Abstract
Introduction
Rolandic epilepsy, a childhood epilepsy associated with language impairments, was investigated for language-related cortical abnormalities.
Methods
Twenty-four children with rolandic epilepsy and 24 controls (age 8–14 years) were recruited and underwent the Clinical Evaluation of Language Fundamentals test. Structural MRI was performed at 3 T (voxel size 1 × 1 × 1 mm3) for fully automated quantitative assessment of cortical thickness. Regression analysis was used to test for differences between patients and controls and to assess the effect of age and language indices on cortical thickness.
Results
For patients the core language score (mean ± SD: 92 ± 18) was lower than for controls (106 ± 11, p = 0.0026) and below the norm of 100 ± 15 (p = 0.047). Patients showed specific impairments in receptive language index (87 ± 19, p = 0.002) and language content index (87 ± 18, p = 0.0016). Cortical thickness was reduced in patients (p < 0.05, multiple-comparisons corrected) in left perisylvian regions. Furthermore, extensive cortical thinning with age was found in predominantly left-lateralized frontal, centro-parietal and temporal regions. No associations were found between cortical thickness and language indices in the regions of aberrant cortex.
Conclusion
The cortical abnormalities described represent subtle but significant pathomorphology in this critical phase of brain development (8–14 years) and suggest that rolandic epilepsy should not be considered merely a benign condition. Future studies employing longitudinal designs are prompted for further investigations into cerebral abnormalities in RE and associations with cognitive impairment and development.
Keywords: Cortical thickness (quantitative), Benign rolandic epilepsy of childhood with centro-temporal spikes, Developmental trajectory, Language impairment
Highlights
-
•
Children with RE exhibit aberrant cortex in left perisylvian language regions.
-
•
Cortical abnormalities comprise reduced cortical thickness.
-
•
Extensive regions of earlier onset of cortical thinning were also observed.
-
•
Cortical development may provide an important new subject of research in RE.
1. Introduction
Rolandic epilepsy (RE) is an idiopathic focal epilepsy with most frequent onset at 7–10 years of age (Gomez and Klass, 1983; Panayiotopoulos et al., 2008). The epileptic focus is typically located in the lower motor and/or somatosensory cortex (rolandic area) (Koutroumanidis, 2007). RE is also known as benign (rolandic) epilepsy (of childhood) with centro-temporal spikes (BECTS), which reflects both the typical spontaneous remission of seizures during adolescence and the characteristic location of the epileptiform activity on the electroencephalogram (EEG) (Loiseau and Duché, 1989).
Although the seizure semiology of RE is relatively mild (Lerman and Kivity, 1975; Loiseau et al., 1992), recent evidence suggests serious comorbidities in selected cases and has put the assumed purely benign nature of RE under debate (Nicolai et al., 2006; Vinayan et al., 2005; Völkl-Kernstock et al., 2009; Weglage et al., 1997). An often reported comorbidity of RE is language impairment (Monjauze et al., 2005; Northcott et al., 2007; Overvliet et al., 2010; Papavasiliou et al., 2005). It has been suggested that the diagnosis of language impairment may even precede that of RE (Overvliet et al., 2011a).
Even though the sensorimotor and language system are mutually involved in for instance speech production (in which complex articulatory movement and auditory feedback are required), the link between RE and problems in purely cognitive aspects of language such as reading is less trivial (Carlsson et al., 2000; Clarke et al., 2007). The existence of such an association is suggested by that fact that a significant correlation has been demonstrated between problems in motor and problems in language development (Gündüz et al., 1999; Overvliet et al., 2011b). This suggests the existence of a mechanism through which epileptiform activity originating from the sensorimotor cortex might enter and disturb the language system as a whole, the neuronal pathways of which are as yet unknown.
Structural imaging has been used in attempts to identify cerebral abnormalities in RE. Several authors concluded that distributed subtle structural abnormalities on clinical MRI are common in RE (Eeg-Olofsson et al., 2000; Gelisse et al., 2003; Lundberg et al., 1999), but not specific for this disorder (Boxerman et al., 2007). However, these studies did not include healthy controls, were of qualitative nature, and were not tailored for systematic abnormalities (i.e. consistent over subjects with respect to location). In this context, quantitative approaches might seem advantageous. In recent years, quantitative techniques to study cortical thickness have been developed (Fischl and Dale, 2000; Kim et al., 2005). These techniques allow local analysis of the entire cortex and are less influenced by inter-individual gyral variations than traditional voxel-based whole-brain methods, such as voxel-based-morphology (VBM) (Mueller et al., 2009). In a group of children with frontal lobe epilepsy, cortical thickness analysis has been successfully applied; in a study of Widjaja et al (Widjaja et al., 2011), regions of thinner cortex were found both within and beyond the frontal lobe. Also in other types of epilepsy, such as temporal lobe epilepsy in adults, reduced cortical thickness has been reported beyond the lobe of the primary seizure focus (Mueller et al., 2009).
The goal of the current study is to investigate whether abnormalities in cortical thickness can be found in RE, both within and beyond the sensorimotor cortex. Furthermore, we investigated whether such abnormalities are localized in classical left perisylvian language areas and are associated with language impairment as assessed using neuropsychological testing.
2. Materials and methods
2.1. Study population
A total of 24 children (9 girls) with a clinical diagnosis of RE were selected as recently described (Overvliet et al., in press), see also the selection criteria below. The average age at testing was 11.3 years (range: 8–14 years) and the age at epilepsy onset (7.3 ± 2.2 years) was typical (Panayiotopoulos et al., 2008). An age and gender-matched healthy control population of 24 children (10 girls) was included. The average age of the controls at testing was 10.6 years (range: 8–14 years; t-test for group age difference: p = 0.15). Of the patients, 20/24 were right handed; of the controls 22/24. For further subject characteristics, see Table 1.
Table 1.
Characteristics of the study participants.
RE stands for rolandic epilepsy, AED stands for anti epileptic drug. Note that age at onset and epilepsy duration are difficult to accurately establish given the mild and nocturnal nature of RE seizures.
| Subject characteristics | RE | Controls |
|---|---|---|
| N | 24 | 24 |
| Age [y] | 11.3 ± 1.9 | 10.6 ± 1.8 |
| Age at epilepsy onset [y] | 7.3 ± 2.2 | n.a. |
| Epilepsy duration [y] | 2.4 ± 2.0 | n.a. |
| Gender (male/female) | 15/9 | 14/10 |
| Handedness (r/l/ambidexter) | 20/3/1 | 22/2/0 |
| Number of AEDs (0/1/> 1) | 8/11/5 | n.a. |
2.1.1. Selection criteria
Children with RE were selected based on EEG criteria and seizure semiology (Berroya et al., 2005; Panayiotopoulos et al., 2008). EEG criteria include the presence of spike and slow wave complexes occurring as individual paroxysms or in repetitive clusters with a maximum in the mid temporal and/or central electrodes and with a temporal-frontal dipole field. Additional independent central, mid temporal, parietal or occipital spike wave foci in the same or other hemisphere were allowed. To exclude severe cases (Landau–Kleffner syndrome (LKS) or LKS-like), interictal epileptiform activity was required to be present < 85% of the time during non-REM sleep. With respect to seizure semiology, seizures with anarthria, hemiclonia involving the face and/or unilateral extremities, or secondarily generalized seizures were considered. In case of poorly observed nocturnal seizures, post-ictal signs of a generalized seizure or confirmation of post-ictal hemiparesis was sufficient for inclusion in case of otherwise typical EEG.
The children with RE were tested by the Wechsler Intelligence Score for Children, third edition (WISC-III), and all had a full-scale IQ > 70. None of the healthy controls had (a history of) dyslexia, learning disorders or psychiatric disorders, or attended special education. Children were excluded if they had dental braces (MRI quality) or were somewhat afraid in the scanner. Healthy controls were excluded in case of suspicion of language impairment (see language assessment).
A board certified neuroradiologist specialized in epilepsy (PH) reviewed all scans and no structural abnormalities were found.
All parents (or guardians) and children gave written informed consent prior to study participation. The study was approved by the ethical review boards of both participating institutions and has ClinicalTrials.gov identifier NCT01335425.
2.2. Language assessment
To assess language performance, the Clinical Evaluation of Language Fundamentals, Fourth edition (CELF-4), Dutch version, was used (Paslawski, 2005; Semel et al., 2010). The CELF-4 is considered the gold standard for the identification of language disorders or delays in children and yields several age-corrected indices. Among these are the core language score (norm value, mean ± standard deviation: 100 ± 15), which is a global measure for language performance and can serve as a screening measure (e.g. exclusion of language impaired controls). More specific language indices were obtained in the group of children with RE only, including receptive language index (listening and understanding), expressive language index (expressing oneself, speaking), and language content index (semantic development).
2.3. MRI acquisition
Structural T1-weighted MRI was performed at 3.0 T (Philips Achieva system; Philips Medical System, Best, The Netherlands) using an eight-element receive-only head coil. Acquisition settings were: 1 × 1 × 1 mm3 voxel size, 3D fast spoiled gradient echo sequence, echo time/repetition time/inversion time 3.8/8.3/1022 ms and acquisition time 8 min.
2.4. Cortical thickness analysis
Cortical thickness analysis was performed using the Freesurfer image analysis software package (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). Freesurfer tessellates the interface between gray and white matter and between gray matter and cerebrospinal fluid (CSF) based on image intensity (gradients) in a highly robust and fully automated fashion. The shortest distance between the two surfaces represents an estimate of the cortical thickness (at approximately 300,000 nodes). Freesurfer was also used to spatially register the cortical thickness maps to Freesurfer standard space, and to perform general linear model (GLM) analysis for group comparisons and to find predictors for cortical thickness variations. To account for residual registration errors and to strengthen the assumption of Gaussian distribution of the data, the thickness maps were smoothed using a Gaussian kernel (full-with-at-half-maximum 10 mm). As on average males have a somewhat thicker cortex than females (Raznahan et al., 2011), all analyses were gender corrected.
Matlab (R2008a, The MathWorks, Natick, MA) was used to perform additional data visualizations. Additionally, Matlab was used to perform robust quadratic fits for cortical thickness–age relationships (Fig. 2).
Fig. 2.
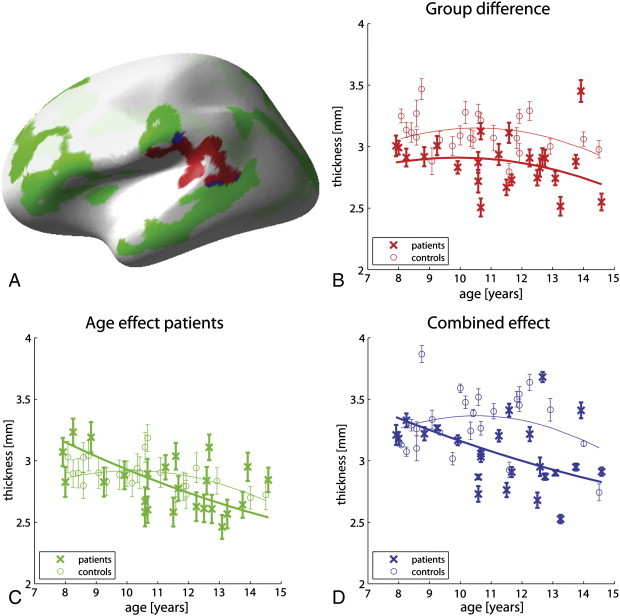
Aberrant cortical development in rolandic epilepsy.
A: Color-coded depiction of abnormalities in cortical thickness in children with rolandic epilepsy. Regions in which patients show reduced cortical thickness are depicted in red, regions in which patients display cortical thinning with age are given in green, and overlap between the two is depicted in blue. For these 3 types of regions, cortical thickness is plotted against age in the same colors in subfigures B, C and D, respectively, for both the patient and the control cohorts. Subfigures C and D reveal linear trends of decreasing cortical thickness with age for the patient group only; the control cohort seems to be in the transition from cortical thickening to cortical thinning . Error bars indicate 1 standard error of spatial variance and for visualization purposes, quadratic fits are provided in addition to the data points.
2.5. Statistical analysis
Group comparisons of core language score and comparisons of patient specific indices to the CELF-4 norm values were performed using two-sided Student's t-tests (SPSS, version 17); p-values below 0.05 were considered significant. The cortical thickness group comparison and associations of cortical thickness with age and language indices were investigated using Freesurfer's build-in GLM tool, Qdec. All Qdec results (at approximately 300,000 nodes) were corrected for multiple comparisons using the built-in tool for assessment of the cluster size p-values. These multiple-comparisons corrected results were considered significant for p < 0.05.
3. Results
3.1. Neuropsychological assessment (CELF-4)
The core language score of the patients (92 ± 18) was under the norm score of 100 (p = 0.047) and lower than that of the healthy controls (106 ± 10.5, p = 0.0026). The patients scored below norm on all subtests. The deficits were significant in receptive language index (87 ± 19, p = 0.002) and language content index (87 ± 18, p = .0016) and a trend of reduced expressive language index was found (92 ± 18, p = .054).
3.2. Thinner cortex in rolandic epilepsy
In the left hemisphere, a perisylvian region was identified in which patients had a thinner cortex than controls (Fig. 1A, age corrected). This region was located predominantly in the supramarginal gyrus and partly covered the bank of the superior temporal sulcus, the superior temporal gyrus and the lower postcentral gyrus.
Fig. 1.
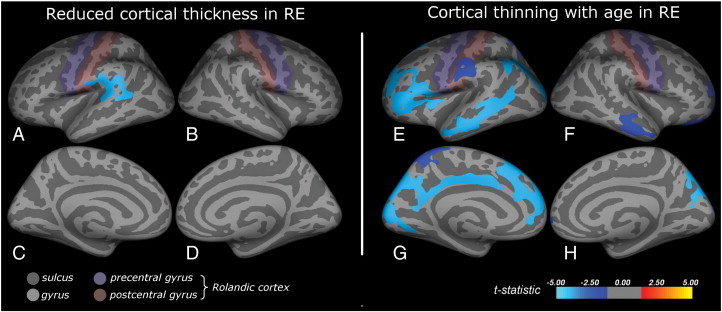
Cortical abnormalities in rolandic epilepsy.
Inflated brain visualizations of the regions showing abnormal cortical morphology in RE. Subfigures A–D display reduced cortical thickness in RE (age and gender corrected), subfigures E–H depict regions showing cortical thinning for increasing age, an effect which was only found in the patients (gender corrected). Reduced cortical thickness was found predominantly in the supramarginal gyrus and partly covered the bank of the superior temporal sulcus, the superior temporal gyrus and the lower postcentral gyrus (A). Cortical thinning with age was predominantly found in the left hemisphere and involved the inferior frontal gyrus, the inferior postcentral and the supramarginal gyrus and the middle temporal gyri at the lateral side (E), and the cuneus, precuneus and cingulate cortex medially (G).
No aberrant regions were found in the right hemisphere and no regions were found in which the patients had a thicker cortex than the controls.
3.3. Cortical thinning with age in rolandic epilepsy
The effect of age on cortical thickness was subsequently investigated for both groups separately. The patients exhibited widespread cortical thinning with age in predominantly the left hemisphere (Fig. 1E, G). The left frontal region covered superior and rostral middle frontal areas and parts of the pars triangularis and opercularis of the inferior frontal gyrus and the insula (Fig. 1E). The left parietal region partly covered the supramarginal gyrus and also the lower part of the postcentral gyrus. The left temporal region largely covered the middle temporal gyrus and a part of the bank of the superior temporal sulcus, whereas the posterior region covered parts of the inferior parietal and lateral occipital areas. This region extended medially (Fig. 1G) to cover parts of the cuneus and precuneus, the pericalcarine and lingual cortex, and the cingulate cortex. In the right hemisphere (Fig. 1F, H), several smaller regions were found in the rostral middle frontal and lateral orbitofrontal cortex, the lateral temporal, the superior parietal and the lateral occipital cortex.
No regions were found showing cortical thickening with age and also no (linear) age effect was found in the controls. In fact, whereas the patients show consistent cortical thinning, the controls seem to be in the transition from cortical thickening to cortical thinning (Fig. 2).
3.4. Correlations between cortical thickness and language indices
The association between cortical thickness and language indices was investigated per group (age corrected). No associations were found within the regions of abnormal cortical thickness and/or aberrant age effect described above. Instead, in the patients, higher core language scores were associated with lower cortical thickness in the left inferior occipital lobe, more specifically the lingual and lateral occipital cortex (Pearson correlation r = − 0.65, p < 0.001). Similar effects were found for the receptive language index, the expressive language index and the language content index, while no effects were found in the controls.
4. Discussion
In this study, we set out to detect cortical abnormalities in children with RE and potential associations with language (performance).
4.1. Main findings
We found reduced cortical thickness in patients compared to controls, not only within the seizure onset zone (rolandic cortex), but also beyond, in perisylvian regions of the left hemisphere. More extensive and distributed cortical abnormalities were observed when taking into account the effect of age, which demonstrated cortical thinning as a function of age, predominantly in the left hemisphere and in patients only. Language impairment in RE was confirmed for multiple language domains, particularly concerning receptive language and language content.
4.2. Reduced cortical thickness
Reduced cortical thickness in epilepsy is not specific for RE and has been demonstrated before in both adult and paediatric patients (Mueller et al., 2009; Widjaja et al., 2011). In this study, we found reduced cortical thickness not only in the rolandic cortex, but also in the supramarginal and superior temporal gyrus of the left hemisphere. We speculate that this is secondary pathology (i.e. not coinciding with the epileptic zone) and, given its location (Wernicke's area), might be related to language impairment. To explain reduced cortical thickness outside the seizure onset zone, previously the existence of an underlying network has been suggested to propagate epileptiform activity to other cortical regions and to induce distal atrophy (Mueller et al., 2009; Widjaja et al., 2011). An alternative explanation is that both cortical abnormalities and seizures are symptoms of an underlying pathology (benign childhood seizure susceptibility syndrome; BCSSS (Panayiotopoulos, 1993; Panayiotopoulos et al., 2008)).
4.3. Cortical thinning with age in RE
Widely distributed morphological abnormalities were found when studying cortical thickness as a function of age. Gradual cortical thinning for increasing age was found predominantly in the left hemisphere in several frontal, centroparietal, temporal, and medial regions in the patients only. Again, not only the laterality of these abnormalities (left hemisphere) suggests a link with language impairment, but also their specific localization in the left inferior frontal, supramarginal and middle temporal gyri (Broca's area, Wernicke's area and regions relevant for reading, respectively) (Backes et al., 2005; Deblaere et al., 2002).
4.4. Abnormal developmental trajectory
Upon further investigation, cortical thickness was also dependent on age in the controls, which showed cortical thickening at the beginning of the study age window and cortical thinning towards the end (Fig. 2). Cortical thinning is a normal phase of preadolescent brain development and reflects optimization-driven pruning of neurons and synapses in the underlying white matter (Andersen, 2003; Lenroot and Giedd, 2006; Muftuler et al., 2011). As such, cortical thinning does not reflect pathology per se, but can also be an aspect of normal maturation. However, the fact that the patients showed consistent cortical thinning over the entire study age window (8–14 years) whereas the controls seemed to be in the transition from cortical thickening to thinning might imply early onset of cortical thinning in the patients, which might represent actual pathomorphology. During the preadolescent phase of rapid brain development, proper neuronal cues (e.g. hormones, neurotropic factors, environmental demands) are essential for typical differentiation. Especially at the age range under investigation, strong region-specific maturational changes occur in the rolandic gray matter, which present an increased susceptibility to deviations from the normal developmental trajectory by improper signaling (Andersen, 2003; Lenroot and Giedd, 2006). Moreover, during development, preadolescent influences are incorporated into the (further) maturation of anatomy and function as they determine set points for adult function, with possibly lasting effects (Andersen, 2003). Localized early onset of cortical thinning in RE might represent a deviation from the normal developmental trajectory in the corresponding regions, possibly induced by improper neuronal signaling as a result of the typical preadolescent seizures and/or epileptiform activity. We combined our findings with information from literature on normal cortical development to construct a hypothetical trajectory for cortical development of aberrant regions in RE (Fig. 3) (Raznahan et al., 2011).
Fig. 3.
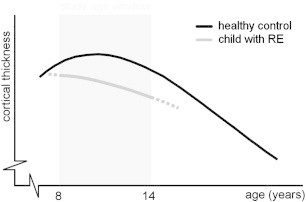
Schematic overview of cortical development.
Hypothetical cortical thickness developmental trajectory of affected regions in Rolandic epilepsy compared to healthy controls. The normal developmental trajectory follows an inverted U-shaped curve, wherein initial cortical thickening is followed by cortical thinning. In RE, several regions display both reduced cortical thickness and aberrantly early onset of cortical thinning (at or even before 8 years of age), other regions show one of these effects separately, see Fig. 2. Normal developmental trajectory adapted from (Raznahan A et al., 2011).
4.5. Mechanisms for impairment
The phase of preadolescent cortical thinning is preceded by a tremendous overshoot of neurons and connections, during which the brain is established as an over-complete network (Andersen, 2003; Lenroot and Giedd, 2006; Muftuler et al., 2011). The subsequent pruning process removes redundant neurons and connections to optimize the network for environmental needs, the level of redundancy determining the degree of adaptation. In RE, the early onset of cortical thinning in specific regions, alone or in combination with reduced cortical thickness per se, might represent locally suboptimal network formation and/or pruning and might actually be the mechanism behind impaired language function. Dedicated research, e.g. linking cortical thickness to the integrity of the underlying network, is needed to test this hypothesis, see also the following section.
4.6. Lack of association between abnormal cortex and language impairment
The current study did not observe an association between cortical thickness and language performance for regions of aberrant cortex. Possibly this association was not found since it is indirect, i.e. mediated by the underlying network integrity, and might only become apparent in case of severe network breakdown, which is probably not the case in RE. Techniques offering a more direct/closer window on the underlying network, such as diffusion weighted and functional imaging probing functional and structural network integrity, respectively, might prove more sensitive in establishing such associations in future research (Besseling et al., 2013; Jones, 2010; Jones et al., 2012; van den Heuvel and Hulshoff Pol, 2010).
4.7. Methodological considerations
In our cross-sectional study, the effect of age could only be assessed by virtue of the variability in age of the subjects included. Our findings suggest (but do not prove) aberrant time courses of cortical development in RE. For future research, longitudinal designs are prompted to further investigate abnormalities in cortical development in individual RE patients. Furthermore, we can only speculate that abnormal cortical development during preadolescence reflects network impairments in the underlying white matter which may persist into adulthood, however, explicit network assessment and inclusion of adults in remission from RE are needed to validate these claims. For future research, we propose to follow up over many years, from the moment of seizure onset (or even before, e.g. based on the identification of a predictive profile of language impairment (Overvliet et al., 2011a, 2011b)) until well into adulthood. In addition to timely inclusion, adequate assessment of characteristics such as seizure frequency is expected to be especially challenging in such longitudinal studies of RE, given its mild and typically nocturnal seizure semiology. We also suggest the acquisition of diffusion weighted or functional imaging to assess structural and functional connectivity, respectively (Jones et al., 2013; van den Heuvel and Hulshoff Pol, 2010).
4.8. Clinical outlook
RE is commonly regarded as benign and consequently children with RE typically do not receive special care (Lerman and Kivity, 1975; Loiseau et al., 1983; Loiseau et al., 1992). Because of the increasing awareness of significant comorbidities in RE, its classification as benign is under debate (Hughes, 2010; Nicolai et al., 2006; Vinayan et al., 2005; Völkl-Kernstock et al., 2009; Weglage et al., 1997). In the current study, we report widespread cortical abnormalities in language areas in children with RE who were selected based on EEG criteria and seizure semiology and not on language performance. These findings might signify that language impairment is more general in RE than commonly assumed. Indeed, it has been demonstrated that (seizure free) siblings of children with RE are at increased risk for language disorders (Clarke et al., 2007), suggesting a shared genetic basis for language impairment and seizures in RE. When health care professionals are willing to generally regard RE as malign to a certain extent (i.e. not only in atypical cases), they might be more inclined to subject children with RE to treatment instead of the often applied “wait and see” strategy. Furthermore, the current study demonstrates the relevance of the effect of age in RE and proposes to adopt the view that RE represents a deviation from the normal developmental trajectory of the brain in a critical period of brain maturation. The earlier this deviation occurs, the more severe the consequences, with children of age at seizure onset below 6 years being having the lowest language performance (Jurkeviciene et al., 2012). This warrants further research into whether it is possibly to exploit the increased brain plasticity in this critical period as a window of opportunity to redirect aberrant development onto a normal trajectory (Andersen, 2003). A possible approach is to stimulate language network formation by speech therapy, however this has not systematically been studied yet (Besag, 2006). Alternatively, improper neuronal signaling might be reduced by suppression of seizures and/or epileptiform activity using anti epileptic drugs (Porras-Kattz et al., 2011), although in clinical practise this is controversial in RE as it is difficult to differentiate adverse effects of medication from disease effects (Hughes, 2010).
In conclusion, for the first time specific cortical abnormalities consistent over subjects were observed in children with RE. The abnormalities were localized predominantly in language mediating brain regions of the left hemisphere and involve areas of reduced cortical thickness and of early onset of cortical thinning of patients compared to controls. Future longitudinal research is prompted to further investigate developmental abnormalities in RE, e.g. investigating whether the cortical abnormalities represent a predictive cerebral marker for language impairment risk during and potentially after the active seizure period.
Acknowledgments
This work was supported by the Dutch Epilepsy Foundation (NEF). The author JJ was funded by VENI research grant 916.11.059 from the Netherlands Organization for Scientific Research (NWO) and the Netherlands Organization for Health Research and Development (ZonMw). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank Esther Peeters for her help with the MRI data acquisitions, Marc Geerlings for continuing ICT back-up and the Dutch Epilepsy Foundation for funding author AA.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Backes W.H. Language activation distributions revealed by fMRI in post-operative epilepsy patients: differences between left- and right-sided resections. Epilepsy Research. 2005;66(1–3):1–12. doi: 10.1016/j.eplepsyres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Berroya A.M. Spike morphology, location, and frequency in benign epilepsy with centrotemporal spikes. Journal of Child Neurology. 2005;20(3):188–194. doi: 10.1177/08830738050200030401. [DOI] [PubMed] [Google Scholar]
- Besag F.M. Cognitive and behavioral outcomes of epileptic syndromes: implications for education and clinical practice. Epilepsia. 2006;47(Suppl. 2):119–125. doi: 10.1111/j.1528-1167.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- Besseling R.M.H. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. NeuroImage: Clinical. 2013;2:239–246. doi: 10.1016/j.nicl.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerman J.L. Is rolandic epilepsy associated with abnormal findings on cranial MRI? Epilepsy Research. 2007;75(2–3):180–185. doi: 10.1016/j.eplepsyres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson G. Neuropsychological long-term outcome of rolandic EEG traits. Epileptic Disorders. 2000;2(Suppl. 1):S63–S66. [PubMed] [Google Scholar]
- Clarke T. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48(12):2258–2265. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. Journal of Cognitive Neuroscience. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Deblaere K. Developing a comprehensive presurgical functional MRI protocol for patients with intractable temporal lobe epilepsy: a pilot study. Neuroradiology. 2002;44(8):667–673. doi: 10.1007/s00234-002-0800-4. [DOI] [PubMed] [Google Scholar]
- Eeg-Olofsson O., Lundberg S., Raininko R. MRI in rolandic epilepsy. Epileptic Disorders. 2000;2(Suppl. 1):S51–S53. [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelisse P. Abnormal neuroimaging in patients with benign epilepsy with centrotemporal spikes. Epilepsia. 2003;44(3):372–378. doi: 10.1046/j.1528-1157.2003.17902.x. [DOI] [PubMed] [Google Scholar]
- Gomez M.R., Klass D.W. Epilepsies of infancy and childhood. Annals of Neurology. 1983;13(2):113–124. doi: 10.1002/ana.410130202. [DOI] [PubMed] [Google Scholar]
- Gündüz E., Demirbilek V., Korkmaz B. Benign rolandic epilepsy: neuropsychological findings. Seizure. 1999;8(4):246–269. doi: 10.1053/seiz.1999.0293. [DOI] [PubMed] [Google Scholar]
- Hughes J.R. Benign epilepsy of childhood with centrotemporal spikes (BECTS): to treat or not to treat, that is the question. Epilepsy & Behavior. 2010;19(3):197–203. doi: 10.1016/j.yebeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Jones D. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging in Medicine. 2010;2(3):341–355. [Google Scholar]
- Jones D.K., Knosche T.R., Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Jurkeviciene G. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. European Journal of Paediatric Neurology. 2012;16(6):653–661. doi: 10.1016/j.ejpn.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Kim J.S. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Koutroumanidis M. Panayiotopoulos syndrome: an important electroclinical example of benign childhood system epilepsy. Epilepsia. 2007;48(6):1044–1053. doi: 10.1111/j.1528-1167.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lerman P., Kivity S. Benign focal epilepsy of childhood. A follow-up study of 100 recovered patients. Archives of Neurology. 1975;32(4):261–264. doi: 10.1001/archneur.1975.00490460077010. [DOI] [PubMed] [Google Scholar]
- Loiseau P., Duché B. Benign childhood epilepsy with centrotemporal spikes. Cleveland Clinic Journal of Medicine. 1989;56(Suppl. Pt 1):S17–S22. doi: 10.3949/ccjm.56.s1.17. (discussion S40-2) [DOI] [PubMed] [Google Scholar]
- Loiseau P. Long-term prognosis in two forms of childhood epilepsy: typical absence seizures and epilepsy with rolandic (centrotemporal) EEG foci. Annals of Neurology. 1983;13(6):642–648. doi: 10.1002/ana.410130610. [DOI] [PubMed] [Google Scholar]
- Loiseau P., Duché B., Cohadon S. The prognosis of benign localized epilepsy in early childhood. Epilepsy Research. Supplement. 1992;6:75–81. [PubMed] [Google Scholar]
- Lundberg S. Hippocampal asymmetries and white matter abnormalities on MRI in benign childhood epilepsy with centrotemporal spikes. Epilepsia. 1999;40(12):1808–1815. doi: 10.1111/j.1528-1157.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Monjauze C. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain and Language. 2005;92(3):300–308. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Mueller S.G. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. NeuroImage. 2009;46(2):353–359. doi: 10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muftuler L.T. Cortical and subcortical changes in typically developing preadolescent children. Brain Research. 2011;1399:15–24. doi: 10.1016/j.brainres.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy & Behavior. 2006;8(1):56–70. doi: 10.1016/j.yebeh.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Northcott E. Memory and phonological awareness in children with benign rolandic epilepsy compared to a matched control group. Epilepsy Research. 2007;75(1):57–62. doi: 10.1016/j.eplepsyres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: review of the literature. Epilepsy & Behavior. 2010;19(4):550–558. doi: 10.1016/j.yebeh.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M. Impaired language performance as a precursor or consequence of rolandic epilepsy? Journal of the Neurological Sciences. 2011;304(1):71–74. doi: 10.1016/j.jns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M. Correlation between language impairment and problems in motor development in children with rolandic epilepsy. Epilepsy & Behavior. 2011;22(3):527–531. doi: 10.1016/j.yebeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Overvliet, G.M., et al., in press. Clinical evaluation of language fundamentals in rolandic epilepsy, an assessment with CELF-4. European Journal of Paediatric Neurology. [DOI] [PubMed]
- Panayiotopoulos C.P. Benign childhood partial epilepsies: benign childhood seizure susceptibility syndromes. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56(1):2–5. doi: 10.1136/jnnp.56.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos C.P. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain. 2008;131(Pt 9):2264–2286. doi: 10.1093/brain/awn162. [DOI] [PubMed] [Google Scholar]
- Papavasiliou A. Written language skills in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy & Behavior. 2005;6(1):50–58. doi: 10.1016/j.yebeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Paslawski C. The clinical evaluation of language fundamentals, fourth edition (CELF-4): a review. Canadian Journal of School Psychology. 2005;20:129–134. [Google Scholar]
- Porras-Kattz E. Magnesium valproate in learning disabled children with interictal paroxysmal EEG patterns: preliminary report. Neuroscience Letters. 2011;492(2):99–104. doi: 10.1016/j.neulet.2011.01.065. [DOI] [PubMed] [Google Scholar]
- Raznahan A. How does your cortex grow? Journal of Neuroscience. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E., Wiig E.H., Secord W.A. 3 ed. Pearson; Amsterdam: 2010. Clinical Evaluation of Language Fundamentals 4, Nederlandse versie. [Google Scholar]
- van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. European Neuropsychopharmacology. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vinayan K.P., Biji V., Thomas S.V. Educational problems with underlying neuropsychological impairment are common in children with benign epilepsy of childhood with centrotemporal spikes (BECTS) Seizure. 2005;14(3):207–213. doi: 10.1016/j.seizure.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Völkl-Kernstock S. Speech and school performance in children with benign partial epilepsy with centro-temporal spikes (BCECTS) Seizure. 2009;18(5):320–326. doi: 10.1016/j.seizure.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Weglage J. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Developmental Medicine and Child Neurology. 1997;39(10):646–651. doi: 10.1111/j.1469-8749.1997.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Widjaja E. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52(9):1685–1691. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]


