Summary
We characterized cytokine profiles of CD4+ T-helper (h) cells in adults and young children to ascertain if responses occur to next-generation candidate vaccine antigens PspA, PcpA, PhtD, PhtE, Ply, LytB of Streptococcus pneumonia (Spn) and Protein D and OMP26 of non-typeable Haemophilus influenzae (NTHi). Adults had vaccine antigen-specific Th1 - and Th2 cells responsive to all antigens evaluated whereas young children had significant numbers of vaccine antigen-specific CD4+ T cells producing IL-2, (p=0.004). Vaccine antigen-specific CD4+ T-cell populations in adults were largely of effector (TEM) and/or central memory (TCM) phenotypes as defined by CD45RA−CCR7+ or CD45RA−CCR7− respectively; however among young children antigen-specific IL-2 producing CD4+ T cells demonstrated CD45RA+ expression (non-memory cells). We conclude that adults have circulating memory CD4+T cells (CD45RA−) that can be stimulated by all the tested Spn and NTHi protein vaccine candidate antigens, whereas young children have a more limited response.
Keywords: Streptococcus pneumonia, non-typeable Haemophilus influenzae, CD4+ T-helper (h) cell, IFN-y, IL-2, memory T cells
Introduction
Adults and children are frequently affected by infections caused by Streptococcus pneumoniae (Spn) and non-typeable Haemophilus influenzae (NTHi), accounting for an increased incidence of pneumonia, invasive Spn disease, acute sinusitis and acute otitis media [1–3]. For prevention of Spn infections, protein-conjugated capsular polysaccharide vaccines have been introduced to provide capsular serotype-specific protection but protection from those vaccines erodes over time as new capsular types emerge to replace previously prevalent strains [4–6]
Therefore, a search is ongoing to identify next-generation vaccine proteins of Spn and NTHi that are conserved among all the strains, and immunogenic in adults and young children. Among the vaccine candidates, pneumococcal surface protein A (PspA), pneumococcal histidine triad proteins -PhtD and PhtE, a choline binding protein-PcpA, a murein hydrolase (–LytB) and a non-toxic pneumolysin derivative PlyD1, have emerged as most likely to proceed to clinical trials in humans [7–11]. Besides that, at this time there is no licensed vaccine to prevent NTHi infections such as acute otitis media, sinusitis, bronchopneumonia and acute exacerbations of chronic bronchitis [12]. Protein D has been used as a carrier in a conjugate Spn polysaccharide vaccine, shown to be immunogenic in young children and to possibly have efficacy in reducing acute otitis media caused by NTHi [13] but it is yet awaiting approval from regulatory authorities as an NTHi vaccine. OMP26 is another highly conserved protein NTHi vaccine candidate that reduces NTHi infections in animal models [14, 15].
CD4+ T lymphocytes have been shown to be important for protective immunity against Spn and NTHi infections in mice [16–18]. In both humans and mice, CD4+ T lymphocytes comprise functionally distinct populations characterized by specific cytokine profiles produced in response to antigens [19, 20]. In adults and older children (median age 5 years), antigen specific CD4+ T-cells reduce Spn nasopharyngeal colonization [21, 22]. Moreover, in adults an effective T-cell response has been associated with protection from invasive pneumococcal disease (IPD) and chronic obstructive pulmonary disease (COPD) caused by Spn and NTHi, respectively [23, 24]. However, there are no data that demonstrate the nature of CD4+ T lymphocyte responses to Spn and NTHi among younger children, and their comparative analysis with adults.
In this study we characterized and compared circulating antigen-specific CD4+ T lymphocyte populations responsive to six Spn and two NTHi antigens in adults and young children. The objectives were to determine (1) whether CD4+T lymphocytes in the circulation that were elicited by natural exposure to Spn and NTHi are capable of producing cytokine responses against vaccine protein antigens expressed by Spn and NTHi; (2) whether the functionality of cytokine producing CD4+ T-cells were disparate between adults and young children; and (3) whether phenotypic profiles of antigen-specific CD4+T-cells were different between adults and young children.
Material and Methods
Subjects and Samples
Eleven healthy adults (five females and six males; median age 32.5 yrs) and 17 young children (6 months to three years old, median age 20 months old) were involved in the study. Adults were pediatricians and pediatric nurses who had frequent interactions with children having ear infections. The children were part of a cohort recruited as part of a 5-year NIH supported prospective study of immunity to NTHi. Enrolled children were healthy and from a middle class socio-demographic population in Rochester NY. The children selected had culture-proven Spn and/or NTHi nasopharyngeal or oropharyngeal colonization and adults were presumed to have natural colonization based on detectable serum antibody, prior to collection of blood for peripheral blood mononuclear cells (PBMC) isolation. None of the subjects had experienced invasive Spn infections or lobar pneumonia. Written informal consent was obtained through a protocol approved by the Rochester General Hospital IRB.
Venous blood was collected in heparinized tubes and immediately transferred from the clinic to the laboratory. PBMCs were isolated using a Ficoll gradient according to the manufacturer’s instruction (GE Healthcare) and then washed with 1× phosphate buffered saline (PBS), re-suspended at a concentration of 1×107 cells/ml in cell recovery freezing media (Gibco) and frozen in liquid nitrogen until used.
Antigens and Antibodies
Pneumococcal protein antigens that were used for T-cell stimulation included: surface protein, PspA (EF5668), two pneumococcal histidine triad proteins (PhtD, PhtE), an autolysin (LytB), a choline binding protein (PcpA) and a detoxified derivative of pneumolysin (PlyD1). All the pneumococcal antigens were provided by Sanofi-Pasteur. NTHi antigens used were Protein D and OMP26 and were gifts from GlaxoSmithKline, UK and Dr. Jenelle Kyd, University of Canberra Australia, respectively.
Antibodies used for staining were anti-CD3 Qdot 605 (clone UCHT1, Invitrogen), anti-CD4 APC Alex Fluor 750 (clone RPA T4, eBiosciences), PE-Cy5 anti-CD69 (clone FN50, BD biosciences), PE-Texas Red anti- CD45RA (clone MEM56, Invitrogen), anti-CCR7 PerCP/Cy5.5 conjugate (clone TG8/CCR7, Biolegend), PE-Cy7 conjugated anti-IFN-γ (clone B27, BD biosciences), Pacific blue conjugated anti-IL17A (clone BL168, Biolegend), Alexa fluor 700 anti-IL2 (clone MQ1-17H12, Biolegend), APC conjugated anti-IL13 (clone JES10-5A2, Biolegend), Alexa fluor 488 conjugated anti-IL10 (clone JES3-9D7, Caltag), PE conjugated anti-IL4 (clone 8D4-8, BD Biosciences). Anti-CD28 and anti-CD49d antibodies (clones L293 and L25 respectively) were obtained from BD Biosciences.
PBMC Stimulation for detection of intracellular cytokine
Prior to stimulation, frozen PBMCs were quickly thawed in a 37°C water bath followed by slowly adding complete culture medium (RPMI 1640 supplemented with 10% of FBS, 2mM L-glutamine, 0.1 mM sodium pyruvate, nonessential amino acids, 100U/mL penicillin, 100µg/mL streptomycin). After thawing, cells were counted before distributing them in a 96-well plate and 80–90% cells were found viable. Cells were then washed and rested overnight in complete culture media in 24-well plates. The duration of stimulation (6 hours) was based on earlier work with young child CD4+ T cells and PBMCs were stimulated using a standardized protocol [25]. The optimal concentration of Spn and NTHi antigens was assessed within the range of 0.1 to 10 and 1µg/ml was selected.
Briefly, cells were counted again and placed in a 96-well flat bottom plate and stimulated with either 1µg/ml final concentration of individual Spn or NTHi antigen or Staphylococcal enterotoxin B (SEB, used as a positive control). An optimal dosage for stimulation was determined by absence of detectable cell toxicity, measured by the use of tryptan blue staining and/or flow cytometry analysis after propidium iodide staining (data not shown). Cells were then incubated for 2h at 37° C in the presence of 5% CO2 for antigen processing. After 2 hours, Golgi transport inhibitors (BD Biosciences) were added to preserve cytokines intracellularly and incubated for an additional 4 hours. Anti-CD28 and anti-CD49d (1ug/ml) antibodies were also added to provide co-stimulation and enhance the detection of antigen specific response as described earlier [26, 27].
Surface and intracellular staining for flow Cytometric Analysis
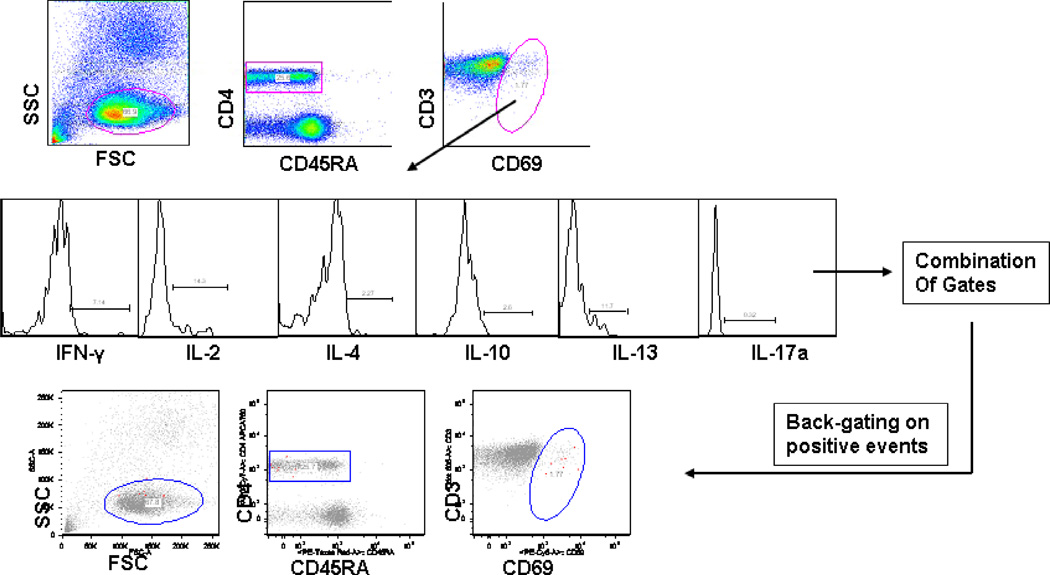
An intracellular cytokine-staining assay (ICS) was used to evaluate the antigen specific CD4+ T-cell subsets. Post stimulation, cells were transferred to 96-well V-bottom plates and washed once with FACS buffer (PBS with 0.5%FBS). Cells were then incubated with various cell surface antibodies for 30 minutes, followed by washing with FACS buffer. Cells were then permeabilized with fixation and permeabilization solution for 20-minutes and washed 3- times with permeabilization buffer (both BD Biosciences). A cocktail of antibodies was used to stain intracellularly captured cytokines as a result of stimulation. Because of the down regulation of CD3 on T-cell surface post stimulation, CD3 staining was carried out intracellularly. After intracellular staining, cells were further washed 3-times with 1× permeabilization buffer and finally with FACS buffer before re-suspending them into the FACS tubes. A custom made BD LSR II flow cytometer equipped with 3 lasers and 12 fluorescent detectors was used to collect 0.2–1 × 106 events for each sample and data was analyzed using FLOW JO (Tree Star) software. Cells were first gated on lymphocytes based on forward- and side-scatter properties followed by sequential gating on CD4+ T-cells. Since CD69 was used as an activation marker, cells were further gated onto CD3+CD69+ and individually to each of the cytokine on a histogram. Because of the low frequencies of cytokine-positive cells in children, Boolean gate combinations were used to evaluate true cytokine producing cells. Boolean gate combinations showed that all the cells contributing to the background were eliminated in the all cytokine-negative population. Furthermore true responders were confirmed by extensive back gating. We used CD4+ T cells as a counting gate and percentages of cytokine producing cells were calculated based on the total number of acquired CD4+ T cells in the respective sample. The percentage of responding cells was calculated by dividing cytokine-positive cells by the total CD4+ T-cell counts for each stimulation.
Cytometric Bead array (CBA) for detection of secreted cytokines
For CBA assay, PBMCs were thawed and rested overnight as described previously before stimulating them with either 1µg/ml of individual antigens or SEB for 18–20h. After stimulation, supernatants were collected and cytokines were measured using a CBA kit for Th-1 and Th-2 cytokine detection (BD Biosciences) as described by the manufacturer. Acquisition template provided by BD was used to acquire cytometric beads on a LSRII flow cytometer and data was analyzed using BD FACS DIVA software and Flowjo. Standard graphs were plotted and unknown sample values were extrapolated on linear regression plots with GraphPad Prism 5 software.
Statistical Analysis
We used a rank based procedure, related to the Friedman blocked rank design, to determine those antigens with consistently high or low responses to specific cytokines. In the blocked rank design (see below) within each individual each antigen is assigned a rank based on its normalized response. The average rank for each antigen across individuals may then be calculated. To assess the statistical significance of the average ranks a bootstrap procedure was used. Re-sampling individuals with replacement created a replicated sample, equal to the original sample size. For each antigen, an empirical distribution of the average rank was estimated using N replications (here N = 5000). The results are reported by summarizing the frequency within the estimated bootstrap sampling distribution with which the average rank of a specific antigen was within the K lowest or highest [28].
In a second method, for each pair of antigen and response the N differences (antigen response – no antigen response) were calculated, a signed-rank test was performed against equality. Only pairs for which at least 5-nonzero differences were available were tested, since this is the minimum needed for a p-value < 0.05. The Benjamini-Hochberg procedure was used to estimate the false discovery rate (FDR) in each analysis. In addition to testing specific antigen-response pairs, a test for non-homogeneity of response across antigens was performed for each cytokine, using the Friedman test for blocked rank designs. Interpreting subjects as the blocking variable adjusts for subject specific random effects.
P-values for cytokine differences for each stimulation by flow cytometry and CBA data among children and adults were calculated with the two-tailed Mann-Whitney test.
Results
Intracellular cytokine staining and multiparameter flow cytometry were used to identify functional CD4+ T cells in the PBMC samples stimulated with various Spn and NTHi antigens. For analysis, we gated on all CD4+ T cells regardless of their CD45 expression, followed by gating on CD3+CD69+ cells (Figure 1). Cells were further gated according to cytokines produced and these were combined with Boolean gating. This gating strategy was helpful for the detection of even rare frequencies of functional cells. In Table 1 antigen responses within the bootstrapped sampling distribution with significantly lower or higher ranked responses are shown.
Figure 1. Flow cytometric detection of CD4+ T-cell cytokine expression in PBMCs.
PBMCs from adults (n=11) and young children (n=17) were stimulated with various pneumococcal and NTHi protein antigens and analyzed by ICS. Individual cytokine producing CD4+ T-cells in each sample were sequentially gated on total CD45RA followed by CD69+ cells as shown in the example figure from a child. Several Boolean gate combinations were produced for cytokine positive cells and CD4+ T cell frequencies producing individual cytokines but not others were quantified as described earlier [25] (A). Individual cytokine combinations as produced by Boolean gating were further validated by back-gating on CD69-high cells (shown in red) (B).
Table 1.
Estimated ordering of average antigen response within bootstrapped sampling distribution in adults and children. Confidence sets indicating significantly lower or higher ranked responses are highlighted.
| Cytokine Responses |
Adults | Children | ||||||
|---|---|---|---|---|---|---|---|---|
| Significantly Lower |
Significantly Higher |
Significantly Lower |
Significantly Higher | |||||
| IFN-γ | No stim | 0.954 | PsPA | 0.979 | PhtE | 0.965 | OMP26 | 0.955 |
| PcpA | 0.955 | PD | 0.979 | |||||
| PlyD1 | 0.987 | OMP26 | 0.971 | |||||
| IL-2 | No stim | 0.950 | PhtD | 0.972 | PLYD1 | 0.978 | PsPA | 0.973 |
| PhtE | 0.999 | PD | 0.993 | PD | 0.983 | |||
| PlyD1 | 0.998 | OMP26 | 0.946 | OMP26 | 0.968 | |||
| IL-4 | No stim | 0.959 | PcpA | 0.984 | PsPA | 0.992 | ||
| LytB | 0.981 | PhtD | 0.975 | |||||
| OMP26 | 0.973 | |||||||
| IL-10 | LytB | 0.985 | PsPA | 0.967 | PD | 0.923 | ||
| OMP26 | 0.956 | OMP26 | 0.969 | |||||
| IL-13 | PcPA | 0.991 | PsPA | 0.993 | PhtE | 0.991 | PsPA | 0.981 |
| PlyD1 | 0.955 | PhtD | 0.968 | OMP26 | 0.990 | |||
| LytB | 0.950 | PD | 0.987 | |||||
| OMP26 | 0.985 | |||||||
CD4+T-cell responses to Spn and NTHi protein antigens in adults
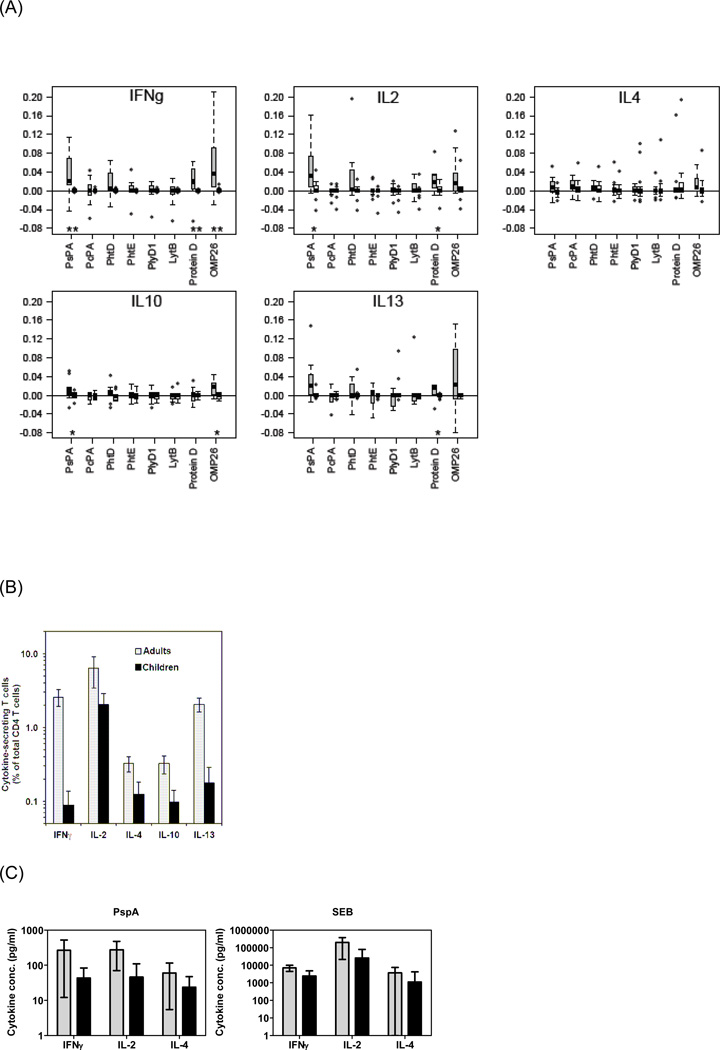
Adult IFNγ, IL-2 and IL-4 producing CD4+ T cells showed significant responses to all 6 Spn and both NTHi proteins compared to non-stimulated controls (Table 1). Ranking among the antigens studied identified IFNγ responses as higher to Spn antigens PspA and to NTHi protein D and OMP26. Evaluation of rank among the antigens for significantly lower responses from FNγ-producing cells identified PcpA and PlyD1 (Table 1). Ranking among the antigens studied identified IL-2 responses as higher to Spn antigens PspA and PhtD and to NTHi protein D (Table 1). Evaluation of rank among the antigens for significantly lower responses from IL-2 producing cells identified PhtE and PlyD1 (Table 1). Variations in rank among the antigens for stimulation of significantly higher and significantly lower responses from IL-4, IL-10 and IL-13 producing cells were also observed in adults (Table 1). The estimated false discovery rate for these significant test outcomes is 15.2%, using the Benjamin-Hochberg procedure. In effect, the expected number of false positives is approximately 1. The IL-4, IL-10 and IL-13 responses were generally lower than IL-2 and IFNγ responses. No IL-17 responses were detected in adults (data not shown). Thus the adult responses to the Spn and NTHi proteins, presumably induced by asymptomatic nasopharyngeal colonization or prior mucosal infections (acute otitis media, sinusitis or bronchopneumonia), were dominated by Type 1 (IFNγ, IL-2) or primed, uncommitted (IL-2) responses [29], with a lower level of Type 2 cytokines, and no detectable Th17 responses. In addition, all cytokines exhibited significantly non-homogeneous responses with respect to antigen exposures (IFNγ, p < 0.0001; IL2, p < 0.0001; IL4, p = 0.0087; IL10, p = 0.021; IL13, p = 0.0003).
Characteristics of CD4+T-cell responses to Spn and NTHi protein antigens in very young children
Overall, compared with negative controls, IL-2 (p = 0.004) and IFNγ (p = 0.04) producing cells exhibited significantly higher responses to vaccine antigen stimulations in young children. Table 1 shows the ranking of studied vaccine antigens for significantly higher and lower responses from the identified CD4+ T cell cytokine-producing populations, Ranking among the antigens with regard to IFNγ responses showed OMP26 to stimulate relatively higher responses and PhtE to stimulate relatively lower responses compared to other antigens studied. Ranking of IL-2 responses showed PspA, protein D and OMP26 to stimulate relatively higher responses and PlyD1 to stimulate relatively lower responses compared to other antigens (Table 1). IL-4 background frequencies were high and the responses to Spn and NTHi antigens were mostly in the same range as the negative controls. Relatively higher IL-10 responses were detected in response to OMP26. Relatively higher IL-13 responses were detected in response to PspA, but backgrounds were high. SEB positive controls showed that in the ICS assay, we could detect IL-4, IL-10, and IL-13 expressions in most of the young child samples but the more convincing responses were IL-2 and IFNγ. IL-17 producing CD4+ Th-cells were infrequent and were present only in a few children (data not shown).
Divergence of CD4+T-cell responses to Spn and NTHi protein antigens in young children versus adults
IFNγ, IL-2 and IL-10 responses to PspA (p = 0.001, 0.018, 0.043), IFNγ, IL-2 and IL-13 responses to Protein D (p = 0.004, 0.035, 0.021, and IFNγ and IL-10 responses to OMP26 (p = 0.003, 0.014) were higher among adults compared to young children (Fig 2A). The estimated false discovery rate for these eight significant differences is 21.7%, i.e. one or two of these results are likely to be falsely significant. The differences in IFNγ responses were particularly striking (Figure 2A) as these were the highest responses in adults, and the p-values were <0.005 for all three proteins. Other cytokine responses were low in adults and very low to undetectable in young children (Fig. 2A). The differences in the cytokine responses among adults and young children were maintained even when in vitro stimulation time points were extended further (data not shown).
Figure 2. Frequencies of Spn and NTHi specific CD4+ T-cells among young children and adults.
Boxplots of bacterial antigen-specific CD4+ T-cells producing each cytokine among adults and children are compared after subtraction of unstimulated values. Grey boxplots: Adults; open boxplots: Children. “*” = P < 0.05; “**” = P < 0.005, Wilcoxon rank sum test between adults and children. [Edges of box represents 25th and 75th percentiles, median indicated within box. “Whiskers” extend to most extreme observations within 1.5 IQR of the box (IQR = 75th – 25th percentile). Outliers beyond whiskers and within plot range are indicated separately]. (B) Responses to SEB stimulation. (C) Secretion of cytokines into the culture supernatant was measured by cytometric bead array (CBA) after stimulation with PspA or SEB (backgrounds from unstimulated cultures were subtracted).
The capacity of PBMCs from adults and young children to produce cytokines was compared by stimulation with SEB. Significantly higher frequencies of cells producing Th1 cytokines IFNγ (p=0.002) and IL-2 (p=0.001) were detected among adults compared to young children (Fig 2B). After SEB stimulation, CD4+ T-cells demonstrated variability in IL-4 production among adults and young children but overall adults showed higher responses (p<0.05) (Fig 2B). There was significantly higher IL-13 & IL-10 producing CD4+ T-cells among adults compared to young children after SEB stimulation (p<0.05) (Fig 2B). Adults also had a significantly (p< 0.05) increased capability to up-regulate IL-17a producing CD4+ T-cells after SEB stimulation compared to young children (Fig 2B). The results of SEB stimulation for IFN-γ, IL-2 and IL-4 were verified by the CBA method (Fig 2C).
Memory phenotypes of Spn and NTHi specific CD4+ T-cells among very young children and adults
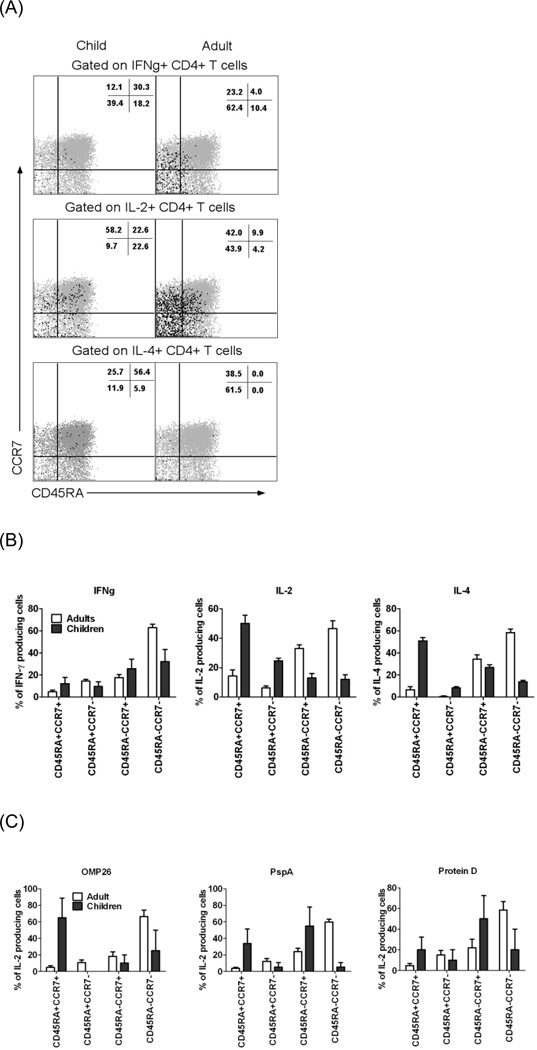
Four major and distinct antigen-experienced CD4+ T-cell subsets could be discerned based on the expression of CD45RA and CCR7 (Fig 3A) - naive (CD45RA+CCR7+), effector (CD45RA+CCR7−), central memory (CD45RA−CCR7+) and effector memory (CD45RA−CCR7−). As expected in adults, effector cytokines IFNγ and IL-4 were expressed mainly (about 60%) by effector memory CD4 T cells after SEB stimulation, whereas young children’s CD4 T cells producing these two cytokines were less restricted to this subset (Figure 3B).
Figure 3. Heterogeneity in phenotypes of responding CD4+ T-cells among very young children and adults.
A. PBMC from adults (n=11) and children (n=17) were stimulated with SEB, analyzed by ICS, and cytokine-producing CD4 T cells were divided according to the expression of CD45RA and CCR7. Representative samples from one adult and one child are shown (black dots). B. The percentages of total cytokine-producing cells contributed by each subpopulation after SEB stimulation are shown. C. The percentages of total IL-2-producing cells responding to three bacterial antigens are shown. P values adult vs. young children <0.05.
Cells producing IL-2 in response to three bacterial antigens (PspA, OMP26 and Protein D) were compared between adults and young children (as IL-4 and IFNg responses were very low only IL-2 responses were compared). Consistent with the SEB results, most of the IL-2-secreting adult T cells specific for the three antigens were in the effector memory subset, whereas this preference was reduced in the young children’s responses (Fig. 3C). Thus the separation of CD4 subpopulations based on CCR7 and CD45RA expression may be less informative in young children.
Discussion
To our knowledge there are no previous reports evaluating and comparing T cell responses in adults and young children to vaccine protein candidates of Spn and NTHi. Since efforts to develop improved vaccines to prevent Spn and NTHi infections are underway it is important to provide foundational data on Th1 and Th2 cellular immune responses to key protein antigens. Adults, who have a more mature immune system and who have more accumulated natural exposures to Spn and NTHi, had significantly higher antigen-specific CD4+ T-cells compared to young children. In fact, in adults all tested vaccine candidate antigens stimulated CD4+ T-cell responses, predominantly with a Th-1 profile. Therefore, vaccination with the studied antigens would likely to boost higher CD4+ T-cell responses among adults.
CD4+ T-cell responses in young children to Spn and NTHi antigens were most prominent from IL-2-producing cells. IFN-γ responses were lower than IL-2 but detected. IL-4, IL-10 and IL-13 producing cells were too low to be confident that their detection was accurate among young children. Of the antigens evaluated NTHi protein D and OMP26 were more consistent in stimulating CD4+ T cell responses compared to any of the Spn protein antigens in young children. Vaccination of young children would likely require several priming doses to stimulate CD4+ T-cell responses since the frequency of such cells circulating in the blood of young children appears to be low following natural exposure to the organisms.
Our results suggest that young children age 6 to 36 months have reduced Th1 and Th2 responses similar to the immaturity seen in neonates [30, 31]. Our prior work evaluating CD4+ T cell responses to diphtheria toxoid, tetanus toxoid and acellular pertussis antigens also showed a more neonatal-like immune response in young children in response to routine pediatric vaccinations compared to adult responses [25].
Studies involving neonatal immunity in humans rely on plentiful umbilical cord blood, whereas immune maturation from the neonate to the young child has received very limited study. Indeed what happens and when between the neonatal period and age 5 years old, when children show more “adult-like” immune responses, has been largely unexplored. Therefore, our present study included a diverse age group of young children. We developed novel statistical methodology to analyze the significance of observations despite the low frequency of antigen specific CD4+ T-cells in young children.
Previous studies using vaccination and/or infection models have demonstrated that distinct populations of antigen-experienced T-cells may be associated with long-term protection [32–34]. Among all the cytokines analyzed in the young children, we found that frequencies of IL-2 producing CD4+ T-cells were highest. Since, IL-2 is the primary cytokine that aids in cellular proliferation and young children’s T-cell pool is mainly of naive and effector phenotypes (Fig 3), we expected to see and saw IL-2 production in response to antigenic stimulation in these children.
We observed low antigen-specific IFN-γ-producing CD4+ T-cell responses among young children to all of the Spn and NTHi antigens, and significantly reduced numbers in response to the positive control SEB (Fig 2A & B). Since SEB activates T-cells by cross-linking the variable region of the β-chain (Vβ) on the T-cell receptor [35], deficiency of IFN-γ production in response to SEB suggests inherently reduced Th1 responses in the young children’s overall T-helper cell pool. Our observation regarding SEB has been reported previously [36].
Enumerating IL-4 expressing T-cell frequencies by the intracellular staining method was challenging but responses in adults were detectable. A significant IL-4 response in young children could not be discerned. IL-10 and IL-13- producing CD4+ T cell frequencies in response to several antigens as well as SEB were increased in adults. This could be attributed to age dependent maturation of individual cytokine production by T-cells as reported for IFN-γ [36].
The divergence of Th-responses into Th1 & Th2 that was discovered about two decades ago has now been expanded by discovery of a completely different Th-subset of CD4+ T-cells known as Th-17. This CD4+ Th-subset has been characterized by the production of cytokines IL-17 and IL-21 [37, 38]. Studies in mice have demonstrated a protective role of the Th-17 subset in the eradication of Spn by an antibody independent mechanism [39]. The role of IL-17 secreting T cells in protection of humans against Spn has not been previously described [38]. Another study reported lower IL-17 production in response to whole cell antigen (WCA) of group B streptococcus among children compared to adults [40]. In contrast, a higher level of IL-17 was detected in children from Bangladesh that may have been induced by pneumococcal pneumonia. We found that IL-17a producing CD4+ T-cells were rarely detected or were completely absent in response to individual Spn and NTHi antigens in both young children and adults. The different observations in various studies may be due to differences in exposure to pneumococci. Moreover, IL-17 secreting T cells may reside primarily in regional lymph nodes and not circulate in the blood; we did not assess this possibility [37].
The memory phenotypic profiles of antigen-induced CD4+T-cells were divergent among adults and young children. As expected, young children had higher percentages of CD45RA+ naïve (CCR7+) and effector (CCR7−) CD4+ T-cells as compared to adults (data not shown). Both in young children and adults, the highest percentages of IFN-γ producing cells were of the effector memory phenotype (CD45RA−CCR7−). On the other hand, antigen-experienced IL-2 expressing CD4+ T-cells are more likely to have central memory phenotypes [32]. In our study population IL-2 expressing CD4+ T-cells were present in the effector memory phenotype among adults, while the majority of IL-2 producers in very young children were of naive phenotypes (Fig 3). A similar pattern was found for IL-13 and IL-4 producing cells whereas the majority of IL-10 secreting T-helper cells were of the effector memory phenotype among both young children and adults (Fig 3). Cumulative exposure to environmental Spn and NTHi may contribute to persistent immune activation and therefore a predominance of effector T-cells as seen for all the cytokines in adults and IFN-γ for young children. We observed that IL-2, IL-4 and IL-13 expressing CD4+ T-cells had a naive phenotype CD45RA−CCR7− in young children. Because of nasopharyngeal colonization, young children may have early differentiated antigen-specific CD4+T-cells that have not lost CD45RA expression, as was previously observed during anti-mycobacterial vaccination studies [32, 33].
The exact mechanisms responsible for the limited responsiveness of CD4+ T-cells in young children after vaccination is not known but may involve multiple factors as outlined in studies of neonatal mice and limited studies in young children [29]. Young children may have (1) immunologically immature APCs that fail to stimulate T-cells to the same extent as observed in adults [17, 20], (2) maternal antibodies that interfere with normal processing of antigen or signal through Fc receptors [30, 31], (3) lower toll like receptor expression [32], (4) altered innate responses that impact T-cell activation [25, 41] or (5) a predilection to Th2 dominated responses [33].
In conclusion, this report demonstrates the frequencies of Spn and NTHi antigen specific CD4+ T-cells among adults compared to young children following natural exposure to the bacteria. Quantitative and qualitative differences in results were observed that are informative regarding the vaccine candidates studied. Using a multi-parameter flow cytometry approach, we found divergence in functional, phenotype and memory cell populations. Young children age 6 months to three years old had features resembling a “neonatal” immune profile in transition to an “adult-like” pattern. Cellular responses following vaccination with purified, increased amounts of these proteins (perhaps with adjuvant) might yield more robust responses. Identified aspects contributing to the divergence between the age populations should represent target areas for evaluation of immune-response enhancing approaches.
Highlights.
Adultshad higher CD4+ T-cell responses to Spn and NTHiantigens compared to very young children.
CD4+ T-cell responses of very young children to Spn and NTHiantigens were Th-2 predominant.
Very young children have reduced Th1 and Th2 responses similar to neonates IL-17a producing CD4+ T-cells were rarely detected in response to Spn and NTHi antigens.
Memory phenotypic profiles of antigen-induced CD4+T-cells were divergent in adults and children.
Acknowledgements
This study was supported by NIH NIDCD RO1 08671 and sanofi pasteur. We thank Janet Casey, MD, and the nurses and staff of Legacy Pediatrics and the collaborating pediatricians; Sanofi Pasteur for pneumococcal antigens; GlaxoSmithKline for protein D; and Dr. Jennelle Kyd, University of Canberra, Australia for OMP26.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bogaert D, de Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 1995;332(19):1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 3.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect. Dis. 2010;10(3):195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 4.Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr. Infect. Dis. J. 2004;23(9):824–828. doi: 10.1097/01.inf.0000136871.51792.19. [DOI] [PubMed] [Google Scholar]
- 5.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 2010;29(4):304–309. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 7.Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 2002;45(5):1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 8.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 2008;8(6):497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 9.Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 2008;76(6):2767–2776. doi: 10.1128/IAI.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamou JE, Heinrichs JH, Erwin AL, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 2001;69(2):949–958. doi: 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briles DE, Tart RC, Swiatlo E, et al. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin. Microbiol. Rev. 1998;11(4):645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gkentzi D, Slack MP, Ladhani SN. The burden of nonencapsulated Haemophilus influenzae in children and potential for prevention. Curr. Opin. Infect. Dis. 2012;25(3):266–272. doi: 10.1097/QCO.0b013e32835310a4. [DOI] [PubMed] [Google Scholar]
- 13.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 14.Bakaletz LO, Kennedy BJ, Novotny LA, Duquesne G, Cohen J, Lobet Y. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 1999;67(6):2746–2762. doi: 10.1128/iai.67.6.2746-2762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyd JM, Cripps AW. Potential of a novel protein, OMP26, from nontypeable Haemophilus influenzae to enhance pulmonary clearance in a rat model. Infect. Immun. 1998;66(5):2272–2278. doi: 10.1128/iai.66.5.2272-2278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 2005;102(13):4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect. Immun. 2004;72(10):5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snapper CM, Shen Y, Khan AQ, et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 2001;22(6):308–311. doi: 10.1016/s1471-4906(01)01926-3. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann TR, Schumacher JH, Street NF, et al. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol. Rev. 1991;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 21.Mureithi MW, Finn A, Ota MO, et al. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J. Infect. Dis. 2009;200(5):783–793. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Bagrade L, Bernatoniene J, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J. Infect. Dis. 2007;195(8):1194–1202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 23.King PT, Hutchinson PE, Johnson PD, Holmes PW, Freezer NJ, Holdsworth SR. Adaptive immunity to nontypeable Haemophilus influenzae. Am. J. Respir. Crit Care Med. 2003;167(4):587–592. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 24.de Bree GJ, Daniels H, Schilfgaarde M, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J. Infect. Dis. 2007;195(11):1718–1725. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SK, Pichichero ME. Functional deficits of pertussis-specific CD4(+) T cells in infants compared to adults following DTaP vaccination. Clin. Exp. Immunol. 2012;169(3):281–291. doi: 10.1111/j.1365-2249.2012.04613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitcher CJ, Quittner C, Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5(5):518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 27.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 1998;161(10):5284–5295. [PubMed] [Google Scholar]
- 28.McCall MN, Almudevar A. Affymetrix GeneChip microarray preprocessing for multivariate analyses. Brief. Bioinform. 2011 doi: 10.1093/bib/bbr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divekar AA, Zaiss DM, Lee FE, et al. Protein vaccines induce uncommitted IL-2-secreting human and mouse CD4 T cells, whereas infections induce more IFN-gamma-secreting cells. J. Immunol. 2006;176(3):1465–1473. doi: 10.4049/jimmunol.176.3.1465. [DOI] [PubMed] [Google Scholar]
- 30.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25–26):3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 31.Velilla PA, Rugeles MT, Chougnet CA. Defective antigen-presenting cell function in human neonates. Clin. Immunol. 2006;121(3):251–259. doi: 10.1016/j.clim.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares AP, Scriba TJ, Joseph S, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 2008;180(5):3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tena-Coki NG, Scriba TJ, Peteni N, et al. CD4 and CD8 T-cell responses to mycobacterial antigens in African children. Am. J. Respir. Crit Care Med. 2010;182(1):120–129. doi: 10.1164/rccm.200912-1862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu W, Chen S, Sharp M, et al. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent very young children. J. Immunol. 2004;172(5):3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 35.Fleischer B. Superantigens. APMIS. 1994;102(1):3–12. doi: 10.1111/j.1699-0463.1994.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanna-Wakim R, Yasukawa LL, Sung P, et al. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J. Infect. Dis. 2009;200(12):1921–1927. doi: 10.1086/648375. [DOI] [PubMed] [Google Scholar]
- 37.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 38.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect. Immun. 2010;78(1):32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 2006;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ, Qadri F, Malley R. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine. 2012;30(26):3897–3907. doi: 10.1016/j.vaccine.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr. Res. 2009;65(5 Pt 2):98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]