Abstract
People with autism spectrum disorders (ASD) may show unusual reactions to unexpected changes that appear in their environment. Although several studies have highlighted atypical auditory change processing in ASD, little is known in this disorder about the brain processes involved in visual automatic change detection. The present fMRI study was designed to localize brain activity elicited by unexpected visual changing stimuli in adults with ASD compared to controls. Twelve patients with ASD and 17 healthy adults participated in the experiment in which subjects were presented with a visual oddball sequence while performing a concurrent target detection task. Combined results across participants highlight the involvement of both occipital (BA 18/19) and frontal (BA 6/8) regions during visual change detection. However, adults with ASD display greater activity in the bilateral occipital cortex and in the anterior cingulate cortex (ACC) associated with smaller activation in the superior and middle frontal gyri than controls. A psychophysiological interaction (PPI) analysis was performed with ACC as the seed region and revealed greater functionally connectivity to sensory regions in ASD than in controls, but less connectivity to prefrontal and orbito-frontal cortices. Thus, compared to controls, larger sensory activation associated with reduced frontal activation was seen in ASD during automatic visual change detection. Atypical psychophysiological interactions between frontal and occipital regions were also found, congruent with the idea of atypical connectivity between these regions in ASD. The atypical involvement of the ACC in visual change detection can be related to abnormalities previously observed in the auditory modality, thus supporting the hypothesis of an altered general mechanism of change detection in patients with ASD that would underlie their unusual reaction to change.
Keywords: Autism spectrum disorders, Visual change detection, fMRI, Functional connectivity, Anterior cingulate cortex
Highlights
► Brain processes involved in visual change detection in ASD remain unknown. ► This fMRI study was designed to localize brain activity elicited by visual changes. ► Larger activity in modality-specific sensory cortex and ACC in ASD ► Atypical functional connectivity between ACC and visual cortex in ASD ► Findings suggest an abnormal “amodal” change detection process in ASD.
1. Introduction
First described by Leo Kanner (1943), the autistic syndrome is a severe and pervasive neurodevelopmental disorder defined broadly by characteristic difficulties in social interaction, communication and repetitive and restricted behaviors (RRB) (DSM-IV-R; APA, 2000). RRB cover a wide range of heterogeneous behavioral manifestations such as motor stereotypies, sensory-related behaviors, circumscribed interests, rituals, echolalia and excessive sensitivity to change (Cuccaro et al., 2003; Militerni et al., 2002; Szatmari et al., 2002; Turner, 1999). Although this group of symptoms has to be observed in a person to diagnose autism, the resistance to change has been less often investigated than the social or communication deficits. However, sensitivity to any change occurring in the environment is a fundamental feature of ASD that appears to be a durable treatment-resistant symptom, which prevents the individual from adapting and thus results in major difficulties in daily life (Gabriels et al., 2005). Clinical reports of individuals with ASD show that they have strong reactions to changes in the environment, suggesting that they may detect changes differently than typically developing people. Consistent with clinical observation of intense reactions to environmental changes, several studies have also shown unusual perceptive functions across sensory modalities (Ashwin et al., 2009; Ben-Sasson et al., 2009; Khalfa et al., 2004; Leekam et al., 2007a,b; Reynolds and Lane, 2008). Particularly in the visual modality, many studies have reported unusual perception in ASD, hypo-functioning (attraction to light, intense look at objects or people, movements of fingers or objects in front of the eyes, fascination with reflections and/or brightly colored objects, running hands around the edges of objects) or hyper-functioning (focus on tiny pieces of dust/particles, dislike of the dark and bright lights, dislike of sharp flashes of light, look down most of the time, covering/closing eyes at bright lights) (Bogdashina, 2003; Leekam et al., 2007a,b) both being sometimes observed in the same subject. Taken together, these features suggest that resistance to change in people with ASD may reflect basic abnormalities in the processing of sensory information, and especially in the automatic processing of changing stimuli.
Behavioral studies of change detection have shown that the ability to detect targets increased with increasing developmental level for typical children, but remained constant over the same developmental range for children with ASD, pointing to an atypical developmental trajectory for change-detection in ASD (for review see Simmons et al., 2009). Attentional abnormalities have been proposed to contribute to atypical reactions to change in autism: increased distractibility might generate heightened reactivity to seemingly meaningless stimuli, while overly focused attention might contribute to the development of restricted pattern of interests or activities (Allen and Courchesne, 2001; Goldstein et al., 2001; Keehn et al., 2012; Lovaas et al., 1979; Simons and Rensink, 2005). Although attentional abnormalities have been shown to be involved in RBB in ASD, the pre-attentional processing involved in automatic change detection that initiates the orientation of attention towards relevant events, has not been determined in the visual modality.
A popular means to study the neural correlates of automatic change detection is the use of an oddball paradigm where a sequence of repetitive standard stimuli is presented with infrequent unpredictable deviant stimuli. Classically, in the auditory modality, electrophysiological and fMRI studies report that generators of automatic change detection are located bilaterally in the supratemporal part of the auditory cortex with additional generators in the prefrontal cortex (Celsis et al., 1999; Doeller et al., 2003; Garrido et al., 2009; Gomot et al., 2006; Molholm et al., 2005; Opitz et al., 2002; Rinne et al., 2005; Schall et al., 2003; Schonwiesner et al., 2007). Atypical auditory change processing in ASD has been described, in both electrophysiological and fMRI studies (Gomot et al., 2002; Gomot et al., 2006) highlighting normal activity in the auditory cortex but unusual activation in the ACC, a region known to be involved in attention switching and in the distribution of attentional resources (Daffner et al., 2003). The ACC is involved in the detection of non-routine situations and is thought to trigger the lateral prefrontal cortices to engage further attentional top–down cognitive processes (Carter, 2000). Gomot et al. (2006) suggested that atypical activation of the ACC could prevent appropriate allocation of pre-attentional processes to changing events. The normal activity in the sensory cortices associated with the abnormal involvement of non-specific regions such as the ACC in ASD suggested the existence of atypical change processing that would operate independent of the sensory modality.
Visual change detection process has been investigated in several electrophysiological studies in control participants using various deviants such as colors (Czigler et al., 2004), form (Maekawa et al., 2005), motion (Kremlacek et al., 2006; Urban et al., 2008), spatial frequency (Kimura et al., 2006; Sulykos and Czigler, 2011) and orientation (Astikainen et al., 2008; Kimura et al., 2010; Sulykos and Czigler, 2011). Previous fMRI (Yucel et al., 2007) and electrophysiological studies showed the main contribution of the occipital areas (Kimura et al., 2010; Urakawa et al., 2010), associated with prefrontal areas (Clery et al., 2012; Czigler et al., 2004; Heslenfeld, 2003; Urakawa et al., 2010) in automatic visual change processing. Other electrophysiological studies have further revealed sources in prefrontal regions. Kimura et al. (2010, 2011) performed source analysis of responses to visual changes and showed generators of vMMN in the visual extra-striate region (BA19) and in the medial, lateral and ventro-lateral prefrontal cortex (right orbitofrontal region (BA47 and BA11)). Concordant with these findings, the prefrontal area has been described as one of the multi-modal cortical areas sensitive to sensory changes in fMRI (Downar et al., 2000) and MEG (Tanaka et al., 2009) studies.
To date, no study has reported brain correlates of automatic visual change process in ASD. The present work investigated brain activations elicited by visual change in adults with ASD to determine whether there are abnormalities comparable to those reported in the auditory modality, and thus whether unusual reactions to change might be underlain by atypical general change processing independent of sensory modality. To localize brain activations elicited by unattended visual change in healthy adults, we designed an fMRI study using a passive three stimulus oddball paradigm, adapted from Besle et al. (2005). Stimuli consisted of the dynamic deformation of a circle into an ellipse either horizontally or vertically, resulting in two different shapes and thus involving two visual dimensions: object shape and motion direction. Based on previous electrophysiological, magnetoencephalographical and functional neuroimaging studies reviewed above, a region of interest (ROI) analysis approach was selected. We expected larger activity in the following regions in response to deviant and novel stimuli compared to standard stimuli: BA 17/18/19 (occipital visual region), BA 39/40 (temporo-parietal junction), BA 6/8 (dorsolateral premotor cortex), and BA 11 (orbitofrontal cortex). The dynamic stimuli used are expected to elicit activation along the two visual pathways, thus ROIs in BA 7 (dorsal stream) and BA 20 (ventral stream) were included. Finally, the ACC has been repeatedly found to be involved in novelty processing (Clark et al., 2000; Kiehl et al., 2001), and atypical involvement of this region has been described during change detection in the auditory modality (Gomot et al., 2006). Therefore, we also examined the BA 32/24 during automatic detection of novel and deviant stimuli. To further investigate ACC involvement during automatic change detection, a psychophysiological interaction analysis was done with ACC as the seed region.
2. Materials and methods
2.1. Participants
Twelve adults with high functioning autism or Asperger syndrome aged (mean age (years) ± SD: 28 ± 7; 11 males and 1 female) participated in the experiment. Diagnosis was made according to DSM-IV-R criteria (APA, 2000) and by using the Autism Diagnostic Observation Schedule-Generic (ADOS-G, fourth module) (Lord et al., 2000) (social interaction + communication scores mean ± SD: 10 ± 4; threshold for ASD = 7). Diagnosis was complemented by the Autism Spectrum Quotient (AQ) (Baron-Cohen et al., 2001) (mean ± SD: 38 ± 7; threshold for ASD = 32). Intellectual quotients (IQ) were assessed by the Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, 1997), which provided overall intellectual (mean ± SD) (IQ: 114 ± 21), verbal (vIQ: 119 ± 18) and performance (nvIQ: 101 ± 22) quotients. Adults with ASD were age matched with seventeen healthy volunteer adults (mean age (years) ± SD: 27 ± 6; 15 males and 2 females), none of whom had a previous history of neurological or psychiatric problems. All participants had normal or corrected-to-normal vision and none were receiving psychotropic medication. The Ethics Committee of the University Hospital of Tours approved the protocol. Written informed consent from all participants was obtained.
2.2. Stimuli and experimental design
Change detection process was studied through an oddball paradigm with three different types of stimuli, using an event-related fMRI paradigm. The stimuli consisted of the deformation of a circle into an ellipse either horizontally (Standard) or vertically (Deviant) or into another, always novel, non-meaningful shape (Novel), adapted from Besle et al. (2005) (Fig. 1). The amount of deformation in either direction relative to the diameter was 33% and lasted 140 ms. Between each deformation, the circle remained present on the screen. Each stimulus was constituted of seven successive images presented in 140 ms (i.e. 20 ms per image) which resulted in apparent motions in the stimuli. Sequences included ‘Standard’, ‘Deviant’ (probability of occurrence P = 0.03) and ‘Novel’ (probability of occurrence P = 0.03) stimuli. The total number of stimuli was 1395. To control for effects related to the stimulus features, 2 runs were performed in which Standards and Deviants were counterbalanced. In order to present the visual stimuli outside the focus of attention, a primary task was required. Subjects were asked to fixate at a central cross and to respond to its disappearance (target stimuli: 7% of the trials) by pressing a button with the right thumb as quickly as possible. This disappearance had a duration of 120 ms and occurred unpredictably within a standard trial, was desynchronized relative to the standard onset and could not occur during a standard preceding a deviant trial. Visual stimuli were presented with a constant interstimulus interval of 650 ms. Three resting periods of 15 s each (involving a black screen watching) were presented at the beginning, at the middle and at the end of the sequence.
Fig. 1.
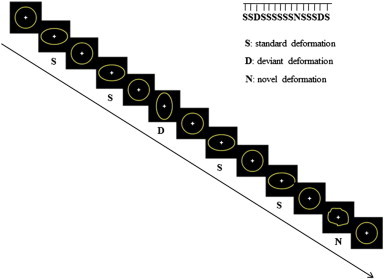
Experimental design. Dynamic stimuli consisted on the deformation of a circle into an ellipse either horizontally (standard deformation) or vertically (deviant deformation) or into a new shape (novel deformation).
2.3. Behavioral responses
For each subject, the reaction times (in ms) and response accuracy were measured by taking into account the rates of hits, false alarms to non-target stimuli and missed targets, according to the formula: (targets − missed targets) ∗ 100 / (targets + false alarms).
2.4. fMRI procedure
2.4.1. Data acquisition
Magnetic resonance data were acquired on a 1.5-T Siemens Magnetom scanner (Siemens AG, Erlangen, Germany). Structural image were a 3-D anatomical T1-weighted sequence (repetition time: 1970 ms; echo time: 3.93 ms; inversion time = 1100 ms; FOV: 256; matrix size: 256 voxel size: 1 × 1 × 1 mm3). Data were acquired in the sagittal plane. Functional images were collected using a T2*-weighted gradient-echo EPI sequence with TR = 2.5 s, TE = 50 ms, and flip angle = 90°. The acquisition volume consisted of 29 interleaved axial (AC/PC) slices with slice thickness = 4 mm and interslice gap = 0 mm. The matrix was 64 × 64 with a 220 cm field of view, yielding in an in-plane resolution of 3.4 × 3.4 mm.
2.4.2. Image pre-processing
Image preprocessing was performed using statistical parametric mapping software (SPM5, Wellcome Department of Cognitive Neurology, London, http://www.fil.ion.ucl.ac.uk/spm). Functional volumes were first time corrected, motion corrected by spatial realignment to the first volume and then normalized to the MNI reference brain (courtesy of the Montreal Neurological Institute). The normalized functional images were finally spatially smoothed with an 8 mm FWHM (full-width half-maximum) Gaussian kernel. The six estimated movement parameters were included as covariates in the design matrix.
2.4.3. Statistical analyses
The statistical analysis of the variations of the BOLD signal was based on the application of the general linear model to time series of the task-related functional activations (Friston et al., 1995). Trials for all events (Target, Standard, Deviant, Novel, Rest) were modeled separately by a canonical hemodynamic response function and its first-order temporal derivative. The three standard stimuli following a resting period as well as the standard stimulus following a rare stimulus (deviant stimulus, novel stimulus or target) were modeled as separate events. Contrast images (Standard–Rest, Deviant–Standard and Novel–Standard) consisting of statistical parametric maps (SPMs) of t statistics at each voxel were then produced for each individual.
Analyses were completed using different methods. A whole brain analysis was used for the standard minus rest contrast. Individual SPM(t) were entered into a second level group analysis permitting inferences about condition effects across subjects that generalize to the population (i.e., random effects analysis). SPM(t) statistics were computed for this contrast to examine areas of activation for the group as a whole (Control + Autism), with a threshold of P < 0.01 false discovery rate (FDR) corrected for multiple comparisons (Genovese et al., 2002).
For the Deviant–Standard and Novel–Standard contrasts analyses were performed by investigating a priori regions of interest (ROIs) using the WFU PickAtlas toolbox (version 2.4, Maldjian et al., 2003) within SPM5. We based our regions of interests (ROIs) on findings from previous studies that localized generators of visual automatic change detection process. BA 17-18-19 (occipital cortex), BA 39–40 (posterior parietal cortex), BA 6–8 (anterior premotor cortex), BA 11 (orbitofrontal cortex), BA 20 (ventral stream) and BA 7 (dorsal stream) and the ACC were examined (BA 32/24). These ROIs were defined in MNI space. The locations of significant activations were expressed in Talairach coordinates (Talairach and Tournoux, 1988), using a non-linear transformation, as implemented in the WFU PickAtlas and based on the method developed by Matthew Brett (http://www.imaging.mrc-cbu.cam.ac.uk/imaging/Mni2Talairach). The differences between the two groups were evaluated by computing for each contras the SPM(t) using a two sample Student's t. Statistical threshold was set at P < 0.05 with small volume correction (Worsley et al., 1996).
2.4.4. Psychophysiological interaction analysis
The PPI analysis is a seed-region-based measure that establishes predictive linkages of neural activity in one cortical area based on the activity in the chosen seed region within the experimental or psychological context (Friston et al., 1997). PPI can reveal the interactive effect between the experimental condition and the predictive activity from the seed region. Although PPI analysis cannot provide detailed information about mutual modulatory facilitations among multiple cortical regions, it nevertheless provides data on how the activities in one region influence those in other brain regions, which served to test the specific hypothesis of the present study. We conducted a PPI analysis to estimate dynamic coupling between ACC and the other ROIs previously defined during visual deviance detection. For each participant, the ACC time-series was derived by extracting the first eigenvariate time series (“volumes of interest” within SPM5) from a sphere of 8 mm radius centered in the seed coordinates obtained in the ROI analysis for the deviant minus standard contrast. These time series were mean-corrected and high-pass filtered to remove low-frequency signal drifts. PPI analyses were then carried out for each subject by creating a design matrix with the interaction term, the psychological factor, and the physiological factor as regressors. For each subject, voxel-wise PPI effects were estimated, and statistical parametric maps (SPMs) were produced for the PPI term. The resulting contrast images were used in a second-level PPI group analysis, comparing the PPI contrast images between adults with ASD and controls in a two-sample t-test. Statistical threshold was set at P < 0.05 with small volume correction (Worsley and Friston, 1995). The locations of significant activations were expressed in Talairach coordinates (Talairach and Tournoux, 1988), using a non-linear transformation, as implemented in the WFU PickAtlas.
3. Results
3.1. Behavioral results
Both groups performed the distraction task well, indicating that they looked at the screen and perceived the visual stimuli; no significant between groups difference was found, neither in response accuracy (Ctrl: 98.4% ± 1.2; ASD: 96.2% ± 6.4; n.s) nor in reaction times (Ctrl: 453 ms ± 78; ASD: 459 ms ± 62; n.s).
3.2. fMRI results
3.2.1. Activations common to both groups
3.2.1.1. Standard stimuli
Combining data from all control and ASD participants, the whole brain analysis of the contrast between standard and rest conditions produced significant activation of posterior brain regions (P < 0.01, FDR corrected). Left and right middle occipital gyri (BA 18, left: x = − 28, y = − 99, z = 3 and right: x = 26, y = − 93, z = 0) were more activated in the standard condition than during rest.
3.2.1.2. Deviance detection
Main findings from the ROI analysis of brain activation elicited by deviant stimuli compared to standard stimuli in both groups combined (Controls + ASD) are listed in Table 1 (with P levels after small volume corrections) and illustrated in Fig. 2. Generically activated regions mainly included the left anterior premotor cortex (BA 6/8). Left-lateralized activation was also seen in the orbital cortex (BA 11) and the left posterior parietal cortex (BA 7). Finally, activations of the left posterior parietal cortex (BA 7) were elicited by visual deviant stimuli.
Table 1.
Main results from the ROI analysis of brain activations elicited in both groups by deviant stimuli compared to standard stimuli (N = 29; control + autism; P < 0.05, small volume correction). R = right; L = left.
| Functional comparison | Region | BA | Structures | Talairach coordinates |
Voxels | t | P | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Dev > Sta | Anterior premotor cortex | 8 | L middle frontal gyrus | − 44 | 10 | 21 | 159 | 3.29 | 0.001 |
| 6 | L medial frontal gyrus | − 6 | 42 | 33 | 119 | 2.75 | 0.005 | ||
| Orbitofrontal cortex | 11 | L medial frontal gyrus | − 2 | 34 | − 12 | 107 | 2.93 | 0.003 | |
| Occipital cortex | 19 | R middle occipital gyrus | 38 | − 84 | 21 | 73 | 2.66 | 0.006 | |
| 19 | L middle occipital gyrus | − 40 | − 74 | 42 | 47 | 2.45 | 0.010 | ||
| Posterior parietal cortex | 7 | L precuneus | − 38 | − 72 | 46 | ||||
| Nov > Sta | Occipital cortex | 19 | R middle occipital gyrus | 38 | − 85 | 21 | 287 | 3.49 | 0.001 |
| 19 | L middle occipital gyrus | − 30 | − 83 | 19 | 270 | 2.92 | 0.003 | ||
| Anterior premotor cortex | 8 | L middle frontal gyrus | − 50 | 14 | 42 | 151 | 3.15 | 0.002 | |
| 6 | L medial frontal gyrus | − 4 | 48 | 34 | 109 | 3.02 | 0.003 | ||
| Orbitofrontal cortex | 11 | L orbital gyrus | − 2 | 42 | − 19 | 93 | 2.98 | 0.003 | |
| 11 | R orbital gyrus | 2 | 42 | − 19 | 65 | 2.85 | 0.004 | ||
| Posterior parietal cortex | 7 | L superior parietal lobule | − 18 | − 30 | 38 | 9 | 2.30 | 0.014 | |
Fig. 2.
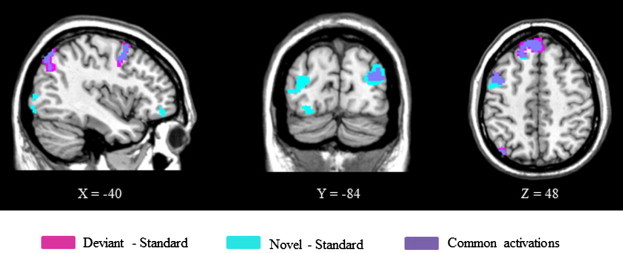
Activation maps showing common pattern of activations in both groups (N = 29). Main findings from the ROI analysis of brain activation elicited by deviant stimuli compared to standard stimuli are shown in pink and by novel stimuli compared to standard stimuli are shown in blue. Common activations are shown in purple. Voxels with activation significant at P < 0.05 after small volume correction.
3.2.1.3. Novelty detection
Table 1 also shows the main findings from the ROI analysis of brain activations elicited by novel stimuli compared to standard stimuli in both groups combined. Significant bilateral activations were revealed in the occipital cortex (BA 19) and in orbitofrontal cortex (BA 11). Left-lateralized activations were found in the middle frontal gyrus (BA 6/8). Finally, only the visual dorsal stream displayed significant activation (BA 7) (Fig. 2).
3.2.1.4. Salience effect
Although brain activity elicited by visual deviant and novel stimuli appeared very comparable, a degree-of-deviance effect was observed (Fig. 2); compared to deviant stimuli, novel stimuli elicited greater activity in bilateral occipital regions (BA 19) and in orbitofrontal cortex (BA 11).
3.2.2. Activation differences between groups
3.2.2.1. Standard stimuli
ROI analysis was performed using the WFU PickAtlas toolbox (version 2.4, Maldjian et al., 2003) within SPM5. Two spheres were drawn centered around the peaks of activation common to both groups but no significant between groups differences were found for the standard vs. rest contrast.
3.2.2.2. Deviant and novel stimuli
The same between group differences were observed in response to deviant and novel stimuli (Table 2, Fig. 3). Compared to adults with ASD, controls revealed greater activity in bilateral anterior premotor cortex (BA 6/8) and in the right orbitofrontal cortex (BA11). The bilateral temporal cortex was more activated in controls than in ASD during visual change perception. Conversely, adults with ASD showed stronger BOLD signal in bilateral visual areas (BA 18/19) and the ACC (BA 32/24).
Table 2.
Group comparisons of the main findings from the ROI analysis of brain activations elicited by deviant stimuli compared to standard stimuli and by novel stimuli compared to standard stimuli (P < 0.05, small volume correction). R = right; L = left.
| Functional comparison | Region | BA | Structures | Talairach coordinates |
Voxels | t | p | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Controls > ASD | |||||||||
| Dev > Sta | Anterior premotor cortex | 6 | R medial frontal gyrus | 6 | − 22 | 56 | 751 | 4.03 | < 0.001 |
| 6 | L medial frontal gyrus | − 2 | − 23 | 55 | |||||
| 8 | L middle frontal gyrus | − 38 | 12 | 47 | 222 | 2.49 | 0.010 | ||
| Orbitofrontal cortex | 11 | R middle frontal gyrus | 39 | 37 | − 7 | 152 | 2.83 | 0.004 | |
| Inferior temporal cortex | 20 | R inferior temporal gyrus | 53 | − 20 | − 19 | 35 | 3.50 | 0.001 | |
| 20 | L inferior temporal gyrus | − 59 | − 18 | − 20 | 30 | 2.89 | 0.004 | ||
| Nov > Sta | Anterior premotor cortex | 6 | L medial frontal gyrus | − 2 | − 23 | 55 | 560 | 3.85 | < 0.001 |
| 6 | R medial frontal gyrus | 4 | − 26 | 60 | |||||
| 8 | L middle frontal gyrus | 42 | 24 | 43 | 101 | 2.97 | 0.003 | ||
| Orbitofrontal cortex | 11 | R middle frontal gyrus | 38 | 38 | − 6 | 281 | 3.43 | 0.001 | |
| Inferior temporal cortex | 20 | L inferior temporal gyrus | − 61 | − 18 | − 18 | 43 | 3.70 | < 0.001 | |
| 20 | R inferior temporal gyrus | 53 | − 20 | − 19 | 33 | 2.89 | 0.004 | ||
| ASD > controls | |||||||||
| Dev > Sta | Occipital cortex | 18 | R cuneus | 20 | − 68 | 7 | 492 | 3.82 | < 0.001 |
| 18 | L cuneus | − 10 | − 70 | 7 | 369 | 2.95 | 0.003 | ||
| 19 | L superior occipital gyrus | − 40 | − 84 | 24 | |||||
| Posterior parietal cortex | 7 | L superior parietal lobule | − 14 | − 59 | 62 | 86 | 3.14 | 0.002 | |
| Anterior Cingulate cortex | 32 | R anterior cingulate gyrus | 4 | 18 | 18 | 75 | 2.16 | 0.020 | |
| Nov > Sta | Occipital cortex | 18 | R cuneus | 20 | − 68 | 7 | 1024 | 3.85 | < 0.001 |
| 19 | R middle occipital gyrus | 38 | − 74 | 2 | |||||
| 18 | L cuneus | − 12 | − 68 | 7 | 609 | 3.41 | 0.001 | ||
| 19 | L superior occipital gyrus | − 32 | − 90 | 23 | |||||
| Posterior parietal cortex | 7 | L superior parietal lobule | − 16 | − 59 | 62 | 206 | 3.68 | 0.001 | |
| 7 | R superior parietal lobule | 12 | − 56 | 64 | 178 | 3.23 | 0.002 | ||
Fig. 3.
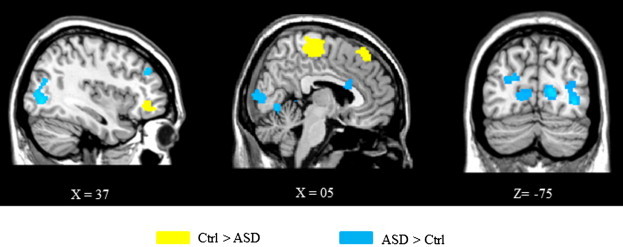
Group comparisons of the main findings from the ROI analysis of brain activation elicited by deviant stimuli compared to standard stimuli. Regions more activated in the control group than in ASD are shown in yellow and conversely, regions more activated in ASD than in controls are shown in blue. Voxels with activation significant at P < 0.05 after small volume correction.
3.2.3. PPI analysis
Using PPI analyses, we found significant between group differences in the connectivity maps of the seed region (Table 3, Fig. 4). While the ACC displayed functional connectivity during deviancy detection with the orbitofrontal cortex (BA 11), the anterior premotor cortex (BA 6), the posterior parietal cortex (BA 7) and the inferior temporal cortex (BA 7) in the control group, this seed region only showed functional connectivity with occipital regions (BA 18/19) and posterior parietal cortex (BA 7) in ASD.
Table 3.
Group comparisons of the main findings from the PPI analysis using the ACC as seed during deviance detection (P < 0.05, small volume correction). R = right; L = left.
| Functional comparison | Region | BA | Structures | Talairach coordinates |
Voxels | t | p | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Controls > ASD | |||||||||
| Dev > Sta | Orbitofrontal cortex | 11 | R medial frontal gyrus | 8 | 40 | − 15 | 310 | 4.15 | < 0.001 |
| 11 | L medial frontal gyrus | − 2 | 32 | − 17 | 134 | 3.50 | 0.001 | ||
| Anterior premotor cortex | 6 | L middle frontal gyrus | − 42 | 9 | 55 | 113 | 3.79 | 0.001 | |
| Posterior parietal cortex | 7 | L superior parietal lobule | − 34 | − 73 | 46 | 79 | 3.42 | 0.002 | |
| Inferior temporal cortex | 20 | R inferior temporal gyrus | 63 | − 18 | − 18 | 54 | 3.09 | 0.003 | |
| 20 | L inferior temporal gyrus | − 64 | − 18 | − 22 | 51 | 3.02 | 0.004 | ||
| ASD > controls | |||||||||
| Dev > Sta | Occipital cortex | 18 | L middle occipital gyrus | − 32 | − 80 | 11 | 381 | 3.22 | 0.002 |
| 19 | R superior occipital gyrus | 32 | − 78 | 26 | 296 | 3.40 | 0.002 | ||
| 19 | R fusiform gyrus | 32 | − 76 | − 9 | 222 | 4.37 | < 0.001 | ||
| 18 | L cuneus | − 6 | − 94 | 26 | 34 | 2.65 | 0.008 | ||
| Posterior parietal cortex | 7 | L superior parietal lobule | − 32 | − 48 | 59 | 28 | 3.00 | 0.004 | |
Fig. 4.
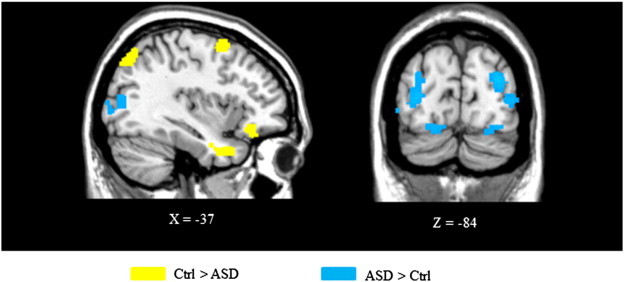
Group comparisons of the main findings from the PPI analysis using the ACC as seed during deviance detection. Regions more activated in the control group than in ASD are shown in yellow and conversely, regions more activated in ASD than in controls are shown in blue. Voxels with activation significant at P < 0.05 after small volume correction.
4. Discussion
The present study is the first to demonstrate brain activations associated with automatic visual change detection in adults with ASD. An oddball paradigm with standard, deviant and novel stimuli was used to localize brain correlates of passive visual change processing, according to the salience of the change.
Combined results across participants highlight the involvement of both occipital (Kimura et al., 2010; Urakawa et al., 2010; Yucel et al., 2007) and frontal (Czigler et al., 2004; Kimura et al., 2011) regions in visual change detection. Both deviant and novel stimuli elicited activations in occipital (BA 19), posterior parietal (BA 7), anterior premotor (BA 6/8) and orbitofrontal (BA 11) cortices. A salience effect was observed, as novel stimuli elicited greater activity in the sensory occipital regions (BA 19) than deviant stimuli, suggesting that novelty detection involves an additional activation of sensory areas to process unexpected change in shape and in motion.
The main result of the group comparison during visual change detection was that adults with ASD showed stronger activity in the bilateral occipital cortices (BA 18/19) than controls. Several neuroimaging studies have revealed stronger task-related activity in visual cortex in autism, shown as higher levels of activity associated with visual information processing (Belmonte and Yurgelun-Todd, 2003; Brown et al., 2005). Atypical perceptual processing, often manifested as enhanced perceptual performance (Dakin and Frith, 2005), is now included as an associated feature of the autistic phenotype (Belmonte et al., 2004). Autistic visual strengths are consistently reported for the Block Design subtest of the Wechsler Intelligence Scales (Caron et al., 2006; Shah and Frith, 1993), the Embedded Figures Task (Jolliffe and Baron-Cohen, 1997), visual search tasks (Joseph et al., 2009; Kemner et al., 2008; O'Riordan, 2004; O'Riordan et al., 2001), and visual discrimination tasks (Bertone et al., 2005; Plaisted et al., 1998). In addition, an increasing number of studies have demonstrated early sensory processing advantages or atypicalities in stimulus dimension extraction in ASD, with examples including crowding (Baldassi et al., 2009; Keita et al., 2010), contour and texture processing (Pei et al., 2009; Vandenbroucke et al., 2008) and spatial frequency processing (Jemel et al., 2010; Milne et al., 2009). Higher activity in the occipital cortex in ASD was also reported in an fMRI study in relation to increased search efficiency during a visual search task (Keehn and Joseph, 2008). These results suggest that ASD behavioral advantages might arise from stronger and more pervasive engagement of sensory processing mechanisms. These behavioral characteristics, along with other aspects of the autistic perceptual phenotype, have been summarized in the Enhanced Perceptual Functioning Model (EPF) (Mottron et al., 2006). Assuming generally stronger physiological engagement of the visual system in autism, this model predicts superior perceptual performance and a wider role for perceptual processes in autistic cognition.
Associated with this greater occipital activation, adults with ASD showed less activity than controls in anterior premotor (BA 6) and orbitofrontal cortices (BA 11). It is well established that damage to orbitofrontal cortex (OFC) and adjacent medial prefrontal cortex can result in impairments to flexibly modulate action selection in the face of changing contingencies (Fellows and Farah, 2005; Hornak et al., 2004). The FEF (BA 6/8) regions have been shown to play a major role in the voluntary shift of visual attention and to be particularly important for top-down regulated attentional processes (Donner et al., 2000; Goebel et al., 1998; Schall, 2002; Wojciulik and Kanwisher, 1999). This literature suggests that the brain activations observed in orbitofrontal regions and anterior premotor cortex are related to the inhibition of motor responses to task-irrelevant stimulus deviations. Reduced frontal activity in association with larger occipital activity in ASD has also been reported for tasks incorporating a broad range of cognitive and perceptual components, including embedded figure detection (Ring et al., 1999), attention shifting (Belmonte and Yurgelun-Todd, 2003), saccades to visual targets (Luna et al., 2002), working memory (Koshino et al., 2005), visuomotor learning (Muller et al., 2003) or face processing (Hubl et al., 2003). Based on neuroimaging evidence of anatomical and functional connectivity disruptions in autism, a connectivity bias theory has been proposed in ASD (Just et al., 2004; Thai et al., 2009; Wicker et al., 2008). This theory suggests that the behavioral markers of autism are directly or indirectly caused by limitations in communication between frontal and posterior brain regions, and predicts that these limitations will impact those tasks that require extensive coordinated functioning of frontal and posterior processing centers. The theory accounts for restricted repetitive and stereotyped patterns of behavior in terms of the inability of the frontal executive system to exert control over posterior processing centers. Thus the theory posits a biological mechanism, frontal–posterior connectivity disruption, which may be able to explain diverse impairments that characterize ASD (Schipul et al., 2011).
The third main group differences in brain structures involved in visual change perception were the greater activity observed in the ACC in ASD than in controls. The ACC is a complex structure which has been functionally and anatomically dissociated into 3 subdivisions: affective, cognitive and motor. The abnormal activity we found in ASD was located in the cognitive part of the ACC. This cognitive subdivision is part of a distributed attentional network and has been assigned with various functions, including modulation of attention or executive functions by influencing sensory or response selection (or both); monitoring competition, complex motor control, novelty and error detection; and anticipation of cognitively demanding tasks (Carter et al., 1999; Posner and Rothbart, 1998) (Cabeza et al., 1997; Devinsky et al., 1995; Elliott and Dolan, 1998; Garavan et al., 2002; Menon et al., 2001; Posner and Petersen, 1990; Reischies et al., 2005; Taylor et al., 2007). In view of the processes engaged during the paradigm we used, abnormal activity in the ACC is an interesting finding as this area is thought to play a crucial role in stimulus evaluation (Bush, et al., 2000). Anterior cingulate cortex is theorized to belong to a recency system that abandons older, stored information in order to capture new potentially relevant information (Ebmeier et al., 1995). Greater activation of the ACC in passive visual change detection may be related to an atypical attention switch towards the changing stimuli in ASD, in the sense that this structure would be involved in the capture of any new event whether it is relevant or not. The ability to attend selectively to meaningful sources of information while ignoring irrelevant sources is essential for competent and adaptive functioning. This may explain why individuals with ASD appear to ignore salient stimuli in the environment in favor of relatively discrete and apparently meaningless stimuli (Allen and Courchesne, 2001; Keehn et al., 2012), but may also contribute to the exceptional perceptual abilities observed in some individuals with ASD. This might be a maladjustment in so far as it leads to distress at small changes in the environment (Happe and Frith, 2006).
There is growing evidence of both functional and structural abnormalities of the ACC in ASD. Abnormal ACC activation has been observed during a range of cognitive tasks (Bogte et al., 2007; Gomot et al., 2006; Haznedar et al., 1997; Henderson et al., 2006; Russell and Jarrold, 1998; Sokhadze et al., 2010; Sokhadze et al., 2012; Vlamings et al., 2008). Among these studies Thakkar et al. (2008) suggested that ACC abnormalities compromise response monitoring and thereby lead to behaviors that are rigid and repetitive rather than flexible and responsive to contingencies. There is also evidence of reduced volume of the ACC (Haznedar et al., 1997; Haznedar et al., 2000) and of reduced fractional anisotropy in the white matter adjacent to the anterior cingulate gyri, suggesting a disruption of neural connections between this region and other brain structures (Barnea-Goraly et al., 2004). Aberrant connectivity is also suggested by findings of decreased functional connectivity with other brain regions (Kana et al., 2007). In order to investigate the role and the functional connectivity of this structure with other brain areas in our paradigm, we conducted PPI analyses, with the ACC as the seed region. Results showed that in ASD, the ACC was more functionally connected to sensory regions than to prefrontal and orbitofrontal cortices as seen in controls. Anatomically, recent studies of axonal connectivity of area 32 of ACC and prefrontal areas revealed an exuberance of thin axons that course over short or medium distances in the ASD brain, which may lead to occupation of sites normally available to the considerably sparser long-distance pathways (Zikopoulos and Barbas, 2010). Reduction in the strength of long-distance pathways in ASD may thus be secondary to the excessive short-range connections of ACC. Again, this connectivity bias may help in explaining why individuals with ASD do not adequately shift attention when necessary, and engage in repetitive and inflexible behavior (Gomot and Wicker, 2012). The atypical involvement of the ACC in visual change detection can be related to previous results of Gomot et al. (2006) investigating the change detection in ASD in the auditory modality. However, the authors reported atypical inhibitory mechanisms in this region that could prevent appropriate allocation of pre-attentional processes to changing events. Our results thus cannot be interpreted as highlighting the same atypical involvement of the ACC in automatic change detection, regardless of the sensory modality. However, it could be hypothesized that inappropriate allocation of pre-attentional resources interferes with change detection and may contribute to intolerance of change observed in ASD.
In the same vein, several reports suggested that individuals with ASD focus their attention on less contextually relevant aspects of a visual scene and notice details which are often ignored by typical observers. The ability to detect changes in a visual scene has therefore been investigated in ASD using the change blindness paradigm that makes it possible to assess unnoticed change effects (Beck et al., 2001; Rensink et al., 1997). However, analysis of the few studies in this domain reveals inconsistent findings showing either superior levels of task performance (Smith and Milne, 2009), similar error detection rate (Fletcher-Watson et al., 2008) or decreased levels of performance in ASD (Kikuchi et al., 2009) the latter being mainly related to a default in context facilitation effect (Fletcher-Watson et al., 2006; Loth et al., 2008). These findings suggest a weaker influence of schematic expectations on spontaneous attention to change in individuals with ASD, but highlight the fact that the brain processes engaged during both noticed and unnoticed changes need further study in this population.
In conclusion we found atypical brain correlates of automatic visual change detection in adults with ASD. Stronger sensory activation has been highlighted in association with reduced frontal activity in ASD, congruent with the idea of atypical connectivity between these regions described in the literature.
Acknowledgments
This research was supported by grants from the “Fondation Orange” and the “Région Centre” and by the CHRU Bretonneau, Tours (PHRR05-FBB EPCA). We thank all the volunteers for their time and effort spent participating in this study.
Experimental part of this study was performed on CERMEP — imagerie du vivant, Bron, F-69677, France, imaging facilities.
Special thanks are due to Pierre Fonlupt for his contribution in the experimental design and to Michael Lombardo for his help in the functional connectivity analysis. We also thank Dr Catherine Fischer for her contribution and Margot Taylor for her helpful comments.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Allen G., Courchesne E. Attention function and dysfunction in autism. Frontiers in Bioscience. 2001;6:105–119. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- APA . American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual-IV—Text Revision. [Google Scholar]
- Ashwin E., Ashwin C., Rhydderch D., Howells J., Baron-Cohen S. Eagle-eyed visual acuity: an experimental investigation of enhanced perception in autism. Biological Psychiatry. 2009;65(1):17–21. doi: 10.1016/j.biopsych.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Astikainen P., Lillstrang E., Ruusuvirta T. Visual mismatch negativity for changes in orientation—a sensory memory-dependent response. European Journal of Neuroscience. 2008;28(11):2319–2324. doi: 10.1111/j.1460-9568.2008.06510.x. [DOI] [PubMed] [Google Scholar]
- Baldassi S., Pei F., Megna N., Recupero G., Viespoli M., Igliozzi R. Search superiority in autism within, but not outside the crowding regime. Vision Research. 2009;49(16):2151–2156. doi: 10.1016/j.visres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A.L. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Beck D.M., Rees G., Frith C.D., Lavie N. Neural correlates of change detection and change blindness. Nature Neuroscience. 2001;4(6):645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Belmonte M.K., Yurgelun-Todd D.A. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Research. Cognitive Brain Research. 2003;17(3):651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Belmonte M.K., Cook E.H., Jr., Anderson G.M., Rubenstein J.L., Greenough W.T., Beckel-Mitchener A. Autism as a disorder of neural information processing: directions for research and targets for therapy. Molecular Psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Carter A.S., Briggs-Gowan M.J. Sensory over-responsivity in elementary school: prevalence and social–emotional correlates. Journal of Abnormal Child Psychology. 2009;37(5):705–716. doi: 10.1007/s10802-008-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone A., Mottron L., Jelenic P., Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128(10):2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Besle J., Fort A., Giard M.H. Is the auditory sensory memory sensitive to visual information? Experimental Brain Research. 2005;166(3–4):337–344. doi: 10.1007/s00221-005-2375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdashina O. Jessica Kingsley; London, UK: 2003. Sensory Perceptual Issues in Autism and Asperger Syndrome: Different Sensory Experiences, Different Perceptual Worlds. [Google Scholar]
- Bogte H., Flamma B., van der Meere J., van Engeland H. Post-error adaptation in adults with high functioning autism. Neuropsychologia. 2007;45(8):1707–1714. doi: 10.1016/j.neuropsychologia.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Brown C., Gruber T., Boucher J., Rippon G., Brock J. Gamma abnormalities during perception of illusory figures in autism. Cortex. 2005;41(3):364–376. doi: 10.1016/s0010-9452(08)70273-9. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Grady C.L., Nyberg L., McIntosh A.R., Tulving E., Kapur S. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. Journal of Neuroscience. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M.J., Mottron L., Berthiaume C., Dawson M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain. 2006;129(7):1789–1802. doi: 10.1093/brain/awl072. [DOI] [PubMed] [Google Scholar]
- Carter C. Images in neuroscience. Cognition: executive function. The American Journal of Psychiatry. 2000;157(1):3. doi: 10.1176/ajp.157.1.3. [DOI] [PubMed] [Google Scholar]
- Carter C., Botvinick M.M., Cohen J.D. The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Celsis P., Boulanouar K., Doyon B., Ranjeva J.P., Berry I., Nespoulous J.L. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. NeuroImage. 1999;9(1):135–144. doi: 10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- Clark V.P., Fannon S., Lai S., Benson R., Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. Journal of Neurophysiology. 2000;83(5):3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Clery H., Roux S., Besle J., Giard M.H., Bruneau N., Gomot M. Electrophysiological correlates of automatic visual change detection in school-age children. Neuropsychologia. 2012;50(5):979–987. doi: 10.1016/j.neuropsychologia.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Cuccaro M.L., Shao Y., Grubber J., Slifer M., Wolpert C.M., Donnelly S.L. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry Hum. Dev. 2003;34(1):3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Czigler I., Balazs L., Pato L.G. Visual change detection: event-related potentials are dependent on stimulus location in humans. Neuroscience Letters. 2004;364(3):149–153. doi: 10.1016/j.neulet.2004.04.048. [DOI] [PubMed] [Google Scholar]
- Daffner K.R., Scinto L.F., Weitzman A.M., Faust R., Rentz D.M., Budson A.E. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. Journal of Cognitive Neuroscience. 2003;15(2):294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Dakin S., Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Morrell M.J., Vogt B.A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Doeller C.F., Opitz B., Mecklinger A., Krick C., Reith W., Schroger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. NeuroImage. 2003;20(2):1270–1282. doi: 10.1016/S1053-8119(03)00389-6. [DOI] [PubMed] [Google Scholar]
- Donner T., Kettermann A., Diesch E., Ostendorf F., Villringer A., Brandt S.A. Involvement of the human frontal eye field and multiple parietal areas in covert visual selection during conjunction search. European Journal of Neuroscience. 2000;12(9):3407–3414. doi: 10.1046/j.1460-9568.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Ebmeier K.P., Lawrie S.M., Blackwood D.H., Johnstone E.C., Goodwin G.M. Hypofrontality revisited: a high resolution single photon emission computed tomography study in schizophrenia. Journal of Neurology, Neurosurgery & Psychiatry. 1995;58(4):452–456. doi: 10.1136/jnnp.58.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Dolan R.J. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. NeuroImage. 1998;8(1):17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- Fellows L.K., Farah M.J. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebral Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S., Leekam S.R., Turner M.A., Moxon L. Do people with autistic spectrum disorder show normal selection for attention? Evidence from change blindness. British Journal of Psychology. 2006;97(4):537–554. doi: 10.1348/000712606X114057. [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S., Leekam S.R., Findlay J.M., Stanton E.C. Brief report: young adults with autism spectrum disorder show normal attention to eye-gaze information-evidence from a new change blindness paradigm. Journal of Autism and Developmental Disorders. 2008;38(9):1785–1790. doi: 10.1007/s10803-008-0548-8. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Turner R., Frackowiak R.S. Characterizing evoked hemodynamics with fMRI. NeuroImage. 1995;2(2):157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gabriels R.L., Cuccaro M.L., Hill D.E., Ivers B.J., Goldson E. Repetitive behaviors in autism: relationships with associated clinical features. Research in Developmental Disabilities. 2005;26(2):169–181. doi: 10.1016/j.ridd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garrido M.I., Kilner J.M., Stephan K.E., Friston K.J. The mismatch negativity: a review of underlying mechanisms. Clinical Neurophysiology. 2009;120(3):453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goebel R., Linden D.E., Lanfermann H., Zanella F.E., Singer W. Functional imaging of mirror and inverse reading reveals separate coactivated networks for oculomotion and spatial transformations. Neuroreport. 1998;9(4):713–719. doi: 10.1097/00001756-199803090-00028. [DOI] [PubMed] [Google Scholar]
- Goldstein G., Johnson C.R., Minshew N.J. Attentional processes in autism. Journal of Autism and Developmental Disorders. 2001;31(4):433–440. doi: 10.1023/a:1010620820786. [DOI] [PubMed] [Google Scholar]
- Gomot M., Wicker B. A challenging, unpredictable world for people with autism spectrum disorder. International Journal of Psychophysiology. 2012;83(2):240–247. doi: 10.1016/j.ijpsycho.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Gomot M., Giard M.H., Adrien J.L., Barthelemy C., Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39(5):577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- Gomot M., Bernard F.A., Davis M.H., Belmonte M.K., Ashwin C., Bullmore E.T. Change detection in children with autism: an auditory event-related fMRI study. NeuroImage. 2006;29(2):475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Happe F., Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Haznedar M.M., Buchsbaum M.S., Metzger M., Solimando A., Spiegel-Cohen J., Hollander E. Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. The American Journal of Psychiatry. 1997;154(8):1047–1050. doi: 10.1176/ajp.154.8.1047. [DOI] [PubMed] [Google Scholar]
- Haznedar M.M., Buchsbaum M.S., Wei T.C., Hof P.R., Cartwright C., Bienstock C.A. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. The American Journal of Psychiatry. 2000;157(12):1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Henderson H., Schwartz C., Mundy P., Burnette C., Sutton S., Zahka N. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain and Cognition. 2006;61(1):96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslenfeld D.J. Visual mismatch negativity. In: Polich J., editor. Detection of Change: Event-related Potential and fMRI Findings. Kluwer Academic; Boston USA: 2003. pp. 41–59. [Google Scholar]
- Hornak J., O'Doherty J., Bramham J., Rolls E.T., Morris R.G., Bullock P.R. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hubl D., Bolte S., Feineis-Matthews S., Lanfermann H., Federspiel A., Strik W. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Jemel B., Mimeault D., Saint-Amour D., Hosein A., Mottron L. VEP contrast sensitivity responses reveal reduced functional segregation of mid and high filters of visual channels in autism. Journal of Vision. 2010;10(6):1–13. doi: 10.1167/10.6.13. [DOI] [PubMed] [Google Scholar]
- Jolliffe T., Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? Journal of Child Psychology and Psychiatry. 1997;38(5):527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Joseph R.M., Keehn B., Connolly C., Wolfe J.M., Horowitz T.S. Why is visual search superior in autism spectrum disorder? Developmental Science. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana R.K., Keller T.A., Minshew N.J., Just M.A. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62(3):198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Keehn B., Joseph R.M. Impaired prioritization of novel onset stimuli in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2008;49(12):1296–1303. doi: 10.1111/j.1469-7610.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- Keehn B., Müller R.A., Townsend J. Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews. 2012;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keita L., Mottron L., Bertone A. Far visual acuity is unremarkable in autism: do we need to focus on crowding? Autism Research. 2010;3(6):333–341. doi: 10.1002/aur.164. [DOI] [PubMed] [Google Scholar]
- Kemner C., van Ewijk L., van Engeland H., Hooge I. Brief report: eye movements during visual search tasks indicate enhanced stimulus discriminability in subjects with PDD. Journal of Autism and Developmental Disorders. 2008;38(3):553–557. doi: 10.1007/s10803-007-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalfa S., Bruneau N., Roge B., Georgieff N., Veuillet E., Adrien J.L. Increased perception of loudness in autism. Hearing Research. 2004;198(1–2):87–92. doi: 10.1016/j.heares.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kiehl K.A., Laurens K.R., Duty T.L., Forster B.B., Liddle P.F. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38(1):133–142. [PubMed] [Google Scholar]
- Kikuchi Y., Senju A., Tojo Y., Osanai H., Hasegawa T. Faces do not capture special attention in children with autism spectrum disorder: a change blindness study. Child Development. 2009;80(5):1421–1433. doi: 10.1111/j.1467-8624.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- Kimura M., Katayama J., Murohashi H. An ERP study of visual change detection: effects of magnitude of spatial frequency changes on the change-related posterior positivity. International Journal of Psychophysiology. 2006;62(1):14–23. doi: 10.1016/j.ijpsycho.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ohira H., Schroger E. Localizing sensory and cognitive systems for pre-attentive visual deviance detection: an sLORETA analysis of the data of Kimura et al. (2009) Neuroscience Letters. 2010;485(3):198–203. doi: 10.1016/j.neulet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Kimura M., Schroger E., Czigler I. Visual mismatch negativity and its importance in visual cognitive sciences. Neuroreport. 2011;22(14):669–673. doi: 10.1097/WNR.0b013e32834973ba. [DOI] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kremlacek J., Kuba M., Kubova Z., Langrova J. Visual mismatch negativity elicited by magnocellular system activation. Vision Research. 2006;46(4):485–490. doi: 10.1016/j.visres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Leekam S., Tandos J., McConachie H., Meins E., Parkinson K., Wright C. Repetitive behaviours in typically developing 2-year-olds. Journal of Child Psychology and Psychiatry. 2007;48(11):1131–1138. doi: 10.1111/j.1469-7610.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- Leekam S., Nieto C., Libby S.J., Wing L., Gould J. Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders. 2007;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Loth E., Carlos Gomez J., Happe F. Detecting changes in naturalistic scenes: contextual inconsistency does not influence spontaneous attention in high-functioning people with autism spectrum disorder. Autism Research. 2008;1(3):179–188. doi: 10.1002/aur.19. [DOI] [PubMed] [Google Scholar]
- Lovaas O.I., Koegel R.L., Schreibman L. Stimulus overselectivity in autism: a review of research. Psychological Bulletin. 1979;86(6):1236–1254. [PubMed] [Google Scholar]
- Luna J.D., Artal N., Reviglio V.E., Juarez C.P. LASIK-induced optic neuropathy. Ophthalmology. 2002;109(5):817–818. doi: 10.1016/s0161-6420(02)00981-8. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Goto Y., Kinukawa N., Taniwaki T., Kanba S., Tobimatsu S. Functional characterization of mismatch negativity to a visual stimulus. Clinical Neurophysiology. 2005;116(10):2392–2402. doi: 10.1016/j.clinph.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militerni R., Bravaccio C., Falco C., Fico C., Palermo M.T. Repetitive behaviors in autistic disorder. European Child & Adolescent Psychiatry. 2002;11(5):210–218. doi: 10.1007/s00787-002-0279-x. [DOI] [PubMed] [Google Scholar]
- Milne E., Griffiths H., Buckley D., Scope A. Vision in children and adolescents with autistic spectrum disorder: evidence for reduced convergence. Journal of Autism and Developmental Disorders. 2009;39(7):965–975. doi: 10.1007/s10803-009-0705-8. [DOI] [PubMed] [Google Scholar]
- Molholm S., Martinez A., Ritter W., Javitt D.C., Foxe J.J. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex. 2005;15(5):545–551. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulieres I., Hubert B., Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Muller R.A., Kleinhans N., Kemmotsu N., Pierce K., Courchesne E. Abnormal variability and distribution of functional maps in autism: an FMRI study of visuomotor learning. The American Journal of Psychiatry. 2003;160(10):1847–1862. doi: 10.1176/appi.ajp.160.10.1847. [DOI] [PubMed] [Google Scholar]
- Opitz B., Rinne T., Mecklinger A., von Cramon D.Y., Schroger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. NeuroImage. 2002;15(1):167–174. doi: 10.1006/nimg.2001.0970. [DOI] [PubMed] [Google Scholar]
- O'Riordan M.A. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. [DOI] [PubMed] [Google Scholar]
- O'Riordan M.A., Plaisted K.C., Driver J., Baron-Cohen S. Superior visual search in autism. Journal of Experimental Psychology. Human Perception and Performance. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Pei F., Baldassi S., Procida G., Igliozzi R., Tancredi R., Muratori F. Neural correlates of texture and contour integration in children with autism spectrum disorders. Vision Research. 2009;49(16):2140–2150. doi: 10.1016/j.visres.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Plaisted K., O'Riordan M., Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. Journal of Child Psychology and Psychiatry. 1998;39(5):765–775. [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1998;353(1377):1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischies F.M., Neuhaus A.H., Hansen M.L., Mientus S., Mulert C., Gallinat J. Electrophysiological and neuropsychological analysis of a delirious state: the role of the anterior cingulate gyrus. Psychiatry Research. 2005;138(2):171–181. doi: 10.1016/j.pscychresns.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rensink R.A., O'Regan J.K., Clark J.J. To see or not to see: the need for attention to perceive changes in scenes. Psychological Science. 1997;8:368–373. [Google Scholar]
- Reynolds S., Lane S.J. Diagnostic validity of sensory over-responsivity: a review of the literature and case reports. Journal of Autism and Developmental Disorders. 2008;38(3):516–529. doi: 10.1007/s10803-007-0418-9. [DOI] [PubMed] [Google Scholar]
- Ring H.A., Baron-Cohen S., Wheelwright S., Williams S.C., Brammer M., Andrew C. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain. 1999;122(7):1305–1315. doi: 10.1093/brain/122.7.1305. [DOI] [PubMed] [Google Scholar]
- Rinne T., Pekkola J., Degerman A., Autti T., Jaaskelainen I.P., Sams M. Modulation of auditory cortex activation by sound presentation rate and attention. Human Brain Mapping. 2005;26(2):94–99. doi: 10.1002/hbm.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J., Jarrold C. Error-correction problems in autism: evidence for a monitoring impairment? Journal of Autism and Developmental Disorders. 1998;28(3):177–188. doi: 10.1023/a:1026009203333. [DOI] [PubMed] [Google Scholar]
- Schall J.D. The neural selection and control of saccades by the frontal eye field. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2002;357(1424):1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall U., Johnston P., Todd J., Ward P.B., Michie P.T. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. NeuroImage. 2003;20(2):729–736. doi: 10.1016/S1053-8119(03)00398-7. [DOI] [PubMed] [Google Scholar]
- Schipul S.E., Keller T.A., Just M.A. Inter-regional brain communication and its disturbance in autism. Frontiers in Systems Neuroscience. 2011;5:1–11. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwiesner M., Krumbholz K., Rubsamen R., Fink G.R., von Cramon D.Y. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cerebral Cortex. 2007;17(2):492–499. doi: 10.1093/cercor/bhj165. [DOI] [PubMed] [Google Scholar]
- Shah A., Frith U. Why do autistic individuals show superior performance on the block design task? Journal of Child Psychology and Psychiatry. 1993;34(8):1351–1364. doi: 10.1111/j.1469-7610.1993.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Simmons D.R., Robertson A.E., McKay L.S., Toal E., McAleer P., Pollick F.E. Vision in autism spectrum disorders. Vision Research. 2009;49(22):2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Simons D.J., Rensink R.A. Change blindness: past, present, and future. Trends in Cognitive Science. 2005;9(1):16–20. doi: 10.1016/j.tics.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Smith H., Milne E. Reduced change blindness suggests enhanced attention to detail in individuals with autism. Journal of Child Psychology and Psychiatry. 2009;50(3):300–306. doi: 10.1111/j.1469-7610.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- Sokhadze E., Baruth J., El-Baz A., Horrell T., Sokhadze G., Carroll T. Impaired error monitoring and correction function in autism. Journal of Neurotherapy. 2010;14(2):79–95. doi: 10.1080/10874201003771561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze E.M., Baruth J.M., Sears L., Sokhadze G.E., El-Baz A.S., Casanova M.F. Prefrontal neuromodulation using rTMS improves error monitoring and correction function in autism. Applied Psychophysiology and Biofeedback. 2012;37(2):91–102. doi: 10.1007/s10484-012-9182-5. [DOI] [PubMed] [Google Scholar]
- Sulykos I., Czigler I. One plus one is less than two: visual features elicit non-additive mismatch-related brain activity. Brain Research. 2011;1398:64–71. doi: 10.1016/j.brainres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Szatmari P., Merette C., Bryson S.E., Thivierge J., Roy M.A., Cayer M. Quantifying dimensions in autism: a factor-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):467–474. doi: 10.1097/00004583-200204000-00020. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P., editors. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Georg Verlag Jan; Stuttgart: 1988. [Google Scholar]
- Tanaka E., Kida T., Inui K., Kakigi R. Change-driven cortical activation in multisensory environments: an MEG study. NeuroImage. 2009;48(2):464–474. doi: 10.1016/j.neuroimage.2009.06.037. [DOI] [PubMed] [Google Scholar]
- Taylor S.F., Stern E.R., Gehring W.J. Neural systems for error monitoring: recent findings and theoretical perspectives. The Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Thai N.J., Longe O., Rippon G. Disconnected brains: what is the role of fMRI in connectivity research? International Journal of Psychophysiology. 2009;73(1):27–32. doi: 10.1016/j.ijpsycho.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Thakkar K.N., Polli F.E., Joseph R.M., Tuch D.S., Hadjikhani N., Barton J.J. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131(9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Annotation: repetitive behaviour in autism: a review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–849. [PubMed] [Google Scholar]
- Urakawa T., Inui K., Yamashiro K., Kakigi R. Cortical dynamics of the visual change detection process. Psychophysiology. 2010;47(5):905–912. doi: 10.1111/j.1469-8986.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- Urban A., Kremlacek J., Masopust J., Libiger J. Visual mismatch negativity among patients with schizophrenia. Schizophrenia Research. 2008;102(1–3):320–328. doi: 10.1016/j.schres.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke M.W., Scholte H.S., van Engeland H., Lamme V.A., Kemner C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain. 2008;131(4):1013–1024. doi: 10.1093/brain/awm321. [DOI] [PubMed] [Google Scholar]
- Vlamings P.H., Jonkman L.M., Hoeksma M.R., van Engeland H., Kemner C. Reduced error monitoring in children with autism spectrum disorder: an ERP study. European Journal of Neuroscience. 2008;28(2):399–406. doi: 10.1111/j.1460-9568.2008.06336.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Third ed. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wicker B., Fonlupt P., Hubert B., Tardif C., Gepner B., Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social Cognitive and Affective Neuroscience. 2008;3(2):135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciulik E., Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23(4):747–764. doi: 10.1016/s0896-6273(01)80033-7. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Friston K.J. Analysis of fMRI time-series revisited—again. NeuroImage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Marrett S., Neelin P., Vandal A.C., Friston K.J., Evans A.C. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yucel G., McCarthy G., Belger A. fMRI reveals that involuntary visual deviance processing is resource limited. NeuroImage. 2007;34(3):1245–1252. doi: 10.1016/j.neuroimage.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B., Barbas H. Changes in prefrontal axons may disrupt the network in autism. Journal of Neuroscience. 2010;30(44):14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


