Abstract
Emerging data illustrate a pivotal role for activation of β-cell endoplasmic reticulum stress pathways in diabetes pathophysiology. The purpose of this review is to appraise the evidence for β-cell endoplasmic reticulum stress in human type 1 and 2 diabetes, review the molecular signaling pathways involved in the unfolded protein response and endoplasmic reticulum stress signaling, and to provide data from polyribosome profiling to illustrate the impact of ER stress on the mRNA translation response. Finally, we will discuss existing and novel therapeutic strategies that target β-cell ER stress and discuss their use in rodent and human type 1 and 2 diabetes.
Keywords: Islet, diabetes, mRNA translation, polyribosome, ER stress
Introduction
Diabetes mellitus is a disease of disordered glucose homeostasis that affects nearly 347 million individuals worldwide. Approximately 90% of diabetes cases can be categorized as Type 2 diabetes (T2D), which results from a combination of insulin resistance, dysregulated hepatic gluconeogenesis, and impaired β-cell function. Type 1 diabetes (T1D) comprises the remaining 10% of diabetes cases and results from autoimmune destruction of the pancreatic β-cell. Despite somewhat disparate etiologies and inciting factors, altered β-cell secretory function and survival play a central role in the pathophysiology of both major forms of diabetes.
T2D develops within the context of a modern “Westernized” lifestyle, the hallmarks of which are overnutrition, obesity, and inadequate physical activity. Genetic studies suggest that T2D is largely a polygenic disorder. However, a number of genome-wide association studies point to the presence of gene polymorphisms that impart β-cell susceptibility and predispose certain individuals towards β-cell failure and the development of diabetes in settings where insulin sensitivity is compromised [1–4]. Clinical studies suggest this broad category of β-cell failure is comprised of alterations in β-cell mass as well as insulin secretory function [5–10], while provocative new preclinical data suggest that alterations in β-cell identity may also play a role in the T2D phenotype. In parallel with rising rates of obesity in developing countries, epidemiological data demonstrate a significant increase in the prevalence of T2D globally [11,12].
T1D results from a cascade of events that culminates in autoimmune destruction of the insulin-producing β-cells of the islets of Langerhans. These events are initiated after nonspecific injury to the β-cell, such as occurs in response to viral infection. Injury to the β-cell results in exposure of autoantigens and subsequent activation of CD4+ and CD8+ T cells by macrophages and other antigen presenting cells. A complex and destructive interplay ensues that is amplified by the secretion of pro-inflammatory cytokines such as interleukin 1β (IL-1β), tumor necrosis factor α (TNFα), and interferon γ (IFNγ) from macrophages, T cells, and perhaps the β-cell itself, enabling a vicious cycle of necrotic and apoptotic β-cell death [13,14]. Type 1 diabetes is usually associated with the presence of circulating auto-antibodies directed against β-cell antigens, including anti-glutamic acid decarboxylase (GAD65), anti-islet, anti-insulin, and anti-Zinc transporter 8 (ZnT-8) antibodies [15–18]. T1D risk is associated with the presence of certain high-risk class I and II major histocompatibility complex alleles, while T1D genome-wide association studies have identified polymorphisms in a number of genes that regulate autoimmunity. Interestingly, emerging data show that many of the genes implicated and previously thought to regulate immune function are also expressed in the islet and impact β-cell function as well as the intrinsic risk of β-cell susceptibility in T1D [19–22].
The metabolic milieu of T1D and T2D is characterized by high levels of glucose as well as pro-inflammatory cytokines and chemokines, while concomitant obesity in either disease is associated with elevated serum levels of free fatty acids and other deleterious lipid intermediates. In the case of T1D, viral infection of the β-cell may initiate disease in genetically at risk individuals. These toxic factors can harm the β-cell through several intersecting pathways including autophagy, increased oxidative stress, mitochondrial dysfunction, epigenetic dysregulation, and amyloid formation. Emerging data also illustrate a pivotal role for the activation of endoplasmic reticulum stress in diabetes. In recent years, ER stress signaling and its contribution towards the progression of pancreatic β-cell dysfunction in T1D and T2D have been the focus of intense investigation (reviewed in [22–26]). The purpose of this report is to provide updated evidence for the presence of β-cell ER stress in human T1D and T2D, briefly review the molecular signaling pathways involved in the unfolded protein response and ER stress, and provide data illustrating the central role of translation in the adaptive ER stress response. Finally, we will discuss existing and novel therapeutic strategies that target β-cell ER stress and discuss their use in rodent and human T2D and T1D.
The unfolded protein response and endoplasmic reticulum stress
The mammalian β-cell functions as a highly specialized metabolic factory that is unique in its ability to continually sense nutrient and metabolic homeostasis and respond with appropriate levels of insulin secretion. Because of the need to synthesize and secrete large quantities of insulin, the β-cell possesses a number of specialized characteristics. Among these is a highly developed endoplasmic reticulum (ER), which plays a central role in protein biosynthesis and maturation and Ca2+ storage [23,27,28]. Translation of new proteins occurs on ribosomes associated with the ER, and nascent proteins undergo final folding and maturation within the lumen of the ER. The ER is extremely sensitive to disruptions in cellular homeostasis. Under conditions of stress, proteins may fail to fold correctly, resulting in an accumulation of excess unfolded proteins. When this occurs, a multi-faceted and protective cascade termed the unfolded protein response (UPR) is activated. In brief, the goal of the UPR is to decrease delivery of new proteins to the ER, restore cellular homeostasis, and ultimately increase the protein-folding capacity of the ER. However, if the inciting stress is unresolved, continual stimulation of the UPR can lead to activation of apoptotic pathways and β-cell death. This transition is referred to as endoplasmic reticulum (ER) stress.
Within the β-cell, the UPR can be initiated following exposure to glucotoxic, lipotoxic, or inflammatory conditions, in the setting of hyperinsulinemia, or in response to the accumulation of proinsulin and/or islet amyloid. These components of the diabetic milieu can lead to activation of deleterious signaling pathways, disruption of the cellular redox and/or energy state, or depletion of ER Ca2+ and/or cellular chaperones [23–26,29–33]. At the initiation of the UPR, the ER chaperone immunoglobulin heavy chain binding protein (BiP) dissociates from the luminal side of the ER activating three parallel yet interacting arms of the UPR response. These pathways are regulated by IRE1α, ATF6, and PERK. IRE1α activation leads to alternative splicing of XBP1 to generate spliced XBP1, which serves as a transcriptional activator of genes whose products regulate protein maturation, folding, and ER export [34,35]. IRE1α also functions to degrade mRNAs, thereby limiting the production of new proteins. Emerging data suggest that activation status of IRE1α is key node that dictates whether ER stress can be remediated or has transitioned to an irremedial state [36,37].
ATF6 is a basic leucine zipper transcription factor, which translocates to the Golgi following activation by BiP dissociation, where it is cleaved and activated by site 1 protease and site 2 protease (S1P and S2P). ATF6 subsequently translocates to the nucleus, where it binds to the ER stress response elements in genes whose products function as ER chaperones[1–4]. The final arm of this UPR transduction pathway involves PERK, which phosphorylates eIF2α. In contrast to the global translation inhibition, the PERK/eIF2α pathway also promotes translation of several specific mRNAs such as activating transcription factor 4 (ATF4) and CHOP, which contain 5’-upstream open reading frames [5–10].
Continued activation of the UPR without resolution of the inciting event ultimately results in apoptosis through eventual activation of CHOP. CHOP is a pro-apoptotic transcription factor that serves as the common effector arm for the apoptotic response [11,12]. Stress induced activation of other factors like c-Jun N-terminal kinase and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) may synergize to contribute to this apoptotic death response [13,14].
Translation of mRNAs in eukaryotes
The presence of misfolded proteins in the ER is a primary trigger for the mobilization of folding chaperones and attempts by the β-cell to limit new protein production through inhibition of mRNA translation. These “remedial” mechanisms are necessary to provide the cell the necessary time to recover from the prevailing stress, while still ensuring that the proteins produced are sufficiently active for the interim function and survival of the β-cell. The inhibition of protein production during ER stress is paramount in mitigating the protein load upon the ER. In addition, the translation of mRNAs at ribosomes is considered to be among the most energy consuming processes in the cell and, as such, suppression of translation provides a rapid means to conserve energy in the setting of cellular stress.
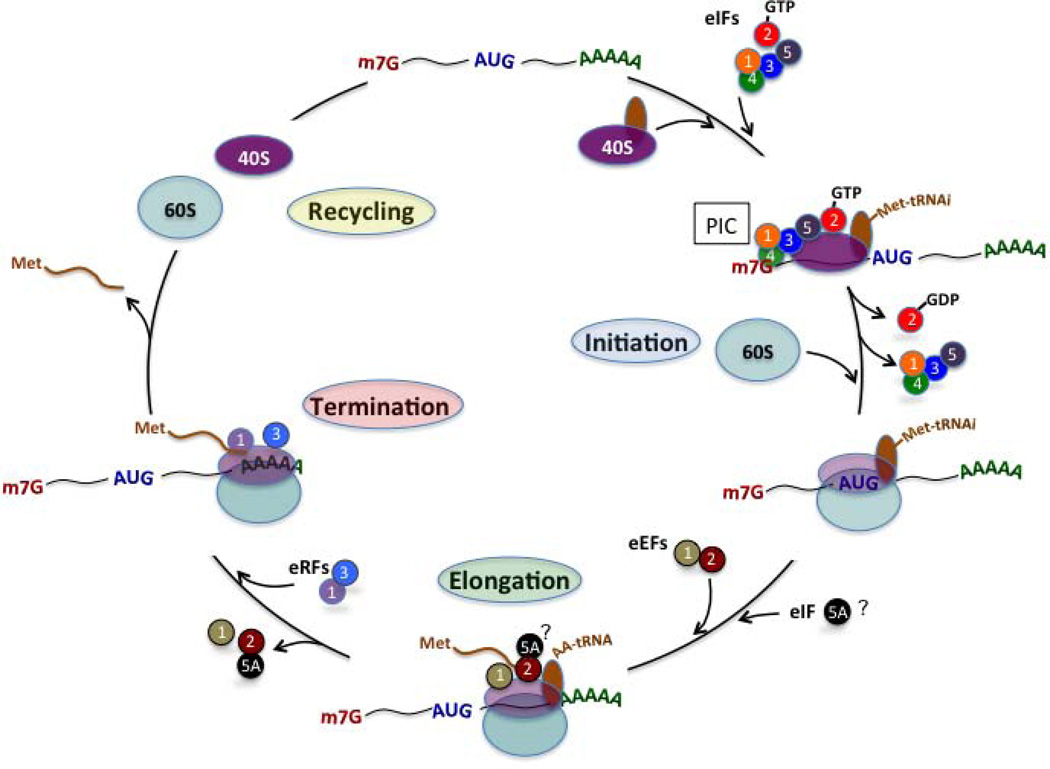
Four major phases are recognized in the translation of mRNAs at ribosomes: initiation, elongation, termination, and ribosome recycling (see Fig. 1). The factors responsible for each of these phases are distinct and minimally overlapping, suggesting that each phase has the potential for independent regulation. Most mRNAs produced in eukaryotes contain a 5’-methylguanylate (m7G) “cap,” which is a feature important for translation initiation. Initiation begins with the binding of initiation factors (eIFs 1, 1A, 2, 3, 4A, 4E, and 4G) to the 5’-m7G cap and the subsequent “scanning” of the 5’-untranslated region of the message for an AUG codon by the initiator Met-tRNA-loaded 40S ribosomal subunit. This complex of initiation factors and the loaded 40S ribosomal subunit, known as the pre-initiation complex (PIC), forms the full 80S initiation complex when hydrolysis of GTP bound by eIF2 signals the entry of the 60S ribosomal subunit. The full 80S initiation complex subsequently enters the elongation phase, where GTP-hydrolyzing reactions mediated by the elongation factors eEF2 and eEF1 ensure the growth of the polypeptide chain. During the elongation phase a third factor, eIF5A, is thought to interact with eEF2 to enhance the rate of translation elongation of at least a subset of mRNAs [15–18]. Translation termination takes place when the elongating 80S ribosome encounters a stop codon (UAA, UAG, UGA). The eukaryotic release factors eRF1 and eRF3 participate in the termination process, which also requires GTP hydrolysis (by eRF3) [19–22]. Finally, recycling is a process that occurs after termination, wherein ribosomal subunits dissociate and are available for initiation of a new mRNA strand. The specific factors and their regulation during translation recycling in mammalian cells remain less well understood compared to bacteria and yeast [22–26].
Figure 1. mRNA translation in mammalian cells.
The figure shows the general scheme of mRNA translation for capped (m7G) mRNAs with poly-A tails (AAAA) and a start codon (AUG). Four general phases of translation are shown: Initiation, Elongation, Termination, and Recycling. The figure shows individual ribosomal components (40S and 60S) and specific initiation factors (eIFs), elongation factors (eEFs), and release factors (eRFs) that participate in the mRNA translation process. PIC, pre-initiation complex.
Regulation of mRNA translation initiation during ER stress in islet β-cells
Under conditions of various stressors, including ER stress and nutrient depletion, inhibition of mRNA translation in islet β-cells occurs primarily at the initiation phase. Regulation of initiation is typically achieved by processes (usually phosphorylation events) that affect PIC formation or the formation of the active 80S ribosomal complex (for a review, see ref. [23,27,28]). PIC formation is classically regulated by the eIF4E-binding proteins (4E-BPs). Hypophosphorylated 4E-BPs bind to eIF4E, thereby preventing association of eIF4E with the 5’-m7G cap. Upon increased phosphorylation, the 4E-BPs dissociate from eIF4E and subsequently promote PIC formation. In the absence of 4E-BP1 and 4E-BP2, mutant mice develop hypersensitivity to diet-induced obesity, presumably a result of losing the normal “brake” for translation initiation provided by the 4E-BPs [23–26,29–33]. A major 4E-BP kinase is the mammalian target of rapamycin (mTOR), which lies downstream of the phosphatidylinositol-3 kinase (PI3K)/Akt pathway and is a sensor of energy homeostasis in the cell [34,35]. Thus, when energy is abundant and mTOR is active, 4E-BPs are hyperphosphorylated and remain dissociated from eIF4E, and translation initiation of capped mRNAs proceeds. Under conditions of ER stress, it has been suggested that the levels of hypophosphorylated 4E-BP1 are increased, leading to suppression of eIF4E function and subsequent suppression of translation initiation [36,37].
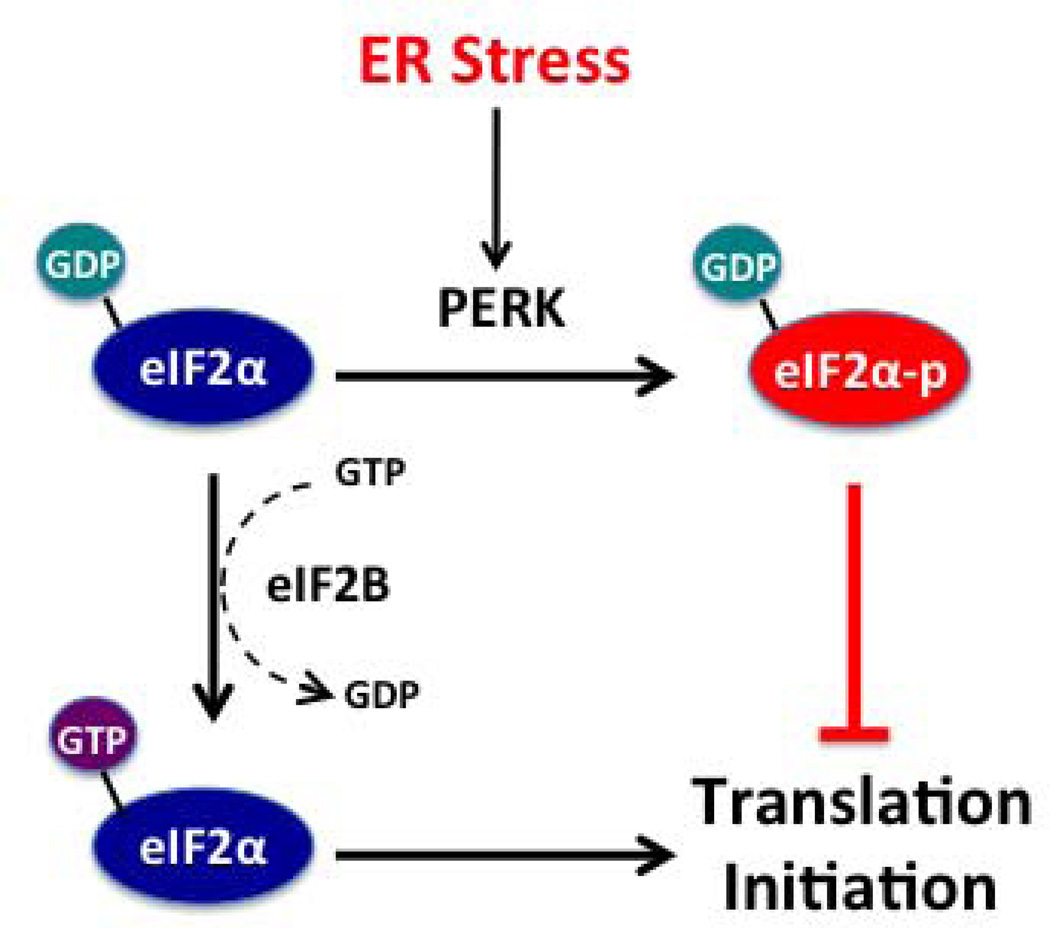
A second major point of regulation during translation initiation is in the formation of the active 80S complex. Formation of the active 80S complex requires hydrolysis of eIF2-bound GTP to GDP. eIF2 is a heterotrimeric protein consisting of α, β, and γ subunits. Phosphorylation of the α-subunit at Ser51 stabilizes the eIF2-GDP complex, preventing a subsequent exchange reaction that would otherwise regenerate eIF2-GTP [38,39] (see Fig. 2), a result of which is impaired formation of the active 80S complex necessary for initiation. Phosphorylation of Ser51 of eIF2α can be accomplished by multiple different kinases, each of which is sensitive to different prevailing stressors. In the case of ER stress, the kinase PERK phosphorylates eIF2α (eIF2α-P). Other known eIF2α kinases include GCN2 (general control nonrepressable 2, activated by amino acid starvation), PKR (protein kinase R, activated by double-stranded RNA), and HRI kinase(heme-regulated eukaryotic initiation factor 2α kinase, activated by heme deficiency) [40]. The importance of eIF2α Ser51 phosphorylation in the setting of ER stress was highlighted in studies of mice harboring a S51A mutation of eIF2α. Whereas homozygous S51A mutant mice die in the neonatal period with severe hypoglycemia [41], global heterozygous S51A mutants exhibit glucose intolerance and a β-cell secretory defect upon high fat diet feeding [42]. This β-cell secretory defect was attributed to excessive β-cell mRNA translation, ER distension, and reduced insulin secretory granule content [42]. Similar findings were observed in mice with β-cell-specific expression of the S51A mutation of eIF2α [43].
Figure 2. Mechanism of eIF2α-mediated translation initiation.
The GDP bound to eIF2α is exchanged for GTP by eIF2B, allowing for eIF2α to recycle and permit translation initiation. When eIF2α is phosphorylated by the enzyme PERK, which itself is activated by ER stress, the bound GDP can no longer exchange with GTP and translation initiation is blocked.
Analogously, deletion of the eIF2α kinase PERK in mice results in animals with unmitigated proinsulin biosynthesis, β-cell ER distension, and progressive diabetes [44,45]. Collectively, these studies emphasize the importance of attenuation of translation initiation by eIF2α-P as a means to remediate ER stress. Whereas the majority of cellular mRNAs are inhibited from translation initiation during ER stress, a subset of mRNAs whose encoded proteins are necessary for stress remediation must not only escape this translational inhibitory process, but in fact have their translation enhanced. Important examples of such mRNAs include those encoding activating transcription factors 4 and 5 (ATF4, ATF5), growth arrest and DNA damage-inducible protein 34 (GADD34), and CHOP [5,7,46]. Paradoxically, eIF2α-P promotes the translation initiation of these mRNAs through a mechanism involving the bypass of an upstream open reading frame (uORF). The uORFs in these mRNAs are translation initiation sites for the PIC under unstressed conditions, such that their translation prevents translation initiation at the actual ORFs for these proteins. Thus, the proteins are either not produced or produced at low levels. However, when phosphorylation of eIF2α occurs, initiation at the uORFs is inhibited, and subsequent ribosome scanning results in the re-initiation of translation from the true ORF [27]. In this way, eIF2α-P functions in a binary manner during ER stress, causing inhibition of general mRNA translation initiation while promoting the translation initiation of specific stress-responsive mRNAs.
Analyzing translational control in the β-cell: polyribosomal profiling
Several methods exist for analysis of specific or global mRNA translation, including luciferase assay readouts from transfected cells [47], analysis of RNA bound to ribosomal proteins [48,49], measurement of radioactive and nonradioactive amino acid incorporation into total protein [47,50], and measurement of changes to the levels and phosphorylation states of individual translational factors [47]. An attractive approach to assess directly the association of specific transcripts with ribosomal machinery is analysis of mono- and polyribosome-associated RNA from whole cell lysates. The technique, variably referred to as polysome or polyribosome profiling (PRP) [47], involves the preparation of whole cell RNA lysate from primary cells or cell lines followed by passage of the lysate through a 10–50% sucrose gradient at ultracentrifugation speeds (~40,000 rpm). The gradient is then fractionated and monitored for total RNA (by UV absorbance at 254 nm). Fig. 3A (solid line) shows results of a typical PRP experiment using unstressed MIN6 insulinoma cells incubated at 20 mM glucose. The profile shows the RNA peaks associated with the 40S and 60S ribosomal subunits and the 80S monoribosome (the species that would enter the translation elongation phase). The peaks appearing after the 80S monoribosome are identified as “polyribosomes” containing 2, 3, 4, 5, and higher numbers of 80S species associated with individual RNA strands. The mRNAs appearing in the polyribosome peaks are thought to represent transcripts that are in the active phase of translation elongation (barring any process or drug—e.g. cycloheximide—that would cause “stalling” of ribosomes at this phase).
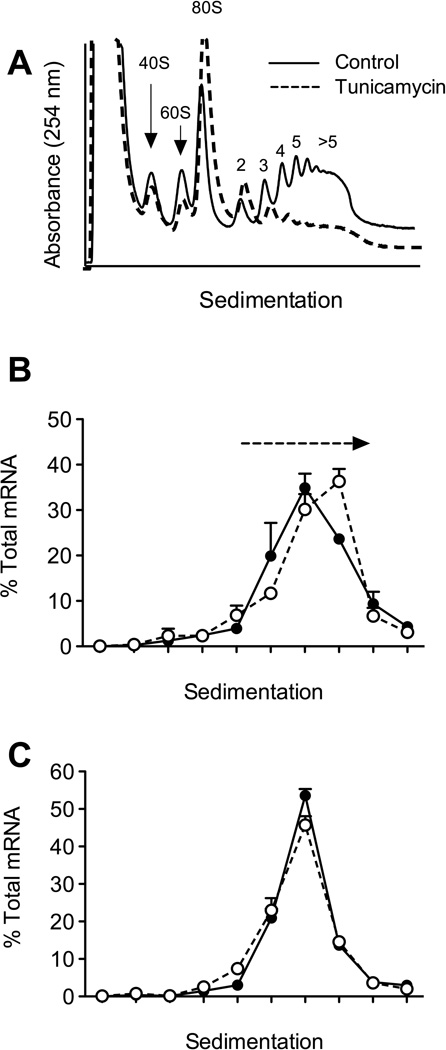
Figure 3. Tunicamycin induces translation initiation blockade.
Mouse-derived MIN6 β-cellswere either incubated with 5 µM Tunicamycin or carrier (Control, dimethylsulfoxide) for 24 hours, then extracts were subjected to polyribosome profiling (PRP) analysis through sucrose gradients as described in ref. 35. Fractions from the PRP analysis were collected and subjected to RNA isolation and reverse transcription real-time PCR analysis. A, PRP profiles of Control (solid line) and Tunicamycin-treated (dashed line) MIN6 β-cell s. B, fractions from the PRP analysis of Control (solid line) and Tunicamycin (dashed line)-treated MIN6 cells were quantitatively analyzed for Atf4 mRNA. Dashed arrow indicates the rightward shift of Atf4 message to higher polyribosomes following Tunicamycin treatment. C, fractions from the PRP analysis of Control (solid line) and Tunicamycin (dashed line)-treated MIN6 cells were quantitatively analyzed for Ins1/2 mRNA.
A significant advantage of the PRP technique is that it allows the identification of ribosome redistribution following cellular stress. Tunicamycin inhibits glycosylation of newly synthesized proteins, leading to protein misfolding, eIF2α phosphorylation, and ER stress. As shown in Fig. 3A (dashed line), when MIN6 β-cells were treated with 5 µM tunicamycin for 24 h, we observed an increase in the RNA associated with the 80S monoribosome and a reduction in the total RNA associated with the polyribosome fraction. These results are consistent with the known consequences of ER stress on translation initiation, wherein phosphorylation of eIF2α leads to a general blockade in translation initiation, but no changes to translation elongation. As such, the increase in the 80S monoribosome peak is indicative of a block at the initiation phase, leaving 80S monoribosomes unable to enter the elongation phase. The ribosomes that have already entered the elongation phase (the polyribosomes) continue their traverse along the mRNAs, causing their “run-off” and eventual polyribosome depletion [7].
As discussed previously, whereas general translation initiation is blocked in the setting of ER stress, a subset of mRNAs—particularly those with uORFs, such as Chop and Atf4—have their translation enhanced by eIF2α. To assess the polyribosome occupancy of specific mRNAs, we subjected individual fractions from the sucrose gradient fractionation to quantitative RT-PCR for Atf4 and the gene encoding preproinsulin (Ins1/2). As shown in Fig. 3B, the Atf4 mRNA shows evidence of a rightward shift toward polyribosomes during tunicamycin-induced ER stress, whereas the Ins1/2 mRNA shows no changes (Fig. 3C). Taken together, the data in Fig. 3 is consistent with the expected observation that ER stress induces a block in general mRNA translation initiation, while simultaneously promoting the translation initiation of ER chaperone-encoding mRNAs.
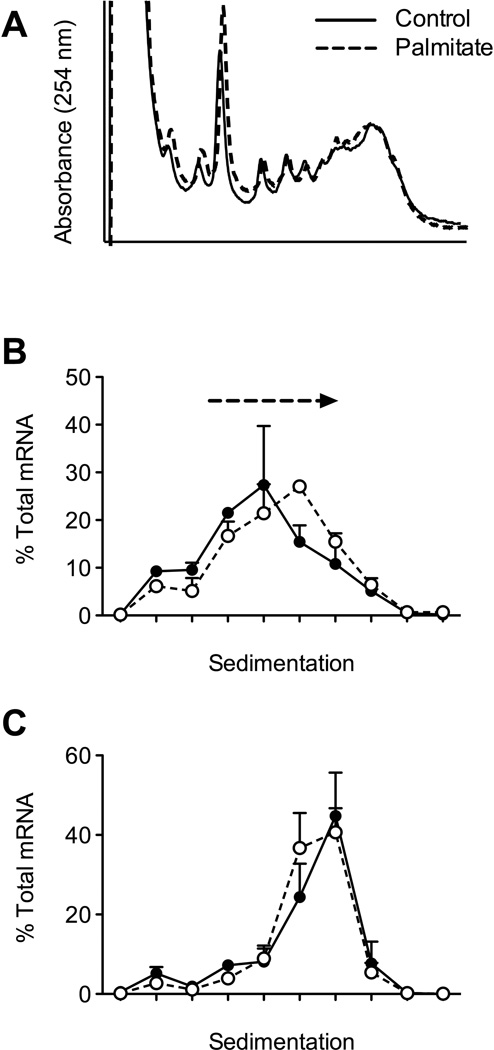
Translational effects of physiologic stressors on primary pancreatic islets can also be assessed by PRP. As shown in Fig. 4A (solid line), mouse islets exhibit a PRP similar to MIN6 β-cells, with the notable difference that the 80S peak appears larger and the polyribosome region appears smaller. This general profile is reflective of the general post-mitotic state of primary pancreatic islets compared to the tumorigenic cell lines, where protein synthesis is generally limited to specific proteins (such as insulin, amylin) that are necessary for the normal function of β-cells. Palmitate is a free fatty acid that is elevated in the setting of diet-induced obesity, and has been shown (in the presence of elevated glucose) to result in β-cell ER stress [51,52]. When islets are treated with the free fatty acid palmitate (0.5 mM for 4 hours), there is no striking change in the PRP (Fig. 4A), but a shift in Atf4 mRNA toward higher polyribosomes was observed (Fig. 4B). As with tunicamycin incubation, no changes in polyribosome occupancy by Ins1/2 mRNA was observed with palmitate incubation (Fig. 4C). Collectively, the data in Fig. 4 are suggestive that palmitate induces polyribosome occupancy changes in specific mRNAs that are similar to those observed with ER stress inducers.
Figure 4. Palmitate induces a polyribosomal shift of Atf4 mRNA in mouse islets.
CD1 mouse islets were isolated by methods approved by the Indiana University Institutional Animal Care and Use Committee, and were either incubated with 0.5 mM Palmitate or carrier (Control) for 24 hours, then extracts were subjected to polyribosome profiling (PRP) analysis through sucrose gradients as described in ref. 35. Fractions from the PRP analysis were collected and subjected to RNA isolation and reverse transcription real-time PCR analysis. A, PRP profiles of Control (solid line) and Palmitate-treated (dashed line) mouse islets. B, fractions from the PRP analysis of Control (solid line) and Palmitate (dashed line)-treated mouse islets were quantitatively analyzed for Atf4 mRNA. Dashed arrow indicates the rightward shift of Atf4 message to higher polyribosomes following Tunicamycin treatment. C, fractions from the PRP analysis of Control (solid line) and Palmitate (dashed line)-treated mouse islets were quantitatively analyzed for Ins1/2 mRNA.
Evidence for ER stress in human diabetes
Evidence of endoplasmic reticulum stress in human diabetes is primarily based on protein and mRNA expression of ER stress markers as well as electron micrographic evidence of changes in ER appearance. The presence of ER stress markers in human T2D has been demonstrated using fixed pancreatic sections and isolated islets from cadaveric donors. When islets from donors with T2D were cultured in high glucose, expression of the ER stress markers BiP and XBP-1 were significantly upregulated compared to islets from nondiabetic donors. Interestingly, there was also an increase in overall ER size in islets from donors with diabetes, which was felt to be consistent with an early adaptive response to ER stress. Perinuclear CHOP staining was significantly increased in pancreatic sections from obese donors with diabetes compared to obese non-diabetic donors. However, this report failed to detect CHOP in sections from subjects with T1D [53]. Islets from cadaveric donors with T1D and T2D have also exhibited isolated positive staining for activating transcription factor 3 (ATF3) [54]. A recent study demonstrated increased staining for BiP, XBP-1, and CHOP was in pancreatic sections from 13 human donors with T1D, providing the first comprehensive evaluation of a combination of ER stress markers in human T1D [55].
A prominent challenge is to identify ER stress in living individuals as a means to identify changes to ER stress in response to therapy. Recently, PCR-based assays have been developed that measure hypomethylated β-cell derived insulin DNA in serum as an indicator of β-cell death [56–58]. Whereas these assays have been studied in T1D, they have not yet been correlated with the presence of ER stress in model systems. Nevertheless, these assays could provide means to identify β-cell response to therapies that target ER stress. Another approach is to measure the relative amounts of secreted proinsulin as a percentage of total insulin or C-peptide secretion. Under unstressed conditions, proinsulin processing (into insulin and C-peptide) occurs prior to secretion, such that relatively little intact proinsulin is secreted. Elevations in the secretion of proinsulin relative to fully processed insulin may be indicative of β-cell ER dysfunction manifesting as alterations in protein folding and processing [59]. Elevations in proinsulin/insulin or proinsulin/C-peptide can readily be identified in large cohorts of subjects with T2D, and the ratio is improved in response to pioglitazone and IL-1β receptor antagonist therapy [60,61].
Analysis of serum from pre-diabetic non-obese diabetic (NOD) mice revealed elevations in the proinsulin/insulin ratio that occur in parallel with activation of islet ER stress [62]. Elevations in serum proinsulin and the ratio of serum proinsulin/C-peptide have also been reported in individuals with new onset T1D, while improvement in this ratio is seen during periods of disease remission and following treatment with certain immunomodulatory therapies [63–67].
Therapeutic approaches to target β-cell ER stress
Given the central role of ER stress in diabetes pathophysiology, the ability of existing T2D therapies to mitigate ER stress have been tested in a number of models. Thiazolidinediones (TZDs), agonists of the nuclear receptor PPAR-γ, prevent the onset of diabetes in humans with pre-diabetes and a history of gestational diabetes [68,69]. While these agents have been classically viewed as peripheral insulin sensitizers, an abundance of clinical and pre-clinical data demonstrate an ability of PPAR-γ agonists to improve β-cell function in humans and rodents [70]. There is also evidence that TZDs positively impact β-cell ER stress signaling in rodent models. Mice with deficiency of Wolfram syndrome protein 1 (WFS1) in combination with the agouti mutation are prone to the development of β-cell ER stress. Administration of PPAR-γ agonists in this model both improved glucose homeostasis and reduced islet ER stress signaling [71]. In the T2D db/db mouse model, pioglitazone restored islet euchromatin architecture and reduced activation of ER stress signaling pathways. These effects were secondary, in part, to restoration of islet pancreatic and duodenal homeobox factor 1 (Pdx1) expression [72]. In fact, the homeodomain transcription factor Pdx1 appears to play a pivotal role in the maintenance of β-cell ER health. When challenged with high fat diet, Pdx1 haploinsufficient mice experience a failed compensatory gain in β-cell mass and exhibit increased ER stress and apoptosis. Interestingly, Pdx1 is a direct transcriptional regulator of the WFS1 gene as well as other ER-associated genes [73].
PPAR-γ also acts as a direct transcriptional regulator of the sarco endoplasmic reticulum Ca2+ ATPase 2b (SERCA2b) gene, and TZDs restored SERCA2b expression in db/db mouse islets and INS-1 cells treated with high glucose and the pro-inflammatory cytokine IL-1β. Decreased activation of ER stress and death pathways occurred concomitant with this effect to improve β-cell calcium homeostasis [72,74]. Additional effects of TZDs include the prevention of amyloid-induced apoptosis, decreased oxidative stress, and decreased NF-κB activity in response to glucolipotoxity and cytokine signaling, as well as decreased activation of JNK [70].
Given the anti-inflammatory effects of TZDs and their ability to modulate NF-κB transcriptional responses, there has been interest in applying these drugs to T1D with the anticipated outcome being that TZDs might have dual effects to modulate immune function and inflammation and preserve β-cell health. In this regard, TZDs have shown beneficial effects to preserve β-cell function in humans with latent autoimmune diabetes of adulthood (LADA) [75]. Furthermore, a number of preclinical studies in NOD mice have demonstrated a decrease in the conversion to diabetes and suppression of Th1-mediated immune responses [76,77], Whereas these studies suggest an effect of TZDs to prevent diabetes onset in “at-risk” humans and rodents, the impact of these drugs to specifically target β-cell ER stress in T1D has not been described.
The incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) act through G-protein coupled receptors on the β-cell plasma membrane to activate adenylyl cyclase, increase intracellular cyclic AMP (cAMP), and stimulate insulin secretion. GLP-1 receptor agonists and dipeptidyl peptidase-IV (DPP-IV) inhibitors (which secondarily raise circulating levels of incretins by preventing their degradation) were developed to enhance insulin secretion in T2D. A number of studies suggest these agents also possess pro-survival and anti-apoptotic activities [78]. Consequently, the ability of these agents to protect against ER stress has been studied in models of chemically-induced ER stress and T2D. Db/db mice treated with the GLP-1 agonist exendin-4 demonstrated decreased islet expression of Chop and the spliced form of Xbp1 [79]; the GLP-1 receptor agonist liraglutide has shown similar effects in this same model [80]. In studies in vitro, exendin-4 protected against lipotoxicity-induced ER stress through induction of BiP and the anti-apoptotic protein JUNB in rat β-cells [81]. Exendin-4 has also been shown to protect against thapsigargin- and tunicamycin-induced ER stress through upregulation of Atf4 and the spliced form of Xbp1 [79], and to protect against cyclopiazonic acid-induced ER stress through effects to block mitochondrial apoptosis [81]. Similarly, chronic administration of vildagliptin, a DPP-IV inhibitor, was shown recently to restore β-cell mass, Pdx1 expression, and alleviate ER stress in C/EBPB (Chop) transgenic mice [82].
Given potent effects to improve β-cell health, there is growing interest in applying incretin therapies to the treatment and/or prevention of T1D. In addition to their potential effects on enhancing GLP-1 levels, DPP-IV inhibitors have addition effects that may be especially relevant to T1D. CD26 is a DPP-IV-like membrane-associated peptidase that is expressed in lymphocytes. CD26 regulates development and maturation of T cells as well as cytokine production and T-cell dependent antibody production. Therefore, in addition to inhibiting protease degradation of incretins, the inhibition of CD26 by DPP-IV inhibitors has been shown to have additional effects to diminish Th1 cytokine production and enhance levels of immune-tolerogenic regulatory T cells [83,84], effects that are particularly advantageous in developing and established T1D. DPP-IV inhibitors have been tested in both prevention and reversal strategies in animal models of T1D. The DPP-IV inhibitor linagliptin reduced insulitis and diabetes incidence by approximately 50% in NOD mice [85], and the related inhibitor MK-626 was shown to enhance levels of regulatory T cells in NOD mice [86]. Established T1D was successfully reversed in 57% of NVP-DPP728-treated NOD mice after 2 weeks of treatment and in 73% of mice after 6 weeks of treatment [87]. Despite these positive metabolic effects, the ability of these drugs to specifically address ER stress in preclinical models of T1D has not been tested.
Emerging evidence from human clinical trials suggest that GLP-1 receptor agonists may modestly improve a number of metabolic parameters including fasting glycemia, hemoglobin A1c, and glucose excursions in individuals with established T1D [88,89]. By contrast, despite encouraging pilot data [90], a recent double-blind, randomized-parallel 20-week study of 141 subjects with established T1D showed no significant effect of the DPP-IV inhibitor sitagliptin on hemoglobin A1c or insulin dose [91]. A definitive strategy is still needed to test whether DPP-IV inhibition in humans is capable of modulating intrinsic β-cell stress pathways and/or immune activation in a manner that promotes β-cell survival in developing T1D. The administration of these drugs in a prevention trial that enrolled individuals with high genetic risk of T1D and measurable circulating autoantibodies would address lingering questions about the usefulness of these drugs in T1D.
In addition to existing diabetes therapies, the ability of novel therapeutic approaches to reduce β-cell ER stress is actively being tested. Given extensive evidence suggesting a central role for cytokine signaling in T1D and T2D ER stress, there has been considerable interest in employing IL-1β receptor antagonist (IL-1Ra). In T2D, IL-1Ra showed positive effects on glycemia and had sustained effects to lower markers of systemic inflammation and the proinsulin/insulin ratio [60,61]. Interestingly, animal data from NOD mice suggests this agent may be best used in a combinatorial approach in T1D. In fact, IL-1Ra combined with anti-CD3 monoclonal antibodies reversed diabetes and markers of pancreatic inflammation NOD mice, whereas IL-1Ra alone was unable to reverse diabetes in the same model [92]. Treatment with IL-1Ra in recent onset T1D is currently being tested, but early reports from TrialNet and AIDA studies suggest no beneficial effects of IL-1Ra in recent onset T1D [93]. Although these data suggest that IL-1Ra therapy may be more beneficial in T2D, further research is needed to see if this approach specifically targets β-cell ER stress and to determine the ideal timing of IL-1Ra in both forms of diabetes. In the case of T1D, additional studies may be needed to determine if/how to use IL-1Ra therapies in combination with other immunomodulatory drugs.
Chemical chaperones are small osmotically active molecules that support protein refolding and stabilization. The two most widely studied chemical chaperones are 4-phenylbutyric acid (PBA) and tauroursodeoxycholic acid (TUDCA), which are both currently FDA approved. PBA is used for urea-cycle disorders, while TUDCA is approved in liver cholestasis [26]. These drugs have been extensively studied in models of insulin resistance and diet induced obesity, where they enhance insulin action, diminish liver ER stress, and decrease hypothalamic ER stress [94,95]. In studies in vitro, PBA protected against palmitate-induced ER stress and death in INS-1 cells [96]. In rats infused with high glucose, PBA and TUDCA protected against hyperglycemia-induced β-cell dysfunction and reduced expression of ER stress markers [97]. Interestingly, PBA was shown recently to prevent lipid-induced alterations in insulin secretion in a small human study [98]. The efficacy of these agents in models of T1D has not been specifically tested.
Strategies to specifically target components of the UPR or ER stress pathways are also being pursued. Elegant chemical biological work has identified IRE1α as a pivotal switch that regulates the balance between survival and apoptosis following ER stress initiation. One of the initial steps of the UPR involves IRE1α kinase autophosphorylation and activation of RNase activity of IRE1α to produce the spliced form of Xbp1 mRNA. Under more severe ER stress conditions, the RNase activity of IRE1α causes decay of ER-localized mRNAs, including RNAs that encode protein chaperones. Methods to thereby restrain IRE1α autophosphorylation may prevent this important and deleterious transition [37].
Imatinib and sunitinib are small molecule tyrosine kinase inhibitors used in a number of cancers including chronic myelogenous leukemia, gastrointestinal stromal tumors, and renal cell carcinoma. These agents are also reported to have immunomodulatory effects [99]. Several case series and reports have demonstrated improvement in glycemia in patients with T1D and T2D who are treated with these drugs [100,101]. In mouse models and studies in vitro, these drugs also appear to reduce ER stress. Imatinib induced diabetes remission in db/db mice primarily through improvements in insulin resistance and reduction of ER stress in liver and adipose tissue. Whereas insulin secretion per se was unaffected by imatinib, there was a concomitant improvement in β-cell mass in treated db/db mice [102]. Imatinib and sunitinib have both been shown to prevent the onset and induce remission of diabetes in NOD mice. Interestingly, an initial compound screen demonstrated that imatinib and sunitinib inhibited IRE1α trans-autophosphorylation and acted as IRE1α kinase-inhibiting RNase attenuators [37]. Collectively, these results raise the possibility that some of the anti-diabetic effects of these drugs in T1D and T2D may occur through modulation of β-cell ER stress. Screening studies identified two distinct classes of kinase inhibitors that are able to modulate the divergent outputs of IRE1α [103]. Future experiments to test these inhibitors in models of T1D and T2D should provide important insight into their potential to affect ER stress and improve β-cell survival and function.
Conclusions
Pancreatic β-cell dysfunction plays a prominent role in human T1D and T2D, and emerging data highlight a central role for activation of ER stress pathways in this process. There is growing evidence that a handful of existing diabetes therapies may target components of β-cell ER stress, while novel therapeutic approaches to target precisely the components of ER stress signaling are actively being developed and tested (Table 1). Clinical testing of novel compounds will be greatly enhanced by developing and validating new ways to noninvasively identify ER stress and/or β-cell ER stress, while preclinical testing of new therapies will be enhanced by the addition of assays like polyribosomal profiling that specifically define changes in protein translation initiation and elongation.
Table 1.
Therapeutic Approaches to Targeting β-cell ER Stress in Diabetes.
| Existing diabetes drugs |
Anti- inflammatory therapies |
Chemical chaperones |
Drugs that target components of the ER stress cascade |
|---|---|---|---|
| PPAR-γ agonists | IL-1β receptor antagonists | PBA | IRE1α kinase-inhibiting RNase attenuators (KIRAs) |
| GLP-1 agonists | TUDCA | ||
| DPP-IV inhibitors |
Acknowledgements
Research in Dr. Evans-Molina’s lab is supported by NIH grants K08 DK080225, R03 DK 089147, and R01 DK093954 and by grants from the Juvenile Diabetes Research Foundation, the George and Frances Ball Foundation, and the Ball Bros. Foundation. Research in Dr. Mirmira’s lab is supported by NIH grants R01 DK060581and R01 DK083583 and by grants from the Juvenile Diabetes Research Foundation, the George and Frances Ball Foundation, the Ball Bros. Foundation, and the Manpei Suzuki Diabetes Foundation of Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- T2D
Type 2 diabetes mellitus
- T1D
Type 1 diabetes mellitus
- IL-1β
interleukin-1β
- TNFα
tumor necrosis factor α
- IFNγ
interferon γ
- UPR
unfolded protein response (UPR)
- ER
endoplasmic reticulum
- BiP
immunoglobulin heavy chain binding protein
- eIF2α
eukaryotic initiation factor 2α
- IRE1α
inositol-requiring enzyme 1α
- ATF6
activating transcription factor 6
- PERK
PKR-like ER kinase
- XBP1
X-box binding protein 1
- eIF2B
eukaryotic initiation factor 2B
- CHOP
C/EBP homologous protein
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- m7G
methylguanylate
- PBA
4-Phenylbutyric Acid
- TUDCA
tauroursodeoxycholic acid
- PIC
pre-initiation complex
- 4E-BPs
eIF4E-binding proteins
- eIF4E
eukaryotic initiation factor 4E
- ATF4
activating transcription factor 4
- uORF
upstream open reading frame (uORF)
- PRP
polyribosome profiling
- Pdx1
pancreatic and duodenal homeobox 1
- PPAR-γ
peroxisome proliferator-activated receptor γ
- SERCA2b
sarco endoplasmic reticulum Ca2+ ATPase 2b
- TZDs
thiazolidinediones
- NOD
non-obese diabetic
- GLP-1
glucagon-like peptide 1
- DPP-IV
dipeptidyl peptidase-4
- IL-1Ra
IL-1β receptor antagonist
Footnotes
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Matsuda T, Kido Y, Asahara S-I, et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010;120:115–126. doi: 10.1172/JCI39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia. 2008;51:1100–1110. doi: 10.1007/s00125-008-1025-9. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J Biol Chem. 2004;279:43046–43051. doi: 10.1074/jbc.M408466200. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 6.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festa A, Williams K, D'Agostino RJ, Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes. 2006;55:1114–1120. doi: 10.2337/diabetes.55.04.06.db05-1100. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, McGrath B, Cavener DR. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mak BC, Wang Q, Laschinger C, et al. Novel function of PERK as a mediator of force-induced apoptosis. J Biol Chem. 2008;283:23462–23472. doi: 10.1074/jbc.M803194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginter E, Simko V. Global prevalence and future of diabetes mellitus. Adv Exp Med Biol. 2012;771:35–41. doi: 10.1007/978-1-4614-5441-0_5. [DOI] [PubMed] [Google Scholar]
- 13.Allagnat F, Fukaya M, Nogueira TC, et al. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death Differ. 2012;19:1836–1846. doi: 10.1038/cdd.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Torre D. Immunobiology of beta-cell destruction. Adv Exp Med Biol. 2012;771:194–218. doi: 10.1007/978-1-4614-5441-0_16. [DOI] [PubMed] [Google Scholar]
- 15.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Skyler JS, Herold KC, Palmer JP. The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1. Diabetes. 2012;61:1331–1337. doi: 10.2337/db11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias CAO, Gregio APB, Rossi D, et al. eIF5A interacts functionally with eEF2. Amino Acids. 2012;42:697–702. doi: 10.1007/s00726-011-0985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregio APB, Cano VPS, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 19.Zhouravleva G, Frolova L, Le Goff X, et al. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995;14:4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of proinflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. doi: 10.1016/j.cell.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 22.Dever TE, Green R. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb Perspect Biol. 2012;4:a013706. doi: 10.1101/cshperspect.a013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 24.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 25.Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic β-cell dysfunction. Diabetes Obes Metab. 2010;12:76–82. doi: 10.1111/j.1463-1326.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 26.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12:108–115. doi: 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 27.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bygrave FL, Benedetti A. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium. 1996;19:547–551. doi: 10.1016/s0143-4160(96)90064-0. [DOI] [PubMed] [Google Scholar]
- 29.Le Bacquer O, Petroulakis E, Paglialunga S, et al. Elevated sensitivity to diet-induced obesity and insulin resistance in mice lacking 4E-BP1 and 4E-BP2. J Clin Invest. 2007;117:387–396. doi: 10.1172/JCI29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes. 2009;58:906–916. doi: 10.2337/db08-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwiazda KS, Yang TLB, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–E701. doi: 10.1152/ajpendo.90525.2008. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. A role for aberrant protein palmitoylation in FFA-induced ER stress and β-cell death. Am J Physiol Endocrinol Metab. 2012;302:E1390–E1398. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012;81:767–793. doi: 10.1146/annurev-biochem-072909-095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi S, Ishihara H, Yamada T, et al. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 2008;7:269–276. doi: 10.1016/j.cmet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Han D, Lerner AG, Vande Walle L, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prostko CR, Brostrom MA, Malara EM, Brostrom CO. Phosphorylation of eukaryotic initiation factor (eIF) 2 alpha and inhibition of eIF-2B in GH3 pituitary cells by perturbants of early protein processing that induce GRP78. J Biol Chem. 1992;267:16751–16754. [PubMed] [Google Scholar]
- 39.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in β-cells. Diabetes Obes Metab. 2010;12:99–107. doi: 10.1111/j.1463-1326.2010.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 41.Scheuner D, Song B, McEwen E, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 42.Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 43.Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P, McGrath B, Li S, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]
- 47.Teske BF, Baird TD, Wek RC. Methods for analyzing eIF2 kinases and translational control in the unfolded protein response. Methods Enzymol. 2011;490:333–356. doi: 10.1016/B978-0-12-385114-7.00019-2. [DOI] [PubMed] [Google Scholar]
- 48.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.König J, Zarnack K, Luscombe NM, Ule J. Protein-RNA interactions: new genomic technologies and perspectives. Nat Rev Genet. 2011;13:77–83. doi: 10.1038/nrg3141. [DOI] [PubMed] [Google Scholar]
- 50.Starck SR, Green HM, Alberola-Ila J, Roberts RW. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem Biol. 2004;11:999–1008. doi: 10.1016/j.chembiol.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–859. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- 52.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 53.Huang CJ, Lin CY, Haataja L, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 54.Hartman MG, Lu D, Kim M-L, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 56.Lebastchi J, Deng S, Lebastchi AH, et al. Immune therapy and β-cell death in type 1 diabetes. Diabetes. 2013;62:1676–1680. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akirav EM, Lebastchi J, Galvan EM, et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A. 2011;108:19018–19023. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Husseiny MI, Kuroda A, Kaye AN, Nair I, Kandeel F, Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. PLoS ONE. 2012;7:e47942. doi: 10.1371/journal.pone.0047942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner DF. On the discovery of precursor processing. Methods Mol Biol. 2011;768:3–11. doi: 10.1007/978-1-61779-204-5_1. [DOI] [PubMed] [Google Scholar]
- 60.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 61.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1-receptor antagonist treatment in type 2 diabetes mellitus. Diabetes Care. 2009;32:1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snorgaard O, Hartling SG, Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of Type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 33:36–42. doi: 10.1007/BF00586459. [DOI] [PubMed] [Google Scholar]
- 64.Scholin A, Nystrom L, Arnqvist H, et al. Proinsulin/C-peptide ratio, glucagon and remission in new-onset Type 1 diabetes mellitus in young adults. Diabet Med. 2011;28:156–161. doi: 10.1111/j.1464-5491.2010.03191.x. [DOI] [PubMed] [Google Scholar]
- 65.Snorgaard O, Kjems LL, Roder ME, Hartling SG, Dinesen B, Binder C. Proinsulin immunoreactivity in recent-onset IDDM: the significance of insulin antibodies and insulin autoantibodies. Diabetes Care. 1996;19:146–150. doi: 10.2337/diacare.19.2.146. [DOI] [PubMed] [Google Scholar]
- 66.Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat. 1982;19:351–358. doi: 10.1007/BF02629258. [DOI] [PubMed] [Google Scholar]
- 67.Roder ME. Disproportionately elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses. The Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1994;79:1570–1575. doi: 10.1210/jcem.79.6.7989457. [DOI] [PubMed] [Google Scholar]
- 68.DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 69.Xiang AH, Peters RK, Kjos SL, et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:1036–1047. doi: 10.1111/j.1463-1326.2010.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akiyama M, Hatanaka M, Ohta Y, et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia. 2009;52:653–663. doi: 10.1007/s00125-009-1270-6. [DOI] [PubMed] [Google Scholar]
- 72.Evans-Molina C, Robbins RD, Kono T, et al. PPAR-{gamma} activation restores islet function in diabetic mice through reduction of ER stress and maintenance of euchromatin structure. Mol Cell Biol. 2009;29:2053–2067. doi: 10.1128/MCB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sachdeva MM, Claiborn KC, Khoo C, et al. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci U S A. 2009;106:19090–19095. doi: 10.1073/pnas.0904849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kono T, Ahn G, Moss DR, et al. PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents β-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol. 2012;26:257–271. doi: 10.1210/me.2011-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Z, Zhou Z, Li X, Huang G, Lin J. Rosiglitazone preserves islet β-cell function of adult-onset latent autoimmune diabetes in 3 years follow-up study. Diabetes Res Clin Pract. 2009;83:54–60. doi: 10.1016/j.diabres.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 76.Beales PE, Pozzilli P. Thiazolidinediones for the prevention of diabetes in the nonobese diabetic (NOD) mouse: implications for human type 1 diabetes. Diabetes Metab Res Rev. 2002;18:114–117. doi: 10.1002/dmrr.262. [DOI] [PubMed] [Google Scholar]
- 77.Augstein P, Augstein P, Dunger A, et al. Prevention of autoimmune diabetes in NOD mice by troglitazone is associated with modulation of ICAM-1 expression on pancreatic islet cells and IFN-γ expression in splenic T cells. Biochem Biophys Res Commun. 2003;304:378–384. doi: 10.1016/s0006-291x(03)00590-4. [DOI] [PubMed] [Google Scholar]
- 78.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Yusta B, Yusta B, Baggio LL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Shimoda M, Kanda Y, Hamamoto S, et al. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia. 2011;54:1098–1108. doi: 10.1007/s00125-011-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunha DA, Ladriere L, Ortis F, et al. Glucagon-like peptide-1 agonists protect pancreatic β-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–2862. doi: 10.2337/db09-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu S, Hosooka T, Matsuda T, et al. DPP4 inhibitor vildagliptin preserves beta-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J Mol Endocrinol. 2012;49:125–135. doi: 10.1530/JME-12-0039. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z, Christensson M, Forslöw A, De Meester I, Sundqvist K-G. A CD26-controlled cell surface cascade for regulation of T cell motility and chemokine signals. J Immunol. 2009;183:3616–3624. doi: 10.4049/jimmunol.0804336. [DOI] [PubMed] [Google Scholar]
- 84.Reinhold D, Bank U, Bühling F, et al. Inhibitors of dipeptidyl peptidase IV (DP IV, CD26) induces secretion of transforming growth factor-beta 1 (TGF-beta 1) in stimulated mouse splenocytes and thymocytes. Immunol Lett. 1997;58:29–35. doi: 10.1016/s0165-2478(97)02716-8. [DOI] [PubMed] [Google Scholar]
- 85.Jelsing J, Vrang N, van Witteloostuijn SB, Mark M, Klein T. The DPP4 inhibitor linagliptin delays the onset of diabetes and preserves β-cell mass in non-obese diabetic mice. J Endocrinol. 2012;214:381–387. doi: 10.1530/JOE-11-0479. [DOI] [PubMed] [Google Scholar]
- 86.Cabrera SM, Colvin SC, Tersey SA, Maier B, Nadler JL, Mirmira RG. Effects of combination therapy with dipeptidyl peptidase-IV and histone deacetylase inhibitors in the NOD mouse model of type 1 diabetes. Clin Exp Immunol. 2013;172:375–382. doi: 10.1111/cei.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian L, Gao J, Hao J, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151:3049–3060. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- 88.Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011;165:77–84. doi: 10.1530/EJE-11-0330. [DOI] [PubMed] [Google Scholar]
- 89.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual β-cell function. Diabetes Care. 2011;34:1463–1468. doi: 10.2337/dc11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellis SL, Moser EG, Snell-Bergeon JK, Rodionova AS, Hazenfield RM, Garg SK. Effect of sitagliptin on glucose control in adult patients with Type 1 diabetes: a pilot, doubleblind, randomized, crossover trial. Diabet Med. 2011;28:1176–1181. doi: 10.1111/j.1464-5491.2011.03331.x. [DOI] [PubMed] [Google Scholar]
- 91.Garg SK, Moser EG, Bode BW, et al. Effect of sitagliptin on post-prandial glucagon and GLP-1 levels in patients with type 1 diabetes: investigator-initiated, double-blind, randomized, placebo-controlled trial. Endocr Pract. 2012;19:19–28. doi: 10.4158/EP12100.OR. [DOI] [PubMed] [Google Scholar]
- 92.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes. 2012;61:145–154. doi: 10.2337/db11-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boerschmann H, Walter M, Achenbach P, Ziegler AG. Survey of recent clinical trials of the prevention and immunointervention of type 1 diabetes mellitus. Dtsch Med Wochenschr. 2010;135:350–354. doi: 10.1055/s-0030-1249169. [DOI] [PubMed] [Google Scholar]
- 94.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Akerfeldt MC, Howes J, Chan JY, et al. Cytokine-induced beta-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57:3034–3044. doi: 10.2337/db07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang C, Koulajian K, Schuiki I, et al. Glucose-induced beta cell dysfunction in vivo in rats: link between oxidative stress and endoplasmic reticulum stress. Diabetologia. 2012;55:1366–1379. doi: 10.1007/s00125-012-2474-8. [DOI] [PubMed] [Google Scholar]
- 98.Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60:918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mokhtari D, Welsh N. Potential utility of small tyrosine kinase inhibitors in the treatment of diabetes. Clin Sci. 2010;118:241–247. doi: 10.1042/CS20090348. [DOI] [PubMed] [Google Scholar]
- 100.Oh JJ, Hong SK, Joo YM, et al. Impact of sunitinib treatment on blood glucose levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2012;42:314–317. doi: 10.1093/jjco/hys002. [DOI] [PubMed] [Google Scholar]
- 101.Templeton A, Brändle M, Cerny T, Gillessen S. Remission of diabetes while on sunitinib treatment for renal cell carcinoma. Ann Oncol. 2008;19:824–825. doi: 10.1093/annonc/mdn047. [DOI] [PubMed] [Google Scholar]
- 102.Han MS, Chung KW, Cheon HG, et al. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes. 2009;58:329–336. doi: 10.2337/db08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Perera BGK, Hari SB, et al. Divergent allosteric control of the IRE1α endoribonuclease using kinase inhibitors. Nat Chem Biol. 2012;8:982–989. doi: 10.1038/nchembio.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]