Abstract
Background
During progressive SIV infection, the ability of innate mononuclear phagocytes to function when responding to the invading pathogen has yet to be determined.
Methods
We generated single-stranded RNA (ssRNA) oligonucleotides from the infecting strain of virus and utilized them to stimulate mononuclear phagocytes from blood and lymph nodes of naïve and SIVmac251 infected rhesus macaques.
Results
Soon after infection and continuing through to chronic disease, plasmacytoid dendritic cells (pDC), monocytes, and macrophages from SIV infected macaques were less able to produce pro-inflammatory cytokines after exposure to virus-derived TLR agonists. In contrast, myeloid dendritic cells (mDC) became hyper-responsive during acute and stable chronic infection.
Conclusions
pDC, monocytes, and macrophages may not instigate continued immune activation by recognizing the ssRNA from SIV as they are left dysfunctional after infection. Conversely, mDC functionality may be beneficial as their hyper-responsiveness is related to slowed disease progression.
Keywords: Innate immunity, HIV/AIDS, Nonhuman primate
Introduction
Simian immunodeficiency virus (SIV) infection in non-natural hosts is an important model system that parallels the disease course of human immunodeficiency virus (HIV) infection in humans [1]. HIV and SIV disease progression has been linked with chronic immune activation and in particular continued stimulation of the innate immune system has been suggested to be a contributing cause [2-6]. Exploring the roles of mononuclear phagocytes in HIV and SIV infection is crucial as their functions span the gap between the innate and adaptive immune responses [7]. The mononuclear phagocyte family consists of monocytes, macrophages and dendritic cells (DC), specifically plasmacytoid DC (pDC) and myeloid (mDC). Each of these cell types is able to sense invading pathogens through pathogen associated molecular pattern (PAMP) recognition receptors, including toll-like receptors (TLRs) [8]. Plasmacytoid DC recognition of viral single-stranded RNA (ssRNA) by TLR7 induces copious amounts of IFN-α production [9-13]. Additionally, TLR8 activation in monocytes and mDC through ssRNA exposure leads to production of multiple pro-inflammatory cytokines including TNF-α, IL-12 and IL-6 [9].
It has been suggested that persistent recognition of viral genomes by TLR7 in pDC results in chronic production of IFN-α and leads to chronic immune activation [2, 14, 15], but these cells are significantly depleted after HIV or SIV infection [16-20]. The functionality of the remaining pDC has been explored but the results remain conflicting [21-27]. In progressive disease, circulating mDC are also depleted, while elevated levels have been shown to be related to stable disease [16, 17, 28-30]. The remaining mDC have been shown to retain their ability to respond to TLR8 activation [24, 27, 30], but thus far a link between mDC responsiveness to the invading pathogen and disease progression has yet to be found. After pathogenic SIV infection, monocytes have been shown to experience increased turnover rates that can be positively correlated with disease progression [31, 32]. Published evidence exploring monocyte and macrophage ability to respond to TLR activation remains conflicting, as dysfunction and hyper-activation have both been reported [24, 33].
Previously, the innate function of pDC has been explored by using synthetic TLR agonists [21, 24, 27], which may not accurately reflect activation by pathogens. This highlights the need for more relevant TLR agonists that directly test the ability for mononuclear phagocytes to respond to the invading pathogen itself. Because it has been shown that poly-uridine rich sequences from the HIV-1 genome are able to specifically stimulate TLR7 and TLR8 in pDC and mDC [9, 14], we identified and generated SIVmac251-derived single-stranded RNA (ssRNA) oligonucleotides and used them to stimulate either circulating or lymphatic mononuclear phagocytes ex vivo. We characterized the functionality of each mononuclear phagocyte population during acute, post-acute, and chronic infection when responding to our virus-derived TLR agonist. We found that soon after infection, pDC, monocytes, and macrophages all became hyporesponsive to virus-derived TLR ligands, whereas mDC appear more sensitive to stimulation. We were able to link mDC function with a positive prognosis of disease as animals that had hyper-responsive mDC at viral set point remained disease free one year after infection [34].
Materials and methods
Animals, virus, anti-retroviral therapy (ART), and samples
All experiments involving animals were performed with appropriate oversight and approval by the Institutional Animal Care and Use Committee of the University of Pittsburgh. For acutely infected samples, ten adult male Indian origin rhesus macaques (Macaca mulatta) were infected through intravenous inoculation of 100 TCID50 of SIVmac251 (generously provided by Preston Marx, Tulane University, LA). Five of these animals were administered anti-retroviral therapy (ART) consisting of 9-R-2-phosphonomethoxypropyl adenine (PMPA), emtricitabine (FTC; both PMPA and FTC provided by Michael Miller, Gilead Science, Foster City, CA), and L-000870812 (provided by Daria J. Hazuda, Merck Research Laboratories Rahway, NJ) beginning at 7 days post-infection as described [34]. Periodic samplings of peripheral blood leukocytes, peripheral blood mononuclear cells, and lymph nodes were processed into single cell suspensions as previously described [19] and necropsies were performed after 5 weeks of infection. Chronically infected samples that had been previously characterized and archived [30] were analyzed for responsiveness at viral set-point (10 weeks post-infection).
SIVmac251-derived ssRNA oligonucleotides
HIV-1-derived RNA40 and RNA41 have been described elsewhere [9]. The SIVmac251 consensus sequence was scanned for regions that were 20 nucleotides long, >50% uridine-rich and were not predicted to make hairpin loops. The oligonucleotides were named according to their location in the genome. The corresponding control sequences were generated to replace uridines with adenines and all oligonucleotides were ordered from Invitrogen with desalting purification. For ex vivo stimulations, 10 μg of each ssRNA oligonucleotide was coupled to DOTAP and incubated in low-volume mixed cultures for two hours. The culture volumes were then increased and brefeldin A was added for the remaining five hours. The appropriate control stimulation using the A-variant oligonucleotide was included for gating purposes [34].
Characterization of mononuclear phagocyte functionality
Flow cytometric analysis was utilized to characterize the functionality of each cell type after stimulation. Monocytes were identified in the blood as HLA-DR+ CD3− CD20− CD14+, while macrophages were defined as HLA-DR+ CD3− CD20− CD163+ in lymphatic tissue. pDC were identified as HLA-DR+ CD3− CD20− CD14/163− CD11c− CD123+. mDC were HLA-DR+ CD3− CD20− CD14/163− CD11c+ CD123−. Intracellular TNF-α and IFN-α were identified through flow cytometry as described elsewhere [34].
Results
Virus-derived ssRNA oligonucleotides are potent stimulators of mononuclear phagocytes
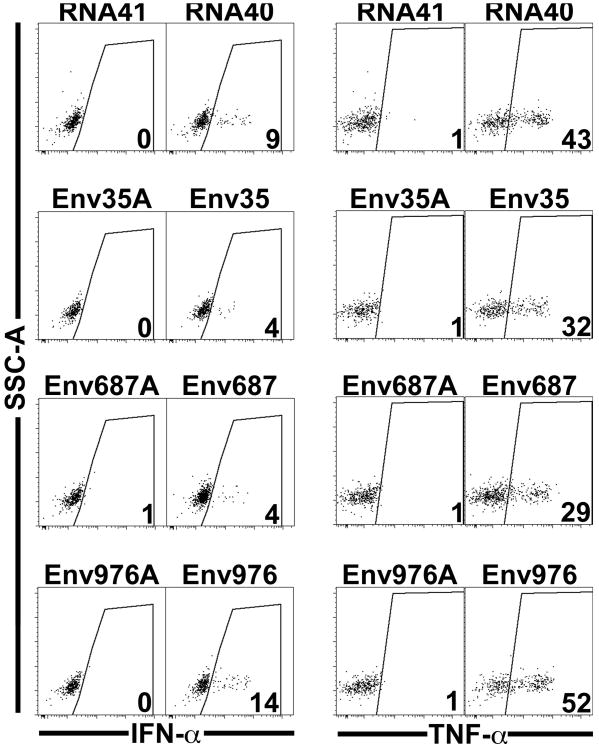
As depicted in Figure 1, we identified >50% poly-uridine rich sequences that were 20 nucleotides long and named them according to their location in the SIVmac251 genome. Eleven locations in the SIVmac251 genome were identified to fit these criteria. Four of the sequences were predicted to create hairpin loops and were therefore deemed inappropriate for our application as they would likely become double-stranded RNA. Of the remaining seven, we selected three sequences (Env35, Env687, and Env976) and their corresponding adenine-containing variants (Env35A, Env687A, and Env976A) to synthesize and be evaluated as our TLR7 and TLR8 stimulants. We then tested the stimulatory capacity of each sequence to cause circulating pDC taken from naïve rhesus macaques to produce TNF-α and IFN-α. As seen in Figure 2, each uridine-rich oligonucleotide was specifically able to stimulate pDC to produce IFN-α and TNF-α as compared to the adenine-containing variant. The HIV-1 version RNA40 caused 9% of pDC to produce IFN-α and 43% to produce TNF-α. Of our SIVmac251-derived agonists, Env976 yielded the most robust stimulation of pDC given 14% produced IFN-α and 52% produced TNF-α. We further quantified the stimulatory capacity of Env976 to activate pDC, monocytes, and mDC in blood and pDC, macrophages, and mDC in lymph nodes taken from naïve rhesus macaques [34]. We determined that blood pDC were able to produce IFN-α and TNF-α, whereas circulating monocytes and mDC were only able to produce TNF-α [34]. In contrast in lymphatic tissue, pDC and, to a larger extent, macrophages were both able to produce IFN-α and TNF-α, whereas mDC only made TNF-α [34].
Figure 1. Location of virus-encoded TLR agonists in the SIVmac251 genome.
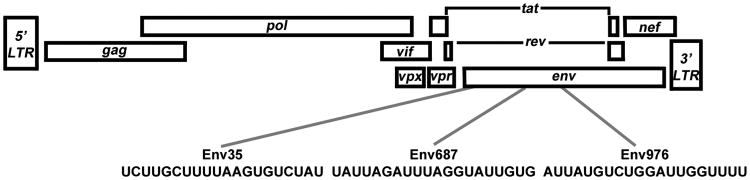
The poly-uridine rich sequences found in SIVmac251 are given and named for their location.
Figure 2. SIVmac251-derived ssRNA oligonucleotides stimulate pDC from SIV-naïve rhesus macaques to produce cytokines at varying levels.
PBMC were exposed to TLR7 and TLR8 agonists derived from either HIV-1 (RNA40) or SIVmac251 (Env35, Env687, Env976) or the respective control oligonucleotides (RNA41, Env35A, Env687A, Env976A). Plasmacytoid DC were then identified through flow cytometry as MHC-II+ CD3− CD20− CD14/163− CD11c− and CD123+. Each representative dot plot shows the percentage of pDC staining positively for each cytokine.
Mononuclear phagocytes experience divergent changes in functionality upon SIV infection when responding to virus-derived TLR agonists
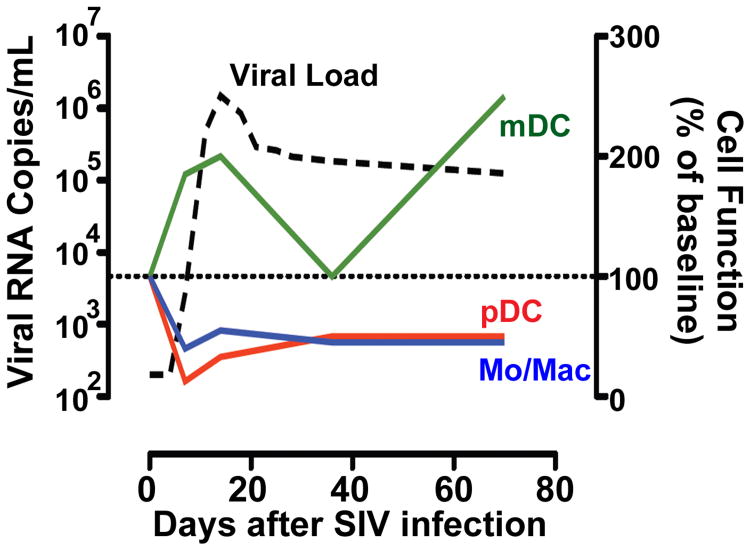
We next wanted to determine how mononuclear phagocytes taken from SIVmac251 infected rhesus macaques function in response to ex vivo exposure to TLR agonists derived from the infecting virus. Very soon after infection, pDC, monocytes, and macrophages experienced a rapid decrease in their ability to produce pro-inflammatory cytokines upon exposure to Env976 as published elsewhere [34] and summarized in Figure 3. The dysfunction of circulating pDC and monocytes was evident seven days post-infection, and pDC and macrophages from biopsied lymph nodes were rendered less functional at 14 days post-infection, effects that were largely reversed with 4 weeks of ART. This dysfunction was maintained at viral set point (ten weeks post-infection) in a separate cohort of animals that were followed long term for disease progression. In stark contrast, mDC exhibited a transient surge in production of TNF-α after exposure to Env976 during acute infection that was diminished by five weeks post-infection. Furthermore, in a separate cohort of animals that were monitored for progressive or stable disease, hyper-responsive mDC were found at viral set point only in the stably infected animals, suggesting that mDC hyper-responsiveness early on may be beneficial to control disease [34].
Figure 3. SIV infection of rhesus macaques renders pDC, monocytes, and macrophages less responsive, whereas mDC become hyper-responsive, upon exposure to virus-derived TLR ligands.
As the plasma viral load (dashed line) becomes detectable, changes in the functional responses of each cell type (right axis) are evident when compared to pre-infection (dotted line). As early as 7 days after infection, plasmacytoid DC (red), monocytes, and macrophages (blue) are left hypo-responsive. In contrast, mDC (green) become hyper-responsive to stimulation as viral load peaks during acute infection as well as during chronic stable infection.
Discussion
In order to determine how SIV infection impacts innate immune cells and overall immune activation, we generated relevant TLR agonists that would likely be encountered, as they are derived from the invading pathogen. In ex vivo mixed cultures, each ssRNA oligonucleotide was able to stimulate circulating and lymphatic mononuclear phagocytes to produce pro-inflammatory cytokines. Using these oligonucleotides to stimulate TLR7 and TLR8, we were able to conduct a direct comparison of functionality between pDC, monocytes, macrophages, and mDC after pathogenic SIV infection. We were also able to use these physiologically relevant stimuli to explore ex vivo how each innate immune cell may function at fighting chronic SIV infection in vivo.
IFN-α presence has been shown to be beneficial to the host by inhibiting HIV replication, decreasing viral load in patients, and by being elevated in infected patients experiencing elite control without therapy [35-38]. In contrast, IFN-α has also been argued to be detrimental by being implicated in chronic up-regulation of interferon stimulated genes (ISGs) leading to persistent immune activation [2, 15, 39-41]; thus, it is important to determine the ability for IFN-α producing cells to function in response to the invading pathogen in vivo. By seven days post-infection, pDC experience up to a seven-fold mobilization into the blood [20] and by 14 days after infection, macrophages experience a significant accumulation in lymph nodes [34, 42]. Already at these time points and continuing through to chronic infection, these cells are less able to produce IFN-α when exposed to virus-derived TLR agonists [34]. Likewise, there is a peak of type I IFN by two weeks post-infection [43] and our data suggests this peak may not be coming from the traditional IFN-α producers as they are rendered hyporesponsive [34]. This suggests that there are other cell types creating type I IFN that may be inducing chronic immune activation, and data from our lab and others shows that mDC from infected animals may be gaining the ability to produce IFN-α [44, 45].
In stark contrast to pDC and macrophage impairment, mDC become hyper-responsive to virus-derived TLR agonists during acute infection and this phenotype may be beneficial as it is related to disease control [34]. Previous work from our lab has shown that circulating mDC from SIV infected rhesus macaques express more CCR7 which causes them to home to lymphatic tissues [30]. The mDC found in the lymph node of SIV infected macaques express less co-stimulatory molecules suggesting that these cells are semi-mature leaving them readily activated by TLR stimulation [30, 34, 46-48]. This beneficial, hyper-responsive phenotype appears only in the chronic stages of stable disease [34], suggesting that some function of semi-mature, hyper-responsive mDC may be important for initiating an appropriate anti-SIV immune response or controlling continued immune activation. Understanding the beneficial and detrimental roles mononuclear phagocytes play on chronic immune activation may be key in understanding disease control and progression. The use of SIV infection in nonhuman primates as a model for AIDS remains crucial to improve our understanding of early immunologic events that may ultimately set the environment for chronic inflammation.
Acknowledgments
We would like to thank members of the Barratt-Boyes laboratory for all of their support during these experiments and C. Janssen for his veterinary expertise.
Funding: These studies were supported by the National Cancer Institute training grant T32 CA082084 to E.R.W. and the National Institute of Allergy and Infectious Diseases grant R01 AI071777 to S.M.B.B.
References
- 1.Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat Rev Immunol. 2009;9:717–728. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campillo-Gimenez L, Laforge M, Fay M, Brussel A, Cumont MC, Monceaux V, Diop O, Levy Y, Hurtrel B, Zaunders J, Corbeil J, Elbim C, Estaquier J. Non pathogenesis of SIV infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol. 2009 doi: 10.1128/JVI.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, Hirsch V, Lifson J, Brenchley JM, Estes JD. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 10.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 11.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 12.Diop OM, Ploquin MJ, Mortara L, Faye A, Jacquelin B, Kunkel D, Lebon P, Butor C, Hosmalin A, Barre-Sinoussi F, Muller-Trutwin MC. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8:350–360. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, van Lunzen J, Altfeld M. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 16.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 17.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 18.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald-Bocarsly P. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 19.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 20.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinson JA, Roman-Gonzalez A, Tenorio AR, Montoya CJ, Gichinga CN, Rugeles MT, Tomai M, Krieg AM, Ghanekar S, Baum LL, Landay AL. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 23.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–4511. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 24.Chang JJ, Lacas A, Lindsay RJ, Doyle EH, Axten KL, Pereyra F, Rosenberg ES, Walker BD, Allen TM, Altfeld M. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. AIDS. 2012;26:533–541. doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegal F. Interferon-producing plasmacytoid dendritic cells and the pathogenesis of AIDS. Res Initiat Treat Action. 2003;8:10–13. [PubMed] [Google Scholar]
- 26.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, Lehmann PV, Valdez H. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–4916. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 27.Sabado RL, O'Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. Aids. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 29.Jones GJ, Watera C, Patterson S, Rutebemberwa A, Kaleebu P, Whitworth JA, Gotch FM, Gilmour JW. Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients. AIDS. 2001;15:1657–1663. doi: 10.1097/00002030-200109070-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6:e1001235. doi: 10.1371/journal.ppat.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa A, Liu H, Ling B, Borda JT, Alvarez X, Sugimoto C, Vinet-Oliphant H, Kim WK, Williams KC, Ribeiro RM, Lackner AA, Veazey RS, Kuroda MJ. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–2925. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilton JC, Johnson AJ, Luskin MR, Manion MM, Yang J, Adelsberger JW, Lempicki RA, Hallahan CW, McLaughlin M, Mican JM, Metcalf JA, Iyasere C, Connors M. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J Virol. 2006;80:11486–11497. doi: 10.1128/JVI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wonderlich ER, Wijewardana V, Liu X, Barratt-Boyes SM. Virus-Encoded TLR Ligands Reveal Divergent Functional Responses of Mononuclear Phagocytes in Pathogenic Simian Immunodeficiency Virus Infection. J Immunol. 2013;190:2188–2198. doi: 10.4049/jimmunol.1201645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poli G, Orenstein JM, Kinter A, Folks TM, Fauci AS. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- 36.Bednarik DP, Mosca JD, Raj NB, Pitha PM. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc Natl Acad Sci U S A. 1989;86:4958–4962. doi: 10.1073/pnas.86.13.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, Walker R, Deyton L, Davey RT, Jr, Falloon J, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection A randomized, placebo-controlled tria. Ann Intern Med. 1990;112:805–811. doi: 10.7326/0003-4819-112-11-805. [DOI] [PubMed] [Google Scholar]
- 38.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 39.Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009;119:3512–3515. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotger M, Dang KK, Fellay J, Heinzen EL, Feng S, Descombes P, Shianna KV, Ge D, Gunthard HF, Goldstein DB, Telenti A. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog. 2010;6:e1000781. doi: 10.1371/journal.ppat.1000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otani I, Mori K, Sata T, Terao K, Doi K, Akari H, Yoshikawa Y. Accumulation of MAC387+ macrophages in paracortical areas of lymph nodes in rhesus monkeys acutely infected with simian immunodeficiency virus. Microbes Infect. 1999;1:977–985. doi: 10.1016/s1286-4579(99)80515-2. [DOI] [PubMed] [Google Scholar]
- 43.Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kader M, Smith AP, Guiducci C, Wonderlich ER, Normolle D, Watkins SC, Barrat FJ, Barratt-Boyes SM. Blocking TLR7- and TLR9-mediated IFN-α production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathogens. 2013 doi: 10.1371/journal.ppat.1003530. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, Garderet L, Fabiani B, Berger A, Schmitz J, Marie JP, Molina TJ, Pacanowski J, Viard JP, Oksenhendler E, Beq S, Abehsira-Amar O, Cheynier R, Hosmalin A. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood. 2009;113:6112–6119. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
- 46.Dillon SM, Robertson KB, Pan SC, Mawhinney S, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 48.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]