Abstract
Consistent across studies in humans, animals and cells, the application of vibrations can be anabolic and/or anti-catabolic to bone. The physical mechanisms modulating the vibration-induced response have not been identified. Recently, we developed an in vitro model in which candidate parameters including acceleration magnitude and fluid shear can be controlled independently during vibrations. Here, we hypothesized that vibration induced fluid shear does not modulate mesenchymal stem cell (MSC) proliferation and mineralization and that cell’s sensitivity to vibrations can be promoted via actin stress fiber formation. Adipose derived human MSCs were subjected to vibration frequencies and acceleration magnitudes that induced fluid shear stress ranging from 0.04Pa to 5Pa. Vibrations were applied at magnitudes of 0.15g, 1g, and 2g using frequencies of both 100Hz and 30Hz. After 14d and under low fluid shear conditions associated with 100Hz oscillations, mineralization was greater in all vibrated groups than in controls. Greater levels of fluid shear produced by 30Hz vibrations enhanced mineralization only in the 2g group. Over 3d, vibrations led to the greatest increase in total cell number with the frequency/acceleration combination that induced the smallest level of fluid shear. Acute experiments showed that actin remodeling was necessary for early mechanical up-regulation of RUNX-2 mRNA levels. During osteogenic differentiation, mechanically induced up-regulation of actin remodeling genes including Wiskott-Aldrich syndrome (WAS) protein, a critical regulator of Arp2/3 complex, was related to the magnitude of the applied acceleration but not to fluid shear. These data demonstrate that fluid shear does not regulate vibration induced proliferation and mineralization and that cytoskeletal remodeling activity may play a role in MSC mechanosensitivity.
Keywords: Bone, Mechanical Signals, Vibrations, Mesenchymal Stem Cells, Differentiation, Proliferation, In Vitro Cell Culture
1. Introduction
Low-level vibrations (≤2g acceleration magnitude) have been recognized as a mechanical signal than can be anabolic and/or anti catabolic to bone cells both in vivo and in vitro (Kim et al., 2012; Ozcivici et al., 2010; Xie et al., 2006). However, the precise mechanical parameter(s) that is most critical for controlling the cellular response is yet to be identified. In vivo, extracellular matrix strains generated by low-level vibrations are at least two orders of magnitude smaller than those required to elicit a response when the signal frequency is much lower (Xie et al., 2006). Not only are vibration induced matrix deformations small but, unlike strains engendered by low-frequency mechanical signals, they are not also directly related to osteogenesis (Garman et al., 2007).
An alternative parameter, fluid shear, can alter transcriptional activity at laminar (Ponik and Pavalko, 2004), pulsatile (Mullender et al., 2006) and oscillating (Donahue et al., 2003) low-frequency (<15Hz) conditions. During in vivo vibrations, drag forces resulting from dynamic accelerations may produce physiologically relevant levels of fluid shear on bone/fluid interfaces (Coughlin and Niebur, 2012; Dickerson et al., 2008) emphasizing that investigations of in vitro vibration need to consider fluid shear as a potential mechanotransduction mechanism. We previously showed that in vitro vibrations, when applied horizontally rather than vertically, can elicit fluid shear in excess of 1Pa and that COX-2 mRNA levels were not directly related to fluid flow (Uzer et al., 2012).
Regardless of the physical mechanism involved in transmitting mechanical signals to the cell, the cytoskeleton as the continuous structure between chromosome and cell membrane is likely involved in transmitting mechanical cues within a cell (Dahl et al., 2010). Cell membrane and cytoskeleton are mechanically coupled (Wang et al., 1993) and cytoskeletal elements can transform local into global deformations (Helmke and Davies, 2002). Through cytoskeletal distributions, possible out-of-phase motion of the denser and stiffer nucleus during vibration may be capable of initiating mechanotransduction cascades that are similar to those induced by mechanical signals originating at the cell membrane. Considering that the cytoskeleton can directly regulate force transfer by changing its prestress in response to mechanical signals (Arnsdorf et al., 2009; Hu et al., 2005; Sen et al., 2011), the efficacy of vibrations may directly depend on cytoskeletal force transfer.
The aim of this study was to explore the role of fluid shear and cytoskeletal prestress (remodeling) in vibration induced osteogenic differentiation of mesenchymal stem cells (MSCs). To this end, we used a previously developed in vitro model that can precisely control the level of fluid shear that cells are exposed to during vibrations. We hypothesized that vibration induced fluid shear is not critical to enhance MSC proliferation and mineralization and that cellular sensitivity to vibrations can be promoted by actin stress fiber formation in the cytoskeleton.
2. Materials and Methods
2.1 Experimental design
To address the hypothesis, we separated the vibration engendered cellular mechanical input into two components – accelerations and fluid shear stress. Acceleration is a direct measure of the magnitude of the applied vibration while vibration induced fluid shear stress, here simply referred to as fluid shear, is a function of vibration frequency, acceleration and fluid viscosity (Uzer et al., 2012). To generate a broad range of fluid shear values and, at the same time, to create similar levels of fluid shear at different vibration magnitudes, three different accelerations (0.15g, 1g, and 2g) were applied at two different frequencies (100Hz and 30Hz).
Inside the bone marrow cavity and in trabecular regions, the level of shear is highly dependent on marrow viscosity which can change dramatically during aging and osteoporosis when fatty bone marrow replaces red marrow that has a 10-fold smaller viscosity (Bryant et al., 1989; Gimble et al., 1996; Justesen et al., 2001). Here, we increased viscosity of the cell culture medium by adding 6% (v/w) dextran, modulating fluid shear independent of frequency and acceleration (Table 1).
Table 1.
Vibration induced peak fluid shear values as a function of medium viscosity and vibration frequency/acceleration.
| 0.15g | 1g | 2g | |
|---|---|---|---|
| 100Hz (no dextran) | 0.04 Pa | 0.28 Pa | 0.56 Pa |
| 30Hz (no dextran) | 0.14 Pa | 0.94 Pa | 1.88 Pa |
| 30Hz (6% dextran) | 0.39Pa | 2.63 Pa | 5.26 Pa |
Acceleration magnitudes are presented as multiples of g where g is the earth gravitational field (9.81 m/s2).
To investigate the effects of fluid shear and acceleration on early RUNX-2, RANKL and OPG mRNA expression levels and cell viability, cells were exposed to a single application of vibrations (Fig. 1). Lysophosphatidic acid (LPA, Cayman Chemical, MI) was used to test whether the creation of rapid (<2h) actin stress fiber formation (Jaganathan et al., 2007) enhances the cellular response. During these acute experiments, we utilized only 30Hz signals because, unlike 100Hz signals, they generated fluid shear greater than 1Pa, frequently considered a threshold at which fluid shear can influence cell metabolism (Riddle and Donahue, 2009). Cell proliferation was determined after one, two, and three days of vibrations. On Day 2, RUNX-2 levels were also quantified (Fig. 1). Changes in cytoskeletal remodeling (PCR array) and RUNX-2 were profiled after 7d of vibrations (Fig. 1). Mechanically-induced mineralization was assessed after exposing cells to vibrations for 14d (Fig. 1). In contrast to the acute data collections, these experiments included 100Hz and 30Hz frequencies (Fig. 1).
Figure 1.
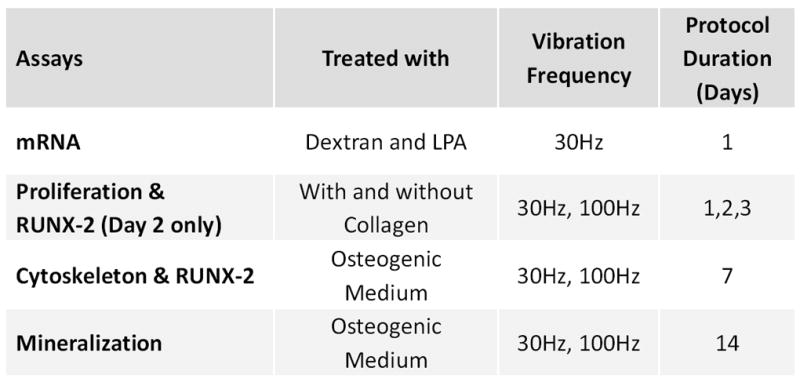
Overview of the experimental design. Vibrations were applied once every 24h. Acceleration magnitudes of 0.15g, 1g and 2g were used for each frequency. Acute effects of vibration on mRNA expression were assessed after a single application of vibration. Vibration related changes on proliferation were measured at day 1, 2 and 3. Cytoskeletal remodeling and mineralization were assessed after 7 and 14 exposures to vibrations, respectively.
2.2 Cell culture
Commercially available adipose derived MSCs (18yr old female, Lifeline Technologies, Walkersville, MD) were cultured in standard flasks (75cm2, Corning Inc., NY) at a density of 5,000 cell/cm2 using Stemlife Basal Medium (Lifeline Technologies) supplemented with penicillin-streptomycin (PS, Gibco, CA) and incubated at 37°C, 5% CO2. Medium was changed every 48h. Cells were sub-cultured prior to reaching 70% confluence. For all experiments, only cells of passage four or less were included. For the experiments evaluating cytoskeletal remodeling and mineralization, osteogenic medium (Osteolife Complete Osteogenic Medium, Lifeline Technologies) was utilized. All other experiments used Stemlife Basal Medium.
2.3 Vibrations and fluid shear
The vibration system that horizontally oscillated cells at RT is described elsewhere (Uzer et al., 2012). Briefly, an actuator was attached to a stainless steel plate mounted on a linear frictionless slide. Vibrations were applied for 30min/d at 0.15, 1, or 2g peak vibration magnitudes using 100Hz or 30Hz frequencies (except acute experiments that only used 30Hz). Non-vibrated control cell culture plates were handled identical to experimental plates except that vibrations were not applied. A validated finite element model determined the levels of peak fluid shear just above the cell layer (Uzer et al., 2012).
2.4 Mineralization
Cells were seeded at a density of 18,000 cells/cm2 in 24-well plates. After 48h, osteogenic medium was introduced and vibration (or sham) treatment commenced for the seven groups (n=5 each). After 14d, cells were fixed with 70% ethanol for 1h, stained with 40mM alizarin red S (Sigma-Aldrich, St Louis, MO), and de-stained with Cetylpyridinium chloride (Sigma). The concentration of total dye was quantified at 590nm.
2.5 Cell proliferation
Cells were seeded on 24-well plates at a density of 7500 cells/cm2. After allowing attachment for 24h, cells were exposed to one of the 6 experimental conditions or sham treatment (n=6 each) over three days. Cell number was determined via XTT cell proliferation assay (ATCC, Manassas, VA) 24h after 1st, 2nd and 3rd vibration treatment. These time points will be referred to as Day 1, Day 2, and Day 3. At each time point, cell viability was checked with a live/dead cell assay (Invitrogen, NY) and visualized under a microscope. On Day 2, RUNX-2 gene expression was assessed (n=3 per group). Additional experiments were performed with collagen coated plates to promote stronger integrin attachment (Hidalgo-Bastida and Cartmell, 2010), potentially enhancing cell responsiveness (Sen et al., 2011). To this end, the same 6 experimental conditions were used (n=4 each) on plates with or without 0.15mg/ml rat tail collagen I (Cell Applications Inc., CA) and cell proliferation was measured at day 2.
2.6 Early gene expression
Immediate effects of changes in fluid shear and increased cellular pre-stress on mRNA levels and cell viability were determined after a single application of vibrations. Cells were plated at 18,000 cell/cm2. After 46h, the medium from each well was aspirated and replaced with medium containing either normal medium, normal medium + 6% (w/v) dextran, or normal medium + 67.5uM LPA. Dishes were then incubated at 37°C and 5% CO2. After 2h, cells were exposed to vibrations once for 30min (n=6 each). Immediately after vibration treatment, cells were lysed with 600ml of TRIzol (Ambion, TX) and stored at -80°C. Total RNA was isolated (RNeasy Mini Kit, Qiagen, CA). RNA quality and concentration were determined (NanodropND-1000, Thermo Scientific, NY). Upon reverse transcription (High Capacity RNA to cDNA kit, Applied Biosystems, CA), RT-PCR was performed (Step-One Plus, Applied Biosystems, CA) using Taqman primer probes (Applied Biosystems) for RUNX-2, RANKL, OPG. GAPDH served as referent. Expression levels were quantified with the delta-delta CT method (Livak and Schmittgen, 2001) and results were reported relative to non-vibrated control.
2.7 Cytoskeletal remodeling
To investigate the role of fluid shear on cytoskeletal remodeling, cells were vibrated for 7d (n=3 each). Long term remodeling of the cytoskeleton was facilitated by culturing cells in osteogenic medium 48h after initial plating. After 7d of vibrations, samples were either analyzed for RUNX2 transcriptional levels or pooled within each group to profile mRNA levels of 84 genes controlling cytoskeletal remodeling using a commercially available PCR array (Human Cytoskeleton Regulators, Qiagen).
2.8 Statistical analysis
Data were presented as mean±SEM. Differences between groups were identified by one-way analysis of variance (ANOVA) followed by Student-Newman-Keul (SNK) post-hoc tests. Two-way ANOVA tested for main effects and interactions between vibration frequency and acceleration as well as mechanical treatment and collagen coating. P-values of less than 0.05 were considered significant and p-values were reported as <0.05, <0.01, or <0.001.
3. Results
3.1 Fluid shear and mineralization
Across vibrations frequencies and accelerations, fluid shear generated by out-of-phase motion of the cell culture medium ranged from 0.04 to 1.88Pa (Table 1). At all 100Hz conditions, fluid shear was less than 1Pa (here referred to as low shear). In these low-shear conditions, all 100Hz groups (0.15g, 1g, and 2g) showed significantly greater (p<0.05) mineralization compared to non-vibrated controls at day 14 (Fig. 2). Acceleration magnitude was not associated with the response. At 30Hz, increasing acceleration from 0.15g to 1g and to 2g raised fluid shear from 0.14 to 0.94 and 1.88Pa (Table 1) but only the 2g signal significantly increased mineralization against controls. Comparing vibration signals across frequencies emphasized that greatly different levels of fluid shear can stimulate similar levels of mineralization; even though the 30Hz-2g signal induced fluid shear (1.88Pa) an order of magnitude greater than the 100Hz-0.15g signal (0.14Pa), there was no significant difference in mineralization between the two groups. The interaction between the factors frequency and acceleration did not reach statistical significance.
Figure 2.
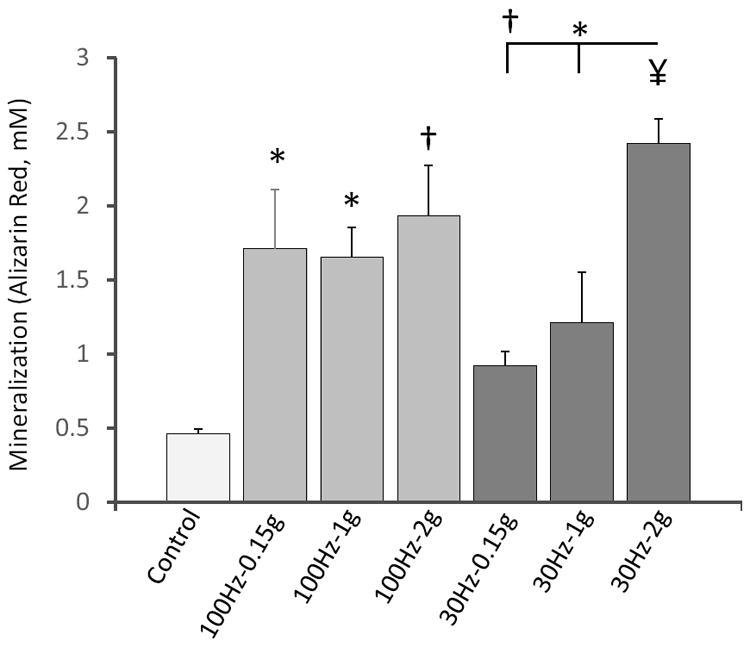
Mineralization levels of cells subjected to vibrations for 14d at a prescribed combination of signal frequency/acceleration and contrasted to non-vibrated control cells. * p<0.05, † p<0.01, ¥ p<0.001 against control and each other.
3.2 Cell proliferation
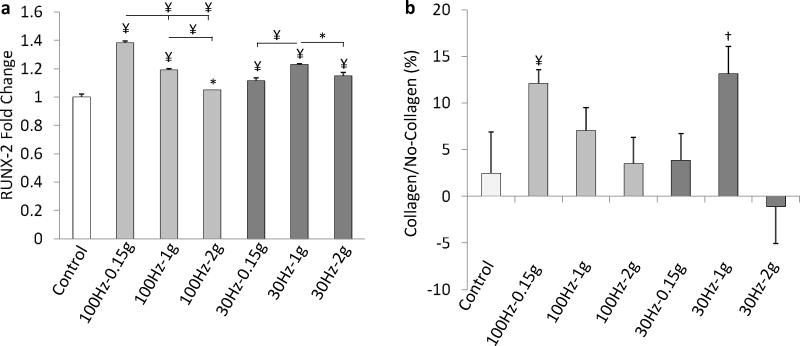
Total cell number was measured 24h after the 1st, 2nd and 3rd vibration exposures, at 100Hz and 30Hz using 0.15g, 1g, and 2g accelerations. No dead cells were detected for any combination of vibration magnitude/frequency at any given time point. Compared to controls, vibrations (averaged across all different regimes) increased cell proliferation by 18%, 20%, and 45% on Day 1, Day 2, and Day 3 (Fig. 3). Averaged across the three days and acceleration groups, 100Hz groups had 15% (p<0.01) more cells than 30Hz groups. Two-way ANOVA demonstrated a strong interaction between vibration frequency and acceleration at every time point (p<0.001). For each day, 0.15g was most effective in raising cell number for 100Hz and 1g was most effective for 30Hz. Across frequencies, the group that was exposed to the least fluid shear (100Hz-0.15g) had a significantly greater (p<0.05) number of cells than any of the other five experimental groups. At 100Hz, increasing acceleration had a negative effect on cell proliferation while at 30Hz, 1g tended to be more effective than 0.15g and 2g. For each frequency, the largest acceleration (2g) gave rise to the smallest increase in cell number. At Day 3 and for each frequency, 2g vibrated cells were 23% (100Hz, p<0.001) and 21% (30Hz, p<0.05) less than the experimental group with the largest proliferation response. RUNX-2 gene expression at Day 2 qualitatively paralleled cell proliferation (Fig. 4).
Figure 3.
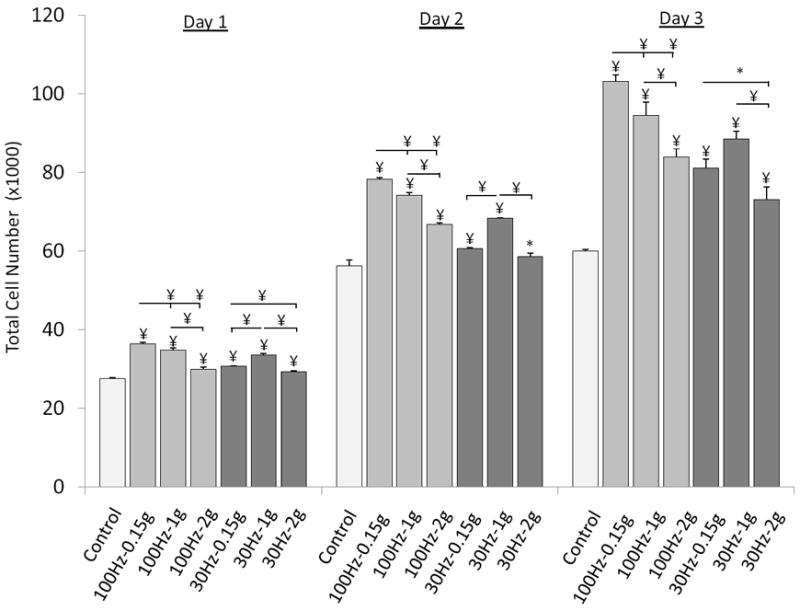
Cell proliferation characterized by total cell number after exposure to 30min/d of vibrations on Day 1 (24h after 1st exposure), Day 2, and Day 3. * p<0.05, † p<0.01, ¥ p<0.001 against control and each other.
Figure 4.
(a) RUNX-2 gene expression normalized to GAPDH at Day 2 for non-collagen coated groups. (b) Cell proliferation at Day 2 as characterized by relative differences between culture plates with and without collagen coating. *p<0.05, †p<0.01, ¥p<0.001 against control and each other.
To test whether stronger cell attachment alters cell proliferation, the cell proliferation experiments were repeated for Day 2 contrasting collagen type I coated with normal plates. Averaged across all groups, the addition of collagen increased total cell number by 7% (p<0.05) but there was no significant difference between control groups (Fig. 4). Qualitatively, cell proliferation profiles were similar between the two groups with significant (p<0.01) differences only for the groups with the greatest response at each frequency (100Hz-0.15g and 30Hz-1g). The interaction between collagen coating and vibration treatment was significant (p<0.05).
3.3 Transcriptional changes
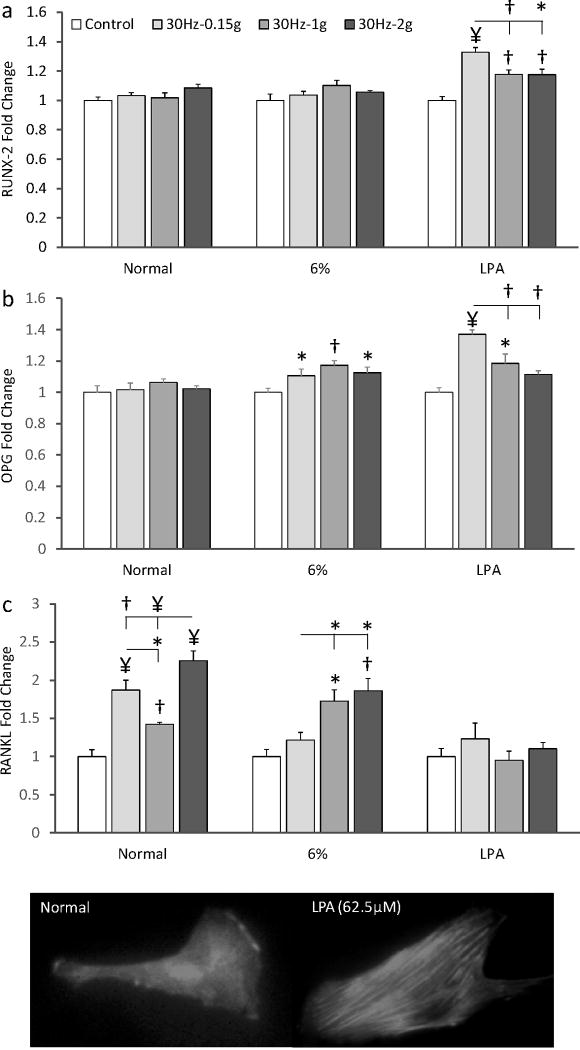
To investigate immediate mRNA regulation of MSCs, transcriptional levels of anabolic (RUNX-2) and (anti)catabolic (RANKL, OPG) genes were quantified upon MSC treatment with specific vibration/fluid shear regimes. Only 30Hz signals were included as fluid shear was low for the 100Hz group. To further raise cell fluid shear, we included groups in which the viscosity of the medium was increased via dextran.
Independent of the level of acceleration (0.15g, 1g or 2g), RUNX-2 and OPG mRNA levels were not significantly different from control groups in normal culture medium (Fig. 4). RANKL expression was significantly increased by all three acceleration magnitudes but the increases did not correlate with fluid shear or acceleration magnitude (Fig. 5).
Figure 5.
Fold-changes in (a) RUNX-2, (b) OPG, and (c) RANKL expression of MSCs exposed to three different mechanical signals in normal medium, high-viscosity medium (6% dextran) and normal medium treated with LPA to increase cell pre-stress. At the bottom, an image of a cell (20×) conditioned in normal growth medium is contrasted with a cell exposed to 62.5 μM LPA for 2 h demonstrating rapid stress fiber formation. *p<0.05, †p<0.01, ¥p<0.001 against control and each other.
The addition of 6% dextran to the culture medium increased fluid shear 2.8-fold for all acceleration magnitudes without compromising cells viability as determined by a live/dead cell assay. The 2.8-fold increase in fluid shear did not up-regulate RUNX-2 expression but OPG levels were significantly greater in all vibrated groups than in controls without any significant differences between the 0.39Pa (0.15g), 2.4Pa (1g) and 5.2Pa (2g) groups (Fig. 5). Under increased shear, RANKL remained up-regulated at 1g (72%, p<0.05) and 2g (86%, p<0.01) but not 0.15g.
Because RUNX-2 was not up-regulated by any of the six mechanical signals above, we tested whether increased cytoskeletal tension is required for osteogenic commitment. LPA increased the number of actin stress fibers after 2h of incubation (Fig. 5) and vibrations were able to stimulate RUNX-2 expression. The largest response relative to non-vibrated controls (32%, p<0.001) occurred at 0.15g, a response that was greater than at 1g (p<0.01) and 2g (p<0.05). OPG levels of pre-stressed cells were also greater in all vibrated groups than in pre-stressed controls with the 0.15g group showing the greatest increase (36%, p<0.001, Fig. 5). No RANKL response was observed after LPA administration.
3. 4 Cytoskeletal remodeling
Since cell pre-stress, but not fluid shear, enabled vibratory signals to modulate osteogenic mRNA expression, we further explored the association between cytoskeletal development and vibrations with a PCR array including 84 key cytoskeletal regulatory genes. RUNX-2 expression was also monitored. Cells were treated with osteo-inductive medium for 7d and exposed once a day to 0.15g, 1g and 2g accelerations at frequencies of 100Hz and 30Hz. RUNX-2 was up-regulated in all vibration groups, however only 30Hz-1g (p<0.01) and 30Hz-2g (p<0.05) groups were significantly greater than controls (Table 2). The cytoskeletal regulator PCR array identified a total of 25 genes showing a 2-fold or greater response to at least one of the six frequency/acceleration combinations. Thirteen genes were up-regulated by more than 4-fold. When stratified into functional categories, 22 out of 25 genes were related to actin remodeling (Table 2). For both frequencies, expression of actin network regulators tended to increase with increasing acceleration magnitude (Table 2). The most highly up-regulated gene pertained to the Wiskott-Aldrich syndrome (WAS) protein, a critical regulator of the Arp2/3 complex (Machesky and Insall, 1998a; Machesky et al., 1999) that enables rapid actin fiber formation (Machesky and Insall, 1998b; Macheseky et al., 1999; Rohatgi et al., 2001) and binding of newly formed actin filaments to the existing actin network (Marchand et al., 2001; Mullins et al., 1998). Averaged across 100Hz and 30Hz groups, WAS was up-regulated 7-fold (0.15g), 28-fold (1g), and 42-fold (2g). Despite the large differences in generated fluid shear, up-regulation of WAS was similar between 100Hz and 30Hz signals.
Table 2.
Transcriptional response of RUNX-2 and cytoskeletal genes.
| Genes | 100Hz | 30Hz | ||||
|---|---|---|---|---|---|---|
| 0.15g | 1g | 2g | 0.15g | 1g | 2g | |
| RUNX-2 | 1.2 | 1.6 | 2.0 | 1.4 | 2.89 | 2.17 |
| ARHGEF11 | 1.3 | 2.5 | 2.5 | 1.3 | 5.6 | 6.0 |
| AURKC | 1.8 | 3.0 | 3.8 | 1.6 | 5.6 | 9.6 |
| CDC42 | 2.1 | 3.0 | 3.4 | 2.0 | 4.6 | 4.3 |
| CDK5R1 | 1.6 | 6.9 | 6.2 | 0.8 | 4.7 | 13.5 |
| CYFIP2 | 2.4 | 11.5 | 13.1 | 2.2 | 23.2 | 18.7 |
| LIM-K2 | 1.3 | 2.6 | 2.4 | 1.0 | 3.3 | 6.7 |
| MAP3K11 | 1.7 | 2.3 | 2.1 | 1.6 | 5.7 | 5.2 |
| MARK2 | 1.3 | 3.1 | 3.2 | 1.0 | 3.6 | 4.8 |
| NCK2 | 1.6 | 3.0 | 3.0 | 1.5 | 5.5 | 6.7 |
| PPP1R12B | 2.4 | 3.3 | 2.4 | 2.0 | 4.7 | 5.0 |
| RADIXIN | 2.7 | 4.2 | 3.8 | 2.0 | 3.9 | 3.7 |
| WAS | N/A | 26.8 | 40.0 | 6.9 | 27.6 | 43.0 |
| WASL | 2.1 | 2.6 | 2.8 | 1.9 | 4.7 | 3.1 |
|
| ||||||
| Functional Categories | ||||||
| Actin Remodeling | 1.6 | 3.8 | 4.5 | 1.8 | 5.5 | 5.9 |
| Calmodulin / Calcineurin | 1.2 | 1.9 | 1.8 | 1.2 | 4.1 | 3.4 |
| Cell Motility / Migration | 1.6 | 2.2 | 2.2 | 1.5 | 3.3 | 3.1 |
| Cell Projections | 1.9 | 3.3 | 3.4 | 1.8 | 5.3 | 4.5 |
| Cell Shape, Size, Polarity | 1.7 | 4.2 | 4.7 | 1.6 | 8.0 | 7.1 |
| Cytokinesis | 1.6 | 2.5 | 2.8 | 1.4 | 4.6 | 6.2 |
| Cytoskeleton Adaptor Activity: | 1.6 | 2.7 | 2.7 | 1.7 | 3.7 | 4.0 |
| G-Protein Signaling | 1.5 | 4.8 | 6.0 | 2.0 | 7.1 | 7.5 |
| Kinases & Phosphatases | 1.6 | 2.3 | 2.3 | 1.5 | 3.6 | 4.0 |
| Microtubules | 1.6 | 6.9 | 6.2 | 0.8 | 4.7 | 13.5 |
| Mean mRNA Activity | 1.6 | 3.5 | 3.7 | 1.5 | 5.0 | 5.9 |
After 7d of vibration in osteogenic medium, expression levels of RUNX-2 and 84 genes were determined. A total of 25 genes showed a greater than 2-fold response to at least one frequency/acceleration combination. In the upper panel, genes with a greater than 4-fold response are included. In the lower panel, the 25 genes were subdivided into 9 functional categories. For both frequencies, the greatest mRNA response was observed for 1g and 2g.
4. Discussion
We investigated the role of fluid shear and cytoskeletal pre-stress in vibration-driven proliferation and osteogenesis of MSCs. Combining accelerations of 0.15g, 1g and 2g with frequencies of 100Hz and 30Hz induced fluid shear magnitudes between 0.04Pa and 1.88Pa near the cell surface. 100Hz vibrations elicited low fluid shear (<1Pa) and MSCs oscillated at this frequency showed a more than 3-fold increase in mineralization independent of the specific acceleration magnitude. At 30Hz, fluid shear magnitudes were much greater (0.14 to 1.88Pa) and only the largest acceleration (fluid shear) magnitude was able to increase mineralization to the same level as the 100Hz (low shear) conditions. Similarly, vibration enhanced cell proliferation under low flow shear conditions. In acute experiments, fluid shear of up to 5Pa was unable to up-regulate RUNX-2 mRNA levels. In contrast, enhancing the actin stress fiber formation via LPA increased RUNX-2 transcriptional levels, with the smallest acceleration (0.15g) and fluid shear (0.04Pa) signal producing the greatest increase in RUNX-2. This association between vibrations and cytoskeletal remodeling was further investigated by profiling 84 regulatory genes. Vibrations almost exclusively up-regulated actin related genes (22 out of 25) and increased expression of these genes was largely correlated with the increases in acceleration magnitude.
A limitation of this study was that not only the levels of fluid shear were different between the 100Hz and 30Hz conditions but also the number of loading cycles. Most previous studies have suggested a threshold behavior of bone cells to the number of loading cycles (Cullen et al., 2001; Qin et al., 1998) and it is unclear whether adjusting the duration of the vibration exposures to produce similar numbers of loading cycles would change the cellular response. Because continuous loading duration may also be an important variable (Xie et al., 2006), we held exposure time constant and used a large range of acceleration magnitudes to create distinct fluid shear levels within a given frequency. The significant interactions we found between vibration acceleration and frequency highlight this interdependence of parameters. Also, a previously developed and experimentally validated FEM revealed the level of generated peak shear stress as a function of oscillation frequency, acceleration, and viscosity but the stress field across the culture well surface was spatially non-uniform (Uzer et al., 2012). However, the location dependency of peak fluid shear that the cells were exposed to should not have influenced the conclusions of this study as the relative differences of fluid stress profiles between individual vibration regimes was spatially similar.
Computational studies have suggested the existence of significant fluid shear levels at the interface of bone marrow and bone surfaces during oscillations (Coughlin and Niebur, 2012; Dickerson et al., 2008). These levels of fluid shear, when applied at low frequencies (<5Hz), can readily stimulate osteocyte and osteoblast signaling (Liu et al., 2008; Tan et al., 2007). However, many fluid flow devices do not permit longer term experiments (Arnsdorf et al., 2009) and when experimental duration is increased, fluid shear does not necessarily lead to increased osteoblastogenesis (Li et al., 2004). Across the experiments performed here, we consistently found that vibrations promoted an osteoblastic phenotype that did not correlate with fluid shear. For instance, the 100Hz-0.15g and 30Hz-2g groups had very large differences in fluid shear (0.04Pa vs 1.88Pa) without apparent differences in the levels of mineralization. Similarly, the lowest level of fluid shear induced by the 100Hz-0.15g group (0.04Pa) elicited the largest increase in proliferation over 3d of measurements. Our data showing that the vibration induced increase in the osteogenic commitment and proliferation of MSC does not depend on fluid shear as a mechanical input is not surprising as previous studies observed osteogenic commitment of MSCs with very small acceleration (fluid shear) magnitudes (Kim et al., 2012; Zhou et al., 2011).
Even when increasing vibration induced fluid shear up to 5Pa, uncommitted MSCs did not increase their levels of osteogenic markers. In contrast, inducing actin stress fiber formation via LPA allowed oscillatory signals of even very small magnitude to up-regulate both RUNX-2 and OPG. Actin stress fiber formation increases tension within the cell (Hammerick et al., 2010; Wall et al., 2007), promoting force transfer (Hu et al., 2005; McGarry and Prendergast, 2004) and perhaps modulating a cell’s sensitivity to vibrations. At the molecular level, LPA can activate RhoA (Riddick et al., 2008) which, together with the number of mature focal adhesions, can amplify mechanically induced β-catenin signaling in MSCs (Sen et al., 2011) and alter the fate of MSCs. Thus, cellular tension may potentiate the ability of vibrations to promote osteogenesis.
All vibration regimes significantly increased cell proliferation. Coating cell culture plates with collagen, promoting cell attachment (Hidalgo-Bastida and Cartmell, 2010) and integrin mediated focal adhesion kinase activity (Takeuchi et al., 1997), caused small increases in cell number that were most pronounced in those groups that were most responsive to vibrations. These data are consistent with studies showing that coating surfaces with molecules such as collagen-I can readily enhance specific functions of MSCs including cell proliferation and differentiation (Inoue et al., 2005; Lindner et al., 2010; Tsai et al., 2010). As the effect of vibrations was modulated by collagen coating, it is conceivable that stronger cellular attachments afforded by substrate collagen coating amplified the physical mechanism by which cells sense vibrations. This hypothesis that the cytoskeleton is intimately involved in transmitting and amplifying the oscillatory mechanical signal, was corroborated by the up-regulation of genes important for cytoskeletal remodeling.
We showed that the regulation of MSC proliferation and differentiation by oscillatory mechanical signals is not modulated by fluid shear as demonstrated by the responsiveness of MSCs to mechanical signals that induced negligible fluid shear stress (0.04Pa). Instead, the mechanically driven osteogenic commitment of undifferentiated MSCs was influenced by the level of cytoskeletal remodeling and regulatory genes for cytoskeletal remodeling during osteogenic differentiation were correlated with the magnitude of applied acceleration (but not fluid shear). These results demonstrate that greatly different levels of acceleration, frequency, and fluid shear can result in similar MSC responses, perhaps suggesting that the yet to be identified mechanism by which cells sense oscillations is only indirectly related to the mechanical cues considered here.
Acknowledgments
Funding by the National Institutes of Health (NIAMS) is gratefully acknowledged. Technical expertise from Sarah Manske and Lester Orlick was greatly appreciated.
Footnotes
Conflict of Interest Statement
Stefan Judex owns (provisional) patents regarding the application of vibrations to the musculoskeletal system.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of Cell Science. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JD, David T, Gaskell PH, King S, Lond G. Rheology of bovine bone marrow. Proc Inst Mech Eng H. 1989;203:71–75. doi: 10.1243/PIME_PROC_1989_203_013_01. [DOI] [PubMed] [Google Scholar]
- Coughlin TR, Niebur GL. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. Journal of Biomechanics. 2012;45:2222–2229. doi: 10.1016/j.jbiomech.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Cullen DM, Smith RT, Akhter MP. Bone-loading response varies with strain magnitude and cycle number. Journal of Applied Physiology. 2001;91:1971–1976. doi: 10.1152/jappl.2001.91.5.1971. [DOI] [PubMed] [Google Scholar]
- Dahl KN, Booth-Gauthier EA, Ladoux B. In the middle of it all: Mutual mechanical regulation between the nucleus and the cytoskeleton. Journal of Biomechanics. 2010;43:2–8. doi: 10.1016/j.jbiomech.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dickerson DA, Sander EA, Nauman EA. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomechanics and Modeling in Mechanobiology. 2008;7:191–202. doi: 10.1007/s10237-007-0085-y. [DOI] [PubMed] [Google Scholar]
- Donahue TLH, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. Journal of Biomechanics. 2003;36:1363–1371. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level Accelerations applied in the absence of weight bearing can enhance trabecular bone formation. Journal of Orthopaedic Research. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19:421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- Hammerick KE, James AW, Huang ZB, Prinz FB, Longaker MT. Pulsed Direct Current Electric Fields Enhance Osteogenesis in Adipose-Derived Stromal Cells. Tissue Engineering Part A. 2010;16:917–931. doi: 10.1089/ten.tea.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke BP, Davies PF. The Cytoskeleton Under External Fluid Mechanical Forces: Hemodynamic Forces Acting on the Endothelium. Annals of Biomedical Engineering. 2002;30:284–296. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Bastida LA, Cartmell SH. Mesenchymal Stem Cells, Osteoblasts and Extracellular Matrix Proteins: Enhancing Cell Adhesion and Differentiation for Bone Tissue Engineering. Tissue Engineering Part B-Reviews. 2010;16:405–412. doi: 10.1089/ten.TEB.2009.0714. [DOI] [PubMed] [Google Scholar]
- Hu SH, Chen JX, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochemical and Biophysical Research Communications. 2005;329:423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Inoue S, Hori Y, Hirano Y, Inamoto T, Tabata Y. Effect of culture substrate and fibroblast growth factor addition on the proliferation and differentiation of human adipo-stromal cells. Journal Of Biomaterials Science. Polymer Edition. 2005;16:57–77. doi: 10.1163/1568562052843366. [DOI] [PubMed] [Google Scholar]
- Jaganathan BG, Ruester B, Dressel L, Stein S, Grez M, Seifried E, Henschler R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells (Dayton, Ohio) 2007;25:1966–1974. doi: 10.1634/stemcells.2007-0167. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kim IS, Song YM, Lee B, Hwang SJ. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J Dent Res. 2012;91:1135–1140. doi: 10.1177/0022034512465291. [DOI] [PubMed] [Google Scholar]
- Li YJ, Batra NN, You L, Meier SC, Coe IA, Yellowley CE, Jacobs CR. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. Journal of Orthopaedic Research. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lindner U, Kramer J, Behrends J, Driller B, Wendler NO, Boehrnsen F, Rohwedel J, Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- Liu DW, Genetos DC, Shao Y, Geist DJ, Li JL, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-[Delta][Delta]CT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Current Biology. 1998a;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Current Biology. 1998b;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J-B, Kaiser DA, Pollard TD, Higgs HN. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat Cell Biol. 2001;3:76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- McGarry JG, Prendergast PJ. A three-dimensional finite element model of an adherent eukaryotic cell. European cells & materials. 2004;7:27–33. doi: 10.22203/ecm.v007a03. discussion 33-24. [DOI] [PubMed] [Google Scholar]
- Mullender MG, Dijcks SJ, Bacabac RG, Semeins CM, Van Loon JJ, Klein-Nulend J. Release of nitric oxide, but not prostaglandin E2, by bone cells depends on fluid flow frequency. Journal of Orthopaedic Research. 2006;24:1170–1177. doi: 10.1002/jor.20179. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proceedings of the National Academy of Sciences. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcivici E, Luu YK, Rubin CT, Judex S. Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5:e11178. doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponik SM, Pavalko PM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. Journal of Applied Physiology. 2004;97:135–142. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- Riddick N, Ohtani K, Surks HK. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. Journal of Cellular Biochemistry. 2008;103:1158–1170. doi: 10.1002/jcb.21488. [DOI] [PubMed] [Google Scholar]
- Riddle RC, Donahue HJ. From Streaming Potentials to Shear Stress: 25 Years of Bone Cell Mechanotransduction. Journal of Orthopaedic Research. 2009;27:143–149. doi: 10.1002/jor.20723. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho H-YH, Kirschner MW, Mayer BJ. Nck and Phosphatidylinositol 4,5-Bisphosphate Synergistically Activate Actin Polymerization through the N-WASP-Arp2/3 Pathway. Journal of Biological Chemistry. 2001;276:26448–26452. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, Oguz I, Rubin C, Burridge K, Rubin J. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29:1829–1836. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and Transforming Growth Factor-β Receptor Down-regulation by Collagen-α2β1 Integrin Interaction Is Mediated by Focal Adhesion Kinase and Its Downstream Signals in Murine Osteoblastic Cells. Journal of Biological Chemistry. 1997;272:29309–29316. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- Tan SD, de Vries TJ, Kuijpers-Jagtman AM, Semeins CM, Elverts V, Klein-Nulend J. Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone. 2007;41:745–751. doi: 10.1016/j.bone.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Tsai K-S, Kao S-Y, Wang C-Y, Wang Y-J, Wang J-P, Hung S-C. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. Journal of Biomedical Materials Research Part A. 2010;94A:673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- Uzer G, Manske S, Chan M, Chiang F-P, Rubin C, Frame M, Judex S. Separating Fluid Shear Stress from Acceleration during Vibrations In Vitro: Identification of Mechanical Signals Modulating the Cellular Response. Cellular and Molecular Bioengineering. 2012;5:266–276. doi: 10.1007/s12195-012-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Rachlin A, Otey CA, Loboa EG. Human adipose-derived adult stem cells upregulate palladin during osteogenesis and in response to cyclic tensile strain. American Journal of Physiology - Cell Physiology. 2007;293:C1532–C1538. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction Across the Cell-surface and Through the Cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Guan X, Zhu Z, Gao S, Zhang C, Li C, Zhou K, Hou W, Yu H. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. Eur Cell Mater. 2011;22:12–25. doi: 10.22203/ecm.v022a02. [DOI] [PubMed] [Google Scholar]