Abstract
Frontotemporal dementia (FTD) is classically considered to be a neurodegenerative disease with cortical changes. Recent structural imaging findings, however, highlight that subcortical and in particular striatal regions are also affected in the FTD syndrome. The influence of striatal pathology on cognitive and behavioural changes in FTD is virtually unexplored. In the current study we employ the Weather Prediction Task (WPT), a probabilistic learning task which taps into striatal dysfunction, in a group of FTD patients. We also regressed the patients' behavioural performance with their grey matter atrophy via voxel-based morphometry (VBM) to identify the grey matter contributions to WPT performance in FTD. Based on previous studies we expected to see striatal and frontal atrophy to be involved in impaired probabilistic learning. Our behavioural results show that patients performed on a similar level to controls overall, however, there was a large variability of patient performance in the first 30 trials of the task, which are critical in the acquisition of the probabilistic learning rules. A VBM analysis covarying the performance for the first 30 trials across participants showed that atrophy in striatal but also frontal brain regions correlated with WPT performance in these trials. Closer inspection of performance across the first 30 trials revealed a subgroup of FTD patients that performed significantly poorly than the remaining patients and controls on the WPT, despite achieving the same level of probabilistic learning as the other groups in later trials. Additional VBM analyses revealed that the subgroup of FTD patients with poor early probabilistic learning in the first 30 trials showed greater striatal atrophy compared to the remaining FTD patients and controls. These findings suggest that the integrity of fronto-striatal regions is important for probabilistic learning in FTD, with striatal integrity in particular, determining the acquisition learning rate. These findings will therefore have implications for developing an easily administered version of the probabilistic learning task which can be used by clinicians to assess striatal functioning in neurodegenerative syndromes.
Keywords: Frontotemporal dementia, Probabilistic learning, Weather Prediction Task, Striatum, Orbitofrontal cortex, Voxel-based morphometry
Highlights
► Probabilistic association memory was investigated in FTD patients. ► FTD patients with slow acquisition rates showed greater striatal atrophy. ► Striatal dysfunction in FTD can be functionally assessed via such tests.
1. Introduction
Frontotemporal dementia (FTD) is a progressive neurodegenerative brain disorder characterised by predominant frontal and temporal neocortical atrophy. Three clinical variants of FTD are generally reported: behavioural variant (bvFTD), semantic dementia (SD) and progressive nonfluent aphasia (PNFA) (Hodges, 2007). Cognitive deficits specific to each variant are related to the pattern of underlying atrophy. bvFTD is characterised by behaviour and personality changes, such as reduced empathy, apathy, social inappropriateness and disinhibition (Piguet et al., 2011) and is associated with atrophy most pronounced in the ventromedial prefrontal cortex regions. The language variants of FTD present either with degradation of semantic knowledge (SD) associated with asymmetric anterior temporal lobe atrophy (generally left > right) or with language production difficulties (PNFA) observed in the context of left inferior frontal lobe and insular atrophy (Knibb et al., 2006; Hodges and Patterson, 2007). Importantly, however, recent evidence has shown atrophy to subcortical brain regions including the striatum (Broe et al., 2003; Chow et al., 2008; Looi et al., 2008; Garibotto et al., 2011), across FTD subtypes.
The cognitive and functional deficits in FTD arising from atrophy in these subcortical structures remain largely unknown. To our knowledge, the only study to date to examine functional deficits associated with striatal dysfunction in FTD employed a probabilistic learning task, the weather prediction task (WPT) (Weickert et al., 2011) and found that the behavioural variant of FTD is particular impaired on this task. The WPT has previously been shown to be a sensitive tool for identifying striatal dysfunction in patients with Parkinson's disease (PD), a neurodegenerative disorder which affects striatal brain regions (Knowlton et al., 1996a). The WPT requires participants to learn which of two outcomes (rain or shine) are predicted by different sets of geometric shapes. Outcomes are probabilistically assigned to each set. Participants must begin by guessing the outcome but over cumulative trials healthy individuals implicitly learn which outcome is most probable for each set. Such implicit learning tasks are thought to be dependent on striatal structures which are proposed to be involved in managing outcome expectancies (de Wit et al., 2007; Hare et al., 2008). Indeed, patients with predominant striatal damage as present in PD and Huntington's disease (HD) are impaired on the WPT task (Knowlton et al., 1996a, 1996b; Shohamy et al., 2004; Perretta et al., 2005). In contrast, patients with explicit memory loss following medial temporal lobe lesions, perform the WPT at the level of healthy control subjects (Knowlton et al., 1994).
Importantly, functional magnetic resonance imaging (fMRI) studies have highlighted that numerous frontal regions, in addition to striatal regions, are activated during successful performance on the WPT [for review see 17]. The importance of fronto-striatal white matter integrity for successful performance on a probabilistic reward learning task (Samanez-Larkin et al., 2012) provides further support that a fronto-striatal circuit may be crucial for probabilistic learning. In light of the evidence for fronto-striatal contributions to probabilistic association learning, it is unknown whether impaired performance on the WPT in FTD (Weickert et al., 2011) is more related to frontal or striatal abnormalities.
The current study aimed at establishing the brain correlates of WPT task performance in FTD. We hypothesised that atrophy specific to orbital frontal and striatal brain regions would be related to performance on the WPT task, corroborating previous patient studies and demonstrating the presence of striatal abnormalities in FTD patients.
2. Methods
2.1. Participants
Fifteen FTD patients participated in the study (bvFTD = 5; SD = 5; PNFA = 5). Patients met the current clinical diagnostic criteria for FTD (Gorno-Tempini et al., 2011; Rascovsky et al., 2011). Only patients with evidence of disease progression and brain atrophy on MRI were included to rule out behavioural-variant phenocopy cases (Kipps et al., 2010). Twelve healthy adults were selected from a healthy volunteer database at Neuroscience Research Australia or were spouses/carers of the FTD patients. Importantly, the healthy adults' WPT performance was matched to the patients. Exclusion criteria included other neurological conditions, a history of significant TBI, alcohol abuse, use of medications with CNS side effects and an Addenbrooke's Cognitive Examination —Revised (ACE-R) score of under 85. Additional exclusion criteria for MRI scanning included the presence of ferrous implants, pacemakers and claustrophobia. All participants underwent a battery of neuropsychological tests including the ACE-R as a general measure of cognitive impairment, the Rey Auditory Verbal Learning Test (RAVLT) as a measure of verbal learning and memory, and the Doors and People test as a measure of nonverbal memory function. All participants provided informed written consent prior to participation in this study. This study was approved by the University of New South Wales and the South Eastern Sydney and Illawarra Area Health Service Human Research Ethics Committees.
2.2. Probabilistic association learning test — WPT
Each participant was administered the probabilistic association learning Weather Prediction Test (Knowlton et al., 1994). The task consists of four cue cards containing patterns of different geometrical shapes presented on a laptop computer screen. In any given trial, one, two or three cue cards are displayed (see Fig. 1 for an example of a trial). Participants were instructed to make a decision to predict ‘rain’ or ‘shine’ based on the combination of the cue cards presented. They were told that they would need to guess at first but would gradually improve at determining which cue card combinations predict rain or shine based on feedback provided. The relation between cue cards and outcomes were determined on a probabilistic basis (see Table 1 for an example of a cue–outcome probability schedule). Stimulus presentations were randomised but each outcome (rain or shine) was limited to five consecutive occurrences. All stimuli were displayed on screen for 4.5 s with an inter-trial interval of .5 s. Participants responded with a left mouse button press by their right hand to choose either rain or shine. After each response the words ‘correct’ or ‘incorrect’ appeared on screen as feedback to the participant. Missed trials were not included in the analyses.
Fig. 1.
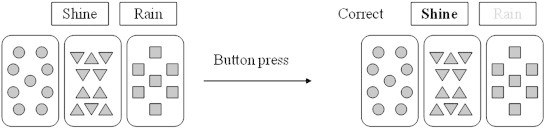
Example of a probabilistic learning task trial.
Table 1.
Probability structure of probabilistic learning (Weather Prediction) test.
| Cue |
||||||
|---|---|---|---|---|---|---|
| Cue pattern | 1 | 2 | 3 | 4 | P(cue combination) | P(outcome) |
| 1 | 0 | 0 | 0 | 1 | .133 | .150 |
| 2 | 0 | 0 | 1 | 0 | .087 | .385 |
| 3 | 0 | 0 | 1 | 1 | .080 | .083 |
| 4 | 0 | 1 | 0 | 0 | .087 | .615 |
| 5 | 0 | 1 | 0 | 1 | .067 | .200 |
| 6 | 0 | 1 | 1 | 0 | .040 | .500 |
| 7 | 0 | 1 | 1 | 1 | .047 | .143 |
| 8 | 1 | 0 | 0 | 0 | .133 | .850 |
| 9 | 1 | 0 | 0 | 1 | .067 | .500 |
| 10 | 1 | 0 | 1 | 0 | .067 | .800 |
| 11 | 1 | 0 | 1 | 1 | .033 | .400 |
| 12 | 1 | 1 | 0 | 0 | .080 | .917 |
| 13 | 1 | 1 | 0 | 1 | .033 | .600 |
| 14 | 1 | 1 | 1 | 0 | .047 | .857 |
Note. For any given trial, 1 of the 14 possible cue pattern combinations displayed above appeared on the computer screen with a probability indicated as: P(cue combination). As shown above, the probability of the cue combinations to predict “sunshine” (outcome 1) was set at P(outcome). Conversely, the probability of the above cue combinations to predict “rain” (or outcome 2) was equal to 1 − P.
2.3. Behavioural analyses
Data were analysed using PASWS 17.0 (IBM, Chicago, Ill., USA). Demographic (age, education), neuropsychological (ACE-R, general cognitive tests) and behavioural (WPT) data were compared across groups using parametric statistical tests. Between-group differences in cumulative percent correct responses for each block of 10 trials were tested using a repeated-measures analysis of variance (ANOVA) with two groups (Controls, FTD) as between-subject variable.
2.4. Imaging acquisition and voxel-based morphometry (VBM) analysis
All participants underwent a brain imaging protocol comprising whole-brain T1 using a 3-Tesla Philips MRI scanner with standard quadrature head coil (16 channels):coronal orientation, matrix 256 × 256, 200 slices, 1 mm isotropic, TE/TR = 2.6/5.8 ms, flip angle α = 19°.
Images were analysed with FSL-VBM, a voxel-based morphometry analysis (Ashburner and Friston, 2000; Good et al., 2001) which is part of the FSL software package http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html (Smith et al., 2004). First, tissue segmentation was carried out using FMRIB's Automatic Segmentation Tool (FAST) (Zhang et al., 2001) from brain extracted images. The resulting grey matter partial volume maps were then aligned to the Montreal Neurological Institute standard space (MNI152) using the nonlinear registration approach FNIRT (Andersson et al., 2007a, 2007b), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). The registered partial volume maps were then modulated to correct for local expansion or contraction by dividing them by the Jacobian of the warp field. Note that the modulation did not include the affine part of the registration so that participants were matched for brain size. The modulated images were then smoothed with an isotropic Gaussian kernel with a standard deviation of 3 mm (FWHM: 8 mm). Finally, a voxelwise general linear model (GLM) was applied and permutation-based non-parametric testing was used to form clusters with the Threshold-Free Cluster Enhancement (TFCE) method (Smith and Nichols, 2009), tested for significance at p < .05 corrected for multiple comparisons via Family-wise Error (FWE) correction across space. Results which did not survive FWE correction were thresholded at p < .001 False Discovery Rate (FDR) corrected and a voxel threshold of at least 50 contiguous voxels.
3. Results
3.1. Demographics and neuropsychological data
The control group were significantly older than the FTD group (p < .05) but were matched to the patients on WPT performance (see below). The groups were matched for years of education. Performance on the general cognitive measure ACE-R was significantly better in healthy controls than in the FTD group (p < .01). Likewise controls significantly outperformed the FTD group on the explicit memory tests Doors A (p < .05), Doors B (p < .05) and the RAVLT total (p < .001), recognition (p < .05) and thirty-minute delay recall (p < .001) (Table 2).
Table 2.
Demographics, characteristics and cognitive test performance of the study samples.
| FTD (n = 15) |
Controls (n = 12) |
F values | |
|---|---|---|---|
| Mean (SD) | |||
| Age | 62.6 (6) | 70.2 (8) | * |
| Education | 13.1 (3) | 11.9 (2) | n.s. |
| Disease duration (years) | 4.8 (3) | N/A | N/A |
| ACE-R | 69.6 (16) | 92.4 (4) | ** |
| RAVLT (total) | 26.5 (17) | 53.5 (6) | *** |
| RAVLT (recognition) | 8.7 (6) | 13.8 (1) | * |
| RAVLT (30 min delay) | 3.3 (8) | 13.9 (5) | *** |
| Doors A | 9.5 (10) | 11.1(1) | * |
| Doors B | 4.2 (4) | 7.4(3) | * |
n.s. = non significant. * = p < .05. ** = p < .01. *** = p < .001.
3.2. Behavioural results
Statistical analyses revealed a significant main effect of trial block [F(2,50) = 7.871, p < .01] but no significant group effect and no significant group × condition interaction (see Fig. 2A).
Fig. 2.
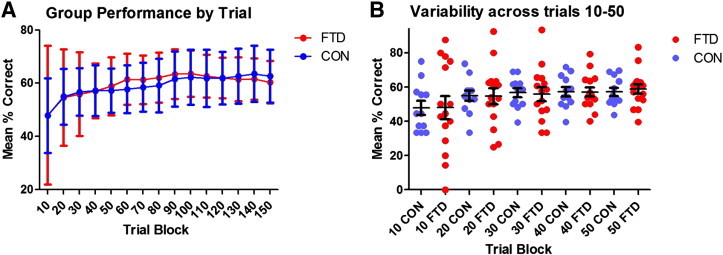
Weather Prediction Task (WPT) performance for FTD patients and controls as A) line graph and B) dot scatterplot; (red = FTD; blue = controls).
Closer inspection of the data revealed that performance improved most in the first 30 trials in both groups. During this early phase of the task, performance in the FTD group was highly variable, with some patients being markedly impaired while others performed at the level of Controls (Fig. 2B). Paradoxically, some FTD patients outperformed controls over the early trials. We analysed the standard deviations of each group by trial block revealing that within the FTD group, there was significantly greater variability in performance during trials 10–30 when compared with trials 40–140 [t(12) = 7.18, p < .01]. The same analysis in the control group revealed no significant difference in variance between early and later trials. The FTD group was therefore dichotomised into individuals with fast versus slow acquisition rates based on their performance in the first 30 trials of the task. For this exploratory analysis, we arbitrarily selected the bottom 5 individuals with consistently slow acquisition rates and the top five individuals with consistently fast acquisition rates over the first 30 trials. Fast and slow acquisition FTD groups contained an even mix of FTD subtypes. There were no significant differences between fast and slow acquisition FTD groups on demographic measures such as age, disease duration, education, ACE-R, RAVLT or Doors A and B. A repeated-measures ANOVA on the first three trial blocks (30 trials) with three groups (FTD fast acquisition; FTD slow acquisition; and controls) revealed a significant main effect of trial block [F(2,38) = 7.089, p = .01], a significant group effect [F(2,19) = 17.122, p < .001] and no significant interactions. Follow-up pair-wise comparisons revealed significant differences between all groups (all p's < .05).
3.3. Voxel-based morphometry analyses
We first contrasted the FTD and control groups to identify the pattern of brain atrophy. Compared to controls, the FTD group exhibited significant grey matter density reductions in frontotemporal cortical as well as striatal subcortical regions (Supplementary Fig. 1). These findings are consistent with previous investigations of brain atrophy in FTD (Rosen et al., 2002; Whitwell and Jack, 2005).
Supplementary Fig. 1.
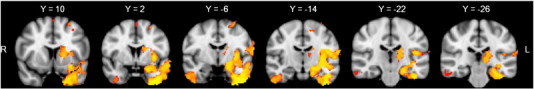
Regions of significant grey matter intensity decrease for FTD patients versus controls at p < .05 FWE corrected.
We first contrasted the FTD and control groups to identify the pattern of brain atrophy. Compared to controls, the FTD group exhibited significant grey matter density reductions in frontotemporal cortical as well as striatal subcortical regions (Supplementary Fig. 1). These findings are consistent with previous investigations of brain atrophy in FTD (Rosen et al., 2002; Whitwell and Jack, 2005).
We then entered performance over the entire task as a covariate in a second VBM analysis. This analysis included all participants and revealed a significant correlation between performance across the entire task and grey matter density in a number of cortical regions including the orbital and medial frontal cortices but no striatal structures (Table 3).
Table 3.
Voxel-based morphometry (VBM) results showing regions of significant grey matter intensity decrease (t-score > 2.41) as a function of WPT performance. Results are reported at p < .001 FDR corrected with a voxel threshold of at least 50 contiguous voxels.
| Regions | Hemisphere (L/R/B) |
MNI coordinates |
Number of voxels | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| All WPT trials | |||||
| Inferior temporal gyrus | L | − 36 | − 8 | − 36 | 157 |
| Anterior supramarginal gyrus | R | 64 | − 26 | 34 | 79 |
| Superior parietal lobule | L | − 26 | − 46 | 48 | 74 |
| Anterior cingulate gyrus | L | − 10 | 10 | 32 | 68 |
| Orbitofrontal cortex/frontal pole | R | 48 | 52 | 0 | 62 |
| First 30 WPT trials | |||||
| Frontal Medial cortex | L | − 4 | 38 | − 26 | 770 |
| Putamen | L | − 24 | 10 | − 8 | 190 |
| Precentral Gyrus | L | − 8 | − 30 | 58 | 91 |
| Frontal pole | R | 46 | 50 | − 2 | 78 |
| Orbitofrontal cortex | R | 28 | 28 | − 12 | 69 |
In light of the greatest implicit learning variability being present in the first 30 trials of the task, performance on this portion of the task was entered as a covariate in a separate VBM analysis which also included all participants. This analysis revealed a significant correlation between performance on the first 30 trials of the WPT and grey matter density in cortical (medial frontal, orbitofrontal, and frontopolar) as well as subcortical (particularly putamen) regions (Table 3 and Fig. 3).
Fig. 3.
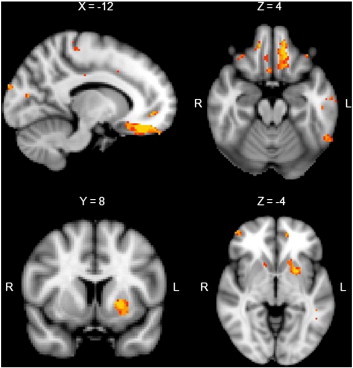
Regions of significant grey matter intensity decrease correlating with performance over the first 30 trials across all subjects. Clusters are overlaid on the MNI standard brain (t = 2.41). Coloured voxels show regions that were significant in the analyses for p < 0.001 FDR corrected and a cluster threshold of 50 contiguous voxels.
A VBM analysis was conducted contrasting the slow acquisition FTD group (i.e., 5 FTD subjects who learned consistently poorly across the early stages of the task) against the healthy controls. This analysis revealed significant grey matter density reduction in the caudate nucleus and putamen, as well as in the orbitofrontal and frontal pole brain regions in the slow acquisition FTD group (Table 4 and Fig. 4A). In contrast, similar analyses with the fast acquisition FTD group revealed that faster acquisition rates on the WPT were associated with atrophy in left medial temporal lobe and surrounding cortical regions (Table 4 and Fig. 4B). A final VBM analysis revealed that the slow acquisition FTD group had significant grey matter density reduction in the putamen, caudate nucleus and orbitofrontal cortex when compared with the fast acquisition FTD group (Table 5 and Fig. 4C).
Table 4.
Voxel-based morphometry (VBM) results showing regions of significant (t > 2.41) grey matter intensity decrease as a function of WPT performance over the first 30 trials in slow and fast acquisition FTD groups. Results are reported at p < .001 FDR corrected with a voxel threshold of at least 50 contiguous voxels.
| Regions | Hemisphere (L/R/B) |
MNI coordinates |
Number of voxels | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Slow acquisition FTD group | |||||
| Putamen, caudate nucleus | L | − 24 | 2 | − 2 | 3086 |
| Temporal fusiform cortex | L | − 34 | 0 | − 50 | 1257 |
| Superior parietal lobule | L | − 34 | − 42 | 40 | 228 |
| Orbitofrontal cortex/frontal pole | L | − 14 | 48 | − 22 | 77 |
| Fast acquisition FTD group | |||||
| Temporal fusiform cortex, inferior temporal gyrus, hippocampus, amygdala | L | − 36 | − 8 | − 50 | 2806 |
| Superior temporal gyrus | L | − 58 | 2 | − 14 | 82 |
Fig. 4.
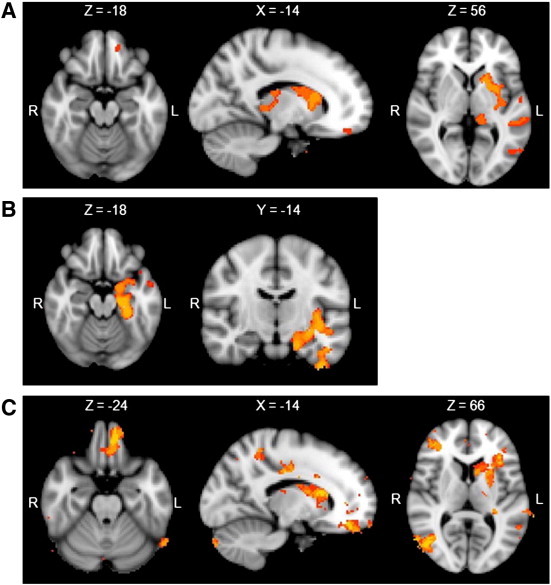
Regions of significant grey matter intensity decrease for: A) controls versus slow acquisition FTD group at p = .001 FWE corrected; B) controls versus fast acquisition FTD groups at p = .05 FWE corrected, C) fast versus slow acquisition FTD groups at p = .001 FDR corrected.
Table 5.
Voxel-based morphometry (VBM) results showing regions of significant (t > 2.41) grey matter intensity decrease for the contrast of slow versus fast acquisition FTD groups. Results are reported at p < .001 FDR uncorrected with a voxel threshold of at least 50 contiguous voxels.
| Regions | Hemisphere (L/R/B) |
MNI coordinates |
Number of voxels | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Superior temporal gyrus/putamen/caudate nucleus | L | − 70 | − 38 | 6 | 7182 |
| Angular gyrus | R | 58 | − 56 | 18 | 2389 |
| Frontal medial cortex | L | − 2 | 32 | − 32 | 1867 |
| Frontal pole | R | 36 | 50 | − 6 | 483 |
| Inferior frontal gyrus | R | 44 | 4 | 18 | 269 |
| Superior frontal gyrus | R | 0 | 50 | 50 | 222 |
| Superior parietal lobule | R | 14 | − 52 | 70 | 96 |
| Middle temporal gyrus | L | − 64 | − 62 | − 6 | 90 |
| Middle temporal gyrus | R | 66 | − 54 | − 2 | 54 |
4. Discussion
To our knowledge, this is the first study to directly relate striatal structural abnormalities to performance measures on a probabilistic association learning task in FTD patients. Our findings show that frontal and striatal atrophy is associated with probabilistic association learning in FTD. Importantly, however, implicit learning performance in a subset of FTD patients who performed particularly poorly during the early stages of the task was more specifically related to atrophy in the putamen and caudate nucleus. These findings suggest that atrophy to frontal as well as striatal brain regions is correlated with performance over the early stages of the WPT, but that striatal regions play a more crucial role in implicit probabilistic learning performance.
On a behavioural level, the current results differ from our previous findings employing the WPT (Weickert et al., 2011). In our previous study, FTD patients were impaired during the later stages of the weather prediction task in comparison to healthy controls. In the present study however, we found no significant difference in performance between groups in the latter stages of the task. This could be explained by the slightly older mean age of the control group used in this study. Instead, a subset of FTD patients in the present study performed particularly poorly during the early stages of the task but performance improved to control levels as the task progressed. Our current results are, however, much more consistent with previous WPT findings, which show that basal ganglia patients perform poorly during the early stages of the WPT, while they improve to control level as the task progresses. For example, Knowlton et al. (1996a) observed that PD patients performed very poorly over the first 50 trials of the WPT but their performance gradually improved as the task progressed past the 50 trial stage. They posit that this could be due to associations having been detected and memorised resulting in later retrieval being dependent upon declarative memory systems, while the early stages of the task are more striatal dependent. There is growing evidence showing that calculation of such outcome expectancies are dependent upon striatal brain regions in healthy participants (Behrens et al., 2007; Hare et al., 2008) which dovetails nicely with our findings.
Similarly, our imaging results support previous findings by showing that striatal regions are important for intact performance on the WPT (Knowlton et al., 1994, 1996a). Nevertheless, Knowlton et al. (1996a) have argued that learning deficits on the WPT are specifically related to striatal pathology after observing that patients with frontal lobe lesions were unimpaired on the task. Our results do not concur with this view as frontal regions and in particular the OFC, medial PFC and frontopolar cortex correlated with performance on the WPT as well. Our findings are therefore much more in line with other studies showing that lesions to the orbitofrontal cortex also significantly impairs performance on the WPT (Chase et al., 2008) as well as recent fMRI studies showing concurrent activations in striatal and frontal brain regions during WPT performance, including medial PFC and OFC activations (Aron et al., 2006; Poldrack and Foerde, 2008).
The subcortical findings of putamen and caudate nucleus atrophy being associated to probabilistic learning support previous findings (Moody et al., 2004). The putamen and other basal ganglia structures have been associated with learning to predict outcomes on the basis of feedback. For example, Shohamy et al. (2004) found PD patients to be impaired on a feedback version of a probabilistic learning task but not on a nonfeedback version. Slowed learning over the early stages of the WPT observed in the current study, could reflect impairments in the ability to learn and predict outcomes on the basis of feedback as a result of atrophy to the putamen. This possibility is supported by the results of the second VBM analysis of this study, which contrasted the subset of FTD patients who showed delayed learning during the early stages of the WPT and controls and found that the slow acquisition FTD group had significant atrophy more specifically in the putamen and caudate nucleus. The results of this analysis suggest that atrophy to striatal regions may lead to a greater deficit on learning performance than atrophy to frontal regions. This further implies that structures within the striatum may be more crucial for probabilistic association learning than frontal regions.
Although we have linked a slowed initial acquisition to atrophy to fronto-striatal regions, it is striking that despite their atrophy all patients reach performance levels equal to controls across all trials. One possible explanation for this surprising observation could be that we saw mostly left lateralised fronto-striatal atrophy being associated with the probabilistic learning system and thus the fronto-striatal structures in the right hemisphere might have been able to compensate the learning deficits over time. Alternatively the results of this study could imply that fronto-striatal atrophy is directly related to the speed that individuals acquire competency over the early stages of the WPT and as such suggests that fronto-striatal structures may subserve acquisition related aspects of the task rather than being important for sustained performance, which needs to be addressed in future studies. It is also interesting to note that two FTD patients (1 bvFTD and 1 SD) outperformed controls over the early stages of the WPT in the present study (Fig. 2B). One potential explanation for this finding could be the interaction or competition between declarative and non-declarative memory systems during the performance of this task (Moody et al., 2004; Poldrack and Rodriguez, 2004). There is evidence that damage to structures subserving declarative memory can lead to improvements in implicit memory performance. This has been reported in Alzheimer's disease patients with explicit memory deficits who significantly outperform both patients with MCI and healthy controls on the WPT (Klimkowicz-Mrowiec et al., 2008). Our findings of MTL atrophy being correlated with faster acquisition over the early stages of the task lend further support this theory, which, however, needs to be confirmed in future studies.
From a clinical perspective, implicit probabilistic association learning might allow the detection of striatal dysfunction in FTD patients on a cognitive level. A combination of structural imaging and behavioural performance on tasks such as the WPT could be a good diagnostic indicator for striatal problems in these patients. This seems particularly helpful for detection of a subgroup of patients having more striatal damage. It is currently not clear what influence this striatal damage has for the symptoms seen in FTD, however FTD patients have been shown to present with severe decision making problems (Gleichgerrcht et al., 2010). Such decision making deficits have usually been attributed to the frontal atrophy and dysfunction seen in this group. Our results raise the question as to whether some striatal damage might also contribute to the decision making deficits seen in these patients, in particular in decision making which requires the prediction of outcomes based on feedback.
There are a few limitations of our study. For example, we did not have neurodegenerative comparison groups with more pure striatal dysfunction, such as Huntington's or Parkinson's patients. It would be further interesting to investigate the longitudinal changes in fronto-striatal atrophy in FTD, i.e. is the striatal atrophy a knock-on effect from the substantial frontal atrophy? Finally, are the different clinical subtypes (bvFTD, SD, PNFA) associated with different levels of frontal or striatal atrophy and how does this impact on their probabilistic learning? Interestingly, not all regions found to correlate with slow acquisition on the WPT in the present study are consistent with the results of previous fMRI studies which suggest that early trials of the WPT are dependent upon PFC and/or hippocampus (Poldrack et al., 2001). This discrepancy needs to be investigated in future studies.
Taken together, we have shown that FTD patients show a great deal of variability in learning ability over the early stages of probabilistic association learning. The variability in WPT performance can be associated with atrophy in fronto-striatal regions, with striatal atrophy being particularly related to the poorest initial learning performance on probabilistic association learning. These findings further corroborate the notion that the WPT is a striatal sensitive task, which however, also requires the intactness of ventromedial prefrontal cortex structures. Identification of probabilistic association learning impairment can therefore been seen as a diagnostic marker of fronto-striatal deficits, which in turn will inform everyday decision making deficits seen in FTD patients.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2012.11.001.
Acknowledgments
We would like to thank the participants and their families, especially those who donated their brain tissue after death. Data for the clinical study was obtained from the FRONTIER clinic at Neuroscience Research Australia, which is funded by an Australian Research Council Centre of Excellence grant and National Health and Medical Research Council of Australia grants. This work was supported by the Australian Research Council [DP110104202 to M.H., FF0776229 to J.R.H.] and the National Health and Medical Research Council of Australia [APP1022684 to O.P.]. M.A.D. is supported by an Australian Rotary Health award.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Hodges J.R. Cambridge University Press; Cambridge: 2007. Frontotemporal Dementia Syndromes. [Google Scholar]
- Piguet O. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurology. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurology. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Knibb J.A., Kipps C.M., Hodges J.R. Frontotemporal dementia. Current Opinion in Neurology. 2006;19(6):565–571. doi: 10.1097/01.wco.0000247606.57567.41. [DOI] [PubMed] [Google Scholar]
- Garibotto V. Subcortical and deep cortical atrophy in frontotemporal lobar degeneration. Neurobiology of Aging. 2011;32(5):875–884. doi: 10.1016/j.neurobiolaging.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Looi J.C. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR. American Journal of Neuroradiology. 2008;29(8):1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe M. Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology. 2003;60(6):1005–1011. doi: 10.1212/01.wnl.0000052685.09194.39. [DOI] [PubMed] [Google Scholar]
- Chow T.W. Magnetic resonance imaging in frontotemporal dementia shows subcortical atrophy. Dementia and Geriatric Cognitive Disorders. 2008;26(1):79–88. doi: 10.1159/000144028. [DOI] [PubMed] [Google Scholar]
- Weickert T.W. Probabilistic association learning in frontotemporal dementia and schizophrenia. Cortex. 2011;21(8):1879–1888. doi: 10.1016/j.cortex.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Knowlton B.J., Mangels J.A., Squire L.R. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- de Wit S. Stimulus-outcome interactions during instrumental discrimination learning by rats and humans. Journal of Experimental Psychology. Animal Behavior Processes. 2007;33(1):1–11. doi: 10.1037/0097-7403.33.1.1. [DOI] [PubMed] [Google Scholar]
- Hare T.A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127(Pt 4):851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Perretta J.G., Pari G., Beninger R.J. Effects of Parkinson disease on two putative nondeclarative learning tasks: probabilistic classification and gambling. Cognitive and Behavioral Neurology. 2005;18(4):185–192. doi: 10.1097/01.wnn.0000187939.81541.1d. [DOI] [PubMed] [Google Scholar]
- Knowlton B.J. Dissociations within nondeclarative memory in Huntington's disease. Neuropsychology. 1996;10(4):538–548. [Google Scholar]
- Knowlton B.J., Squire L.R., Gluck M.A. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1(2):106–120. [PubMed] [Google Scholar]
- Poldrack R.A., Foerde K. Category learning and the memory systems debate. Neuroscience and Biobehavioral Reviews. 2008;32(2):197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin G.R. Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. Journal of Neuroscience. 2012;32(15):5333–5337. doi: 10.1523/JNEUROSCI.5756-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps C.M., Hodges J.R., Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Current Opinion in Neurology. 2010;23(6):628–632. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Good C.D. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. FMRIB technical report TR07JA1. 2007. Non-linear optimisation. ( www.fmrib.ox.ac.uk/analysis/techrep) [Google Scholar]
- Andersson J.L.R., Jenkinson M., Smith S. FMRIB technical report TR07JA2. 2007. Non-linear registration, aka Spatial normalisation. ( www.fmrib.ox.ac.uk/analysis/techrep) [Google Scholar]
- Rueckert D. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Rosen H.J. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Jack C.R., Jr. Comparisons between Alzheimer disease, frontotemporal lobar degeneration, and normal aging with brain mapping. Topics in Magnetic Resonance Imaging. 2005;16(6):409–425. doi: 10.1097/01.rmr.0000245457.98029.e1. [DOI] [PubMed] [Google Scholar]
- Behrens T.E. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10(9):1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Chase H.W. The role of the orbitofrontal cortex in human discrimination learning. Neuropsychologia. 2008;46(5):1326–1337. doi: 10.1016/j.neuropsychologia.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Gluck M.A., Poldrack R.A. Long-term test-retest reliability of functional MRI in a classification learning task. NeuroImage. 2006;29(3):1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T.D. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behavioral Neuroscience. 2004;118(2):438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Rodriguez P. How do memory systems interact? Evidence from human classification learning. Neurobiology of Learning and Memory. 2004;82(3):324–332. doi: 10.1016/j.nlm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Klimkowicz-Mrowiec A. Severity of explicit memory impairment due to Alzheimer's disease improves effectiveness of implicit learning. Journal of Neurology. 2008;255(4):502–509. doi: 10.1007/s00415-008-0717-x. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E. Decision-making cognition in neurodegenerative diseases. Nature Reviews. Neurology. 2010;6(11):611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Interactive memory systems in the human brain. Nature. 2001;414(6863):546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]


