Abstract
A number of studies suggest that the clinical manifestation of neurological deficits in hepatic encephalopathy results from pathologically synchronized neuronal oscillations and altered oscillatory coupling. In the present study spontaneous and evoked oscillatory brain activities were analyzed jointly with established behavioral measures of altered visual oscillatory processing. Critical flicker and fusion frequencies (CFF, FUF) were measured in 25 patients diagnosed with liver cirrhosis and 30 healthy controls. Magnetoencephalography (MEG) data were collected at rest and during a visual task employing repetitive stimulation. Resting MEG and evoked fields were analyzed. CFF and FUF were found to be reduced in patients, providing behavioral evidence for deficits in visual oscillatory processing. These alterations were found to be related to resting brain activity in patients, namely that the lower the dominant MEG frequency at rest, the lower the CFF and FUF. An analysis of evoked fields at sensor level indicated that in comparison to normal controls, patients were not able to dynamically adapt to flickering visual stimulation. Evoked activity was also analyzed based on independent components (ICs) derived by independent component analysis. The similarity between the shape of each IC and an artificial sine function representing the stimulation frequency was tested via magnitude squared coherence. In controls, we observed a small number of components that correlated strongly with the sine function and a high number of ICs that did not correlate with the sine function. Interestingly, patient data were characterized by a high number of moderately correlating components. Taken together, these results indicate a fundamental divergence of the cerebral resonance activity in cirrhotic patients.
Abbreviations: CFF, critical flicker frequency; CON, control; CSI, component similarity index; EEG, electroencephalography; EMG, electromyogram; ERA, event related averages; FUF, fusion frequency; GSI, general similarity index; GW, Gabor wavelet; HE, hepatic encephalopathy; HESA, hepatic encephalopathy scoring algorithm; ICA, independent component analysis; MEG, magnetoencephalography; MELD score, model of end-stage liver disease-score; MSC, magnitude squared coherence; PCA, principal component analysis; SSVEF/SSVEP/SSVER, steady state visual evoked field/potential/response; SW, sine wave
Keywords: Visual steady state evoked fields, Liver cirrhosis, Impaired neuronal oscillations, Critical flicker and fusion frequency, Resting frequency
Highlights
-
•
Cirrhotic patients show a fundamental divergence of cerebral resonance activity.
-
•
Patients show an impaired dynamical adaptation to visual flickering stimulation.
-
•
The MEG findings were significantly related to neuropsychological testing.
1. Introduction
The relationship between liver disease and mental disorders was discovered centuries ago (Morgagni, 1769). However, the effects of the metabolic disturbances associated with liver disease on human brain function remain poorly understood. With a prevalence of 20 to 80% (Kircheis et al., 2007), hepatic encephalopathy (HE) is a common neuropsychiatric disorder frequently following liver cirrhosis. Clinical symptoms associated with HE vary, and range from rather subtle changes in the state of vigilance and altered sleep patterns to very prominent, potentially permanent changes of personality. States of acute HE result in strongly impaired cognitive (confusion, disorientation) and motor (lethargy, stupor, coma) abilities (Butterworth, 2007). Even mild forms of HE can seriously impair a patient's daily functioning (Riordan and Williams, 2010). It is therefore likely that HE induces fundamental dysfunctions in different systems of the human central nervous system (Timmermann et al., 2008). To better understand the consequences of HE on human brain function we studied ongoing and event-related brain oscillations in a sample of 25 patients diagnosed with liver cirrhosis using whole-head magnetoencephalography (MEG).
Recent studies suggest that the clinical manifestation of neurological deficits of HE result from pathologically synchronized neuronal oscillations and altered oscillatory coupling. For instance, ongoing or spontaneous oscillatory activity appears to be slowed in HE patients (Foley et al., 1950; Davies et al., 1991). Moreover, a wide range of studies report a basic deficit in cerebral oscillatory processing in this patient group, in particular in the motor system (Timmermann et al., 2002, 2003, 2005, 2008). For example, Timmermann et al. (2002) and colleagues studied the coupling between the surface EMGs of the hand muscles and oscillations in the primary motor cortex (M1) and found that tremor patients showed excessive EMG-M1 coupling at the individual tremor frequency when elevating their arm. The same group could demonstrate that the smaller the peak frequency of cortico-muscular coherence is the clinically worse the HE (Timmermann et al., 2008). Interestingly, this result was accompanied by another dysfunction within the visual system, namely the impaired ability to perceive oscillating visual stimuli. This finding was in line with the preceding observation by Kircheis et al. (2002). They demonstrated that the critical flicker or fusion frequency (CFF), a neuropsychological behavioral test, is reduced even in mild forms of HE. Timmermann and colleagues suggested that this reduction may reflect a psychophysical marker for slowed oscillatory processing in the patients' human visual system and might thus provide a reliable and easy to handle non-invasive tool to detect mild forms of HE before the disease worsens. At present it is not known whether the behavioral deficits measured in CFF are directly related to the resting-state, ongoing brain activity, indicative of a more fundamental dysfunction, or more specifically relate to abnormal visual processing. Deficient cortical visual processing should be reflected in alterations of steady-state visual evoked potentials (SSVEPs).
SSVEPs can be generated by a blinking or moving visual stimulus at a constant stimulus frequency, which in turn elicits a response in the brain at the same frequency and its even harmonics (Vialatte et al., 2010). In normal healthy volunteers SSVEPs can be found up to 90 Hz (Herrmann, 2001). The main mechanism generating SSVEPs is still open to debate. For example, Capilla et al. (2011) recently suggested that SSVEPS can be explained by temporal superposition of transient event-related potentials. A more common view is supported for instance by Moratti et al. (2007), who provided evidence that the main mechanism generating SSVEPs is not a single trial amplitude modulation, but rather a phase alignment of the ongoing EEG at the driving stimulus frequency. The authors state that this phase alignment could serve as a filtering function that enhances the signal-to-noise ratio in neural systems, which can be achieved by synchronizing neural activity to the stimulus train via phase alignment.
Irrespective of this debate are SSVEPs widely used to study pathological brain dynamics, for instance in Alzheimer's disease and other neurodegenerative disorders, schizophrenia, migraine, depression, epilepsy, stress, anxiety, and autism (Vialatte et al., 2010). Moreover, SSVEPs are also applied to study higher cognitive functions (Janson and Kranczioch, 2011). For instance, Kaspar et al. (2010) used SSVEPs to examine cortical mechanisms underlying object recognition. They showed that SSVEP amplitudes were different for flickering pictures of familiar and unfamiliar objects. The morphology of the difference depended on the flicker frequency, with higher SSVEP amplitudes for familiar objects when the flicker frequency was 12 or 15 Hz, but lower SSVEP amplitudes for familiar objects when flicker frequency was 7.5 Hz. Irrespective of flicker frequency the familiar/unfamiliar effect was found to be rather localized and restricted to posterior electrode sides. At present it remains open whether the effect of object recognition on SSVEPs is preserved in HE patients or other patient groups.
Given the evidence for generally slowed oscillatory brain activity and pathological synchronization in HE (Timmermann et al., 2005) and the proposed impairment in the processing of visual oscillatory (flicker-) stimuli in HE patients (Kircheis et al., 2002; Timmermann et al., 2008), the aim of the present study was to follow-up on these findings and characterize alterations in the behavioral CFF, resting-state brain activity, and steady-state visual evoked fields (SSVEF) responses in an object recognition paradigm in this patient group. We expected to confirm a lowered behavioral CFF in HE patients as compared to a healthy, age-matched control group (CON). Regarding ongoing, resting brain activity, we focused on the prominent alpha activity and predicted that the resting alpha peak frequency would be lower in HE patients as compared to CON. In addition, SSVEFs were collected in an object recognition paradigm, similar to the one used by Kaspar et al. (2010). SSVEF responses obtained in an object recognition paradigm were expected to be significantly different in the HE group compared to CON, and the familiar vs. unfamiliar condition effect in this paradigm was expected to be impaired in HE patients.
2. Materials and methods
2.1. Participants
30 controls (CON, 15 females; age from 19 to 71 years) and 25 patients (HE, 5 females. age from 28 to 73 years) diagnosed with liver cirrhosis participated in the study. One healthy volunteer was almost blind on the left eye but had normal vision on the right eye; the other 29 had normal or slightly impaired vision. 22 of the patients had normal or slightly impaired vision and 3 were almost blind on either the left or the right eye.
The grade of hepatic encephalopathy was determined using the hepatic encephalopathy scoring algorithm (HESA according to Hassanein et al. (2008)). Sixteen of the 25 patients in the HE group were rated with grade 0, seven patients with grade 1 and two patients with grade 2 of hepatic encephalopathy. Moreover, the raw number of neurological and clinical symptoms observed during the HESA procedure was assessed. Patients showed one up to eight symptoms, and the model of end stage liver disease (MELD) score ranged from 8 to 34 (maximum score 40, according to Wiesner et al., 2003). Twenty-four of the patients suffered from alcoholic liver cirrhosis, whereas one patient had an autoimmune hepatitis. Cirrhosis was confirmed by biopsy or by a combination of clinical, biochemical and imaging data. Patients were at least one week and at the most eight years abstinent from alcohol. All patients were recruited from the Internal Medicine Department of the University Hospital Jena. Due to organizational constraints, no additional blood tests were performed on the day of recording, therefore the blood values were taken from the patients' health record. Clinical patient data are provided in Table 1.
Table 1.
Clinical patient data.
| Type | Cirrhosis patients (n = 25) |
||
|---|---|---|---|
| Median (Q25/Q75)/absolute value (% of 18) | Min/max | ||
| Demography | |||
| Age (years) | 58.5 (46.5/68) | 28/73 | |
| Sex | Female | 6 (25%) | |
| Male | 18 (75%) | ||
| Clinical characteristics | |||
| MELD score | 13 (10.75/18.5) | 7/34 | |
| CHILD Pugh | A | 1 (4%) | |
| B | 14 (56%) | ||
| C | 7 (28%) | ||
| HESA points | 3.75 (2.5/5.6) | 1/8 | |
| Etiology for liver cirrhosis | |||
| Alcoholic | 24 (96%) | ||
| Other | 1 (4%) | ||
| Diagnoses | |||
| Ascites | 19 (76%) | ||
| Hepatocellular carcinoma | 4 (16%) | ||
| Diabetes mellitus (type II) | 8 (32%) | ||
| Hepatitis | B | 1 (4%) | |
| NASH | 1 (4%) | ||
| Steatohepatitis | 1 (4%) | ||
| Autoimmune | 1 (4%) | ||
| Spontaneous bacterial peritonitis | 3 (12%) | ||
| Splenomegaly | 3 (12%) | ||
| Esophagus varices | 15 (60%) | Grades I–III | |
Child Pugh was not determined in 2 patients.
All participants gave their written informed consent prior to the experiments. All healthy volunteers were paid 6 Euros/h for participation, whereas patients received no money for participating in the study. The study was approved by the local ethics committee of the University Hospital Jena (2969-11/10).
2.2. Procedure and stimuli
2.2.1. Critical flicker and fusion frequency
The critical fusion frequency (FUF) is defined as the frequency at which a flickering light is perceived by the patient as stable and the critical flicker frequency (CFF) is defined as the frequency at which a flickering light is perceived as flickering. These values were determined with the Schuhfried Test System (Dr. Schuhfried Inc.). After a standardized instruction and a short training period, both values were determined eight times per participant. For a detailed description of the test system, see Timmermann et al. (2008).
2.2.2. MEG recordings
Participants were seated comfortably in a magnetically shielded room. MEG was recorded with a 306-channel (204 gradiometers, 102 magnetometers) helmet-shaped neuromagnetometer (Vectorview, Elekta Neuromag Oy, Helsinki, Finland). MEG data were sampled at 1 kHz, and on-line a low pass filter at 330 Hz was applied. A 3D Digitizer (3SPACE FASTRAK, Polhemus Inc., USA) was used to locate anatomical locations (nasion and preauricular points) and the MEG localization coil sets.
2.2.3. Resting MEG
Resting MEG data were recorded with eyes open and eyes closed. Both conditions were repeated twice, each lasting 120 s. Data collection always started with the eyes open condition. Participants received verbal instructions to open or close their eyes every 120 s.
2.2.4. Object recognition paradigm
The stimulus set consisted of 30 meaningful pictures (condition familiar) from a standard picture library (Hemera Technologies, 1997) and 30 distorted and thus meaningless versions of the same pictures (unfamiliar condition). The spatial frequency of the distorted pictures was equal to the spatial frequency of the standard pictures (Busch et al., 2006; Müller et al., 2006).
Stimuli were presented using Presentation 14.1 (Neurobehavioral Systems, Inc., San Francisco. CA). Pictures were presented centrally, with a size of approximately 7 × 7° in terms of visual angle and on a light-gray background (Müller et al., 2006). A trial consisted of the repeated presentation of a picture for 117 ms followed by gap of 16.7 ms. This resulted in a stimulus rate of 7.46 Hz. Stimulus presentation was repeated for 3000 ms. Each picture was presented twice. Participants were encouraged to avoid eye movements and blinks during the picture presentation and instead use the 1000 ms gap between two trials for blinking. To ensure attention to the picture presentation, a red dot was superimposed on the pictures in 24 of 60 trials. Participants were instructed to press a button as fast as possible when detecting the dot. Across subjects, the order of picture presentations was fully randomized.
2.3. Data analysis
2.3.1. Data preprocessing
In one HE-patient, neither FUF nor CFF was measurable because the patient did not detect the flickering light even at 25 Hz. In three other patients, only FUF was measured due to time constraints (CFF: n = 21, age 28–37, 4 females, FUF: n = 24, age 28–73, 4 females). One HE-patient was not measured in the MEG because of magnetic disturbances (n = 24, age 28–73, 5 females). Due to residual noise, the MEG datasets concerning resting frequency of three healthy volunteers had to be excluded from analysis (n = 29, age 19–69, 14 females). In one healthy volunteer, neither FUF nor CFF was measured due to time constraints (n = 29, age 19–71, 14 females).
All raw MEG data were filtered with Maxfilter Version 2.0.21 (Elekta Neuromag Oy, Finland) using the Signal Space Separation (SSS) method (Taulu and Simola, 2006). Further analysis was restricted to the data of the 102 magnetometers. Magnetometers covered the whole head and no magnetometer channels were excluded from the analysis.
Processing was performed with the open source software fieldtrip (Oostenveld et al., 2011, Version 20110503, running under MATLAB R2010a). Resting MEG data were pseudo-epoched separately for eyes open and eyes closed conditions. Each of the two segments per condition was subdivided into 15 eight-second pseudo-epochs, resulting in 30 eyes open and 30 eyes closed epochs, respectively. The frequency analysis was performed with a Morlet wavelet at a frequency resolution of 0.375 Hz and a time resolution 420 ms at 7.5 Hz. Data were band-pass filtered from 0.3 to 30 Hz. For each participant, we determined the peak amplitude and corresponding frequency value of the observed dominant frequency. In the object recognition paradigm, total power was obtained by digitally band-pass filtering data from 0.3 to 50 Hz. Data were segmented from 9 s pre-flicker to 9 s post-flicker. Trials afflicted with squid jumps were generally rejected.
2.3.2. Independent component analysis and cross-correlation approach
For resting data and evoked activity, respectively, extended infomax independent component analysis (ICA) with principal component analysis (PCA) dimension reduction was performed (Lee et al., 2000), reducing the segmented raw data to 50 independent components (IC). The ICA clearly separated components reflecting artifacts such as heartbeat or vertical eye movements in both HE and control groups. In the control group, one to three components derived from the evoked data set appeared to capture brain activity related to the flickering stimulation. These maximally temporally independent components showed a recognizable quadrupolar pattern in the occipital area and their frequency content reflected the 7.5 Hz stimulation frequency (as an example, cf. Fig. 1). This pattern turned out to be much less prominent in the HE group. In order to quantify this observation for each participant, the IC time courses (IC activations) were averaged across trials, thus creating event-related averages (ERA) for each of the 50 ICs (IC-ERA). The steady-state content of all ICs was assessed by applying a cross-correlation approach to the ERA. To this end, a synthetic Gabor wavelet (GW) was revealed by multiplying a sine wave (SW) of length 2 s representing the stimulation frequency of 7.5 Hz with a Gaussian envelope. The GW was then cross-correlated with each IC-ERA. The resulting correlation coefficient is a measure of the correspondence of the shapes of the reference waveform (GW) and the functional waveform (IC-ERA) (Bandettini et al., 1993).
Fig. 1.
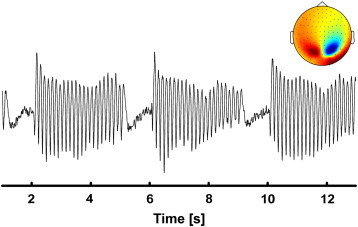
Representative flicker IC time course with its corresponding topography of the 102 magnetometer channels in one control.
The cross-correlation of GW and IC-ERA was calculated for each sampling point, which represents a time-variant measure of cross correlation. For each sampling point of this function, the magnitude-squared coherence (MSC (1)) was calculated, which is defined as the squared cross-spectral density between GW (i) and one IC-ERA (j) divided by the product of the autospectral density of GW and one IC-ERA, respectively.
| (1) |
Coherence values lie between 0 and 1. CSIi,j(f) = 1 indicates a linear relation between GW (i) and IC-ERA (j) at a frequency f (7.5 Hz). We used the maximum value of MSC for each IC-ERA to quantify the maximum possible correlation between GW and IC-ERA. This value was normalized to the cross-spectral density between SW and GW to obtain values from 0 to 1. In total 50 correlation values per participant were obtained and named component similarity index (CSI). CSIs were binned into 10 equally spaced bins ranging from 0 to 1 in steps of 0.1. A weighted sum of all CSIs per participant was calculated multiplying each bin number with the average over all subjects (e.g., number of CSIs (n1) in bin 1 multiplied with the average number of CSIs over all subjects in bin 1 () + number of CSIs in bin 2 (n2) multiplied with the average number of CSIs over all subjects in bin number 2 () and so on; (Eq. (2)). This weighted sum will further be called global similarity index (GSI) and is used to describe the overall grade of coupling of brain activity measured via the MEG to the steady state frequency.
| (2) |
2.3.3. Analysis of the onset and offset behavior
To characterize the onset and offset behavior of the flicker frequency, the total power of the familiar condition was analyzed. Firstly, the spectrum was averaged to the 102 magnetometer channels and the envelope of the 7.5 Hz flicker frequency was extracted. Furthermore, a 12 second epoch was considered, containing three flicker trains which took place before, after and at stimulus onset. The three maximal and minimal power values for the 12 second segment were obtained. These values corresponded to the maximal/minimal power in response to the onset and offset of the center (second of the three) flicker stream and in response to the preceding and following stimulus streams. To obtain a robust value, the three maxima and minima were averaged and their difference calculated for each participant.
2.3.4. Analysis of familiar and unfamiliar trials
The “familiarity effect” of the object recognition paradigm was studied on segments covering 9 s pre-flicker to 9 s post-flicker. Eye blink and heart beat ICs were rejected. Trials for familiar and unfamiliar objects were averaged separately. Evoked power for familiar and unfamiliar trials was obtained by a frequency analysis at a frequency resolution of 0.375 Hz with a time resolution 420 ms at 7.5 Hz with a Morlet wavelet. Data were baseline corrected from − 3 to 0 s before stimulus onset. The peak amplitude of the 7.5 Hz SSVEF was obtained.
2.4. Statistical analysis
2.4.1. Behavioral data
In the object recognition paradigm subjects were asked to respond to an occasionally occurring red dot. The number of correctly detected red dots and mean reaction times were calculated. Data were statistically analyzed with a univariate general linear model (GLM) with the between subject-factor CASE (1 = CON, 2 = HE). Critical fusion (FUF) and flicker frequency (CFF) were also analyzed with a univariate general linear model (GLM) with the between subject-factor CASE (1 = CON, 2 = HE).
2.4.2. Resting MEG
For the two sets of variables “Frequency” and “Amplitude”, the GLM was performed with the within-subject factor EYE (for eyes open and eyes closed) and the between-subject factor CASE (1 = CON, 2 = HE). A univariate GLM was additionally performed using the mean eyes open and eyes closed frequency of each participant comparing patients and controls. For statistical analysis, amplitudes were logarithm transformed.
2.4.3. Evoked data
A multivariate GLM was performed with the 8 dependent variables BIN (as only one of the patients showed a hit at bin 9 and none of the HE patients showed hits at bin 10, bins 8 to 10 were merged) and the fixed factor CASE (1 = CON, 2 = HE) to test differences between patients and healthy volunteers within each category. P-values were ε-corrected according to Greenhouse–Geisser where appropriate. Results were corrected according to Bonferroni and Holm.
For statistical analysis, amplitudes were logarithm transformed (basis 10). To analyze differences between familiar and unfamiliar pictures, paired t-tests were used to compare familiar and unfamiliar responses.
2.4.4. Correlation analyses
For patient data, correlations between FUF/CFF, clinical data, the peak resting frequency, the difference of maximum and minimum and the GSI were calculated by using the Spearman–Rho rank correlation (one-tailed). When FUF/CFF was involved, a partial correlation was used to correct for age, since CFF and FUF decrease with age (Misiak, 1951).
3. Results
3.1. Behavioral data
The red dot was equally well detected in both groups. Eighty-three percent (25/30) of the controls and 88% (22/24) of the patients detected all 24 red dots. Detection category was never below 20/24. The reaction time was significantly lower in the HE group (mean 446.7 ms ± 45.8 SD) in comparison to the control group (mean 424.3 ms ± 14.9 SD, F(1,54) = 6.07; p = 0.017).
Both FUF and CFF were lower in patients (FUF: 32.7 ± 2.6 and CFF: 35.3 ± 4.5) in comparison to controls (FUF: 35.5 ± 3.2 and CFF: 37 ± 3.6), though this difference was more pronounced in FUF (FUF: F(1,51) = 11.54; p = 0.001; CFF: F(1,48) = 2.23; p = 0.14).1
3.2. Resting MEG
Fig. 2 shows the results of the resting MEG. No differences were found concerning the location of the main resting activity. However, the peak frequencies of patients and controls differed significantly. In the CON group, the frequency with the highest power for eyes open was 10.22 ± 1.5 Hz (mean ± SD) and 10.26 ± 1.4 Hz for eyes closed and thus fell clearly in the alpha frequency range. As compared to that the mean ± SD peak frequency for HE was 8.16 ± 1.4 Hz for eyes open and 7.99 ± 1.1 Hz for eyes closed, thus falling into the lower alpha/upper theta frequency range. Peak frequencies were not significantly different between eyes open and eyes closed (F(1,49) = 0.074; p = 0.79). Also the interaction eye ∗ case did not reach significance (F(1,49) = 0.47; p = 0.5). However, the eyes closed alpha frequency was significantly higher in the CON group (F(1,49) = 40.757; p < 0.001).
Fig. 2.
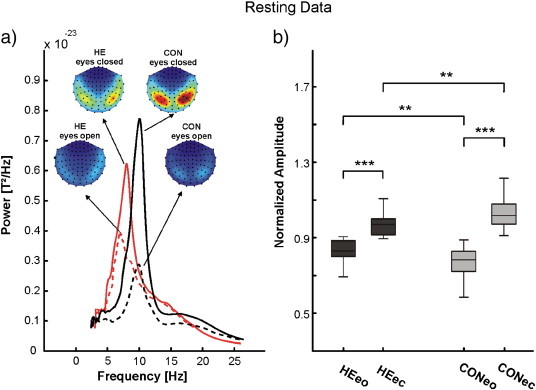
Resting frequency of 102 magnetometers of the eyes open (eo) and eyes closed (ec) conditions in patients (HE) and controls (CON). a) Frequency power plot with the corresponding topographies and b) illustration results of the statistical analysis.
In both groups, the maximum power was significantly greater in the eyes closed condition (F(1,49) = 58.3; p < 0.001). The interaction eye ∗ case was highly significant (F(1,49) = 8.6; p = 0.001), meaning that the amplitude difference between eyes open and eyes closed was greater in the CON group (cf. Fig. 2).
3.3. Evoked data
On the descriptive level, time–frequency analysis revealed clearly distinguishable 7.5 Hz evoked activity and a clear separation from ongoing alpha activity for individuals of the control group. However, in the patient group, evoked activity was often time-invariant and showed no temporal correspondence to the flicker stimulation. That is, in many individuals no gap in the activity during the 1000 ms period between the picture stimulation could be observed (cf. Fig. 3 for exemplary single cases and group data). This was confirmed by the comparison of the maximum/minimum differences in patients and controls. In comparison to the patients, the differences between maximum and minimum were significantly higher in controls (F(1,52) = 9.198; p = 0.004, cf. Fig. 3b).
Fig. 3.
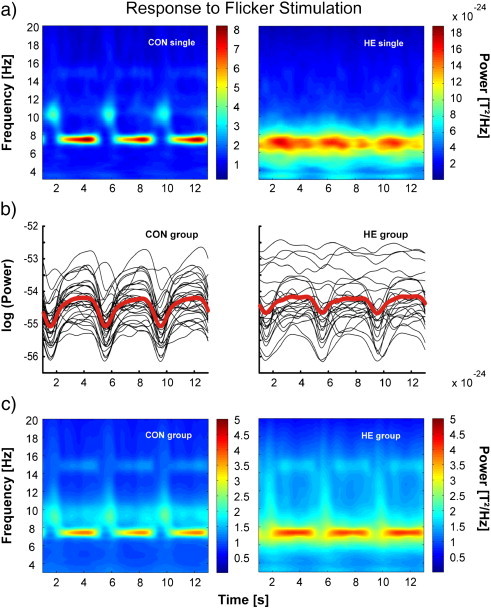
Response to flicker stimulation in patients and controls. a) Total power plot of the steady state response averaged over 102 magnetometers for a representative control and patient. b) Envelopes of the 7.5 Hz frequency of each participant (thin lines) and the mean (thick red line). c) Grand mean total power plots (left) for controls and HE-patients (averaged across familiar pictures) of the steady state response averaged over 102 magnetometers.
Comparing the power of the evoked activities after stimulation for familiar and unfamiliar pictures, power was significantly higher for unfamiliar pictures in comparison to the familiar pictures (cf. Fig. 4). This effect could be found both in controls and patients, though it was clearly stronger in the control group (CON group: t = − 4.256; df = 29; p < 0.001, HE group t = − 2.045; df = 23; p = 0.053), since the mean amplitude difference was smaller in HE patients (0.7 ± 0.37 log(T2/Hz)) in comparison to controls (1 ± 0.38 log(T2/Hz)). On a descriptive level, in 16 of 24 HE patients (66%), power was higher for unfamiliar pictures, in CON it was 24 of 30 (80%). Moreover, it could be observed that the alpha power was lower in the unfamiliar condition (cf. Fig. 4b). The effect was more pronounced in the control group.
Fig. 4.
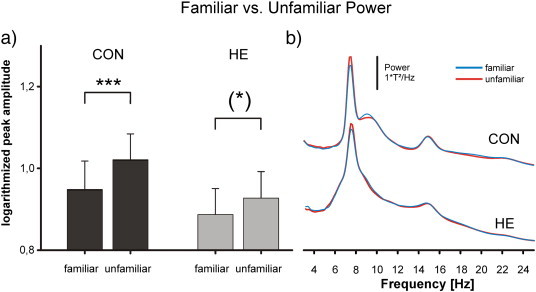
Comparison of familiar and unfamiliar trials. a) Statistical comparison between familiar and unfamiliar responses (evoked power). b) Waveforms of the 102 magnetometer total activity, prominent peak and harmonics.
For the cross-correlation analysis and the GSI, trials of familiar and unfamiliar pictures were merged. On a descriptive level, about 50% of CSIs fell in the first bin (0–0.1) in the control group, indicating very low similarity between the corresponding IC-ERAs and the 7.5 Hz GW. This proportion was clearly reduced in the HE group.2 Also for the control group a higher number of CSIs fell into bins 6 and 7 (0.5–0.7). More CSIs fell into bins 2 to 4 (0.1–0.4) in the HE group as compared to the CON group (cf. Fig. 5b). Statistical analysis revealed significant and by trend differences between groups in bins 1–3 only (see Table 2 and Fig. 4). In correspondence with these results, the GSI was significantly smaller in patients than in controls (F(1,44) = 12.8; p = 0.001, see Fig. 5c).
Fig. 5.
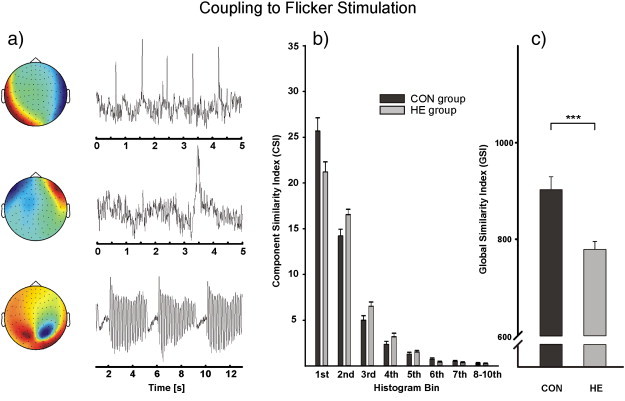
a) Representative IC time courses with their corresponding topographies of the 102 magnetometer channels in one control. Upper part: representative extracted time course of a typical heartbeat artifact. Middle: representative extract time course and topography of a typical eye blink artifact, and lower: typical IC-ERA correspondent to the flickering stimulation b) CSI group average of the two study cohorts for the eight bins. c) GSI group average in patients and controls.
Table 2.
Statistical comparison of the number of CSIs in HE patients and controls.
| F-value | p-Value | Observed power | Difference | Mean (CON) | Mean(HE) | |
|---|---|---|---|---|---|---|
| BIN 1 | F(1,44) = 5.1 | 0.029 | 60% | HE < Con | 26.4 ± 7.6 | 22 ± 4.9 |
| BIN 2 | F(1,44) = 6.8 | 0.012 | 72% | HE > Con | 14 ± 3.9 | 16.6 ± 2.6 |
| BIN 3 | F(1,44) = 2.9 | 0.098 | 37% | HE > Con | 4.8 ± 2.6 | 6 ± 1.9 |
| BIN 4 | F(1,44) = 2.2 | 0.15 | 30% | HE > Con | 2.2 ± 1.6 | 3 ± 1.8 |
| BIN 5 | F(1,44) = 1.1 | 0.3 | 18% | HE > Con | 1.17 ± 0.96 | 1.4 ± 0.7 |
| BIN 6 | F(1,44) = 1.4 | 0.25 | 20% | HE < Con | 0.65 ± 0.75 | 0.42 ± 0.5 |
| BIN 7 | F(1,44) = 1.6 | 0.22 | 23% | HE < Con | 0.5 ± 0.57 | 0.3 ± 0.5 |
| BIN 8-10 | F(1,44) = 0.009 | 0.93 | 5% | HE ≈ Con | 0.29 ± 0.53 | 0.28 ± 0.48 |
Mean represents the mean value of bins in a bin. Trends and significant values are displayed in bold face.
3.4. Correlation between behavioral, clinical and MEG data
In patients, a significant correlation was observed between the resting peak frequency (eyes closed) and CFF (r = 0.6; n = 20; p = 0.003) as well as between the resting peak frequency (eyes closed) and FUF (r = 0.6; n = 23; p = 0.004), indicating that low peak frequencies were associated with low flicker and fusion frequencies. Interestingly, the number of symptoms assessed with the HESA also significantly correlated with CFF (r = − 0.55; n = 20; p = 0.007), with FUF (r = − 0.34; n = 23; p = 0.046) and significantly with the (eyes closed) resting peak frequency (rs = − 0.42; n = 24; p = 0.02), indicating that the higher the number of symptoms, the lower the peak frequencies, FUF and CFF. Generally, the higher the difference between maximum and minimum response in the object recognition paradigm, the higher was the CFF (rs = 0.63; n = 20; p = 0.002) and FUF (rs = 0.44; n = 23; p = 0.076) and the lower the number of symptoms assessed by HESA (rs = − 0.39; n = 24; p = 0.03).
4. Discussion
The present study provides clear evidence that patients with liver cirrhosis show a general slowing of resting-state oscillatory activity and a temporal and spatial blurring of evoked oscillatory activity. This is a clear indication of the presence of highly aberrant brain activity in this patient group. Importantly, the general oscillatory slowing was observed to be tightly linked not only to the severity of the symptoms in terms of clinical parameters, but also to general impairments in visual information processing.
4.1. CFF, FUF and clinical parameters
As already stated in the Results section, CFF and FUF values were lower than reported in the existing literature. This was due to a significantly lower background illumination, which is known to influence the frequency values (Foley, 1956). In liver cirrhosis patients, CFF and FUF were both reduced in comparison to controls. Moreover, our data indicate a tight correlation between CFF/FUF and HESA. The analysis of the flicker-fusion frequency has been used in the study of Alzheimer's disease (Croningolomb et al., 1991), psychoactive drugs (Smith and Misiak, 1976) or multiple sclerosis (Salmi, 1985), but lately also with cirrhotic patients. Beside a possible alteration in the retinal function (Eckstein et al., 1997), evidence has been provided for an impairment in the general functional efficiency of the cerebral cortex (Smith and Misiak, 1976; Kircheis et al., 2002). The observed decrease of the CFF/FUF in our HE patients is well in line with the results of Kircheis et al. (2001a, 2001b, 2001c, 2002) and Timmermann et al. (2008). Timmermann et al. (2002) argues that there exists a positive correlation between the impairment of thalamic or cortical processing of fast visual stimuli and the decline of the CFF. If not, a dysfunction in the cerebral processing of oscillatory visual stimuli is very likely, which is assumed to lead to the observed deficits in HE-patients (Timmermann et al., 2008). Moreover, our data suggest that the decline in CFF and FUF is linked to the worsening of clinical (e.g., MELD score) and psychological parameters (as assessed by the HESA). This finding is in good agreement to previous results (Kircheis et al., 2002), who found a strong relationship between the outcome of various psychometric tests and the flicker-fusion frequencies. These and other authors (Quero and Schalm, 1996) also reported a strong relation of plasma ammonia levels to the flicker-fusion frequency. Nevertheless, our results indicate that flicker-fusion frequency testing in patients with liver cirrhosis is indeed reliable, reproducible and also practicable in terms of clinical feasibility. However, in contrast to electromagnetic measures of brain activity it is not able to reveal the processes that underlie the alterations, i.e., a general slowing of oscillatory activity and the corresponding frequency bands.
4.2. MEG measurements
4.2.1. Resting frequency
The MEG resting data revealed a general slowing of oscillatory activity in liver cirrhosis patients. The amplitude difference between eyes open and eyes closed was found to be significantly smaller in patients compared to controls. Importantly, we also found a positive correlation between resting frequency and CFF/FUF, which highlights the link between behavioral and electrophysiological data.
Our MEG results confirm and complement earlier EEG studies (Foley et al., 1950; Davies et al., 1991) showing a decreased resting frequency in this patient group. The resting EEG basic rhythm is generally interpreted as a global expression of brain function (Amodio et al., 2001) and can therefore be seen as an indicator for the acute or chronic impairment of cerebral processing. This suggestion is corroborated by the observed positive correlation between resting peak frequency and flicker fusion frequency and additionally by the negative correlation between the resting peak frequency with HESA, indicating that a lower flicker fusion frequency is accompanied by clinical and psychological deficits. General background slowing was not only reported from HE patients, but also from patients with schizophrenia (Clementz et al., 1994), mild cognitive impairment and Alzheimer's disease (Zappoli et al., 1995) and bipolar disorder (Basar et al., 2012).
In both study groups, the power of the resting frequency was smaller in the eyes open condition, an effect known since the early days of EEG research (Berger, 1933). However, the power difference between eyes open and eyes closed was less pronounced in the patient group. The normally observed reduction of alpha power in the eyes open condition is assumed to be due to the higher level of alertness and the prominence of beta-waves (Dustman et al., 1962). We hypothesize that an overall reduction of alertness in liver cirrhosis-patients might be responsible for the smaller power difference between eyes open and eyes closed in these patients. However, this would have to be tested separately.
4.2.2. Evoked activity
Along with the resting activity, evoked activity was also found to be significantly impaired in liver cirrhosis patients.
4.2.2.1. Analysis of familiar and unfamiliar pictures
In controls, the evoked power elicited by familiar pictures was significantly smaller than the evoked power elicited by unfamiliar pictures. This result is in line with the results of Kaspar et al. (2010). In liver cirrhosis patients, the “familiarity effect” was also evident, but weaker. It can be hypothesized that the “familiarity effect” is not generated by the steady state evoked response (SSVER) itself, but rather by the modulation of the individual alpha or resting oscillations, which more or less overlap the steady state activity. The greater degree of overlap of stimulation and resting frequency in liver cirrhosis patients might reduce this modulation. Basar et al. (1999) for example showed that the presentation of a stimulus leads to the suppression of alpha activity. This effect could be clearly observed in our data, too: resting activity was only observed within the 1 second pauses between the flicker trains in controls, but the effect was not or less pronounced within the patient group. The fact that the difference between maximum and minimum power of the 7.5 Hz envelope in controls was significantly greater than in liver cirrhosis patients would account for the overlapping theory. However, different authors have shown that there is a close positive relation between the level of alpha suppression and the level of attention (Pfurtscheller, 1989; Mo et al., 2011). This has even been reported for the same familiar and unfamiliar objects by Gruber et al. (2006), showing that unfamiliar objects elicited in fact a stronger alpha suppression than familiar ones. Interestingly, on a descriptive level we also found smaller alpha power for unfamiliar pictures, likely because due to their unfamiliar content, unfamiliar pictures gain more attention than familiar ones. We assume that this effect was less pronounced in the patients' group because alpha suppression itself was less pronounced during flicker stimulation. However, even if the phenomenon of alpha suppression was present, this seems to be uncoupled to the “familiarity effect” itself, since it would result in a higher instead of a lower power elicited by the presentation of familiar pictures. Two explanations could account for our result. On the one hand, the 7.5 Hz activity could be overlapped by theta (5–7 Hz) instead of alpha activity. Theta rhythms are known to be strongly interacting with gamma oscillations and serve to integrate functional networks (Jensen and Colgin, 2007). It is also reported that there is a strong relation to attention (Kahana et al., 2001). On the other hand, unfamiliar pictures might trigger the reaction of networks different to those elicited by familiar pictures in terms of involving a different or a higher number of sources.
4.2.2.2. Cross correlation approach
The results of the cross correlation approach indicated a dynamical adaptation of the visual system to the flickering stimulus in the frequency spectrum in controls: a flicker response at the flicker frequency was clearly evident during flickering, but no response was generated when flickering was interrupted. This pattern was largely absent in liver cirrhosis patients. On the other hand, it could be clearly shown that the entrainment of the driving frequency was significantly weakened in liver cirrhosis patients. The analysis of independent components corroborated these results. Thus, analyses were able to capture aberrant brain activity in liver cirrhosis patients.
Intriguingly, in controls, the SSVER (steady state evoked response) was represented by a small number of distinct independent components (accompanied by a small number of high CSIs since the similarity between the IC-ERA and the Gabor wavelet was high in these components), but a high number of non-correlating components (found in the first bin). This was different in liver cirrhosis patients. These patients showed a high number of medium CSIs, which were found in bins 2–4, which indicated that the similarity between IC-ERA and the Gabor wavelet, while evident, was relatively small. Due to these different patterns in the CSIs the main differences between patients and controls were observed in the first four bins of the CSI. The reason for the lower similarity between IC-ERAs and the Gabor wavelet in patients might again be found in the greater proximity of resting and evoked activity in this group. That is, the greater overlap could result in a greater dependence of the measured signals, which would make a disentanglement of resting and evoked activity with ICA more challenging.
4.2.3. General methodical considerations
4.2.3.1. The cross-correlation analysis
The cross-correlation analysis of IC-ERA and a Gabor wavelet revealed a smaller GSI for liver cirrhosis patients in comparison to controls. In our approach, the number of ICA components that would result from ICA (101 for the present data set) was limited to 50 by running a PCA before the ICA. This reduction of the dimensionality of data can be justified by the fact that the number of true sources is indeed not known. However, the number of possible sources is likely not so very large in a macroscopic viewpoint within a short period (Ikeda and Toyama, 2000). Moreover, according to our experience, the visual evoked sources are very strong and occur within the first 50 components. The same holds for biogenic external (outside the helmet) disturbances (e.g., heart-beat) and internal (inside the helmet) disturbances (e.g., eye-blink). For the coherence analysis, we did not cancel out eye-blink and heart-beat artifacts. Since this kind of artifact was equally observed in controls as well as in patients, we would expect no difference in the outcome in terms of the GSI. However, also non-biogenic internal disturbances often found in patients or elderly people can cause artifacts, which can be observed as a small number of single components. These artifacts afflicted components may occur as the strongest 1–10 components. Future studies should explore in greater detail how the reduction of data dimensionality via PCA affects the GSI results. It would also be interesting to see whether GSI results are affected by the individually different number of components that entered our cross-correlation analysis due to the individually varying amount of non biogenic artifacts.
4.2.3.2. The MEG as a diagnostic tool in encephalopathy
It might seem far-fetched to relate macroscopic electrophysiological data from the MEG and liver function. However, Amodio et al. (2001) states that, from a clinical point of view, the EEG has already been very predictive of both episodes of overt HE and death within one year. Evidence has been given that the EEG provides information on metabolic encephalopathy because it reflects neuronal electrogenesis which, in turn, is quite sensitive both to the influence of nutritive and energy-providing metabolic systems, and to the influence of electrolyte homeostasis and of the clearance of toxic substances (Amodio et al., 2001). This is surely also true for the MEG, which, in comparison to the EEG, might be more suited to the purpose in clinical practice due to the shorter preparation time.
We assume that this kind of non-invasive method could also be a promising approach to study other types of encephalopathy and their worsening or improvement in time. This might be particularly interesting for those types of encephalopathy that are less well understood. Moreover, the long-term cortical consequences in survivors of a septic encephalopathy, for instance, are not well known but this knowledge might help shape future neuropsychological rehabilitation efforts.
4.3. General conclusions
To summarize, we found a massive impairment in oscillatory activity in liver cirrhosis patients both in resting and evoked recording conditions. Behavioral data and cognitive functions could be directly related to the MEG data. We conclude that SSVEFs in particular are a promising tool to study aberrant brain activity in HE patients and should be studied in other not so well-characterized clinical entities and patient groups such as septic encephalopathy. We believe that the GSI and the peak alpha frequency particularly are sensitive diagnostic tools to investigate the status of encephalopathy in these patients.
Acknowledgments
This work has been supported by the German Federal Ministry of Education and Research. We would like to thank Tina Radtke for recording assistance.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Grant: German Federal Ministry of Education and Research, 01 E0 1002. CK was funded by grant KR 3433/2-1 of the German Research Foundation.
FUF and CFF are significantly different from the existing literature. Consulting the Schuhfried Company, it turned out that the background illumination around the test patch was 60% lower than the standard value.
The one patient who consumed alcohol one week prior to the experiment showed the lowest GSI. However, it was not much lower than another patient who had not consumed alcohol for five years.
Contributor Information
Theresa Götz, Email: theresa@biomag.uni.jena.de.
Ralph Huonker, Email: rhuonker@biomag.uni-jena.de.
Cornelia Kranczioch, Email: cornelia.kranczioch@uni-oldenburg.de.
Philipp Reuken, Email: Philipp.Reuken@med.uni-jena.de.
Otto W. Witte, Email: otto.witte@med.uni-jena.de.
Albrecht Günther, Email: albrecht.guenther@med.uni-jena.de.
Stefan Debener, Email: stefan.debener@uni-oldenburg.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- Amodio P., Del Piccolo F., Petteno E., Mapelli D., Angeli P., Iemmolo R., Muraca M., Musto C., Gerunda G., Rizzo C., Merkel C., Gatta A. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. Journal of Hepatology. 2001;35:37–45. doi: 10.1016/s0168-8278(01)00129-5. [DOI] [PubMed] [Google Scholar]
- Bandettini P.A., Jesmanowicz A., Wong E.C., Hyde J.S. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Basar E., Basar-Eroglu C., Karakas S., Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neuroscience Letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E., Guntekin B., Atagun I., Golbasi B.T., Tulay E., Ozerdem A. Brain's alpha activity is highly reduced in euthymic bipolar disorder patients. Cognitive Neurodynamics. 2012;6:11–20. doi: 10.1007/s11571-011-9172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Sechste Mitteilung. Arch of Psychiatry. 1933;99:555–574. [Google Scholar]
- Busch N.A., Herrmann C.S., Muller M.M., Lenz D., Gruber T. A cross-laboratory study of event-related gamma activity in a standard object recognition paradigm. NeuroImage. 2006;33:1169–1177. doi: 10.1016/j.neuroimage.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Butterworth R.F. Neuronal cell death in hepatic encephalopathy. Metabolic Brain Disease. 2007;22:309–320. doi: 10.1007/s11011-007-9072-3. [DOI] [PubMed] [Google Scholar]
- Capilla A., Pazo-Alvarez P., Darriba A., Campo P., Gross J. Steady-state visual evoked potentials can be explained by temporal superposition of transient event-related responses. PloS One. 2011;6:e14543. doi: 10.1371/journal.pone.0014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz B.A., Sponheim S.R., Iacono W.G., Beiser M. Resting EEG in first-episode schizophrenia-patients, bipolar psychosis patients, and their first-degree relatives. Psychophysiology. 1994;31:486–494. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Croningolomb A., Corkin S., Rizzo J.F., Cohen J., Growdon J.H., Banks K.S. Visual dysfunction in Alzheimer's disease — relation to normal aging. Annals of Neurology. 1991;29:41–52. doi: 10.1002/ana.410290110. [DOI] [PubMed] [Google Scholar]
- Davies M.G., Rowan M.J., Feely J. EEG and event related potentials in hepatic-encephalopathy. Metabolic Brain Disease. 1991;6:175–186. doi: 10.1007/BF00996917. [DOI] [PubMed] [Google Scholar]
- Dustman R.E., Boswell R.S., Porter P.B. Beta brain waves as an index of alertness. Science. 1962;137:533–534. doi: 10.1126/science.137.3529.533. [DOI] [PubMed] [Google Scholar]
- Eckstein A.K., Reichenbach A., Jacobi P., Weber P., Gregor M., Zrenner E. Hepatic retinopathia. Changes in retinal function. Vision Research. 1997;37:1699–1706. doi: 10.1016/s0042-6989(96)00318-5. [DOI] [PubMed] [Google Scholar]
- Foley P.J. Effect of background on the critical flicker frequency. Canadian Journal of Psychology. 1956;10:200–206. doi: 10.1037/h0083669. [DOI] [PubMed] [Google Scholar]
- Foley J.M., Watson C.W., Adams R.D. Significance of the electroencephalographic changes in hepatic coma. Transactions of the American Neurological Association. 1950;161–165 [PubMed] [Google Scholar]
- Gruber T., Trujillo-Barreto N.J., Giabbiconi C.M., Valdes-Sosa P.A., Muller M.M. Brain electrical tomography (BET) analysis of induced gamma band responses during a simple object recognition task. NeuroImage. 2006;29:888–900. doi: 10.1016/j.neuroimage.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Hassanein T.I., Hilsabeck R.C., Perry W. Introduction to the hepatic encephalopathy scoring algorithm (HESA) Digestive Diseases and Sciences. 2008;53:529–538. doi: 10.1007/s10620-007-9895-0. [DOI] [PubMed] [Google Scholar]
- Hemera Technologies Hemera Photo Objects. 1997;Volume 1 [Google Scholar]
- Herrmann C.S. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Experimental Brain Research. 2001;137:346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Toyama K. Independent component analysis for noisy data — MEG data analysis. Neural Networks. 2000;13:1063–1074. doi: 10.1016/s0893-6080(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Janson J., Kranczioch C. Good vibrations, bad vibrations: oscillatory brain activity in the attentional blink. Advances in Cognitive Psychology/University of Finance and Management in Warsaw. 2011;7:92–107. doi: 10.2478/v10053-008-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Colgin L.L. Cross-frequency coupling between neuronal oscillations. Trends in Cognitive Sciences. 2007;11:267–269. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Kahana M.J., Seelig D., Madsen J.R. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kaspar K., Hassler U., Martens U., Trujillo-Barreto N., Gruber T. Steady-state visually evoked potential correlates of object recognition. Brain Research. 2010;1343:112–121. doi: 10.1016/j.brainres.2010.04.072. [DOI] [PubMed] [Google Scholar]
- Kircheis G., Knopf C., Wettstein M., Timmermann L., Schnitzler A., Haussinger D. Critical flicker frequency (CFF) for quantification of low grade hepatic encephalopathy. Hepatology. 2001;34:548a–a. doi: 10.1053/jhep.2002.30957. [DOI] [PubMed] [Google Scholar]
- Kircheis G., Oette M., Wettstein M., Timmermann L., Schnitzler A., Haussinger D. Diagnostic value and dynamics of critical flicker frequency in hepatic encephalopathy (HE) Hepatology. 2001;34:187a–a. doi: 10.1053/jhep.2002.30957. [DOI] [PubMed] [Google Scholar]
- Kircheis G., vom Dahl S., Wettstein M., Timmermann L., Schnitzler A., Haussinger D. Hepatic retinopathy as a diagnostic tool for quantification of hepatic encephalopathy (HE) Journal of Hepatology. 2001;34:77. [Google Scholar]
- Kircheis G., Wettstein M., Timmermann L., Schnitzler A., Haussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357–366. doi: 10.1053/jhep.2002.30957. [DOI] [PubMed] [Google Scholar]
- Kircheis G., Fleig W.E., Gortelmeyer R., Grafe S., Haussinger D. Assessment of low-grade hepatic encephalopathy: a critical analysis. Journal of Hepatology. 2007;47:642–650. doi: 10.1016/j.jhep.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Lee T.W., Girolami M., Bell A.J., Sejnowski T.J. A unifying information–theoretic framework for independent component analysis. Computers & Mathematics with Applications. 2000;39:1–21. [Google Scholar]
- Misiak H. The decrease of critical flicker frequency with age. Science. 1951;113:551–552. doi: 10.1126/science.113.2941.551. [DOI] [PubMed] [Google Scholar]
- Mo J., Schroeder C.E., Ding M.Z. Attentional modulation of alpha oscillations in macaque inferotemporal cortex. Journal of Neuroscience. 2011;31:878–882. doi: 10.1523/JNEUROSCI.5295-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S., Clementz B.A., Gao Y., Ortiz T., Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Human Brain Mapping. 2007;28:1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgagni G.B. 1769. The Seats and Causes of Diseases Investigated by Anatomy. (London) [Google Scholar]
- Müller M.M., Andersen S., Trujillo N.J., Valdes-Sosa P., Malinowski P., Hillyard S.A. Feature-selective attention enhances color signals in early visual areas of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14250–14254. doi: 10.1073/pnas.0606668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Spatiotemporal analysis of alpha frequency components with the ERD technique. Brain Topography. 1989;2:3–8. doi: 10.1007/BF01128838. [DOI] [PubMed] [Google Scholar]
- Quero J.C., Schalm S.W. Subclinical hepatic encephalopathy. Seminars in Liver Disease. 1996;16:321–328. doi: 10.1055/s-2007-1007244. [DOI] [PubMed] [Google Scholar]
- Riordan S.M., Williams R. Gut flora and hepatic encephalopathy in patients with cirrhosis. The New England Journal of Medicine. 2010;362:1140–1142. doi: 10.1056/NEJMe1000850. [DOI] [PubMed] [Google Scholar]
- Salmi T. Critical flicker frequencies in MS patients with normal or abnormal pattern VEP. Acta Neurologica Scandinavica. 1985;71:354–358. doi: 10.1111/j.1600-0404.1985.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Misiak H. Critical flicker frequency (CFF) and psychotropic-drugs in normal human subjects. Psychopharmacology. 1976;47:175–182. doi: 10.1007/BF00735818. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Gross J., Kircheis G., Haussinger D., Schnitzler A. Cortical origin of mini-asterixis in hepatic encephalopathy. Neurology. 2002;58:295–298. doi: 10.1212/wnl.58.2.295. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Gross J., Butz M., Kircheis G., Haussinger D., Schnitzler A. Mini-asterixis in hepatic encephalopathy induced by pathologic thalamo-motor-cortical coupling. Neurology. 2003;61:689–692. doi: 10.1212/01.wnl.0000078816.05164.b1. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Butz M., Gross J., Kircheis G., Haussinger D., Schnitzler A. Neural synchronization in hepatic encephalopathy. Metabolic Brain Disease. 2005;20:337–346. doi: 10.1007/s11011-005-7916-2. [DOI] [PubMed] [Google Scholar]
- Timmermann L., Butz M., Gross J., Ploner M., Sudmeyer M., Kircheis G., Haussinger D., Schnitzler A. Impaired cerebral oscillatory processing in hepatic encephalopathy. Clinical Neurophysiology. 2008;119:265–272. doi: 10.1016/j.clinph.2007.09.138. [DOI] [PubMed] [Google Scholar]
- Vialatte F.B., Maurice M., Dauwels J., Cichocki A. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Progress in Neurobiology. 2010;90:418–438. doi: 10.1016/j.pneurobio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Wiesner R.H., Edwards E., Freeman R., Harper A. Does the model for end-stage liver disease (MELD) predict post liver transplant graft survival? Hepatology. 2003;38:370a–371a. [Google Scholar]
- Zappoli R., Versari A., Paganini M., Arnetoli G., Muscas G.C., Gangemi P.F., Arneodo M.G., Poggiolini D., Zappoli F., Battaglia A. Brain electrical activity (quantitative EEG and bit-mapping neurocognitive CNV components), psychometrics and clinical findings in presenile subjects with initial mild cognitive decline or probable Alzheimer-type dementia. Italian Journal of Neurological Sciences. 1995;16:341–376. doi: 10.1007/BF02229172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


