Abstract
Background
The proteasome inhibitor bortezomib sensitizes tumor cells to chemotherapy-induced apoptosis. In preclinical non–small-cell lung cancer (NSCLC) models, p53-dependent growth arrest after bortezomib treatment resulted in reduced cytotoxicity if bortezomib preceded docetaxel. The reverse sequence of docetaxel before bortezomib was associated with increased apoptosis, cleavage of caspase-3 and PARP (poly [ADP-ribose] polymerase), and reduction in Bcl-2. A prospective randomized phase II trial of concurrent versus sequential docetaxel and bortezomib was conducted to assess whether administration sequence resulted in measurable clinical differences.
Patients and Methods
Previously treated patients with advanced NSCLC were randomized to concurrent (CON) or sequential (SEQ) docetaxel (75 mg/m2 intravenous [I.V.]) followed by bortezomib, every 3 weeks. In the CON arm, bortezomib (1.6 mg/m2 I.V.) was given on days 1 and 8, and in the SEQ arm, it was given on days 2 and 8. Previous erlotinib as well as treated or controlled brain metastases were allowed. The primary endpoint was objective response rate (RR); progression-free (PFS) and overall survival (OS) were secondary endpoints.
Results
Eighty-one patients were randomized (40 CON and 41 SEQ). Grade 3+ toxicities were mostly due to myelosuppression. One patient each had grade 4 hyponatremia and syncope. Toxicities were similar between the arms. There was 1 treatment-related death in the SEQ arm. There were 8 partial responders, 4 in each arm, for an overall RR of 10%. Disease control rate was similar in both arms (50% vs. 49%). Median PFS was 12 weeks in the CON arm and 11 weeks in the SEQ arm. Median OS times in the CON and SEQ arms were 13.3 and 10.5 months, respectively.
Conclusion
Docetaxel plus bortezomib given sequentially or concurrently has similar RR and PFS. Median survival in the SEQ arm exceeds published survival estimates for either agent alone or in combination. Any further studies in this population would require molecular characterization of a phenotype most likely to benefit from proteasome inhibitor therapy.
Keywords: Bortezomib, Docetaxel, Proteasome inhibition
Introduction
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer-related death in both males and females in the United States.1 Most patients initially present with advanced or metastatic disease and are often offered first-line palliative platinum-based chemotherapy. Unfortunately, all patients will inevitably develop either intolerable toxicity, chemotherapy resistance, or subsequent disease progression. In this second-line treatment setting, docetaxel has a clear and established role.2,3
Docetaxel stabilizes microtubules against depolymerization by binding to the β-subunit of tubulin, leading to the formation of abnormal microtubule bundles that inhibit cell proliferation and promote cell death. In a phase III prospective randomized trial of docetaxel versus best supportive care in NSCLC patients previously treated with platinum-based chemotherapy, therapy with docetaxel resulted in significant prolongation of survival (7.5 months vs. 4.6 months, P = .01).2 Another randomized phase III trial confirmed this finding, demonstrating that patients who received docetaxel 75 mg/m2 given every 3 weeks had higher RRs, longer time to progression, and better 1-year survival when compared with those treated with ifosfamide or vinorelbine.3 To this day, docetaxel remains a reasonable standard of care in the second-line setting of advanced NSCLC, with no other agents currently demonstrating superiority.
Bortezomib (Velcade®, PS-341) is a boronic acid dipeptide derivative that selectively and potently inhibits the 26S proteasome.4 It has clinically validated activity against plasma cell myeloma and has undergone extensive evaluation in NSCLC. Preliminary in vitro studies established that bortezomib alone induces growth inhibition in A549, H520, H460, H358, and H322 NSCLC tumor cell lines.5–9 When bortezomib is combined with cytotoxic agents in vitro, there is enhanced antitumor effect in NSCLC and other solid tumors.10–12 Our preclinical studies of bortezomib, both as a single agent and in combination with docetaxel, provided a mechanistic rationale for the varied responses seen with differently sequenced combinations of docetaxel and bortezomib.13–15 These studies showed that docetaxel induces accumulation and phosphorylation of the cyclin-dependent kinase p27 and phosphorylation of Bcl-2. The addition of bortezomib to docetaxel maintains p27 induction and decreases levels of Bcl-2, enhancing docetaxel cytotoxicity. The sequence of docetaxel followed by bortezomib also showed a comparative enhancement of apoptosis, as measured by sub-G1 accumulation, in lung cancer cell lines over other schedules.
Our group previously had conducted a phase I trial of docetaxel plus bortezomib in solid tumors, with emphasis on NSCLC, establishing the feasibility and tolerability of this combination.16 Subsequently, a randomized phase II trial of bortezomib with or without docetaxel was completed.17 In that trial, bortezomib plus docetaxel (as compared with bortezomib alone) yielded a higher disease control rate (54% vs. 29%), median time to progression (4 months vs. 1.5 months), and median duration of response (11.3 months vs. 3.8 months). Based on these data, we conducted the randomized phase II study reported here of sequential versus concurrent docetaxel and bortezomib in patients with previously treated advanced NSCLC.
Patients and Methods
Patients
Institutional review boards at each study center approved the trial, and all patients provided written informed consent. Patients with progressive or recurrent NSCLC following treatment with 1 previous platinum-based chemotherapy regimen for advanced or metastatic disease were eligible. Previous neoadjuvant or adjuvant chemotherapy and/or concurrent chemoradiation for early-stage disease as well as previous epidermal growth factor receptor (EGFR) inhibitor therapy also were allowed. Patients were required to have measurable or evaluable disease; Zubrod performance status (PS) of 0 or 1; life expectancy ≥ 3 months; and adequate hematologic, renal, and hepatic function. Patients with previously treated brain metastasis (surgical resection or radiotherapy) were eligible if they were asymptomatic, neurologically stable, and had been off steroids for at least 4 weeks. All enzyme-inducing anticonvulsant medications must have been stopped for at least 2 weeks before treatment and for the duration of the trial. Patients were excluded if they (1) had received docetaxel or bortezomib previously, (2) had grade 2 or higher peripheral neuropathy, (3) were pregnant and/or breastfeeding, or (4) had uncontrolled brain metastases or central nervous system disease.
Study Design and Participating Centers
This multicenter randomized phase II trial of docetaxel plus bortezomib was conducted through the National Cancer Institute (NCI)–sponsored California Cancer Consortium and the Princess Margaret Hospital Consortium. Participating centers were the University of California at Davis, the VA Northern California Health Care System, the City of Hope, the University of Southern California/Norris, the University of Pittsburgh Cancer Institute, and Princess Margaret Hospital. The Data Coordinating Center of the California Cancer Consortium, located at City of Hope, served as the central telephone registry for randomization.
Treatment cycles were 3 weeks in duration. Patients were randomized to either the concurrent arm (CON arm) or the sequential arm (SEQ arm). The CON arm patients received docetaxel 75 mg/ m2 I.V. over 60 minutes immediately followed by bortezomib 1.6 mg/m2 I.V. as a bolus injection over 3–5 seconds on day 1. The SEQ arm patients received docetaxel 75 mg/m2 I.V. on day 1 followed 24 hours later (day 2) by bortezomib 1.6 mg/m2 I.V. as a bolus injection over 3–5 seconds. Bortezomib 1.6 mg/m2 was given I.V. as a bolus injection on day 8 in both arms. Filgrastim support was recommended for prophylaxis of neutropenia and was allowed in cycle 1 at the investigator’s discretion.
Toxicity was assessed before each cycle and graded according to the NCI Common Terminology Criteria (NCI CTC, Version 3.0). Prespecified dose reductions for toxicity were as follows: dose level 1 (docetaxel 60 mg/m2 and bortezomib 1.3 mg/m2) and dose level 2 (docetaxel 55 mg/m2 and bortezomib 1 mg/m2). If day 8 bortezomib was withheld, treatment in the following cycle was at 1 lower dose level. All dose reductions were permanent. Doses that were missed for any reason were not administered at a later time. A maximum of 2 dose level reductions (to dose level 2) were allowed. Patients who had recurrent or persistent dose limiting toxicity despite reduction to dose level 2 were removed permanently from protocol therapy.
Disease response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines.18 Disease control rate was defined as the rate of partial response (PR) plus stable disease (SD; for at least 2 cycles).
Safety, performance status, and efficacy evaluations were performed every 2 cycles (6 weeks), at an end-of-therapy visit after administration of the last bortezomib dose, and during an end-of-study visit at least 3 weeks after the end-of-therapy visit. Criteria for removal from protocol included progressive disease, unacceptable toxicity (as determined by the treating physician and/or the patient), toxicity requiring discontinuation of treatment despite dosage modifications, or treatment delay longer than 3 weeks as a result of toxicity or intercurrent illness.
Statistical Considerations
The primary endpoint was objective or radiologic RR assessed by RECIST. We hypothesized that the sequence of docetaxel given 1 day before bortezomib would result in a higher RR (because of enhanced apoptosis) than the combination given concurrently on day 1. In this randomized phase II trial, 80 patients were planned to be randomized to the 2 schedules (40 patients each). The objective was to select the better regimen with 90% probability, where selection was based on empirical RRs, not necessarily exhibiting a statistically significant difference. This objective corresponded to a 1-sided test at the 0.5 level of significance, with 90% power. With 40 patients per arm, the correct decision would be made with approximately 90% probability if the RRs on the 2 arms were 0.05 versus 0.13, or 0.10 versus 0.20, or 0.15 versus 0.27. Secondary endpoints included overall survival (OS), progression-free survival (PFS), and toxicity.
Results
Patient Demographics
A total of 81 patients were enrolled in the study, 40 in the CON arm and 41 in the SEQ arm. Patient characteristics are summarized in Table 1. Overall, there was a slight predominance of males (53%), median age was 61 years, and 47% had PS of 0. Patients who had received previous erlotinib constituted 18% of patients, while those who had treated brain metastases accounted for only 4%.
Table 1.
Patient Characteristics
| Arm 1 (CON) n = 40 |
Arm 2 (SEQ) n = 41 |
Overall | |
|---|---|---|---|
| Male Sex | 23 (57%) | 20 (49%) | 43 (53%) |
| Zubrod PS = 0 | 17 (42%) | 21 (51%) | 38 (47%) |
| Median Age, Years | 61 | 62 | 61 |
| Previous Erlotinib | 6 (15%) | 9 (21%) | 15 (18%) |
| Brain Metastases | 1 (2.5%) | 2 (4.8%) | 3 (4%) |
| Race/Ethnicity | |||
| White | 32 (80%) | 33 (80%) | 65 (80%) |
| Asian | 3 (8%) | 6 (15%) | 9 (11%) |
| Black | 5 (12%) | 1 (2%) | 6 (7%) |
| Other | 0 | 1 (2%) | 1 (1%) |
| Hispanic ethnicity | 4 (10%) | 4 (10%) | 8 (10%) |
Abbreviations: CON = concurrent; PS = performance status; SEQ = sequential
Safety
There were no unexpected toxicities seen. A summary of grade 3 or higher toxicities by “toxicity term” as possibly, probably, or definitely attributed to treatment is provided in Table 2. There was 1 treatment-related death in a patient who developed grade 4 hematologic toxicities, grade 3 neuropathy, and grade 3 anorexia in cycle 1. There were no significant differences in rates of grade 1–4 hematologic toxicity between the CON and SEQ arms (P = .7, rank sum test, 2-sided). Using a single maximum attributable toxicity for each patient, there were no significant differences (P = .7).
Table 2.
Grade 3 or Higher Toxicitiesa
| Per NCI Common Terminology Criteria Version 3.0 |
Number of Patients | ||
|---|---|---|---|
| Toxicity | Grade | Arm 1 (CON) | Arm 2 (SEQ) |
| Blood/Bone Marrow | |||
| Anemia | 3 | 2 | 0 |
| Leukopenia | 4 | 1 | 2 |
| 3 | 5 | 9 | |
| Lymphopenia | 3 | 10 | 2 |
| Neutropenia | 4 | 7 | 12 |
| 3 | 1 | 2 | |
| Thrombocytopenia | 4 | 0 | 1 |
| 3 | 0 | 2 | |
| Gastrointestinal | |||
| Diarrhea | 3 | 0 | 1 |
| Oral mucositis | 3 | 0 | 1 |
| Nausea | 3 | 1 | 2 |
| Vomiting | 3 | 1 | 2 |
| Pulmonary/Respiratory | |||
| Pneumonia | 3 | 2 | 0 |
| Dyspnea | 3 | 1 | 1 |
| Metabolic/Laboratory | |||
| Creatinine (increased) | 3 | 0 | 1 |
| Hypophosphatemia | 3 | 0 | 1 |
| Hyponatremia | 4 | 0 | 1 |
| 3 | 0 | 1 | |
| Neurology | |||
| Gait abnormality | 3 | 1 | 0 |
| Peripheral sensory neuropathy | 3 | 0 | 1 |
| Syncope | 4 | 1 | 0 |
| General/Other | |||
| Fatigue | 3 | 1 | 3 |
| Anorexia | 3 | 0 | 1 |
| Dehydration | 3 | 0 | 1 |
| Death | 5 | 0 | 1 |
Possibly, probably, or definitely attributed to treatment.
Abbreviations: CON = concurrent; NCI = National Cancer Institute; SEQ = sequential
Efficacy
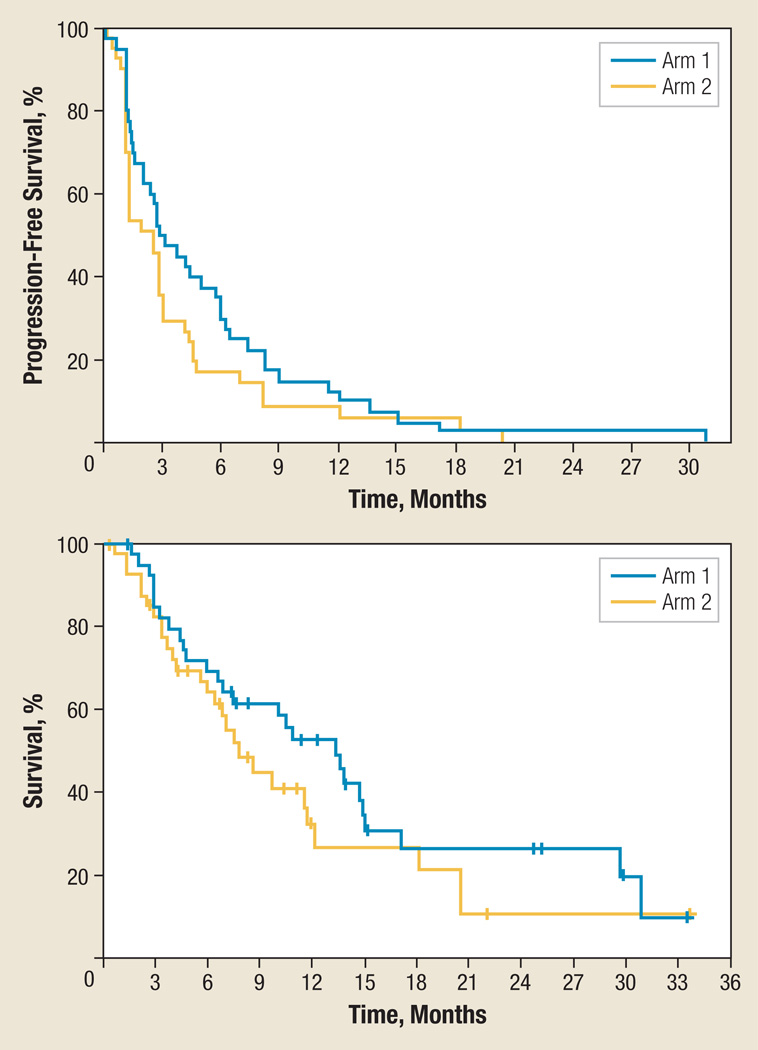
Table 3 summarizes efficacy outcomes. RECIST RR was similar in both arms. Overall, 8 patients had a PR for an overall RR of 10%, with 4 responders in each arm. There were no complete responders. Disease control rate (PR + SD) was also similar between the arms (50% CON arm and 49% SEQ arm). Time on treatment was longer in the CON (2.7 months) versus SEQ (1.4 months) arms. The median number of treatment cycles delivered was 2 in both arms. Progression-free survival (PFS) and OS Kaplan-Meier curves are provided in Figure 1, and data are summarized in Table 3. Median PFS time was 12 weeks in the CON arm and 11 weeks in the SEQ arm. With all the caveats of comparing arms in a randomized phase II trial, there was no statistically significant difference between the arms with regard to PFS (P = .17) nor with regard to OS (P = .23), but the empirical estimates of both favored the CON arm.
Table 3.
Efficacy Parameters
| Efficacy Measure | Arm 1 (CON) |
Arm 2 (SEQ) |
Overall |
|---|---|---|---|
| PR | 4 (10%) | 4 (10%) | 8 (10%) |
| Disease Control Rate (PR + SD) | 20 (50%) | 20 (49%) | 40 (49%) |
| PFS at 6 Months (%) | 30% | 17% | 25% |
| Median OS, Months (95% CI) | 13.3 (7,17) | 7.8 (6,18) | 10.5 (7,14) |
Abbreviations: CON = concurrent; OS = overall survival; PFS = progression-free survival; PR = partial response; SD = stable disease; SEQ = sequential
Figure 1.
Progression-Free and Overall Survival Kaplan-Meier Curvesa
aArm 1 = concurrent, arm 2 = sequential.
Discussion
Inhibition of the 26S proteasome is an established anticancer approach that has been of particular success in hematologic malignancies such as plasma cell myeloma.19 The 26S proteasome is a proteolytic complex involved in the ubiquitin-proteasome catabolic pathway that modulates a multitude of intracellular regulatory proteins, including IκB kinase/nuclear factor-κB (IκB/NF-κB), p53, and the cyclin-dependent kinase inhibitors p21 and p27.20 Proteasome inhibition leads to tumor growth arrest, diminished tumor metastasis, and sensitization of cells to cytotoxic chemotherapy.21,22 Our preclinical studies appeared to support the concept of sequential chemotherapy followed by proteasome inhibition as an optimal approach to induce tumor cell apoptosis, as compared with the reverse sequence or concurrent administration. 13–15 These studies showed that bortezomib therapy results in a timing-specific enhancement of the antineoplastic effect of docetaxel. Specifically, the sequence of docetaxel followed by bortezomib showed a comparative enhancement of apoptosis over other schedules. The in vitro effects of 2-drug treatments with docetaxel plus bortezomib have been evaluated using flow cytometry in the NSCLC lines A549 (p53 wt), and Calu1 (p53 null) cells. When docetaxel was administered first, cells accumulated in M phase, before mitotic catastrophe. The addition of bortezomib enhances docetaxel-related cytotoxicity. In contrast, when bortezomib was delivered before docetaxel, p53-competent cells accumulated in G1 and G2 in response to bortezomib. This resulted in a reversible cytostatic response that potentially limited docetaxel-induced cytotoxicity in the M phase. Notably, simultaneous administration of docetaxel and bortezomib suggested inhibition of docetaxel cytotoxicity in p53 competent cells. In breast cancer, this concept has been validated prospectively with concurrent administration of cytotoxic chemotherapy (eg, anthracyclines) and tamoxifen (which arrests cells in G1), demonstrating antagonistic effects.23
This randomized study does not support the hypothesis that sequential therapy was more effective than does concurrent therapy in which docetaxel precedes bortezomib by a day. The median OS of 7.8 months seen in the sequential arm was similar to previous estimates for either docetaxel alone or docetaxel plus twice-weekly bortezomib in advanced second-line NSCLC.2,3,17 However, it is notable that the median OS of 13.3 months observed in the CON arm of this trial exceeds previous published OS estimates in the second-line setting.
The most likely reason for the trend to better OS in the concurrent arm is chance alone in this randomized phase II trial with a limited sample size. However, there are other potential sources of variation that were not controlled. For example, more patients on arm 2 had received previous EGFR inhibitor therapy, and so in effect, were receiving third-line rather than second-line treatment in this study. This may have led to imbalances in rates of subsequent lines of therapy. When originally designed, this second-line study (which allowed previous erlotinib therapy, a typical third-line agent, as an eligibility criterion) did not mandate reporting of third-line (or beyond) therapies.
It is also possible that imbalances in unseen clinical or molecular prognostic factors could have influenced the results of this trial. For example, p53 mutational status appears to influence results in some preclinical models of docetaxel plus bortezomib. It is known that docetaxel enhances β-tubulin transcription and translation in p53-null H1299 but not in p53 wt A549 cells, and that increased β-tubulin correlates with docetaxel resistance.24 In sequencing studies in NSCLC cell lines, bortezomib induces G1 and G2 arrest in p53 wt cells, which is maintained throughout subsequent docetaxel treatment, likely attenuating docetaxel activity. However, in p53- compromised cells, bortezomib-induced arrest is less apparent and the schedule of treatment less dependent on cytostatic effects. In another preclinical model, induction of apoptosis (assessed by sub-G1 accumulation using flow cytometry) was highest in p53-null PC3 prostate cancer cells.25 In contrast, apoptosis was optimally induced in the p53 wild type LNCaP cell line when both agents were given concurrently. The implication is that p53-null tumors are less likely to benefit from docetaxel with or without concurrent bortezomib, but may be more sensitive to a sequencing strategy.
Conclusion
The combination of docetaxel plus bortezomib is feasible and tolerable in this cohort of patients with pretreated advanced NSCLC. The doublet concurrently given on day 1 has a RR similar to that of sequential therapy but appears to result in OS that exceeds previous published efficacy estimates for either agent alone or in combination. Clinical or molecular parameters that are unaccounted for may have influenced these results. Future studies in advanced NSCLC using proteasome inhibition, either alone or in combination with chemotherapy, will need to define the appropriate molecular phenotype most likely to benefit from such an approach. Until such molecular characterization is performed, additional empirically driven clinical trials in this patient subset are not recommended.
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute through an N01 Early Therapeutics contract (NO1 CM-62209) to the California Cancer Consortium.
The authors thank the CRAs and research nursing staff for their hard work on this trial. We also thank Ms. Stella Khoo Chen, Consortium Manager, for her administrative support.
Disclosures
Athanassios Argiris has received research support from Millennium Pharmaceuticals. Mariana Koczywas is a member of the Speaker’s Bureau for Genentech, Inc. Primo N. Lara, Jr. has received research support from Millennium Pharmaceuticals, Inc., National Cancer Institute/National Institutes of Health, and sanofi-aventis U.S. Primo N. Lara, Jr. has also served as a paid consultant or has been on the Advisory Board of Millennium Pharmaceuticals and sanofi-aventis U.S. Karen Reckamp is a member of the Speaker’s Bureau for Eli Lilly and Company and Genentech, Inc. Frances A. Shepherd has served as a consultant or been on an advisory board for Millennium Pharmaceuticals and sanofi-aventis U.S.
Footnotes
Presented in part at the 2010 American Society of Clinical Oncology Annual Meeting in June 2010 in Chicago, IL, and at the 2nd European Lung Cancer Congress in April 2010 in Geneva, Switzerland.
All other authors have no relevant relationships to disclose.
References
- 1.Jemal A, Siegel R, Ward E. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non–small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 3.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 4.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 5.Ling YH, Liebes L, Ng B, et al. PS-341, a novel proteasome inhibitor, induces Bcl-2 phosphorylation and cleavage in association with G2-M phase arrest and apoptosis. Mol Cancer Ther. 2002;1:841–849. [PubMed] [Google Scholar]
- 6.Ling YH, Liebes L, Zou Y, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–23723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 7.Mortenson MM, Schlieman MG, Virudachalam S, et al. Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapy in the A549 non-small-cell lung cancer cell line. Cancer Chemother Pharmacol. 2004;54:343–353. doi: 10.1007/s00280-004-0811-4. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Ikezoe T, Saito T, et al. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–180. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling YH, Liebes L, Jiang JD, et al. Mechanisms of proteasome inhibitor PS-341-induced G(2)-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res. 2003;9:1145–1154. [PubMed] [Google Scholar]
- 10.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 11.Nawrocki ST, Sweeney-Gotsch B, Takamori R, et al. The proteasome inhibitor bortezomib enhances the activity of docetaxel in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther. 2004;3:59–70. [PubMed] [Google Scholar]
- 12.Denlinger CE, Rundall BK, Keller MD, et al. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg. 2004;78:1207–1214. doi: 10.1016/j.athoracsur.2004.04.029. discussion 07–14. [DOI] [PubMed] [Google Scholar]
- 13.Lara PN, Jr, Davies AM, Mack PC, et al. Proteasome inhibition with PS-341 (bortezomib) in lung cancer therapy. Semin Oncol. 2004;31:40–46. doi: 10.1053/j.seminoncol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Gumerlock PH, Kimura T, Holland WS, et al. Differential in vivo activity of docetaxel plus PS-341 combination therapy in non-small cell lung carcinoma (NSCLC) xenografts. J Clin Oncol. 2004;22(14 suppl) (abstract 7144) [Google Scholar]
- 15.Mack PC, Davies AM, Lara PN, et al. Integration of the proteasome inhibitor PS-341 (Velcade) into the therapeutic approach to lung cancer. Lung Cancer. 2003;41(1 suppl):S89–S96. doi: 10.1016/s0169-5002(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 16.Lara PN, Jr, Koczywas M, Quinn DI, et al. Bortezomib plus docetaxel in advanced non-small cell lung cancer and other solid tumors: a phase I California Cancer Consortium trial. J Thorac Oncol. 2006;1:126–134. [PubMed] [Google Scholar]
- 17.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase ii study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non–small-cell lung cancer. J Clin Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 20.Myung J, Kim KB, Crews CM. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. J Biol Chem. 1999;274:22123–22126. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- 22.Adams J, Palombella VJ, Elliott PJ. Proteasome inhibition: a new strategy in cancer treatment. Invest New Drugs. 2000;18:109–121. doi: 10.1023/a:1006321828515. [DOI] [PubMed] [Google Scholar]
- 23.Albain KS, Barlow WE, Ravdin PM, et al. Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:2055–2063. doi: 10.1016/S0140-6736(09)61523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang JT, Chang GC, Ko JL, et al. Induction of tubulin by docetaxel is associated with p53 status in human non small cell lung cancer cell lines. Int J Cancer. 2006;118:317–325. doi: 10.1002/ijc.21372. [DOI] [PubMed] [Google Scholar]
- 25.Farneth NC, Holland WS, Kenosi T, et al. Proteasome inhibition with bortezomib (PS-341) in combination with docetaxel (Doc) in prostate cancer (CaP) cells and xenografts. J Clin Oncol. 2005;23(16 suppl):239s. (abstract 3192) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.