Abstract
Background
Patients with large, high-grade extremity and truncal soft tissue sarcomas (STS) are at considerable risk for recurrence. A regimen of preoperative chemotherapy consisting of mesna, adriamycin, ifosfamide, and dacarbazine (MAID), interdigitated with radiotherapy (RT), followed by resection and postoperative chemotherapy with or without RT, has demonstrated high rates of local and distant control. The goal of this study is to assess outcomes in a recent cohort of patients treated on this regimen.
Methods
We retrospectively reviewed records of 66 consecutive patients with STS of the extremity or trunk who were treated with the aforementioned regimen from May 2000–April 2011. Clinicopathologic characteristics and patient outcomes were analyzed.
Results
Sixty-six patients were analyzed and were equally divided between grade 2 and 3 tumors. Margins were negative in 57 (89%) patients and positive in 7 (11%) patients. At a median follow-up of 46 months, there were 6 (9%) locoregional and 20 (30%) distant recurrences. The locoregional and distant 5-year recurrence-free survival (RFS) rates were 91% and 64%, respectively. The 5-year overall (OS) and disease-specific survival rates were 86% and 89%, respectively. There were no treatment-related deaths or secondary myelodysplasias. Thirty-four (52%) patients had grade 3 or 4 acute hematologic chemotherapy-related toxicity. There were no statistically significant predictors of OS or RFS.
Conclusions
For a contemporary cohort of patients with high-risk extremity and truncal STS, a regimen of neoadjuvant chemoradiotherapy and surgery continues to result in high rates of survival with tolerable short- and long-term toxicity.
Keywords: sarcoma, neoadjuvant, chemoradiotherapy, chemotherapy, survival, toxicity, recurrence, radiation
Introduction
Contemporary management of extremity and truncal soft tissue sarcomas (STS) revolves around wide local excision in combination with the judicious use of pre- or post-operative radiation therapy1–6 to promote limb salvage, and thereby, to optimize quality of life. This strategy reliably achieves local control in 85–92% of patients. 7 However, the risk of distant metastasis remains a major concern, and this risk is intimately related to tumor grade and size. 8 Thus, there is obvious motivation to include chemotherapy in the treatment approach in an effort to improve metastasis-free and overall survival. 9, 10 In addition, in the neoadjuvant setting, chemotherapy may work synergistically with radiation therapy to decrease the extent of the required surgical resection and, consequently, to promote better functional outcomes. 11
At Massachusetts General Hospital (MGH), we have previously reported high rates of local and distant control in our pilot study of an aggressive regimen of preoperative chemotherapy consisting of mesna, adriamycin, ifosfamide, and dacarbazine (MAID), interdigitated with 44Gy external beam radiation therapy (EBRT) and followed by resection and post-operative chemotherapy with or without additional radiation. 12 This pilot study prompted a multi-institutional Phase II study (RTOG 9514) to evaluate high-risk (≥ 8cm, intermediate or high grade) extremity and truncal STS amenable to complete surgical resection and limb preservation. 13 At a median follow-up of 7.7 years, rates of distant disease-free and overall survival were quite favorable at 64.1% and 71.2%, respectively. 14 We subsequently reported the long-term follow-up of our pilot study of high-risk extremity sarcomas treated from 1989–1999, demonstrating significantly higher 7-year disease-specific (81%) and overall (79%) survival rates when compared to a historic matched control group of patients managed with alternate treatment regimens. 15
However, this aggressive chemoradiotherapy regimen has been associated with noteworthy toxicity, particularly in the multi-institutional phase II study, in which the dose of ifosfamide was 25% higher than in the MGH study and large radiation therapy fields, extending 9 cm proximal and distal to gross disease, were employed. Three (5%) treatment-related deaths, 5 (9.4%) amputations, and a 97% early rate of significant toxicity were reported, although the latter strikingly decreased after 5 years of follow-up. 14 We have not seen such high rates of toxicity in the patients whom we have treated at MGH on this regimen over the past 20 years, and given the encouraging long-term survival data, we currently recommend it for those patients with large, high-grade extremity sarcomas who are deemed fit to tolerate the regimen. In this retrospective study, we report the outcomes of patients with high-risk extremity and truncal STS who participated in this protocol at MGH since completion of the pilot study in 1999.
Patients and Methods
This study was approved by the Institutional Review Board of Partners Healthcare/Massachusetts General Hospital.
Study design and case ascertainment
The radiation oncology database at MGH was retrospectively searched, and 75 consecutive patients with extremity and superficial trunk STS treated with the interdigitated chemoradiotherapy regimen from May 15, 2000 – April 14, 2011 were identified. Nine patients with tumors located primarily in the bone, cartilage, head, neck, retroperitoneum, and brain, or with follow-up of less than 2 months were excluded, yielding 66 patients for this study. Fifty-five patients completed all of the planned therapy, including all six cycles of chemotherapy, 44 Gray of interdigitated radiotherapy, and surgery. Eleven patients completed only a portion of the planned therapy – 2 did not undergo surgery and 9 did not receive all 6 cycles of chemotherapy. These patients were included in this study because we fully intended to treat them on this neoadjuvant protocol, and we surmised that this was the proper way to study all of the potential toxicities of this aggressive therapeutic regimen.
Treatment protocol
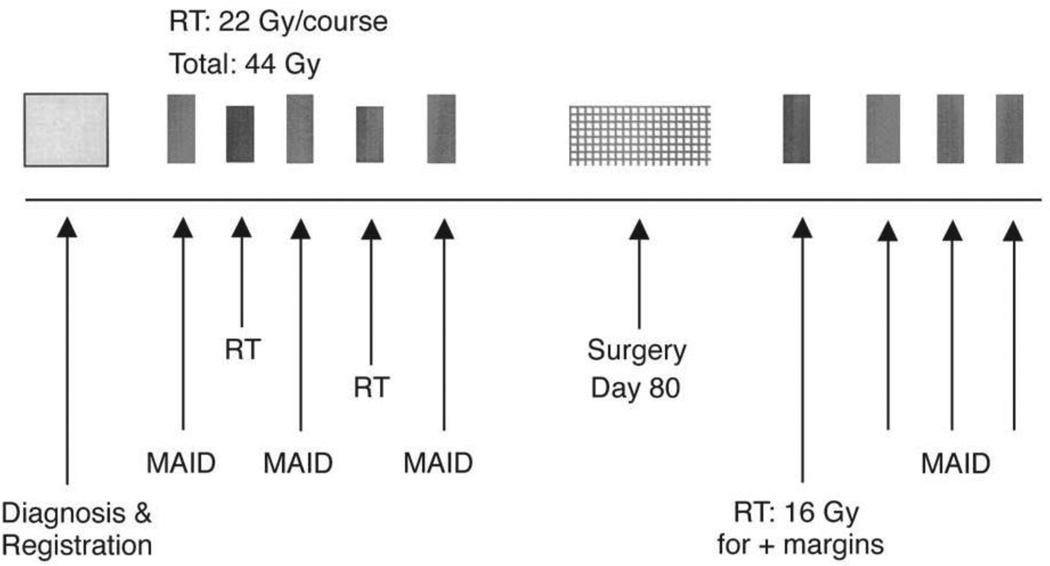
The complete treatment protocol for these patients has been previously described and is depicted in Figure 1. 12 Patients received a total of 6 cycles of chemotherapy, 3 cycles pre-operatively and 3 cycles post-operatively. The 3 pre-operative cycles were alternated with 2 cycles of external beam radiation therapy (courses of 22 Gy each, in fractions of 2 Gy per day). The chemotherapy regimen consisted of mesna (2500 mg/m2/d by continuous i.v. infusion on Days 1 – 4), adriamycin (doxorubicin, 20 mg/m2/d continuous i.v. infusion on Days 1 – 3), ifosfamide (2000 mg/m2/d continuous i.v. infusion on Days 1 – 3), and dacarbazine (250 mg/m2/d continuous i.v. infusion on Days 1 – 4). Restaging CT and/or MRI were performed following pre-operative therapy. Surgery was planned for approximately 80 days after the initiation of the first cycle of chemotherapy and 3 – 6 weeks following the completion of chemoradiotherapy. Surgical resection was undertaken with the intent of wide excision with tumor-free margins and limb salvage. Three cycles of post-operative MAID chemotherapy were given starting 21 – 35 days after surgery, if permitted by wound healing. Post-operative brachytherapy or EBRT, if administered, was done prior to initiating post-operative chemotherapy. A post-operative radiation boost was considered on an individual basis in patients with positive or close (< 1 mm) margins and was not offered to patients demonstrating 100% necrosis of the resected tumor.
Figure 1.
Neoadjuvant MAID chemoradiation treatment protocol.
Clinical and pathologic data retrieval
The electronic medical records of included patients were reviewed and details regarding the presentation, radiologic imaging, histologic characteristics, treatment course, toxicity, complications and vital status were ascertained. All patients had pre-treatment imaging of their primary tumors with either magnetic resonance imaging (MRI) or computed tomography (CT), and the majority of patients had follow-up imaging after three cycles of chemotherapy and radiotherapy, prior to surgery. For those patients with evaluable imaging studies before and after neoadjuvant treatment, radiologic response was recorded according to Response Evaluation Criteria in Solid Tumors (RECIST). 16 The resected tumors were graded according to the National Cancer Institute (NCI) grading system using three-tiers, and tumors with overlapping grades were classified at the higher tier. The resection specimens were bivalved along the long axis of the tumor, and a slab of the long axis was submitted for microscopic examination. Additionally, one section per centimeter was taken of the remaining two halves and submitted for microscopic examination, and the extent of necrosis was assessed relative to the percentage of residual viable tumor based on these representative tumor sections. Pathology reports of surgical specimens were evaluated in conjunction with operative reports to determine the type of resection and margin status. Resections were classified as being R0 (macroscopically complete with negative microscopic margins), R1 (macroscopically complete with positive microscopic margins), or R2 (macroscopically incomplete). A positive microscopic margin was defined as tumor present at the inked surface of the specimen.
Statistical analysis
The primary outcome measures for this analysis were rates of overall survival (OS), disease-specific survival (DSS), overall recurrence free survival (RFS), locoregional- (LRFS) and distant- (DRFS) recurrence-free survival. Secondary outcomes included prognostic factors for the abovementioned primary outcomes. Dates of death for patients with social security numbers were obtained from the Social Security Death Index (SSDI). All patients underwent pre-treatment biopsy (core needle or incisional), and OS was calculated from the date of confirmed pathologic diagnosis to the date of documented death by SSDI. LRFS was calculated from the date of confirmed pathologic diagnosis to the date of first local or regional progression or recurrence. DRFS was calculated from the date of confirmed pathologic diagnosis to the date of distant progression or recurrence. Overall recurrence-free survival (RFS) was calculated from the date of confirmed pathologic diagnosis to the date of first of local, regional or distant progression or recurrence. Censoring occurred at the earlier of date of death or date of last contact. Kaplan-Meier survival estimates were calculated for LRFS, DRFS, RFS, DSS and OS. Univariate Cox proportional hazard regression modeling was used to determine clinical and histologic predictors of overall RFS, DRFS and OS. In the univariable model, patients resected with close (< 1 mm), but negative, margins were considered as R0 resections. All reported p-values are two-sided using a significance threshold of 0.05. Statistical analyses were performed using SAS version 9.2.
Results
Patient and tumor characteristics
Clinicopathologic features of the patients in this series are shown in Table 1. Sixty-six patients were included, of whom 50 (76%) patients were male. The median age at diagnosis was 53 years. The most prevalent histologic type was undifferentiated high grade pleomorphic sarcoma (35%), and the most common location at presentation was the thigh (55%). The pre-treatment median tumor size was 10 cm (range, 4.1 – 35 cm), and all tumors were histologic grade 2 (50%) or 3 (50%). Only one patient’s largest pathologic tumor dimension was less than 5 cm, but this patient’s lesion measured 8.5 cm on pre-treatment MRI, and therefore qualified for this protocol.
Table 1.
Clinicopathologic and treatment characteristics of patients undergoing interdigitated neoadjuvant chemoradiotherapy (N=66)
| n (%) | ||
|---|---|---|
| Gender | ||
| Male | 50 (76) | |
| Age at diagnosis (years) | ||
| Mean | 51 | |
| Median | 53 | |
| Range | 18–73 | |
| Primary vs. recurrent at presentation | ||
| Primary | 63 (95) | |
| Recurrent | 3 (5) | |
| Histology | ||
| Undifferentiated high grade pleomorphic sarcoma | 23 (35) | |
| Liposarcoma | 20 (30) | |
| Synovial sarcoma | 12 (18) | |
| Myxofibrosaroma | 6 (9) | |
| Malignant peripheral nerve sheath tumor | 3 (4) | |
| Myofibroblastic sarcoma | 1 (2) | |
| Angiosarcoma | 1 (2) | |
| Location | ||
| Thigh | 36 (55) | |
| Lower leg | 8 (12) | |
| Axilla/Shoulder | 7 (11) | |
| Buttock | 5 (8) | |
| Arm | 4 (6) | |
| Popliteal space | 2 (3) | |
| Foot | 1 (2) | |
| Back | 1 (2) | |
| Elbow | 1 (2) | |
| Scapula | 1 (2) | |
| Pathologic dimension (cm) | ||
| Mean | 12 | |
| Median | 10 | |
| Range | 2.5–35.5 | |
| Histologic grade | ||
| 1 | 0 (0) | |
| 2 | 33 (50) | |
| 3 | 33 (50) | |
| Surgical margin* | ||
| Negative (≥ 1mm) | 38 (58) | |
| Close (< 1mm) | 19 (29) | |
| Positive | 7 (11) | |
| Percent necrosis* | ||
| 0–25 % | 10 (15) | |
| 26–50 % | 5 (8) | |
| 51–75 % | 12 (18) | |
| 76–100 % | 35 (53) | |
| Unknown | 4 (6) | |
| Extent of resection* | ||
| Radical resection | 62 (94) | |
| Wide local excision | 1 (2) | |
| Amputation | 1 (2) | |
| Surgical reconstruction | 18 (27) ** | |
| Pedicled flap +/− STSG | 8 (12) | |
| Free flap + STSG | 2 (3) | |
| Complex primary closure | 4 (6) | |
| Prophylactic long bone fixation | 5 (8) | |
STSG: Split thickness skin graft, NA: Neoadjuvant, IORT Intraoperative radiation therapy,
2 patients did not receive surgery,
1 patient underwent prophylactic fixation + sartorius muscle flap.
Treatments received
Sixty-four (97%) patients underwent surgical resection, including 62 (94%) who underwent a radical resection, 1 (2%) who underwent wide excision, and 1 (2%) who underwent amputation. Two (3%) patients began the interdigitated treatment but due to excellent clinical and radiographic tumor response, opted to receive definitive chemoradiation and no surgery. Fifty-seven (86%) patients had a R0 resection, and 7 (11%) patients underwent a R1 resection. Pathologic tumor necrosis was > 75% in the majority (53%) of patients. Eighteen (33%) patients underwent complex surgical reconstruction, and prophylactic long bone fixation was performed in 5 (9%) patients.
All patients who completed the regimen received 44Gy interdigitated neoadjuvant radiation therapy, and boost radiation therapy was administered in 9 (14%) patients. Fifty-five (83%) patients received 6 cycles of MAID chemotherapy. Fifty-four (82%) patients underwent 3 cycles pre- and post-operatively, and one patient underwent 2 cycles pre-operatively and 4 cycles post-operatively. Nine (14%) patients started interdigitated chemoradiotherapy treatment during the examined time period but did not complete the regimen due to chemotherapy intolerance (6 patients, 9%) or refusal of post-operative chemotherapy (3 patients, 5%). Of the 6 patients who discontinued chemotherapy during the protocol, 3 stopped due to fever/sepsis, and 1 patient stopped due to each of profound thrombocytopenia, intolerable nausea, and recurrent syncope.
Fifty-eight (88%) patients had evaluable imaging studies to determine tumor response according to RECIST criteria. 16 Seventeen (29%) of 58 patients had a partial response, 36 (62%) patients had stable disease, and 5 (9%) patients had progressive disease. Of the 17 patients who had a partial response, the histologies of their tumors included synovial sarcoma (n = 6), myxoid liposarcoma (n = 5), myxofibrosarcoma (n = 2), pleomorphic sarcoma (n = 2), malignant peripheral nerve sheath tumor (n = 1), and angiosarcoma (n = 1). It should be noted that radiologic “progression” by RECIST criteria in patients undergoing pre-operative chemoradiation for soft tissue sarcomas can reflect significant necrosis of tumor with enlargement of the mass from osmotic effects. 12
Toxicity and complications
There were no reported treatment-related deaths or secondary myelodysplasias. Chemotherapy-related acute hematologic toxicities are shown in Table 2. Twenty-one (32%) patients experienced pre-operative complications (Table 3), including 14 (21%) patients who had chemotherapy-related complications and 5 (8%) patients who experienced venous thromboembolic complications requiring anticoagulation prior to surgery. Thirty-one (47%) patients had post-operative complications (Table 3). According to the Clavien classification, 17 these complications were categorized as class I (n = 2), II (n = 18), IIIa (n = 2), IIIb (n = 7), and IVa (n = 2). Two patients (3%) experienced pathologic fractures, one at 4 months and the second at 4 years after completion of the treatment protocol, both requiring operative fixation.
Table 2.
Acute hematologic toxicities with interdigitated chemoradiotherapy according to Eastern Cooperative Oncology Group Common Toxicity Criteria.
| Criteria | n (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Hematologic | ||||||
| Leukopenia | 18 (27) | 10 (15) | 12 (18) | 8 (13) | 18 (27) | |
| Anemia | 7 (10) | 41 (62) | 16 (24) | 1 (2) | 1 (2) | |
| Thrombocytopenia | 43 (65) | 14 (21) | 3 (5) | 4 (6) | 2 (3) | |
| Infection | 53 (80) | 0 (0) | 7 (11) | 5 (7) | 1 (2) | |
Table 3.
Acute toxicities and complications with interdigitated chemoradiotherapy (N=66)
| n (%) | ||
|---|---|---|
| Pre-operative | 21 (32) | |
| Chemotherapy-related complications | ||
| Admission for febrile neutropenia | 10 | |
| Anemia requiring transfusion | 2 | |
| Steroid induced anxiety/mood disorder/psychosis | 1 | |
| Reactivation shingles | 1 | |
| Radiation-related skin complications | 2 | |
| DVT/PE | 5 | |
| Post-operative | 31 (47) | |
| Wound complications | ||
| Cellulitis | 6 | |
| Wound dehiscence requiring local wound care | 8 | |
| Wound dehiscence requiring I&D/negative pressure wound therapy | 5 | |
| Skin graft loss | 1 | |
| Chemotherapy-related complications | ||
| Admission for febrile neutropenia | 3 | |
| Port-a-cath-related thrombus | 1 | |
| Other | ||
| Pathologic fracture | 2 | |
| Lymphedema | 2 | |
| DVT/PE | 2 | |
| Pneumonia | 1 | |
I&D: incision and drainage, DVT Deep vein thrombosis, PE Pulmonary embolus
Local, regional and distant recurrence and survival
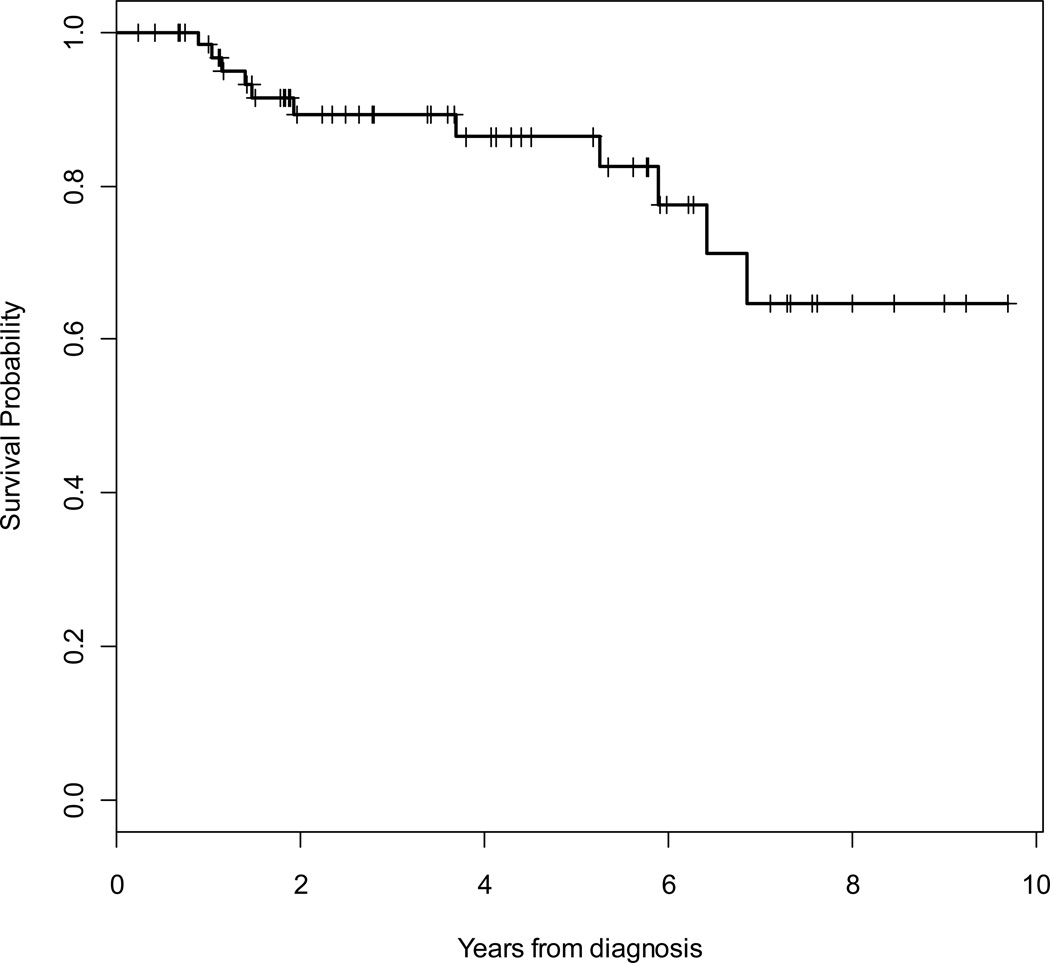
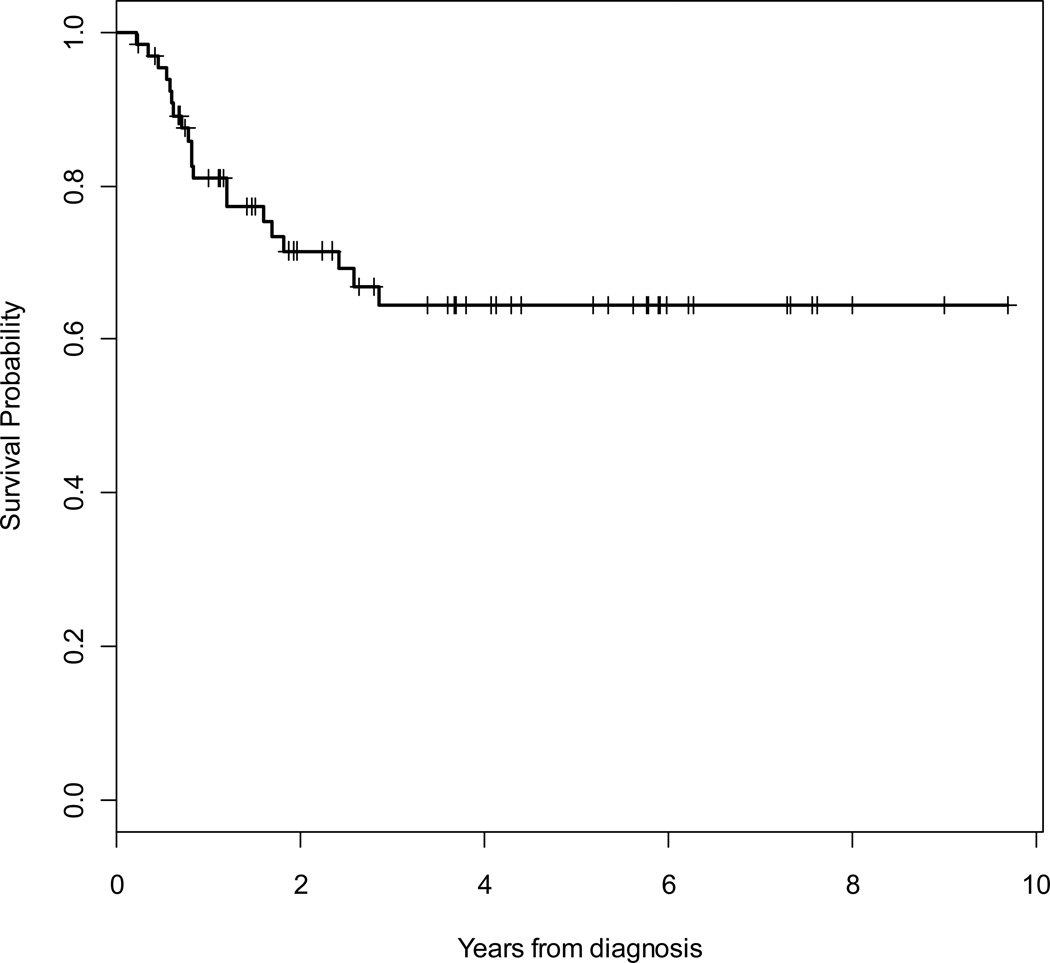
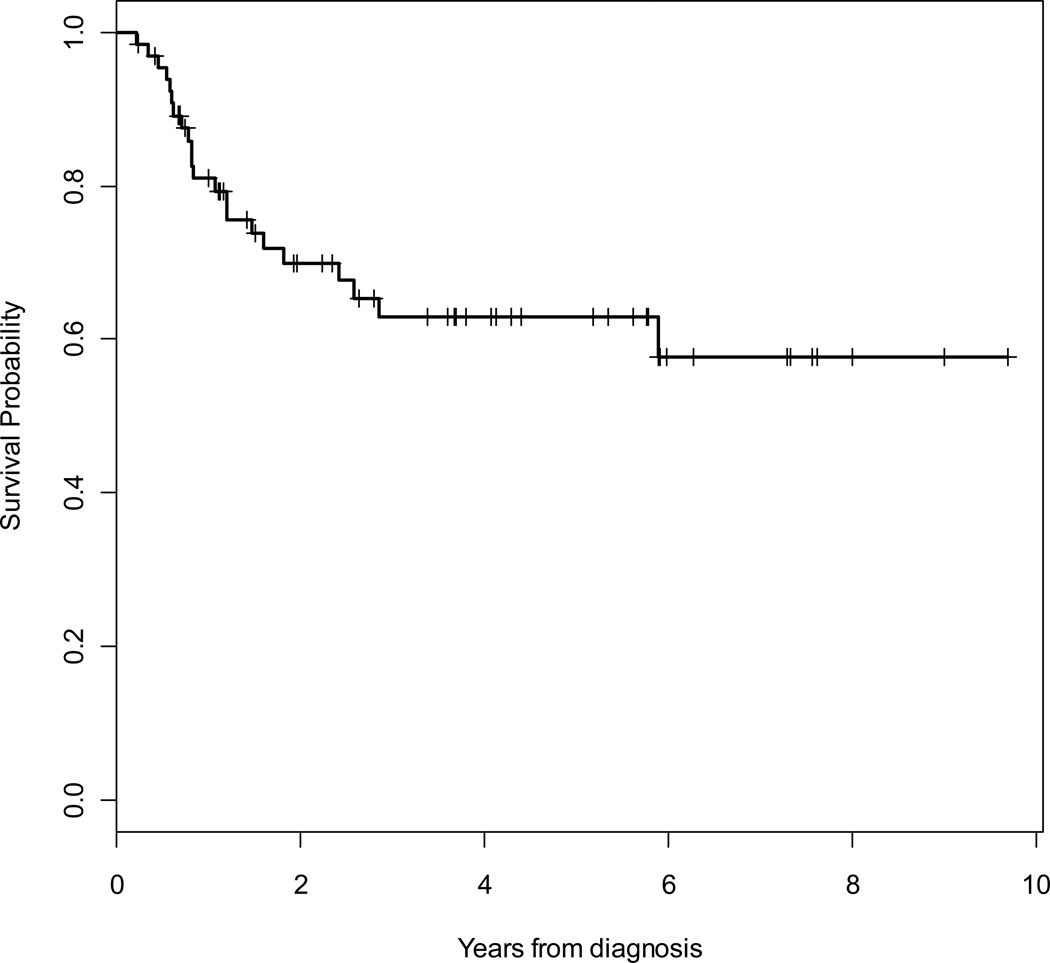
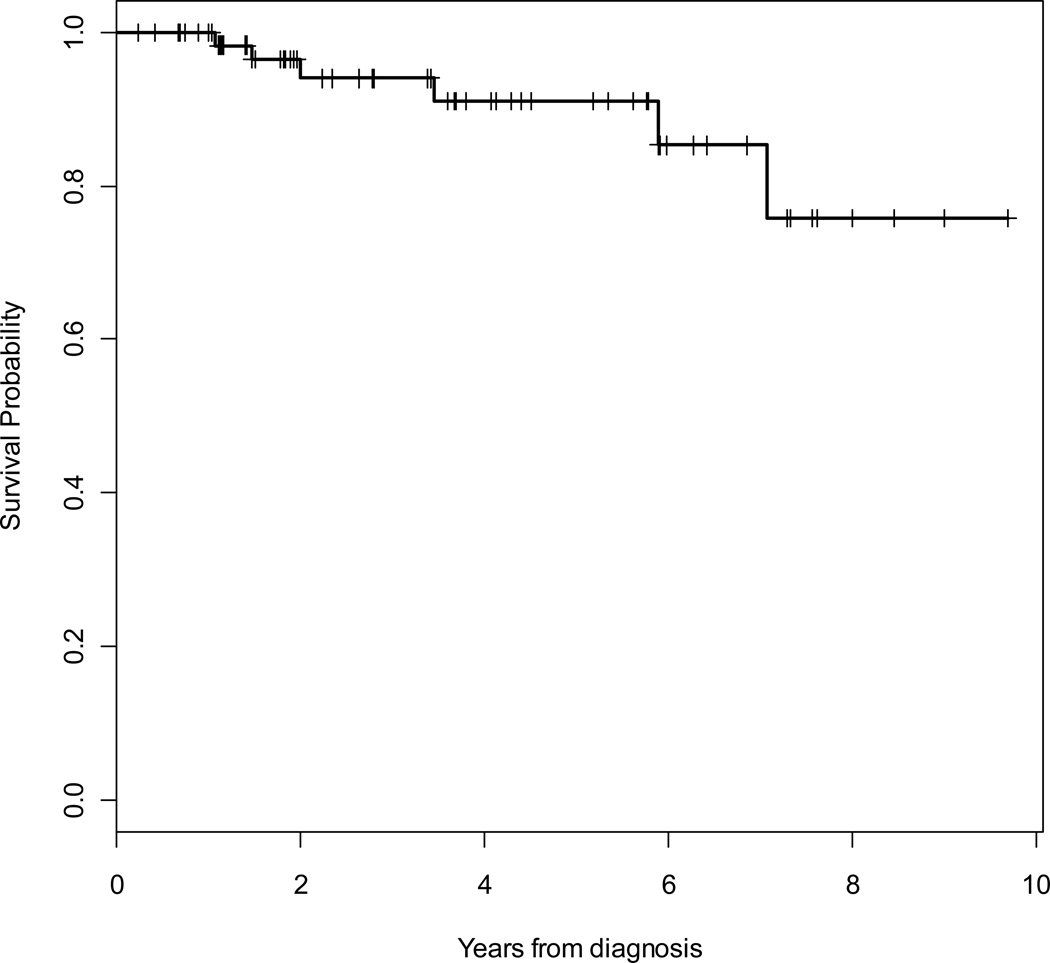
At a median follow-up of 46 months, 11 (17%) patients had died (9 due to sarcoma), 44 (67%) patients were alive without evidence of recurrence, and 11 (17%) patients were alive with local recurrence and/or metastatic disease. Median time to death was 23 months. Overall (OS) and disease-specific (DSS) 5-year survival rates were 86 % (95% CI, 73% – 93%) and 89% (95% CI, 78% – 95%), respectively. The 5-year locoregional- (LRFS) and distant- (DRFS) recurrence-free survival rates were 91% (95% CI, 77% – 97%) and 64% (95% CI, 50% – 76%), respectively. The overall 5-year RFS rate was 63% (95% CI, 49% – 74%). Kaplan Meier curves for OS, RFS, LRFS and DRFS are shown in Figures 2 to 5.
Figure 2.
Kaplan-Meier survival curve for overall survival.
Figure 5.
Kaplan-Meier survival curve for distant recurrence-free survival (DRFS).
Local recurrences occurred in 5 (8%) patients at a median of 41 months after diagnosis. An isolated regional recurrence occurred in 1 (2%) patient at 18 months following diagnosis. Collectively, locoregional recurrences occurred in 6 (9%) patients at a median of 33 months following diagnosis. Distant metastatic disease occurred in 20 (30%) patients at a median of 10 months after diagnosis. Of these, 3 (5%) had multiple sites of disease. Seventeen (26%) patients developed pulmonary metastases, and 5 (8%) patients developed bone metastases. Other sites of metastatic disease included the retroperitoneum, intraabdominal sites, and the liver in one patient each. Thirteen patients underwent surgical metastasectomy (12 pulmonary resections, 1 retroperitoneal resection with IORT). Of these, surgery was accompanied by chemotherapy in 6 patients, by radiation in 1 patient, and by chemotherapy and radiation in 3 patients.
Of the 11 patients who did not complete the intended neoadjuvant chemoradiotherapy regimen, 3 experienced distant recurrences at 7, 7, and 22 months following diagnosis, and 1 experienced a local recurrence at 11 months following diagnosis. Low event rates precluded comparison of outcomes for these patients with the outcomes of the 55 patients who completed the regimen in its entirety. There were no statistically significant predictors of OS, overall RFS, or DRFS. Low event rates for locoregional (6 patients) recurrence precluded the creation of proportional hazard models for these outcomes.
Discussion
In this update to our original pilot study of patients with high-risk extremity and truncal STS treated with an intensive regimen of neoadjuvant chemoradiotherapy, we report promising 5-year rates of overall survival (86%) and disease-specific survival (89%). Although this regimen has been slow to gain wide acceptance due to its toxicity and potential for secondary myelodysplasias, this analysis of the most recent 66 patients at MGH over the past 10 years confirms the modest toxicities that we reported in our initial pilot study.
Given the absence of convincing level I evidence, there is substantial controversy concerning the role of adjuvant or neoadjuvant chemotherapy in the management of patients with high-risk extremity or truncal STS. In fact, there has been just a single randomized phase II study published on the feasibility and outcome of neoadjuvant chemotherapy in adult patients with high-risk STS. 11 This trial was designed by the EORTC to compare high-risk STS of the extremities and trunk treated with surgery alone (SA) versus surgery combined with neoadjuvant (NAC) doxorubicin/ifosfamide. At a median follow-up of 7.3 years, this study demonstrated an 88% rate of limb salvage but no significant differences in disease-free (52% (SA) vs. 56% (NAC), p=0.35) or overall (64% (SA) vs. 65% (NAC), p=0.22) survival. In contrast, several non-randomized phase II studies of neoadjuvant chemoradiotherapy including various combinations of doxorubicin, ifosfamide, dacarbazine, cisplatin, and mitomycin have shown promising local control rates in excess of 90% and 5-year overall survival rates of approximately 70%, including our initial pilot study at MGH as well as the follow-up RTOG Trial 9514. 12, 13, 18, 19 In addition, Grobmyer at al. conducted a retrospective cohort study examining 356 patients with high-grade extremity sarcomas, and they reported improved DSS for patients undergoing neoadjuvant treatment with doxorubicin/ifosfamide/mesna (HR 0.52, 95% CI 0.3–0.92, p=0.02), with the most striking results seen for patients with tumors at least 10 cm in size. 20
There are several potential advantages to administering chemoradiotherapy prior to surgery. The sequencing of chemoradiotherapy prior to surgery enables the assessment of tumor response to treatment and potentially facilitates R0 surgical resection. We saw a partial response rate by RECIST criteria of 29%, which is exactly the same as that reported in the EORTC trial 11 and is similar to the response rate seen in the metastatic setting. Unfortunately, given the retrospective nature of this study, we were not able to determine whether the scope of surgery was changed in those patients who experienced a PR. Furthermore, only 9% of patients had radiologic “progression” (of uncertain clinical significance) during the neoadjuvant chemoradiotherapy, and in no case did tumor progression preclude surgery. In terms of pathologic response, though more than half of the patients had greater than 75% pathologic necrosis documented in the resected specimen, the extent of necrosis did not correlate with outcome. Another potential advantage to the administration of chemotherapy prior to surgery is the eradication of microscopic metastatic disease early in the treatment course. This point is particularly important, as this study demonstrates that distant metastasis is a far greater concern than is locoregional recurrence (30% vs. 9%). Furthermore, the administration of pre-operative (vs. post-operative) therapy improves compliance and maximizes the ability to complete chemoradiation, as patients are not recovering from major surgery and possible wound complications. The alternation of chemotherapy and radiation in the pre-operative period also allows for the mediation of the accumulated toxicities of each modality, as patients are able to partially recover between cycles. Finally, the suggestion that doxorubicin/ifosfamide and radiation, each in isolation, appear to provide improvements in local control lends credence to the possibility that combined modality treatment may additively improve outcomes in STS.
One of the major concerns with the potential widespread use of this regimen, as demonstrated in the multi-institutional RTOG trial, 13 is its significant toxicity. One fatal treatment–related myelodysplastic event occurred 53 months after the completion of treatment in the original pilot study examining this regimen, 12 but no further treatment-related deaths were noted on long-term follow-up (median of 9.3 years). 15 An assessment of toxicities was meticulously undertaken during the pilot study by DeLaney et al. 12 to establish the feasibility and safety of this regimen. Similarly, the RTOG 9514 study reported 3 treatment-related deaths out of 64 patients enrolled (2 due to acute myelogenous leukemia) and an incidence of grade 3 or higher acute toxicities in 97% of patients. 13 This increased toxicity was felt to be due to a 25% higher dose of ifosfamide employed in the RTOG trial, compared to the dosing reported in the original MGH pilot study and reported herein, as well as generous radiation fields. However, after 5 years of follow-up, a marked decrease in treatment-associated toxicity was noted, and no further deaths were reported. 14 In the present study, no treatment-related deaths or secondary mylelodysplasias were noted. Grade 3 and 4 leukocyte, hemoglobin and platelet toxicities were experienced by 39%, 3% and 9% of patients, respectively. These rates are far less than the corresponding 58%, 46% and 29% rates reported in the pilot study. 12 In addition, 83% of patients in the current study completed the treatment regimen in its entirety. Comparatively, only 59% of patients in the RTOG study received all 6 cycles of MAID chemotherapy. Other studies of neoadjuvant chemotherapy and radiation report wound complication rates of 20–50%.11, 18 Thus, morbidity rates in this study appear comparable to those seen in the 2003 pilot study and to other reports of neoadjuvant therapy in STS.
With the recent publication of a randomized, phase III trial from the Italian and Spanish Sarcoma Groups demonstrating non-inferiority of a shorter course (3 cycles versus 5 cycles) of adjuvant chemotherapy with only two agents (epirubicin and ifosfamide) in patients with high-risk STS, 21 one might question why we continue to include dacarbazine in our regimen. Though certainly the use of dacarbazine in addition to mesna, adriamycin, and ifosfamide adds toxicity to the regimen, the majority of this toxicity is gastrointestinal (nausea and vomiting), which is usually easily managed with our current arsenal of antiemetics. In addition, though there are no data directly comparing MAID and AIM in terms of efficacy, there are data showing that the addition of dacarbazine to adriamycin increases the response rate (though not survival) in the metastatic setting. 22 Furthermore, by including dacarbazine, the MAID regimen confers results that are at least as good as AIM with lower doses of adriamycin and ifosfamide, thus limiting the toxicities of these two drugs. Lastly, most adjuvant studies, including the trial referenced above by Gronchi et al., 21 include patients at lower risk for metastatic disease and death (tumors ≥ 5 cm) than the patients to whom we offer the MAID regimen (only tumors ≥ 8cm). Given our encouraging survival data, we are reluctant to switch to a shorter course, less aggressive regimen for such a high-risk patient population, especially in light of the fact that we are comfortable with this regimen and its manageable toxicities.
An additional area of controversy is the role for a postoperative radiation boost to those patients with positive or close surgical margins in an effort to improve long-term local control. We administered a postoperative boost to 9 patients in this series with either close or positive surgical margins, and only one of these patients developed a local recurrence. Of course, these are far too few patients to conclude whether a postoperative boost is necessary, and given the low rate of local recurrences in this study overall (8%), this question will be difficult to definitively answer. Several groups have shown that local control and freedom from amputation after preoperative XRT and a marginal excision is excellent (~ 90%), 4, 23–25 and that the risk of local recurrence is particularly low in certain patient subsets (such as those with planned positive margins against critical neurovascular structures or bone), 26 such that a postoperative boost may not be necessary. 23 Nevertheless, most series continue to document higher rates of local failure in patients with positive surgical margins. 27 Hence, we typically selectively administer a boost to those patients deemed at particularly high risk for local failure, such as the patients included in this series with large, high-grade tumors with positive or very close surgical margins, a group in whom historically one might expect a LR rate of 20 – 30%. Our overall 5-year LRFS rate of 91% and our LR rate of only 11% in the particularly high-risk group chosen to receive a postoperative boost support our current strategy, especially in light of the adverse prognostic impact of a local recurrence on survival. 28
Despite the encouraging findings of this study, there are several important limitations. First, this is a retrospective study conducted at a single institution, and the described regimen is not directly compared to a control group. However, in our initial pilot study, we did compare the outcomes of patients treated on this regimen to a matched historical control population of patients and demonstrated the superiority of this treatment approach to pre-operative XRT alone in terms of several measures of survival. 12 This study is certainly subject to institutional selection biases, in which clinicians preferentially select the healthiest and most motivated patients to undergo a given treatment protocol and thereby encourage the most favorable results. Though only 55 of the patients included in this analysis completed all of the planned therapy, we included the 11 other patients we intended to treat fully on this protocol but who were unable or unwilling to do so, in order to limit to some degree our selection bias. Second, the median follow-up of this study (46 months) is relatively short, and so there remains the question of the durability of the treatment response. However, a previous report from our institution describing the long-term outcome of patients treated with this identical regimen demonstrated encouraging survival rates at a median follow-up of 9.3 years. 15 Finally, this is a complicated treatment regimen that is perhaps best delivered at centers with particular expertise in the multidisciplinary treatment of sarcomas, and so the results reported herein may not be generalizable to the population at large.
In conclusion, this analysis of patients with high-risk extremity and truncal STS treated over the past 10 years at a single institution with an intense regimen of neoadjuvant chemoradiotherapy and surgery confirms survival advantages with relatively modest short and long-term toxicity. These outcomes are similar to, and in some cases better than, those reported in other studies. The challenge lies in finding an optimal chemotherapy regimen, perhaps even multiple different histology-specific regimens rather than a one-size-fits-all approach. Based on the results presented here, all patients with large (≥ 8 cm), high-grade sarcomas who are medically fit and sufficiently motivated to proceed with aggressive treatment, should be considered for neoadjuvant chemoradiotherapy. Ideally, this regimen should be prospectively compared to alternate regimens including complementary systemic agents, such as biologic agents, whose side-effect profiles, tolerability, and resultant outcomes may be superior. 29
Figure 3.
Kaplan-Meier survival curve for overall recurrence-free survival (RFS).
Figure 4.
Kaplan-Meier survival curve for locoregional recurrence-free survival (LRFS).
Acknowledgments
Financial support: Biostatistics Core of Dana-Farber/Harvard Cancer Center supported by NCI Cancer Center Support Grant # NIH 5 P30 CA06516.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures
Conflict of Interest Statement: None declared.
References
- 1.Lindberg RD, Martin RG, Romsdahl MM, Barkley HT., Jr Conservative surgery and postoperative radiotherapy in 300 adults with soft-tissue sarcomas. Cancer. 1981 May 15;47(10):2391–2397. doi: 10.1002/1097-0142(19810515)47:10<2391::aid-cncr2820471012>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002 Jun 29;359(9325):2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 3.Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996 Mar;14(3):859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 4.Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993 Apr;52(4):223–230. doi: 10.1002/jso.2930520405. [DOI] [PubMed] [Google Scholar]
- 5.Sampath S, Schultheiss TE, Hitchcock YJ, Randall RL, Shrieve DC, Wong JY. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma: multi-institutional analysis of 821 patients. Int J Radiat Oncol Biol Phys. 2002 Oct 1;81(2):498–505. doi: 10.1016/j.ijrobp.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998 Jan;16(1):197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Stinson SF, DeLaney TF, Greenberg J, et al. Acute and long-term effects on limb function of combined modality limb sparing therapy for extremity soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1493–1499. doi: 10.1016/0360-3016(91)90324-w. [DOI] [PubMed] [Google Scholar]
- 8.Spiro IJ, Gebhardt MC, Jennings LC, Mankin HJ, Harmon DC, Suit HD. Prognostic factors for local control of sarcomas of the soft tissues managed by radiation and surgery. Semin Oncol. 1997 Oct;24(5):540–546. [PubMed] [Google Scholar]
- 9.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997 Dec 6;350(9092):1647–1654. [PubMed] [Google Scholar]
- 10.Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001 Mar 1;19(5):1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for 'high-risk' adult soft-tissue sarcoma. Eur J Cancer. 2001 Jun;37(9):1096–1103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 12.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003 Jul 15;56(4):1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 13.Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006 Feb 1;24(4):619–625. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 14.Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer. Oct 1;116(19):4613–4621. doi: 10.1002/cncr.25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullen JT, Kobayashi W, Wang JJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. Aug 1;118(15):3758–3765. doi: 10.1002/cncr.26696. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004 Aug;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis KK, Ashman JB, Beauchamp CP, et al. Neoadjuvant chemoradiation compared to neoadjuvant radiation alone and surgery alone for Stage II and III soft tissue sarcoma of the extremities. Radiat Oncol. 6:91. doi: 10.1186/1748-717X-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmonson JH, Petersen IA, Shives TC, et al. Chemotherapy, irradiation, and surgery for function-preserving therapy of primary extremity soft tissue sarcomas: initial treatment with ifosfamide, mitomycin, doxorubicin, and cisplatin plus granulocyte macrophage-colony-stimulating factor. Cancer. 2002 Feb 1;94(3):786–792. doi: 10.1002/cncr.10259. [DOI] [PubMed] [Google Scholar]
- 20.Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004 Nov;15(11):1667–1672. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 21.Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. Mar 10;30(8):850–856. doi: 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- 22.Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987 Jun;5(6):840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 23.Al Yami A, Griffin AM, Ferguson PC, et al. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys. Jul 15;77(4):1191–1197. doi: 10.1016/j.ijrobp.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 24.Dagan R, Indelicato DJ, McGee L, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer. Jun 15;118(12):3199–3207. doi: 10.1002/cncr.26489. [DOI] [PubMed] [Google Scholar]
- 25.Kim B, Chen YL, Kirsch DG, et al. An effective preoperative three-dimensional radiotherapy target volume for extremity soft tissue sarcoma and the effect of margin width on local control. Int J Radiat Oncol Biol Phys. Jul 1;77(3):843–850. doi: 10.1016/j.ijrobp.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 26.Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br. 2001 Nov;83(8):1149–1155. doi: 10.1302/0301-620x.83b8.12028. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe KK, Pollock RE, Ellis LM, Murphy A, Sherman N, Romsdahl MM. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994 Mar 15;73(6):1652–1659. doi: 10.1002/1097-0142(19940315)73:6<1652::aid-cncr2820730617>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007 Apr 1;67(5):1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Riedel RF. Targeted agents for sarcoma: is individualized therapy possible in such a diverse tumor type? Semin Oncol. Oct;38(Suppl 3):S30–S42. doi: 10.1053/j.seminoncol.2011.09.003. [DOI] [PubMed] [Google Scholar]