenomenon of extinction. The Journal of Nervous and Mental Disease. 1948;107:382–387. doi: 10.1097/00005053-194810740-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


