Abstract
Proteomic analysis of sensory organs such as the cochlea is challenging due to its small size and difficulties with membrane protein isolation. Mass spectrometry in conjunction with separation methods can provide a more comprehensive proteome, because of the ability to enrich protein samples, detect hydrophobic proteins, and identify low abundant proteins by reducing the proteome dynamic range. GELFrEE as well as different separation and digestion techniques were combined with FASP and nanoLC-MS/MS to obtain an in-depth proteome analysis of cochlear sensory epithelium from 30-day-old mice. Digestion with LysC/trypsin followed by SCX fractionation and multiple nanoLC-MS/MS analyses identified 3773 proteins with a 1% FDR. Of these, 694 protein IDs were in the plasmalemma. Protein IDs obtained by combining outcomes from GELFrEE/LysC/trypsin with GELFrEE/trypsin/trypsin generated 2779 proteins, of which 606 additional proteins were identified using the GELFrEE/LysC/trypsin approach. Combining results from the different techniques resulted in a total of 4620 IDs, including a number of previously unreported proteins. GO analyses showed high expression of binding and catalytic proteins as well as proteins associated with metabolism. The results show that the application of multiple techniques is needed to provide an exhaustive proteome of the cochlear sensory epithelium that includes many membrane proteins. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium with the data set identifier PXD000231.
Keywords: cochlea, high-resolution mass spectrometry, proteome, sensory epithelium
Introduction
The inner ear, which is encapsulated within a hard bony shell, contains the cochlea, vestibular labyrinths and endolymphatic sac.1 Highly specialized receptors in the sensory epithelium of the organ of Corti contain receptor or hair cells and supporting cells. The hair cells are divided into IHCs and OHCs, which are responsible for transmitting electrical signals to the brain and modulating those signals, respectively.2 Damage to these cells can lead to hearing loss or impairment.1 Hearing loss affects more than 28 million individuals in the United States and approximately half of these cases are thought to be hereditary.3 Genetics may play a role in both noise-induced and age-related hearing loss.
An important step towards understanding an organism’s biology is to determine its genome sequence. However, the sequence does not provide enough information on complex cellular processes, thus, the complement of proteins associated with a particular genome is essential to this understanding.4 Proteomics is a complementary approach that can provide insights into the understanding of complex biological systems by analyzing protein expression, function, modifications, and interactions.5 In order to better understand how the inner ear works and to target potential protein biomarkers for prevention or treatment of hearing impairments, we need to understand the molecular make-up and function of its proteins.
One of the major challenges in using the inner ear for proteomic analysis is its small size, restricted accessibility, and cell type diversity.6 Moreover, key proteins that distinguish its functionality, such as ion channels, transporters and receptors, are membrane proteins.1 Thus, methods are needed to enhance the extraction and preparation of proteins for MS analysis. FASP is advantageous for proteomic analysis of tissues that require detergents to solubilize membrane proteins and that have limited tissue for protein extraction.7 This filtering allows MS analysis of membrane and soluble proteins, and peptide isolation from low molecular weight contaminants, such as nucleic acids.7, 8 Sample enrichment is another proteomic approach that enables analysis of small amounts of tissue. This method involves enriching specific groups of proteins, such as membrane proteins, from complex samples. This technique reduces sample complexity and enhances the detection of low abundant proteins.9, 10 These types of proteins are of particular interest because they are usually of great biological importance.
Combinations of preparative methods with robust and sensitive techniques are also required to maximize the number of identified proteins. MS/MS in combination with multi-dimensional separations have become powerful techniques for peptide and protein identification.11 These separation techniques include SCX, SAX, WAX, RP, SEC, or GELFrEE separation, which can be applied multi-dimensionally to reduce sample complexity, thus enhancing peptide and protein ID and reducing the effect of ion suppression in MS.12 There are two commonly used proteomic techniques. The shotgun technique entails enzymatically digesting proteins in a complex biological sample and then separating using MudPIT. The most widely used multidimensional separation technique is SCX followed by RP liquid chromatography.13 However, other orthogonal techniques have been applied for MudPIT, such as RP followed by RP and WAX or SAX followed by RP.14 In another technique, known as bottom-up proteomics, proteins are first separated and then enzymatically digested prior to LC-MS/MS analysis, thereby providing an increase in separation capacity and dynamic range.13
Several studies of the inner ear have been reported using antibody microarray,15 two-dimensional gel electrophoresis,16-18 DIGE,19 or LC-MS/MS.20-23 These studies have identified a limited number of proteins, particularly membrane proteins. To provide a more exhaustive overview of the mouse cochlear sensory epithelium, we used membrane enrichment, multiple separation techniques, and multi-digestion procedures with nano LC-MS/MS. In addition, we varied the fractionation collection times and the LC-MS/MS gradient times to aid in improving protein and peptide identification. Using these different techniques, 4620 total proteins were identified from the mouse cochlear sensory epithelium.
Materials and Methods
Membrane Fractionation
Cochleae were isolated from eight 30-day-old (P30) CBA/J mice and the tympanic bullae excised, after which the bone, ligament, and stria vascularis were removed, isolating the sensory epithelium along with the modiolus. The cochlear sensory epithelia were washed in 1X PBS, centrifuged for 3 min at 1000g, and the supernatant removed. Cochlear sensory epithelia were sonicated in lysis buffer containing 50 mM Tris-HCl, pH 8.0, 120 mM NaCl, 50 mM NaF, 5 mM EDTA, 500 µg/mL AEBSF, 10 µg/mL leupeptin, 100 µg/mL pepstatin, 2 µg/mL aprotinin and 5 µM okadaic acid using a sonic dismembrator (Model 100; Thermo Fisher). The extract was centrifuged at 750g at 4 °C for 2 min. The supernatant was removed and the pellet was extracted in lysis buffer and centrifuged as above. Both lysates were combined and ultracentrifuged at 100,000g at 4 °C for 60 min. The supernatant was removed and lysis buffer containing 0.1% ASB-14 (Calbiochem) was added to the pellet, vortexed, and incubated for 60 min at 4 °C. The suspension was centrifuged at 16 000g at 4 °C for 5 min and the supernatant retained for digestion and analysis.
Protein Extraction from Sensory Epithelia
Sixteen cochleae from 30-day-old (P30) CBA/J mice were isolated and the sensory epithelia excised and washed as above. The tissue was sonicated and the lysate centrifuged at 750g as above. The supernatant was retained and sonicated in lysis buffer containing 4% (w/v) SDS, 100 mM Tris-HCl, pH 7.6, 0.1 M DTT, 500 µg/mL AEBSF, 10 µg/mL leupeptin, 100 µg/mL pepstatin, 2 µg/mL aprotinin and 1 mg/mL microcystin after which the extract was incubated at RT for 30 min. The sample was heated at 95 °C for 5 min, then cooled at 4 °C for 60 min followed by centrifugation at 16 000g at 25 °C for 10 min. The supernatant was collected and transferred to a new tube.
FASP
The FASP procedure8 was used to remove detergent and perform digestion. Cochlear protein supernatant was concentrated and a 30 µl aliquot of protein extract in 4% SDS, 100 mM Tris-HCl, pH 7.6 and 0.1 M DTT was directly added to a 30 K spin filter and mixed with 200 µL of 8 M urea in Tris-HCl and centrifuged at 14 000g for 15 min. The concentrate was diluted with 200 µL of urea solution and centrifuged at 14 000g for 15 min. Then, 10 µL of 10 × IAA in urea solution was added to the concentrate in the filter and vortexed for 1 min. The spin filter was incubated for 20 min at RT in the dark followed by centrifugation at 14 000g for 10 min. To the concentrate on the filter, 100 µL of urea solution was added and centrifuged at 14 000g for 15 min. This step was repeated 2×. There was 100 µL of 50 mM ABC solution added to the spin filter and centrifuged at 14 000g for 10 min. This step was repeated 2×. Then, 0.4 µg/µL of trypsin was added 1:100 and incubated O/N at 37 °C. Following incubation, 40 µL of 50 mM ABC solution was added and centrifuged at 14 000g for 10 min and repeated once. Finally, 50 µL of 0.5 M NaCl solution was added to the spin filter and centrifuged at 14 000g for 10 min. The filtrate containing the peptides was acidified with trifluoroacetic acid (TFA) and desalted on a C18 MacroSpin column (The Nest Group, Southboro, MA). The concentration of the peptides was determined using a microplate colorimetric assay (BioRad).
Multi-Enzyme Digestion
To increase sequence coverage, multiple enzymes were used in the FASP digestion procedure described previously.24 Following digestion and elution of peptides from the first digestion, spin filters were washed with 40 µL of urea followed with 2X washes of 40 µL of ddH2O. Then, spin filters were washed 3× with 100 µL of 50 mM ABC solution followed by adding 2 µg of endoproteinase trypsin or LysC and incubating O/N. Peptides were eluted and tryptic peptides from the second digestion were pooled with the tryptic peptides from the first digestion. LysC peptides were not pooled, but analyzed separately. Concentrations were determined as mentioned above.
Anion and Cation Exchange Chromatography
Peptides were separated off-line on a 200 × 2.1 mm, 5 µm WAX (AEX) column (linear polyethyleneimine, The Nest Group) using a gradient of 2-40% B over 50 min with a flow rate of 250 µL/min. Solvent A was 5 mM ammonium formate, pH 6.5 in 25% acetonitrile and 75% ddH2O. Solvent B was 500 mM ammonium formate, pH 3.0 in 25% acetonitrile and 75% ddH2O. The peptide fractions were monitored at 280 nm and collected in 2 min fractions. Fractions were resuspended in 15 µL of 0.1% FA for MS analysis.
Peptides were separated off-line on a 200 × 2.1 mm, 5 µm SCX column (Polysulfoethyl A, The Nest Group) using a gradient of 2-40% B over 50 min with a flow rate of 250 µL/min. Solvent A was 5 mM ammonium formate, pH 3.0 in 25% acetonitrile and 75% ddH2O. Solvent B was 500 mM ammonium formate, pH 6.0 in 25% acetonitrile and 75% ddH2O. The separation was monitored at 280 nm and either 4 or 2 min fractions were collected. The fractions were dried using a vacuum centrifuge and resuspended in 500 µL of 50% ddH2O and 50% acetonitrile containing 5% formic acid (FA) to assist with salt removal. Fractions were re-dried and resuspended in 15 µL of 0.1% FA for MS analysis.
Acetone Precipitation
Prior to GELFrEE the protein supernatant was desalted using acetone precipitation. Briefly, three volumes of ice-cold acetone were added to the supernatant. The sample was gently vortexed and incubated on ice O/N. The sample mixture was centrifuged at 15 000g for 15 min at 4 °C and the supernatant removed. The protein pellet was washed 3× with chilled acetone and centrifuged at 14 000g for 5 min at 4 °C. The resulting pellet was air-dried and suspended in 112 µL of ddH2O. The protein concentration was determined using the microplate colorimetric assay.
GELFrEE and Protein Digestion of Fractions
To the desalted protein suspended in 112 µL of nanopure water, 5× sample buffer (0.25 M Tris-HCl pH 6.8, 10% w/v SDS, 50% glycerol, 0.5% w/v bromophenol blue) was added and reduced with 1M DTT at 95 °C for 5 min. There was approximately 350 µg of protein mixture loaded into the GELFrEE chamber (GELFrEE 8100, Protein Discovery) using an 8% Tris-acetate cartridge (Protein Discovery, Knoxville, TN) with a mass range of 3.5-150 kDa. The protein fractions were collected over 2.6 hrs in a total volume of 150 µl per fraction. A 5 µl aliquot of the protein fractions was separated on a 4-15% Tris-HCl gel (BioRad, Hercules, CA) and silver stained with Silver Stain Plus from BioRad to visualize the protein separation.
A modified FASP procedure was used for detergent removal and digestion of the GELFrEE fractions. Briefly, each fraction was directly added to a 30 K spin filter and mixed with 200 µL of 8 M urea in Tris-HCl and centrifuged at 14 000g for 25 min. The concentrate was diluted with 200 µL of urea solution and centrifuged at 14 000g for 12 min and repeated once. Then, 10 µL of 10 × IAA in urea solution was added to the concentrate in the filter, vortexed for 1 min, and incubated for 30 min at RT in the dark. The spin filter was washed as described above for the FASP procedure and each fraction was digested with two consecutive enzymatic digestions. The first digestions were performed with either trypsin or LysC and the second digestions with trypsin. The first and second tryptic digestions were pooled. All digestions were dried in a vacuum concentrator and reconstituted in 20 µL of 0.1% formic acid (FA).
LC-MS/MS
Each of the SCX and WAX fractions was analyzed by nano LC-MS/MS. Prior to separation, 5 µL of each peptide fraction was injected onto a 100 µm × 25 mm sample trap (New Objective, Woburn, MA) to remove salts and contaminants. Chromatographic separation was performed on a 75 µm × 10 cm C18 column (New Objective, Woburn, MA) using a gradient of 2-40% B over 100 min with a flow rate of 200 nL/min on an Eksigent nanoLC (Thermo Scientific Inc.). Solvent A was 95% ddH2O and 5% acetonitrile containing 0.1% FA. Solvent B was 80% acetonitrile and 20% ddH2O containing 0.1% FA. Mass spectrometry data were collected using an LTQ Orbitrap mass spectrometer (Thermo Scientific Inc.). Ten tandem mass spectra were collected for each MS scan.
The tryptic digests from the GELFrEE fractions were resuspended in 15 µL of 0.1% FA and analyzed by LC-MS/MS using a 4 hr gradient on a LTQ Orbitrap MS (Thermo Scientific Inc.). Briefly, 5 µL of each peptide mixture was separated on a 75 µm × 10 cm C18 column (New Objective, Woburn, MA) using a gradient of 3-38% B over 240 min with a flow rate of 200 nL/min. Solvent A was 95% ddH2O and 5% acetonitrile containing 0.1% FA. Solvent B was 80% acetonitrile and 20% ddH2O containing 0.1% FA.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository25 with the dataset identifier PXD000231.
Data Analysis
MS data files were processed with MaxQuant (Version 1.2.2.5, Max Planck Institute) and peak list files were searched by MASCOT search engine against the UniProt mouse database containing both forward and reversed protein sequences and common contaminants such as keratin. The initial parent and fragment ion maximum precursors were set to 6 ppm and 0.5 Da, respectively. The search included a fixed modification of carbamidomethyl of cysteine and variable modifications of oxidation of methionine and protein N-terminal acetylation. The minimum peptide length to be considered for identification was 6 amino acids. The MaxQuant database search score was based on a FDR of 1% for peptides and proteins. All proteins identified from each experimental approach are listed in Supplemental Table 1. GO information of the identified proteins was obtained using UniProt.26 The UniProt GO annotation program provides GO annotations to proteins in the UniProt Knowledgebase. Proteins are assigned in GO terms, which are based on a controlled vocabulary of terms used to describe molecular function, biological process and location of action of a protein in a cell.27
A list of identified peptides containing m/z values for highly abundant proteins were extracted from Scaffold software (Version 3.4.3) and used to generate exclusion lists. The exclusion lists were imported in the LTQ Orbitrap software (Xcalibur, Thermo Scientific Inc.) method file to reject the mass list of the selected peptides.
Results
SCX of Membrane and Whole Lysates
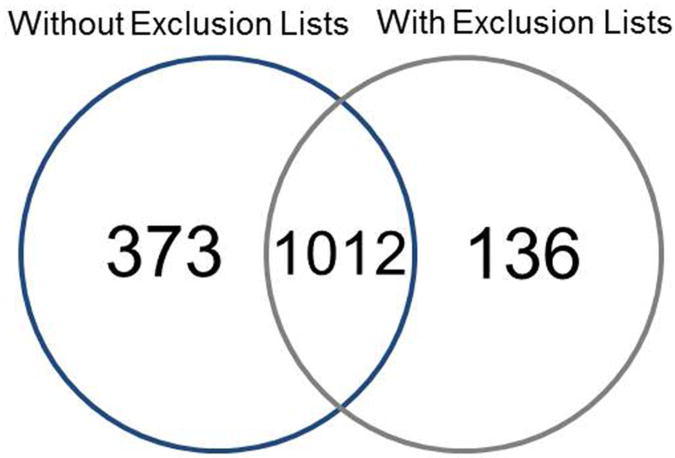
A schematic of the different strategies provides an overview of the steps taken for each experiment (Figure 1). Multidimensional separation with SCX and RP was used to reduce the sample complexity of the enzymatically digested whole lysate, following subcellular fractionation to enrich membrane proteins. The membrane fraction was collected and the proteins digested with trypsin and then fractionated on SCX into 9 fractions. All 9 fractions were analyzed using nano-LC-MS/MS, revealing 267 protein IDs. GO shows that 21% were in the plasmalemma and 24% in the mitochondrion. To characterize both membrane and soluble proteins from the mouse sensory epithelium, a second approach was applied using SCX. The whole protein extract was digested with trypsin and the peptides fractionated on SCX prior to nano LC-MS/MS analysis. A single experiment consisting of nano LC-MS/MS analysis of 9 SCX fractions led to the identification of 1385 proteins. However, it was observed that cochlin and actin accounted for more than 2000 and 1600 collected MS/MS spectra, respectively. To improve peptide and protein identification, the sample was re-injected for analysis using an exclusion list. A total of 1148 proteins were identified, of which 136 were not identified in the previous experiment without the exclusion list (Figure 2). Thus, the combination of proteins identified from the analyses with and without the exclusion list led to GO annotations identifying 18% as plasmalemmal and 22% as mitochondrial proteins.
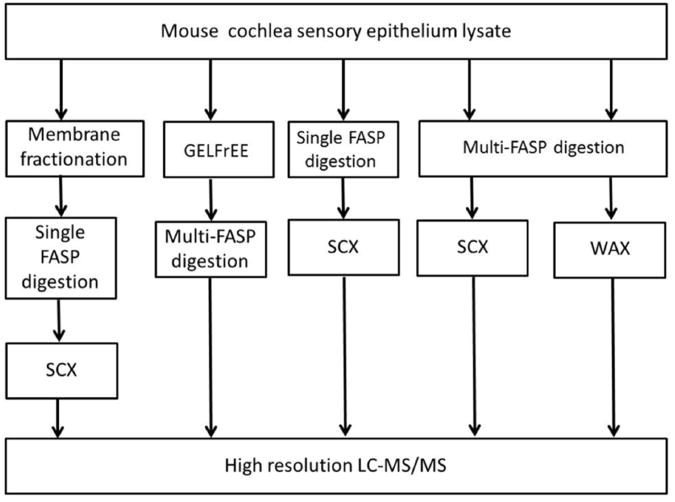
Figure 1.
Diagram depicting the procedures used to determine the proteome of the cochlear sensory epithelium of P30 normal hearing mice.
Figure 2.
Venn diagram of proteins found in the proteome of P30 mouse cochlear sensory epithelium, when using single tryptic digestions with SCX fractionation with and without exclusion lists. Numbers implicate the total number of proteins that are exclusive to or shared in the overlapping regions.
Multiple Digestions and SCX
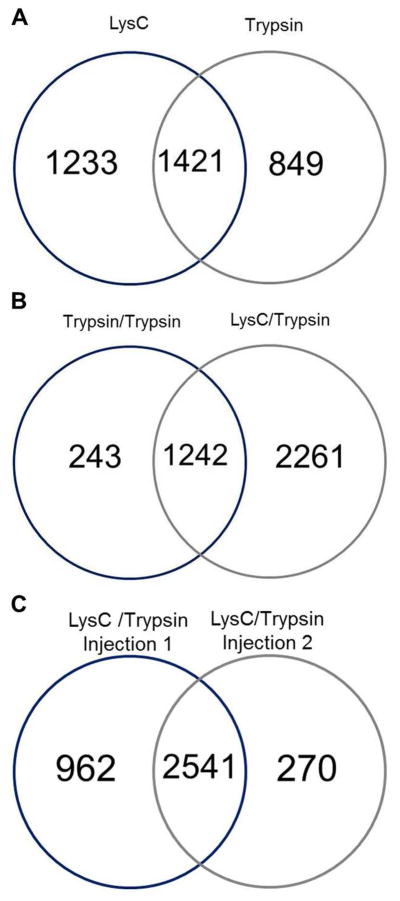
Multi-digestion of whole lysate was used to provide efficient digestion and more protein IDs. SCX was performed as above with the exception that protein samples were digested using a two-step digestion procedure and collected in a smaller time window. In the first multiple digestion strategy, protein extract was first subjected to tryptic digestion followed by a second digestion with trypsin, which were pooled, fractionated on a SCX column into 18 fractions and analyzed by nano LC-MS/MS. A total of 1485 proteins were identified in this experiment. A second strategy employed using LysC in the first digestion followed by a second digestion with trypsin. The LysC and tryptic digestions were fractionated separately on a SCX column and the 18 LysC fractions and 18 tryptic fractions were analyzed by nano LC-MS/MS. The combination of LysC and trypsin digestions produced a total of 3503 proteins, which resulted in the largest number of protein IDs among all the experimental strategies. Analyses of proteins identified revealed that 41% were common to both the LysC and tryptic digestions (Figure 3A). In contrast, a comparison of trypsin/trypsin and LysC/trypsin digestions revealed that 33% of proteins were common to both (Figure 3B). Further comparisons of the trypsin/trypsin and LysC/trypsin strategies using GO annotations for molecular function revealed that LysC/trypsin increased the identification of proteins categorized as ion channel activity and transporter activity by 89% and 113%, respectively. Sequence coverage of the proteins also increased when using the two-step digestion procedure with LysC/trypsin. For example, 42.4% sequence coverage was obtained for voltage-dependent anion-selective channel protein 2 (VDAC-2) in the LysC/trypsin digestion, whereas 19.3% was obtained using the trypsin/trypsin digestion (Supporting Information Table 1).
Figure 3.
Venn diagrams of proteins found in the proteome of P30 mouse cochlear sensory epithelium, when using multiple enzyme digestions. Number of proteins identified when performing a (A) first digestion with LysC and a second digestion with trypsin, (B) first digestion with trypsin or LysC followed by a second digestion with trypsin, and (C) LysC and trypsin digestion with multiple injections of each fraction.
Given the success of the experiment using multi-enzyme digestion with LysC/trypsin followed with SCX fractionation, two replications of nano LC-MS/MS analysis were done. Duplicate analysis of the same sample increased the number of proteins identified. There were 270 newly identified proteins in the second LysC/trypsin injection (Figure 3C), bringing the total number of proteins identified from the duplicate analysis to 3773. Moreover, there were 2541 proteins identified that were shared between the two experiments. GO annotations of protein cellular components identified from SCX preparations of LysC/trypsin digests showed an increase in plasmalemmal and mitochondrial proteins among newly identified proteins in the second LysC/trypsin injection (Supporting Information Figure 1).
WAX
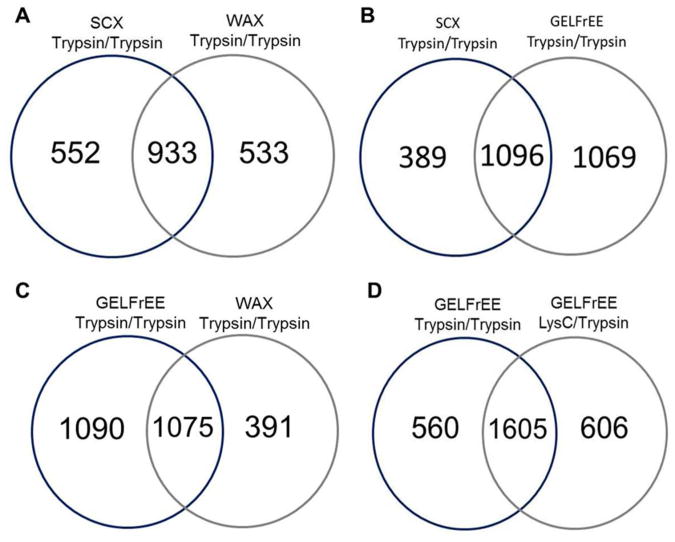
To obtain even greater depth in characterizing the sensory epithelial proteome, a third approach, WAX, was used for peptide fractionation prior to LC-MS/MS. A single experiment using a nano LC-MS/MS analysis of 18 WAX fractions from a trypsin/trypsin digest revealed 1466 protein IDs. A similar experimental approach using trypsin/trypsin digest prior to SCX fractionation identified 1485 proteins (Figure 4A). WAX fractionation generated protein IDs that were 48% similar to SCX fractionation. Analyses using GO biological process annotations showed that proteins involved in biological regulation and metabolism were highly expressed in both the SCX- and WAX-based fractionation. In contrast, an increase in annotations to plasmalemma (17%) was observed for proteins from the WAX-based fractionation compared to a similar SCX-based fractionation experiment (Supporting Information Figure 2).
Figure 4.
Venn diagrams of proteins found in the proteome of P30 mouse cochlear sensory epithelium, when using multiple digestions with different separation techniques. Number of proteins identified when using: (A) trypsin for the first and second digestion followed by either SCX- or WAX-based separation, (B) trypsin as before followed by SCX or GELFrEE separation followed by double trypsin digestion, (C) trypsin as before followed by WAX or GELFrEE followed by double trypsin digestion, or (D) GELFrEE followed by multiple-enzyme digestions using trypsin or LysC in the first digest followed by trypsin in the second digest.
GELFrEE
GELFrEE separation was used to fractionate proteins in the whole lysate based on size, in order to reduce sample complexity prior to digestion. The sample fractionation also helps isolate high abundant proteins, such as cochlin, which interferes with identification of other low abundant proteins. This technique offers separation reproducibility, sample enrichment, high protein recovery, and reduces the distribution of high abundant proteins in a complex protein sample.28 Unlike traditional 2D-PAGE, where proteins are extracted from gel spots, in GELFrEE the protein mixture is fractionated in liquid-phase, which allows for more efficient digestion and higher sample recovery. A silver-stained gel was prepared to visualize the results from GELFrEE fractionation prior to multi-enzyme digestion and LC-MS/MS analysis (Supporting Information Figure 3). A single GELFrEE experiment consisting of 12 fractions followed by LC-MS/MS analysis allowed 2165 protein IDs. SCX- and WAX-based experiments, using a similar digestion approach (trypsin/trypsin digestion), were compared to the GELFrEE experiment. The GELFrEE experiment identified 1069 unique proteins compared to the SCX experiment and 1090 unique proteins compared to the WAX experiment (Figure 4B and 4C). Analyses of proteins using GO annotations of cellular components in the GELFrEE approach showed an increase of proteins in most of the cell components including the mitochondrion, plasmalemma, and cytoskeleton (Supporting Information Figure 4A). GO annotation for molecular function shows binding and catalytic proteins as the most highly represented (Supporting Information Figure 4B).
As a result of the higher number of identified proteins in the SCX experiment using LysC/trypsin, we used a similar multi-digestion approach on the GELFrEE fractions. The LysC/trypsin digestion method applied to GELFrEE fractions resulted in a total of 2211 identified proteins. We also compared the proteins obtained by the LysC/trypsin digestion method to the trypsin/trypsin digestion method of the GELFrEE fractions. The results revealed a total of 1605 proteins that were common to both methods and 606 newly identified proteins that were not previously identified using the trypsin/trypsin method (Figure 4D). Finally, we compared proteins that were common to the three procedures that yielded the greatest number of protein IDs, which included: (1) SCX with LysC/trypsin digestion (2) GELFrEE using trypsin/trypsin digestion, and (3) GELFrEE with LysC/trypsin digestion. There were 1361 proteins common to all three methods, including 9 ion channel α-subunits and several proteins related to inner ear development and function, such as alpha-1 type II collagen, Na(+)/H(+) exchange regulatory cofactor NHE-RF2, and Ras-related C3 botulinum toxin substrate 1 (Supporting Information Table 2). The high number of complementary proteins indicates high reproducibility between these experiments.
GO Analyses of Protein Representations
All proteins identified from the different experimental techniques were combined for a total of 4620 proteins (Supporting Information Table 3), many of which are new to the cochlea (Table 1). GO profiles were obtained for cellular components, molecular functions and biological processes of proteins identified from all experimental techniques (Figures 5 and 6). We observed a distribution of both soluble and membrane proteins. When compared to previous proteomic studies of the inner ear using two-dimensional gel electrophoresis and shotgun proteomic approaches with single enzyme digestion, fewer membrane proteins and ion channel subunits were identified.
Table 1.
A sample of proteins new to the cochlea.
| accession | protein name | protein function |
|---|---|---|
| Q9EPC1 | Actopaxin | actin binding protein |
| Q80WT5 | Aftiphilin | found at synapses, interacts with synaptophysin |
| O08915 | Aip | delays the photoresponse |
| Q6PAM1 | α-taxilin | binds syntaxin |
| Q7TQF7 | Amphiphysin | BAR domain protein, maintain membrane curvature |
| Q6DFX2 | Antxr-2 | capillary morphogenesis protein |
| Q922M7 | Ashwin | implicated in neural patterning |
| P28658 | Ataxin10 | ubiquitous, regulates neural growth |
| Q91YH5 | Atlastin-3 | GTPase involved in ER and Golgi morphogenesis |
| Q4V9Z5 | Bsrp-like | involved in neuronal development |
| Q6XLQ8 | Calumenin | Ca2+-binding protein in ER |
| Q8VHK1 | Caskin | interacts with CASK, which is found in stereocilia |
| Q9Z140 | Copine-6 | intracellular Ca2+ can cause translocation to membrane |
| Q9QXS6 | Drebrin-1 | actin binding protein involved in cell shape |
| Q99JF8 | LEDGF | elevates with stress and regulates heat shock proteins |
| Q8CIV2 | Membralin | found in CNS and tumor cells, function unknown |
| Q8BM06 | Nell-2 | regulates Ca2+-signaling by regulating Ca2+-binding proteins |
| D3Z4E2 | Neuritin | promotes neurite outgrowth, stability and channel expression |
| Q810U3 | Neurofascin | regulates neurite outgrowth and postsynaptic elements |
| Q9CQE1 | NipSnap-related | regulates Trpv6 and Cav channel proteins |
| Q8BK62 | Olfml-3 | glycoprotein, contributes to olfactory and neural development |
| Q8K3K8 | Optineurin | cell trafficking |
| Q9Z0P4 | Paralemmin | controls cell shape, binds to lipid portion of plasmalemma |
| E9Q616 | Protein Ahnak | propeller protein that modulates Cav channels |
| Q6V4S5 | Protein sidekick-2 | regulates synaptic processes, associates with MAGI-1 |
| Q99JR1 | Sideroflexin-1 | tricarboxylate carrier protein with unknown function |
| Q3V2H3 | Sorting nexin-12 | protein trafficking |
| P61807 | Stannin | cell cycle control |
| Q80U23 | Syntaphilin | controls SNARE assembly |
| Q9D1D4 | Tmp-21 | regulates γ-secretase |
| E9QKC6 | Trim-2 | regulates axon polarization |
Figure 5.
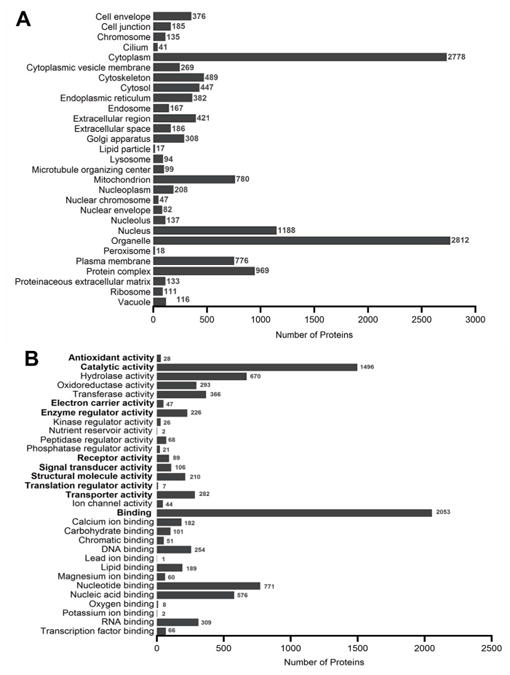
GO profiles for all the proteins identified from SCX, WAX, and GELFrEE sample preparations obtained from normal hearing mouse sensory epithelia. GO profile for (A) cellular components and (B) molecular function. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function. See Supporting Information Table 2 for the proteins.
Figure 6.
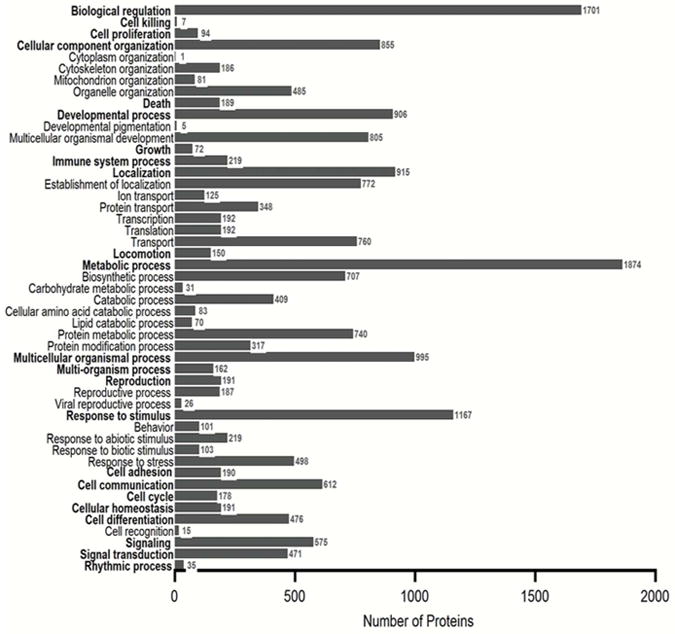
GO biological process profile for all the proteins identified from SCX, WAX, and GELFrEE sample preparations obtained from normal hearing mouse sensory epithelia. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function. See Supporting Information Table 2 for the proteins.
Classifications of proteins identified from the cochlear sensory epithelium were performed using GO analysis. GO annotations were assigned using the UniProt gene ontology program and proteins were classified based on their cellular components, molecular functions, and biological processes. Analyses using the GO annotations for cellular component show that the most highly represented proteins come from organelle (2812) and cytoplasm (2778) classifications and include the nucleus (1188), mitochondrion (780), and plasmalemma (776). The most underrepresented proteins come from the nuclear chromosome (47), cilium (41), peroxisome (18), and lipid particle (17). Analyses using GO annotations for molecular function find both catalytic activity (1496) and binding proteins (2053) highly represented in our data. Among the most highly represented binding proteins are those involved in nucleotide (771), nucleic acid (576), RNA (309), DNA (254), and lipid (189) binding. The most underrepresented include Mg2+ (60), O2 (8), K+, (2), and Pb2+ (1) binding proteins. Highly represented proteins with catalytic activity include hydrolase (670), transferase (366), and oxidoreductase (293). The lowliest represented activities include chromatic binding (51), electron carrier (47), ion channel (44), antioxidant (28), kinase regulator (26), phosphatase regulator (21), and nutrient reservoir (2) activity. Analyses using GO annotations for biological processes revealed proteins involved with biological (1701) and metabolic (1874) processes, followed by multicellular organismal (995) and localization processes (915). Processes that are lowly expressed include cell recognition (15), cell killing (7), developmental pigmentation (5), and cytoplasm organization (1).
Discussion
The combinations of preparative methods for high resolution MS, presented here, resulted in the largest number of proteins identified to date from normal hearing P30 mouse sensory epithelium. While double digestions of LysC and trypsin prior to SCX led to the highest number of protein IDs, each method was valuable in generating an extensive proteome. Proteins related to the function of the inner ear were identified, in addition to previously unidentified proteins.
Multiple Enzyme Digestion Combined with Fractionation
A previous study of the inner ear identified 628 total proteins using single trypsin digestion followed by LC-MS/MS.22 Other studies of the cochlear proteome resulted in far fewer proteins,15, 20-22 including membrane proteins such as ion channel subunits. We initially adopted a standard MudPIT approach using single tryptic digestion; however, we found that using multiple enzymes for digestion, such as LysC and trypsin, increased protein IDs more than 2fold compared to single tryptic digestion experiments. The advantage of multiple enzymes is that different populations of peptides are produced, hence increasing peptide number and protein IDs. Comparisons of the single tryptic digestion method with the trypsin/trypsin multi-digestion method also showed an increase in identified peptides and proteins (Supporting Information Table 1). Although both produced a large number of complementary proteins, there were 498 newly identified proteins using the multi-digestion approach. The higher number of proteins identified with this technique is likely due to differences in the affinity of tryptic cleaving at K/R sites in the peptide sequences produced from the first tryptic digestion compared to peptides produced from the second tryptic digestion.23 Therefore, different peptides are generated, leading to a greater number of protein IDs.
We compared the multi-enzyme digestion approach, LysC/trypsin, of the SCX fractions to the GELFrEE fractions digested with LysC/trypsin. We observed that the SCX-based approach resulted in a higher number of protein IDs as compared to the GELFrEE approach. We believe that the lower number of proteins identified using GELFrEE is due to protein loss resulting from the poor solubility of hydrophobic proteins. In contrast, peptides are more soluble in the solvent used in the SCX-based approach. The loss of hydrophobic proteins in the GELFrEE approach was also evident by the greater number of membrane proteins identified in the SCX-based approach.
Binding and Catalytic Proteins
An analysis of the sensory epithelium proteome revealed an abundance of binding and catalytic proteins. Binding proteins foster the interaction of one molecule with another and include Ca2+ binding proteins, which play a role in modulating or mediating the actions of these ions.29 Calcium ions are important in the physiology of sensory cells, including the transduction of agonist stimuli, intracellular signal transmission, and the modification of synapses.30 Sensory cells of the inner ear require Ca2+ for the transduction of mechanical stimuli into electrical signals via hair cells and for electrical oscillations.31 An example of an identified Ca2+ binding protein is oncomodulin, which is expressed in the OHCs.31 Its function in the inner ear is not completely understood, but it may play a role in Ca2+-sensitive hair cell bundle processes and OHC electromotility.32 In addition, a number of Ca2+ binding proteins were identified that have not been described previously. These are discussed below.
Annotation analysis for molecular function shows the cochlear sensory epithelium to be rich in proteins involved in catalytic activity. Some of these proteins include superoxide dismutase [Cu-Zn], glutathione peroxidase 1, transmembrane protease serine 3, and beta-hexosaminidase subunit beta. Catalytic proteins are essential for continuous protein turnover, a necessary component for the maintenance of cellular homeostasis and for the regulation of multiple cellular functions.33 For example, actin filaments of the stereocilia are renewed every 48 hours.34
Ion channel proteins
Twenty-six different α-subunits were identified, belonging to Na+, Ca2+, and K+ ion channels. Ion channels and transporters play a major role in signal transmission and if damaged can lead to hearing dysfunction.35 Examples include inward rectifier K+ channels Kir4.1 and Kir7.1, and Clic channels 1, 4, 5, and 6. An inward rectifier, IK1 was described first by Marcotti et al., in the inner hair cells of the mouse.36 Our data suggest more than one family of inward rectifiers, Kir 4.1 and 7.1. Additional studies may reveal their expression in different tonotopic regions of the cochlea. Clic5 plays a vital role in stereocilia formation and is necessary for normal organ of Corti development,37 whereas Clic1, 4 and 6 have not been described in the cochlear sensory epithelium. Another identified channel is the Ca2+-activated K+ channel subunit α-1, which is found in OHCs and IHCs.38 BK plays a key role in hair cell tuning in the non-mammalian cochlea and increased hearing sensitivity in mammalian cochlea.39 BK was identified after re-injection of SCX LysC/trypsin fractions, demonstrating that sample re-injection leads to an increase in protein ID. Although there was an overlap in protein IDs when performing reinjections, additional peptides and proteins were identified due to small changes in the chromatography. These changes lead to different peptides for fragmentation and different mass spectra at specified retention times. Finally, a previously undiscovered channel in the cochlea, but recently described in Drosophila, is the Ca2+ channel flower homolog. Drosophila experiments suggest this protein contains three or four transmembrane regions that promote Ca2+ influx, which triggers clatharin-mediated endocytosis at periactive sites of the presynaptic membrane.40
Newly Identified Proteins in Cochlear Sensory Epithelium
Several proteins were identified that were found first in other sensory systems. These include Olfml, Optineurin, and LEDGF/p75). Olfml and similar proteins contain an OLF domain, such as optimedin, myocilin, noelins, latrophilins, are glycoproteins.41 The functions of these proteins are varied and include contributing to olfactory cilia and neural development, as well as a role in glaucoma. However, there are no data describing this protein in the inner ear. Optineurin is a cytolsolic protein that mediates cell trafficking, cell division, and protein secretion.42 LEDGF/p75 was derived from ocular tissues and is elevated with cellular stress, whereupon it binds to promoters that regulate stress genes such as heat shock proteins.43
A number of Ca2+ binding/regulating proteins were identified that have not been described previously in the sensory epithelium. These include calumenin, caskin, Nell2, copine-6, and Nipsnap. Calumenin, an EF-hand Ca2+ binding protein, is found in the endoplasmic reticulum. It is part of a secretory pathway found in the cytosol as well as extracellular space.44 Caskin interacts with Ca2+-calmodulin serine kinase CASK, which is found in stereocilia.45 Caskin functions in regulating scaffolding such as forming the cytomatrix in developing neurons. Nell2 may regulate Ca2+ signaling by regulating Ca2+-binding proteins.46 In addition, it may regulate vesicles at presynaptic release sites. Copine-6 is regulated via intracellular Ca2+ concentrations that can cause its translocation to the membrane. However, its function is poorly understood.47 Nipsnap decreases Trpv6 current while increasing L-type Ca2+ current. This increase leads to phosphorylation of the transcription factor CREB.48
We also identified membrane-associated proteins not previously described in the mouse sensory epithelium, such as sideroflexin-3, amphiphysin 2, and paralemmin-1. These proteins are prominent in development and activity and thus may contribute to normal hearing. Amphiphysin is a member of the BAR domain proteins that generate and maintain membrane curvature.49 This protein can also regulate vesicles as might occur in endocytosis at synapses. Sideroflexins are tricarboxylate carrier proteins in the mitochondrial membrane whose function in vivo is still unclear.50 Paralemmin-1 binds to the lipid portion of the plasma membrane. It has some role in controlling cell shape with a potential for regulating cAMP activity.51 However, very little is known of this protein. Palmdelphin, another member of the paralemmin family, is a cytosolic protein of which there are few data.52
We also identified some proteins that were recently described in the inner ear. We found EMILINS 1, 2 (basilin), 3, and 5, which are predominantly in the extracellular matrix. Basilin was described previously in the basilar membrane of the cochlea.53 EMILIN1, on the other hand, was described recently as an interacting protein with the CNGA3 ion channel in saccular hair cells.54 These authors describe EMILIN1 as a transmembrane protein with a predicted intracellular C-terminus. Their colocalization studies also suggest interactions in the OHCs of rat. In contrast, EMILINS 3 and 5 still await a functional description in the cochlea. Finally, we identified a high mobility group AT-hook 2 protein using four different experimental strategies, including GELFrEE with two different digestion procedures (trypsin/trypsin and LysC/trypsin), a single trypsin digestion followed by SCX with exclusion list, and a trypsin/trypsin digestion followed by SCX. Combining all four experiments produced a total of four unique peptides with 50% sequence coverage. A recent study revealed the expression of this gene, Hmga2, in the transcriptome of the mouse cochlear sensory epithelium.55 The protein has many functions including the transcription of genes by altering DNA confirmation or by regulating transcription factors. Thus, these proteins can control cell differentiation, growth, proliferation, and apoptosis.56
Conclusion
Only a few studies have addressed the proteome of the inner ear. In part, this is due to the small and diverse number of cells, and the challenge of isolating membrane proteins. We have identified 4620 proteins from the mouse cochlear sensory epithelium using FASP combined with GELFrEE and off-line SCX- and WAX-based methods. In addition, peptide and protein IDs, and sequence coverage were increased, by combining multiple enzymes for digestion with sample reinjection and exclusion lists. GO analysis of molecular function showed that among the most highly expressed proteins are those involved in binding and catalytic activity. In addition, many proteins were identified that are currently uncharacterized with respect to their significance and function in the cochlea. Thus, the multiple shotgun and bottom-up proteomic techniques used here provide the most comprehensive cochlear proteome to date. These findings will enhance establishing biomarkers for the prevention and treatment of hearing impairments.
Supplementary Material
Figure S1. GO cellular component profile using protein IDs generated from the second injection of samples obtained using LysC/trypsin digestion and SCX separation. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Figure S2. GO cellular components profile of protein IDs generated using SCX or WAX separation. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Figure S3. Silver stained gel of sensory epithelium GELFrEE fractions, to visualize protein separation in each fraction prior to MS analysis. (M) Protein marker, (1) Fraction 1, (3) Fraction 2, (5) Fraction 5, (7) Fraction 7, (8) Fraction 8, (9) Fraction 9, (10) Fraction 10, (11) Fraction 11, (12) Fraction 12.
Figure S4. GO profile of sensory epithelium proteome for (A) cellular components and (B) molecular function. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Table S1. Single run of lysate from membrane fractionation using SCX-based separation. SCX-based fractionation of proteins identified from tryptic peptides with and without exclusion lists. SCX-based fractionation of proteins identified from LysC/trypsin digestion with multiple injections. SCX-based fractionation of proteins identified from trypsin/trypsin digestion. Proteins identified from GELFrEE separation of mouse sensory epithelium lysate. WAX-based fractionation of proteins identified from trypsin/trypsin digestion.
Table S2. A list of proteins common to the three experimental techniques that generated the largest number of protein IDs. These techniques were: (1) SCX with LysC/trypsin digestion (2) GELFrEE using trypsin/trypsin digestion, and (3) GELFrEE with LysC/trypsin digestion.
Table S3. A complete list of proteins identifed by combining all proteins from the different experiments.
Acknowledgments
The authors thank Dr. Jeremiah Tipton, Director of the Center for Drug Discovery and Innovation (CDDI) Proteomics Core Facility at University of South Florida for his expertise and for the use of the mass spectrometers in this facility. We also thank Margaret Harvey for the cochleae dissections. This work was supported by NIH/NIDCD grant R01 DC004295 to B.H.A.S. The data deposition to the ProteomeXchange Consortium was supported by PRIDE Team, EBI.
Abbreviations
- ABC
ammonium bicarbonate
- AEBSF
4- benzenesulfonyl fluoride hydrochloride
- AEX
anion exchange chromatography
- ASB-14
amidosulfobetaine-14
- Aip
aryl receptor hydrocarbon
- Antxr
anthrax toxin receptor
- BAR
Bin/Amphiphysin/Rvs
- Bsrp
brain specific receptor protein
- cAMP
cyclic adenosine monophosphate
- CASK
Ca2+/CaM-dependent serine protein kinase
- Clic
chloride intracellular channels
- CNGA3
cyclic nucleotide-gated A3
- CREB
cAMP response element binding protein
- DIGE
2D differential gel electrophoresis
- Drebrin
developmentally-regulated brain protein
- DTT
dithiothreitol
- EMILIN
elastin microfibril interface-located protein
- FA
formic acid
- FASP
filter aided sample preparation
- FDR
false discovery rate
- GELFrEE
gel-eluted liquid fraction entrapment electrophoresis
- GO
Gene Ontology
- IAA
iodoacetamide
- ID
identification
- IHC
inner hair cell
- LEDGF
lens epithelium-derived growth factor
- LysC
endoproteinase Lys-C
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MudPIT
multidimensional protein identification technology
- nano LC-MS/MS
nano liquid chromatography-tandem mass spectrometry
- Nell2
neural epidermal growth factor-like like 2
- OHC
outer hair cell
- Olfml
olfactomedin-like
- O/N
overnight
- PBS
phosphate buffered saline
- RP
reversed-phase
- SAX
strong anion exchange
- SCX
strong cation exchange
- SDS
sodium dodecyl sulfate
- SEC
size exclusion
- Tmp21
transmembrane protein 21
- Trim2
tripartite motif containing 2
- Trpv6
transient receptor potential vanilloid 6
- WAX
weak anion exchange
References
- 1.Lang F, Vallon V, Knipper M, Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol - Cell Physiol. 2007;293:C1187–C1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- 2.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 3. [accessed Oct 10, 2012];National Institute on Deafness and Other Communication Disorders. http://www.nidcd.nih.gov/Pages/default.aspx.
- 4.Yarmush ML, Jayaraman A. Advances in proteomic technologies. Annu Rev Biomed Eng. 2002;4:349–73. doi: 10.1146/annurev.bioeng.4.020702.153443. [DOI] [PubMed] [Google Scholar]
- 5.Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–7. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 6.Thalmann I. Inner ear proteomics: A fad or hear to stay. Brain Res. 2006;1091:103–112. doi: 10.1016/j.brainres.2006.01.099. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Meth. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 8.Wisniewski JR, Zielinska DF, Mann M. Comparison of ultrafiltration units for proteomic and N-glycoproteomic analysis by the filter-aided sample preparation method. Anal Biochem. 2011;410:307–9. doi: 10.1016/j.ab.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Le Bihan T, Goh T, Stewart II, Salter AM, Bukhman YV, Dharsee M, Ewing R, Wiśniewski JR. Differential Analysis of Membrane Proteins in Mouse Fore- and Hindbrain Using a Label-Free Approach. J Proteome Res. 2006;5:2701–2710. doi: 10.1021/pr060190y. [DOI] [PubMed] [Google Scholar]
- 10.Huber LA, Pfaller K, Vietor I. Organelle Proteomics. Circ Res. 2003;92:962–968. doi: 10.1161/01.RES.0000071748.48338.25. [DOI] [PubMed] [Google Scholar]
- 11.Aebersold R, Goodlett DR. Mass Spectrometry in Proteomics. Chem Rev. 2001;101:269–296. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- 12.Horvatovich P, Hoekman B, Govorukhina N, Bischoff R. Multidimensional chromatography coupled to mass spectrometry in analysing complex proteomics samples. J Sep Sci. 2010;33:1421–1437. doi: 10.1002/jssc.201000050. [DOI] [PubMed] [Google Scholar]
- 13.Ye M, Jiang X, Feng S, Tian R, Zou H. Advances in chromatographic techniques and methods in shotgun proteome analysis. TrAC, Trends Anal Chem. 2007;26:80–84. [Google Scholar]
- 14.Zhang X, Fang A, Riley CP, Wang M, Regnier FE, Buck C. Multi-dimensional liquid chromatography in proteomics--a review. Anal Chim Acta. 2010;664:101–13. doi: 10.1016/j.aca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamesdaniel S, Hu B, Kermany MH, Jiang H, Ding D, Coling D, Salvi R. Noise induced changes in the expression of p38/MAPK signaling proteins in the sensory epithelium of the inner ear. J Proteomics. 2011;75:410–424. doi: 10.1016/j.jprot.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thalmann I, Hughes I, Tong BD, Ornitz DM, Thalmann R. Microscale analysis of proteins in inner ear tissues and fluids with emphasis on endolymphatic sac, otoconia, and organ of Corti. Electrophoresis. 2006;27:1598–1608. doi: 10.1002/elps.200500768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thalmann I, Rosenthal HL, Moore BW, Thalmann R. Organ of Corti-specific polypeptides: OCP-I and OCP-II. J Acoust Soc Am. 1980;67:S77. doi: 10.1007/BF00455126. [DOI] [PubMed] [Google Scholar]
- 18.Kathiresan T, Harvey M, Sokolowski B. The use of two-dimensional gels to identify novel protein-protein interactions in the cochlea. In: Sokolowski B, editor. Auditory and vestibular research: methods and protocols. Humana; Springer; New York, N.Y: 2009. [DOI] [PubMed] [Google Scholar]
- 19.Zheng QY, Rozanas CR, Thalmann I, Chance MR, Alagramam KN. Inner ear proteomics of mouse models for deafness, a discovery strategy. Brain Res. 2006;1091:113–121. doi: 10.1016/j.brainres.2006.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkan-Miller T, Ulitsky I, Hertzano R, Rudnicki A, Dror AA, Lenz DR, Elkon R, Irmler M, Beckers J, Shamir R, Avraham KB. Integration of Transcriptomics, Proteomics, and MicroRNA Analyses Reveals Novel MicroRNA Regulation of Targets in the Mammalian Inner Ear. Plos One. 2011;6:e18195. doi: 10.1371/journal.pone.0018195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K+-ATPase α1Identified as an Abundant Protein in the Blood-Labyrinth Barrier That Plays an Essential Role in the Barrier Integrity. Plos One. 2011;6:e16547. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CEEM, Wallimann T, Gillespie PG. Hair Bundles Are Specialized for ATP Delivery via Creatine Kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H, Liu M, Pecka J, Beisel KW, Ding SJ. Proteomic Analysis of the Organ of Corti Using Nanoscale Liquid Chromatography Coupled with Tandem Mass Spectrometry. Int J Mol Sci. 2012;13:8171–8188. doi: 10.3390/ijms13078171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiśniewski JR, Mann M. Consecutive Proteolytic Digestion in an Enzyme Reactor Increases Depth of Proteomic and Phosphoproteomic Analysis. Anal Chem. 2012;84:2631–2637. doi: 10.1021/ac300006b. [DOI] [PubMed] [Google Scholar]
- 25.Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O'Kelly G, Schoenegger A, Ovelleiro D, Perez-Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H. The Proteomics Identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013;41:D1063–9. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consortium TU. Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2012;40:D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camon E, Magrane M, Barrell D, Lee V, Dimmer E, Maslen J, Binns D, Harte N, Lopez R, Apweiler R. The Gene Ontology Annotation (GOA) Database: sharing knowledge in Uniprot with Gene Ontology. Nucleic Acids Res. 2004;32:D262–6. doi: 10.1093/nar/gkh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bora A, Anderson C, Bachani M, Nath A, Cotter RJ. Robust Two-Dimensional Separation of Intact Proteins for Bottom-Up Tandem Mass Spectrometry of the Human CSF Proteome. J Proteome Res. 2012;11:3143–3149. doi: 10.1021/pr300057v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JH. Two calcium-binding proteins mark many chick sensory neurons. Neuroscience. 1989;31:697–709. doi: 10.1016/0306-4522(89)90434-x. [DOI] [PubMed] [Google Scholar]
- 31.Kerschbaum HH, Hermann A. Calcium-binding proteins in the inner ear of Xenopus laevis (Daudin) Brain Res. 1993;617:43–49. doi: 10.1016/0006-8993(93)90610-y. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol. 2010;518:3785–3802. doi: 10.1002/cne.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Structural cell biology: Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- 35.Gabashvili I, Sokolowski B, Morton C, Giersch A. Ion Channel Gene Expression in the Inner Ear. J Assoc Res Oto. 2007;8:305–328. doi: 10.1007/s10162-007-0082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548:383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, Gillespie PG, Johnson KR. The Chloride Intracellular Channel Protein CLIC5 Is Expressed at High Levels in Hair Cell Stereocilia and Is Essential for Normal Inner Ear Function. J Neurosci. 2006;26:10188–10198. doi: 10.1523/JNEUROSCI.2166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai Y, Harvey M, Sokolowski B. Identification and Quantification of Full-Length BK Channel Variants in the Developing Mouse Cochlea. J Neurosci Res. 2011;89:1747–1760. doi: 10.1002/jnr.22713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pyott SJ, Meredith AL, Fodor AA, Vázquez AE, Yamoah EN, Aldrich RW. Cochlear Function in Mice Lacking the BK Channel α, β1, or β4 Subunits. J Biol Chem. 2007;282:3312–3324. doi: 10.1074/jbc.M608726200. [DOI] [PubMed] [Google Scholar]
- 40.Yao CK, Lin YQ, Ly CV, Ohyama T, Haueter CM, Moiseenkova-Bell VY, Wensel TG, Bellen HJ. A synaptic vesicle-associated Ca2+ channel promotes endocytosis and couples exocytosis to endocytosis. Cell. 2009;138:947–60. doi: 10.1016/j.cell.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng LC, Han ZG, Ma WJ. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive phylogenetic analysis and gene expression pattern assessment. FEBS Lett. 2005;579:5443–53. doi: 10.1016/j.febslet.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 42.Kachaner D, Genin P, Laplantine E, Weil R. Toward an integrative view of Optineurin functions. Cell Cycle. 2012;11:2808–18. doi: 10.4161/cc.20946. [DOI] [PubMed] [Google Scholar]
- 43.Shinohara T, Singh DP, Fatma N. LEDGF, a survival factor, activates stress-related genes. Prog Retin Eye Res. 2002;21:341–58. doi: 10.1016/s1350-9462(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 44.Hansen G, Vorum H, Jacobsen C, Honoré B. Calumenin but not reticulocalbin forms a Ca2+-dependent complex with thrombospondin-1. A potential role in haemostasis and thrombosis. Mol Cell Biochem. 2009;320:25–33. doi: 10.1007/s11010-008-9895-1. [DOI] [PubMed] [Google Scholar]
- 45.Mburu P, Kikkawa Y, Townsend S, Romero R, Yonekawa H, Brown SD. Whirlin complexes with p55 at the stereocilia tip during hair cell development. Proc Natl Acad Sci U S A. 2006;103:10973–8. doi: 10.1073/pnas.0600923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H, Ha CM, Choi J, Choi EJ, Jeon J, Kim C, Park SK, Kang SS, Kim K, Lee BJ. Ontogeny and the possible function of a novel epidermal growth factor-like repeat domain-containing protein, NELL2, in the rat brain. J Neurochem. 2002;83:1389–1400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- 47.Perestenko PV, Pooler AM, Noorbakhshnia M, Gray A, Bauccio C, Jeffrey McIlhinney RA. Copines-1, -2, -3, -6 and -7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J. 2010;277:5174–89. doi: 10.1111/j.1742-4658.2010.07935.x. [DOI] [PubMed] [Google Scholar]
- 48.Brittain JM, Wang Y, Wilson SM, Khanna R. Regulation of CREB signaling through L-type Ca2+ channels by Nipsnap-2. Channels (Austin) 2012;6:94–102. doi: 10.4161/chan.19415. [DOI] [PubMed] [Google Scholar]
- 49.Mim C, Unger VM. Membrane curvature and its generation by BAR proteins. Trends Biochem Sci. 2012;37:526–33. doi: 10.1016/j.tibs.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Han D, Kin Ting Kam R, Guo X, Chen M, Yang Y, Zhao H, Chen Y. Developmental expression of sideroflexin family genes in Xenopus embryos. Dev Dynam. 2010;239:2742–2747. doi: 10.1002/dvdy.22401. [DOI] [PubMed] [Google Scholar]
- 51.Hultqvist G, Ocampo Daza D, Larhammar D, Kilimann MW. Evolution of the vertebrate paralemmin gene family: ancient origin of gene duplicates suggests distinct functions. Plos One. 2012;7:e41850. doi: 10.1371/journal.pone.0041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu B, Petrasch-Parwez E, Laue MM, Kilimann MW. Molecular characterization and immunohistochemical localization of palmdelphin, a cytosolic isoform of the paralemmin protein family implicated in membrane dynamics. Eur J Cell Biol. 2005;84:853–66. doi: 10.1016/j.ejcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Amma LL, Goodyear R, Faris JS, Jones I, Ng L, Richardson G, Forrest D. An emilin family extracellular matrix protein identified in the cochlear basilar membrane. Mol Cell Neurosci. 2003;23:460–472. doi: 10.1016/s1044-7431(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 54.Selvakumar D, Drescher MJ, Dowdall JR, Khan KM, Hatfield JS, Ramakrishnan NA, Drescher DG. CNGA3 is expressed in inner ear hair cells and binds to an intracellular C-terminus domain of EMILIN1. Biochem J. 2012;443:463–76. doi: 10.1042/BJ20111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smeti I, Assou S, Savary E, Masmoudi S, Zine A. Transcriptomic analysis of the developing and adult mouse cochlear sensory epithelia. Plos One. 2012;7:e42987. doi: 10.1371/journal.pone.0042987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cleynen I, Van de Ven WJ. The HMGA proteins: a myriad of functions (Review) Int J Oncol. 2008;32:289–305. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. GO cellular component profile using protein IDs generated from the second injection of samples obtained using LysC/trypsin digestion and SCX separation. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Figure S2. GO cellular components profile of protein IDs generated using SCX or WAX separation. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Figure S3. Silver stained gel of sensory epithelium GELFrEE fractions, to visualize protein separation in each fraction prior to MS analysis. (M) Protein marker, (1) Fraction 1, (3) Fraction 2, (5) Fraction 5, (7) Fraction 7, (8) Fraction 8, (9) Fraction 9, (10) Fraction 10, (11) Fraction 11, (12) Fraction 12.
Figure S4. GO profile of sensory epithelium proteome for (A) cellular components and (B) molecular function. All categories are counted non-exclusively, when a protein has more than one category for cellular components or molecular function.
Table S1. Single run of lysate from membrane fractionation using SCX-based separation. SCX-based fractionation of proteins identified from tryptic peptides with and without exclusion lists. SCX-based fractionation of proteins identified from LysC/trypsin digestion with multiple injections. SCX-based fractionation of proteins identified from trypsin/trypsin digestion. Proteins identified from GELFrEE separation of mouse sensory epithelium lysate. WAX-based fractionation of proteins identified from trypsin/trypsin digestion.
Table S2. A list of proteins common to the three experimental techniques that generated the largest number of protein IDs. These techniques were: (1) SCX with LysC/trypsin digestion (2) GELFrEE using trypsin/trypsin digestion, and (3) GELFrEE with LysC/trypsin digestion.
Table S3. A complete list of proteins identifed by combining all proteins from the different experiments.