Abstract
Different isoforms of nitric oxide synthase are critically involved in the development of pulmonary failure secondary to acute lung injury. Here we tested the hypothesis that simultaneous blockade of inducible and neuronal nitric oxide synthase effectively prevents the pulmonary lesions in an ovine model of acute respiratory distress syndrome (ARDS) induced by combined burn and smoke inhalation injury. Chronically instrumented sheep were allocated to a sham-injured group (n = 6), an injured and untreated group (n = 6), or an injured group treated with simultaneous infusion of selective inducible and neuronal nitric oxide synthase inhibitors (n = 5). The injury was induced by 48 breath of cotton smoke and a 3rd degree burn of 40% total body surface area. All sheep were mechanically ventilated and fluid resuscitated. The injury induced severe pulmonary dysfunction as indicated by decreases in PaO2/FiO2 ratio and increases in pulmonary shunt fraction, ventilatory pressures, lung lymph flow, and lung wet/dry weight ratio. The treatment fully prevented the elevations in lymph and plasma nitrate/nitrite levels, pulmonary shunting, ventilatory pressures, lung lymph flow, and wet/dry weight ratio and significantly attenuated the decline in PaO2/FiO2 ratio. In conclusion, simultaneous blockade of inducible and neuronal nitric oxide synthase exerts beneficial pulmonary effects in an ovine model of ARDS secondary to combined burn and smoke inhalation injury. This novel treatment strategy may represent a useful therapeutic adjunct for patients with these injuries.
Keywords: acute lung injury, microvascular hyperpermeability, nitric oxide, sheep
Introduction
The presence of concomitant inhalation injury is a major determinant of morbidity and mortality in fire victims (1, 2), contributing to severe impairment of pulmonary function. Despite the establishment of standard treatment strategies including mechanical respiratory support and effective fluid resuscitation management, the mortality rates of these patients remain unacceptably high.
Acute lung injury may result from a direct (aspiration, pneumonia, smoke inhalation, etc.) or an indirect insult (shock, sepsis, transfusion, etc.) Combined burn and smoke inhalation trauma frequently occurs in fire victims and leads to more severe lung injury than either insult alone (2-4). The pulmonary response to this combination trauma is characterized by marked airway hyperemia and pulmonary microvascular hyperpermeability, leading to greatly enhanced transvascular fluid flow into the bronchopulmonary lymphatic vessels and secretion of mucus by airway glands, which together lead to obstructive airway cast formation, ultimately resulting in severe impairment of gas exchange (3-6).
It has previously been demonstrated that excessive formation of nitric oxide (NO) by the inducible NO synthase (iNOS) plays a major role in the pathophysiological alterations following burn and inhalation injury (6-10). More recent evidence suggests that NO produced by increased activity of neuronal NOS (nNOS) is also critically involved in this process (11, 12). Because specific pharmacological blockade of either iNOS or nNOS has been shown to significantly attenuate, but not to fully reverse, pulmonary dysfunction in sheep subjected to combined burn and inhalation injury, we hypothesized that simultaneous iNOS and nNOS inhibitor treatment effectively prevents the pulmonary lesions resulting from this combination injury. We tested this hypothesis in an established ovine model of severe burn and smoke inhalation injury.
Materials and Methods
This study was approved and monitored by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch. The studies were accomplished in the Investigative Intensive Care Unit, a facility certified by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Surgical preparation and injury
Seventeen healthy adult female sheep with a mean body weight of 30-40 kg were included in this study. Following induction of anesthesia with ketamine (500 mg intramuscular, 300 mg intravenously), endotracheal intubation was performed. Anesthesia was maintained using an isoflurane (1.4-1.8 Vol%)-oxygen mixture. The right femoral artery was cannulated with a polyvinylchloride catheter (Intracath, 16-G, 24 inches, Becton Dickinson Vascular Access; Sandy, UT). A thermodilution catheter (model 93A-131-7F, Edwards Critical Care Division; Irvine, CA) was inserted into the right external jugular vein through an introducer sheath (Edwards Lifescience; Irvine, CA) and advanced into the common pulmonary artery. Through the left fifth intercostal space, a Silastic catheter (0.062 inch inner diameter (ID) and 0.125 inch outer diameter (OD), Dow Corning; Midland, MI) was positioned in the left atrium. Through the right fifth intercostal space, a Silastic catheter (0.025 inch ID and 0.047 inch OD) was placed into an efferent lymphatic vessel from the caudal mediastinal lymph node. Ligation of the tail of the caudal mediastinal lymph node and cauterization of the systemic diaphragmatic lymph vessels were performed to remove the systemic lymph contribution.
After a recovery period of 5-7 days, a baseline measurement was performed in spontaneously breathing sheep. Thereafter, the animals were anesthetized using intravenous ketamine 5 mg/kg. Then, tracheotomy was performed and anesthesia was maintained with an isoflurane (1.1-2.0 Vol%)-oxygen mixture. Under deep anesthesia, the animals received combined burn and smoke inhalation injury according to an established protocol which has previously been described in detail (6, 10, 13). The animal's wool was removed, and a 20% total body surface area (TBSA) third-degree (full thickness) flame burn was made on each flank, resulting in a total 40% TBSA burn. The third-degree burn destroys the nerve endings in the skin and is considered a painless injury. We administered 0.03 mg buprenorphine intravenously (Butler Animal Health Supply, Dublin, OH) during the burn and every 12 hours subsequently, to provide analgesia for the edges of the burn, which may be second-degree burns. Inhalation injury was induced using a modified bee smoker filled with 40 g of burning cotton toweling and connected to the tracheostomy tube. The temperature of the smoke was monitored carefully and not allowed to exceed 40°C. Each sheep was insufflated with fours sets of 12 breaths of cotton smoke. A Foley urinary retention catheter was inserted. Anesthesia was then discontinued, and the sheep were allowed to awaken.
Experimental protocol
After injury, the sheep were randomly allocated to the following three study groups: 1) sham-injured, non-treated animals (sham; n = 6), 2) injured, non-treated animals (control; n = 6), and 3) injured animals (n = 5) treated with a simultaneous infusion of the potent and selective iNOS dimerization inhibitor BBS-2 (50 μg/kg/h; ZK-809984, Berlex, Richmond, CA) (14) and the selective nNOS inhibitor 7-nitroindazole (500 μg/kg/h; Sigma-Aldrich, St. Louis, MI) (15). The animals of the sham and control group received the same amount of the vehicles (3 ml/h for the BBS-2 vehicle and 20 ml/h for the 7-NI vehicle). The treatment was started one hour after the injury and continued until the end for the 48-hour experimental period. Both specific inhibitors have been established in several previous studies using the same and a similar sheep model (6, 8, 11, 12, 15-17). The established doses of BBS-2 and 7-NI have been reduced in the current study because in a previous study we reported hazardous effects of simultaneous infusion on splanchnic perfusion (12). All sheep were mechanically ventilated (Servo Ventilator 900C, Siemens, Elema, Sweden) with a tidal volume of 12-15 ml/kg and a positive end expiratory pressure of 5 cm H2O. A higher tidal volume is required for sheep as compared to humans because sheep have a higher dead space to tidal volume ratio (Vd/Vt 0.57 sheep vs. 0.30 human) (18). The FiO2 was set at 1.0 for the first 3 hours post-injury and was then adjusted to maintain sufficient oxygenation (SaO2 >90%, PaO2 80-100 mmHg). The respiratory rate was initially set at 20 breaths per minute and was then adjusted according to blood gas analyses to maintain the PaCO2 within 5 mmHg of the baseline value. All animals were fluid resuscitated with lactated Ringer's solution according to the formula: 4 ml/kg/%TBSA/24 hours. The amount of the 7-NI and BBS-2 infusion or its vehicles was subtracted from the quantity of the Ringer's solution. The sheep had free access to dry food, but not water, to control the fluid balance. At the end of the 48-hour study period, the animals were deeply anesthetized with ketamine (15 mg/kg) and euthanized by intravenous injection of 60 ml saturated potassium chloride. Immediately after expiration, autopsy was performed to harvest lung tissue samples and inspect the bowels for signs of ischemia and integrity.
Hemodynamic measurements
Systemic and pulmonary hemodynamic variables were determined from the femoral and pulmonary artery catheters using pressure transducers (Baxter-Edwards Critical Care, Irvine, CA) and recorded on a hemodynamic monitor (monitor V24C, Philips Medicine System Bollinger, Bollinger, Germany). Cardiac output was measured in triplicate with the thermodilution technique (Monitor 9530, Baxter-Edwards Critical Care).
Blood and lymph analysis
Blood gases and arterial lactate concentrations were measured using a blood gas analyzer (model GEM Premier 3000, Instrumentation Laboratory, Lexington, MA). PaO2 /FiO2 ratio, pulmonary shunt fraction (Qs/Qt), and vascular resistances were calculated using standard equations. Blood was centrifuged and plasma and serum samples frozen at −80°C for the determination of serum aspartate aminotransferase, alanine aminotransferases, bilirubin, lipase, and creatinine (Vitros 5,1 FS, Ortho Clinical Diagnostics, Rochester, NY, USA). Plasma and lymph nitric oxide levels were evaluated by measuring the intermediate and end products, using a nitrate/nitrite (NOx) colorimetric assay kit (Cayman Chemicals, Ann Arbor, MI, USA). The lung lymph NOx production per hour was calculated as the product of lymph NOx concentration and lung lymph flow. Colloid oncotic pressures (ΠP) were determined with a colloid osmometer (model 4420, Wescor; Logan, UT). Protein concentrations (CP) were measured with a refractometer (National Instrument; Baltimore, MD). Lung lymph flow (QL) was collected for an hour and measured with graduated test tubes. Lung permeability index was calculated using the formula QL × (CP lymph / CP plasma) (19).
Lung tissue analysis
The right lung was removed, and a 1-cm thick section was taken from the lower lobe and inflated with 10% formalin for histological examination. Fixed samples were embedded in paraffin, sectioned into 4-μm pieces and stained with hematoxylineosin. A pathologist who was unaware of the group assignments analyzed the samples. Bronchial obstruction was evaluated by estimating the degree of luminal obstruction (0-100%) as previously described (5). From the remaining part of the right lower lobe, bloodless lung wet/dry weight ratio was calculated as an index of lung water content (20).
Statistical analysis
Sigma Stat 3.1 software (Systat Software, Inc., San Jose, CA, USA) was used for statistical analyses. All values are expressed as means ± SEM. Results were compared by a two-way analysis of variance (ANOVA) for repeated measurements with appropriate Student-Newman-Keuls post hoc comparisons to compare differences within and between groups. One-way ANOVA was used to compare groups when measurements were made at only one time point; and the Newman-Keuls procedure was used for post hoc pairwise comparisons. A value of p < 0.05 was regarded as statistically significant.
Results
At baseline, there were no differences among study groups in any of the investigated variables as shown in tables and figures. All animals survived the experimental period.
Respiratory gas exchange and ventilatory pressures
Combined burn and smoke inhalation injury induced severe deteriorations in respiratory gas exchange in the control group, as indicated by a fall in PaO2/FiO2 ratio below 200 mmHg and a significant increase in pulmonary shunt fraction. In the treatment group, the PaO2/FiO2 ratio was moderately decreased toward the sham group, but remained above 350 mmHg throughout the experimental period. The increase in pulmonary shunt fraction seen in controls was abolished in treated animals (Fig. 1). The injury-related elevations in peak and pause airway pressures in the control group were also prevented by the treatment (Fig. 2).
Fig. 1.
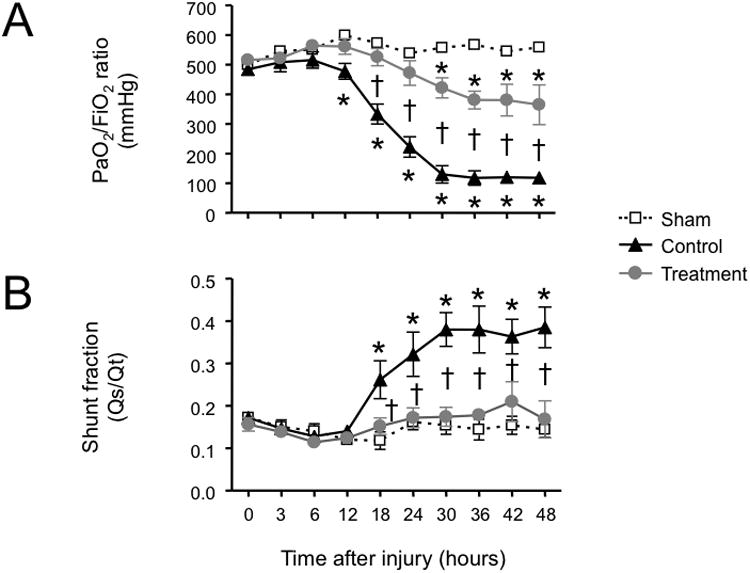
Impact of simultaneous neuronal and inducible nitric oxide synthase inhibition on (A) PaO2/FiO2 ratio and (B) pulmonary shunt fraction (Qs/Qt) in sheep with combined burn and smoke inhalation injury. Data are expressed as mean ± SEM. * P < 0.05 vs. sham; †P < 0.05 treatment vs. control.
Fig. 2.
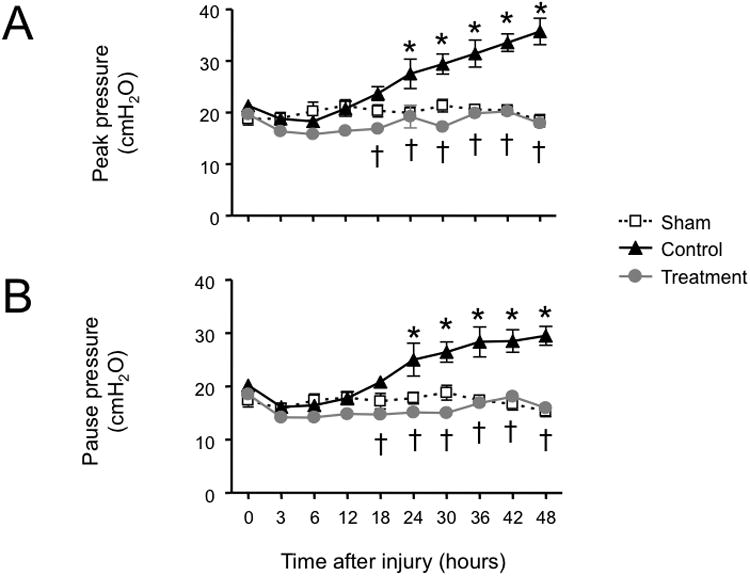
Impact of simultaneous neuronal and inducible nitric oxide synthase inhibition on ventilatory pressures (A, peak airway pressure and B, pause airway pressure) in sheep with combined burn and smoke inhalation injury. Data are expressed as mean ± SEM. * P < 0.05 vs. sham; † P < 0.05 treatment vs. control.
Microvascular permeability and lung water content
In the control group, pulmonary transvascular fluid flux significantly increased after the injury as evidenced by a ninefold elevation in lung lymph flow. Bloodless lung wet/dry weight ratio was also significantly enhanced in control versus sham animals. Both lung lymph flow and wet/dry weight ratio were significantly reduced to the levels of sham animals by the combined NOS inhibitor treatment (Fig. 3). The total amounts of fluids received were 243 ± 7 ml/kg in the sham group, 232 ± 9 ml/kg in the control group, and 238 ± 7 ml/kg in the treatment group (P > 0.05 between groups). Despite identical fluid resuscitation in all study groups, hematocrit and hemoglobin concentrations tended to be elevated in the control group, while these variables remained at baseline levels in treated animals. The injury was further associated with significant decreases in both CP and ΠP in the control group which were partially attenuated in the treatment group. The injury-related increase in lung permeability index seen in the control group was prevented by the (Table 1).
Fig. 3.
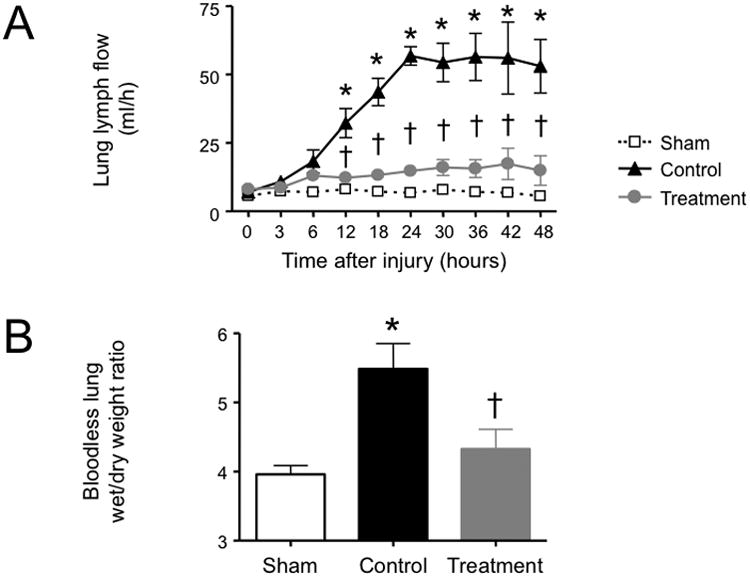
Impact of simultaneous neuronal and inducible nitric oxide synthase inhibition on (A) lung lymph flow and (B) bloodless lung wet/dry weight ratio in sheep with combined burn and smoke inhalation injury. Data are expressed as mean ± SEM. * P < 0.05 vs. sham; † P < 0.05 treatment vs. control.
Table 1. Changes in plasma protein concentration and oncotic pressure, hematocrit, and hemoglobin.
| Time after injury (hours) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 6 | 12 | 18 | 24 | 36 | 48 | |
| CP, g/dl | |||||||
| Sham | 4.7 ± 0.1 | 3.9 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.3 ± 0.1 | 4.3 ± 0.2 | 4.5 ± 0.1 |
| Control | 4.8 ± 0.1 | 3.7 ± 0.1 | 3.6 ± 0.2 | 3.1 ± 0.3a | 2.9 ± 0.3a | 2.8 ± 0.2a | 3.1 ± 0.3a |
| Treatment | 4.7 ± 0.2 | 3.3 ± 0.2 | 3.2 ± 0.2a | 2.8 ± 0.2a | 3.2 ± 0.1a | 3.8 ± 0.1b | 3.9 ± 0.1a,b |
| ΠP, mmHg | |||||||
| Sham | 20.5 ± 0.6 | 19.2 ± 0.7 | 20.3 ± 0.8 | 19.6 ± 0.9 | 19.8 ± 1.0 | 19.6 ± 1.1 | 19.0 ± 1.3 |
| Control | 20.2 ± 1.3 | 15.7 ± 0.6a | 15.5 ± 1.1a | 13.3 ± 0.9a | 12.6 ± 1.1a | 11.0 ± 1.2a | 12.3 ± 1.3 |
| Treatment | 20.3 ± 0.9 | 13.4 ± 1.0a | 14.0 ± 0.6a | 14.3 ± 0.3a | 13.7 ± 0.5a | 15.3 ± 0.5a,b | 15.6 ± 0.6a,b |
| Lung permeability index | |||||||
| Sham | 4.1 ± 0.6 | 5.6 ± 1.7 | 6.6 ± 1.0 | 5.6 ± 0.3 | 4.6 ± 0.5 | 4.9 ± 0.7 | 3.8 ± 0.6 |
| Control | 4.3 ± 0.6 | 10.4 ± 2.8 | 21.1 ± 3.8a | 30.5 ± 4.7a | 43.1 ± 4.5a | 32.9 ± 5.3a | 27.0 ± 4.5a |
| Treatment | 5.0 ± 0.7 | 7.3 ± 0.9 | 7.0 ± 1.4b | 8.3 ± 1.2b | 8.6 ± 1.6b | 7.7 ± 2.6b | 8.3 ± 3.7b |
| Hematocrit, % | |||||||
| Sham | 24 ± 1 | 22 ± 1 | 23 ± 1 | 23 ± 1 | 24 ± 1 | 23 ± 1 | 24 ± 1 |
| Control | 23 ± 1 | 26 ± 2 | 26 ± 2 | 27 ± 2 | 27 ± 2 | 28 ± 3a | 26 ± 2 |
| Treatment | 23 ± 2 | 23 ± 2 | 22 ± 2 | 21 ± 2b | 21 ± 1b | 23 ± 2 | 22 ± 2 |
| Hemoglobin, g/dl | |||||||
| Sham | 8.5 ± 0.3 | 8.0 ± 0.3 | 8.0 ± 0.3 | 7.7 ± 0.4 | 8.4 ± 0.6 | 8.0 ± 0.4 | 8.1 ± 0.5 |
| Control | 8.0 ± 0.5 | 8.8 ± 0.7 | 8.7 ± 0.7 | 9.2 ± 0.8 | 9.3 ± 0.8 | 9.5 ± 0.9 | 8.7 ± 0.8 |
| Treatment | 7.9 ± 0.6 | 8.0 ± 0.5 | 7.7 ± 0.7 | 7.4 ± 0.7 | 7.3 ± 0.8 | 7.8 ± 0.8 | 7.3 ± 0.6 |
CP, plasma protein concentration; ΠP, plasma colloid oncotic pressure. Data are expressed as mean ± SEM.
P < 0.05 vs. sham;
P < 0.05 treatment vs. control.
Bronchial obstruction score
The combination injury was associated with a significant increase in the histologically determined bronchial obstruction score in control versus sham animals. However, the bronchial obstruction score was not significantly elevated versus sham animals in the treatment group (Fig. 4).
Fig. 4.
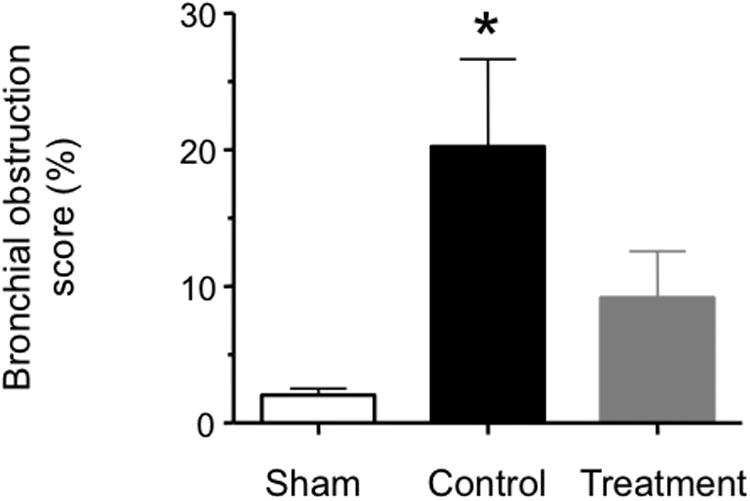
Impact of simultaneous neuronal and inducible nitric oxide synthase inhibition on histologically determined bronchial obstruction in sheep with combined burn and smoke inhalation injury. Data are expressed as mean ± SEM. * P < 0.05 vs. sham.
Lung lymph and plasma nitrate/nitrite concentrations
The lung lymph NOx was significantly increased in the control versus sham group at 12, 24, and 48 hours post-injury. The plasma NOx concentration in the control group was also increased towards sham 12 and 48 hours after induction of the injury. Simultaneous NOS inhibitor treatment prevented the increases in both lung lymph and plasma NOx levels (Fig. 5).
Fig. 5.
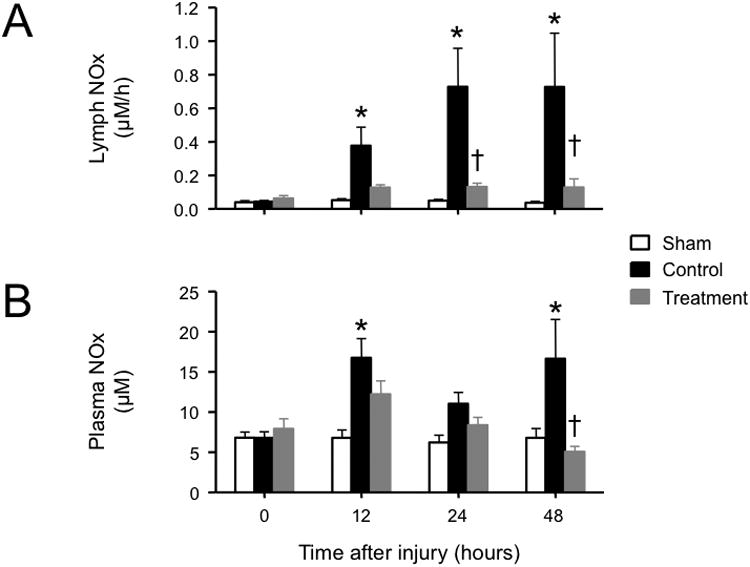
Impact of simultaneous neuronal and inducible nitric oxide synthase inhibition on (A) lung lymph and (B) plasma concentrations of nitrite/nitrate (NOx) in sheep with combined burn and smoke inhalation injury. Data are expressed as mean ± SEM. * P < 0.05 vs. sham; † P < 0.05 treatment vs. control.
Cardiopulmonary hemodynamics and extrapulmonary organ functions
No significant group differences in key variables of systemic and pulmonary hemodynamics were detected, except for an injury-related increase in heart rate in the control and treatment group (Table 2). Serum aspartate aminotransferase and alanine aminotransferase equally increased over time in both injured groups versus the sham group. Serum bilirubin was significantly increased in controls versus the sham and treatment group, but remained within normal range in all groups. There were no significant differences in arterial lactate concentrations, serum lipase, creatinine, or urine output among groups (data not shown). Inspection of the bowels at autopsy revealed no signs of gut ischemia or perforations in all animals.
Table 2. Changes in cardiopulmonary variables.
| Time after injury (hours) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 0 | 6 | 12 | 18 | 24 | 36 | 48 | |
| MAP, mmHg | |||||||
| Sham | 96 ± 3 | 107 ± 3 | 103 ± 4 | 103 ± 2 | 100 ± 2 | 97 ± 4 | 96 ± 3 |
| Control | 96 ± 3 | 114 ± 6 | 105 ± 5 | 103 ± 4 | 102 ± 5 | 102 ± 6 | 104 ± 5 |
| Treatment | 94 ± 4 | 116 ± 5 | 113 ± 7 | 111 ± 5 | 107 ± 5 | 103 ± 3 | 106 ± 5 |
| HR, bpm | |||||||
| Sham | 83 ± 3 | 92 ± 3 | 117 ± 10 | 113 ± 8 | 107 ± 5 | 101 ±5 | 90 ± 3 |
| Control | 91 ± 5 | 124 ± 10a | 118 ± 6 | 145 ± 7a | 148 ± 6a | 153 ± 8a | 131 ± 5a |
| Treatment | 99 ± 4 | 125 ± 9a | 139 ± 12 | 126 ± 9 | 122 ± 2 | 132 ± 7a | 129 ± 5a |
| CI, l/min/m2 | |||||||
| Sham | 6.3 ± 0.2 | 7.3 ± 0.2 | 7.1 ± 0.4 | 7.2 ± 0.4 | 6.4 ± 0.4 | 7.5 ± 0.6 | 6.0 ± 0.5 |
| Control | 6.4 ± 0.2 | 7.4 ± 0.5 | 6.6 ± 0.8 | 7.1 ± 0.6 | 6.3 ± 0.5 | 6.6 ± 0.7 | 6.7 ± 0.4 |
| Treatment | 6.5 ± 0.3 | 6.6 ± 0.5 | 7.7 ± 0.9 | 7.0 ± 0.5 | 7.2 ± 0.6 | 7.3 ± 0.2 | 7.6 ± 0.7 |
| LAP, mmHg | |||||||
| Sham | 9 ± 0 | 12 ± 2 | 13 ± 2 | 11 ± 1 | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| Control | 8 ± 1 | 10 ± 1 | 9 ± 1 | 11 ± 1 | 11 ± 1 | 10 ± 1 | 12 ± 2 |
| Treatment | 9 ± 0 | 11 ± 1 | 11 ± 1 | 12 ± 1 | 12 ± 1 | 11 ± 1 | 10 ± 1 |
| PCWP, mmHg | |||||||
| Sham | 11 ±0 | 16 ± 1 | 17 ± 1 | 16 ± 1 | 15 ± 1 | 16 ± 1 | 16 ± 1 |
| Control | 11 ± 1 | 14 ± 1 | 14 ± 1 | 16 ± 1 | 16 ± 1 | 14 ± 1 | 14 ± 1 |
| Treatment | 13 ± 0 | 16 ± 1 | 17 ± 1 | 17 ± 1 | 17 ± 1 | 17 ± 0 | 15 ± 0 |
| MPAP, mmHg | |||||||
| Sham | 19 ± 0 | 28 ± 1 | 28 ± 1 | 27 ± 0 | 25 ± 1 | 29 ± 1 | 25 ± 1 |
| Control | 19 ± 1 | 26 ± 1 | 26 ± 2 | 27 ± 1 | 29 ± 1 | 27 ± 1 | 28 ± 2 |
| Treatment | 21 ± 0 | 29 ± 2 | 27 ± 2 | 27 ± 2 | 27 ± 1 | 27 ± 1 | 26 ± 1 |
CI, cardiac index; HR, heart rate; LAP, left atrial pressure; MAP, mean arterial pressure; MPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure. The data are expressed as mean ± SEM.
P < 0.05 vs. sham;
P < 0.05 treatment vs. control.
Discussion
The key finding of the present study is that simultaneous iNOS and nNOS blockade efficiently attenuated the development of burn and smoke-induced acute lung injury in sheep. Notably, the injury-related pulmonary ventilation/perfusion mismatch and increases in ventilatory pressures, transvascular fluid flux, and lung water content were completely prevented. In addition, the deterioration of pulmonary oxygenation was more effectively mitigated by simultaneous iNOS and nNOS blockade than with single-drug strategies tested in previous experiments with the same animal model (6, 12).
NO is an endogenous vasodilator generated from L-arginine through catalysis by three different genetic NOS isoforms. While iNOS (NOS-2) is up-regulated by diverse stress stimuli such as oxidative burst and systemic inflammation (21), the constitutively synthesized isoenzymes nNOS (NOS-1) and endothelial NOS (eNOS or NOS-3) were believed to be involved in various physiologic processes (22). In this regard, a previous experiment demonstrated that iNOS is increasingly expressed in bronchial ciliated cells, basal cells, mucus gland cells, and epithelial type I cells in sheep lungs following combined burn and smoke inhalation injury, whereas no change in nNOS and eNOS expressions was observed during a 48-hour time course (7). However, recent evidence suggests that not only iNOS, but also nNOS-derived NO may be critically involved in the pathophysiology of several diseases including acute lung injury (11, 12, 23, 24). As an essential regulator of vascular tone and microcirculatory blood flow, including vascular permeability (25), NO is thought to be critically involved in the regulation of bronchial blood flow (26). In acute lung injury, disturbances of NO synthesis in the lung tissue contribute to the loss of hypoxic pulmonary vasoconstriction and a subsequent ventilation/perfusion mismatch (12, 27). In conjunction with NO-induced pulmonary microvascular hyperpermeability, increased transvascular fluid flux, and pulmonary edema formation (10), it comes to the development of pulmonary failure with a severely disturbed respiratory gas exchange and systemic hypoxemia (3).
Previous studies have demonstrated that pharmacological blockade of either iNOS or nNOS attenuated the degree of experimental burn and smoke-induced acute lung injury (6, 8-12). However, it remained undetermined if iNOS and nNOS inhibition have synergistic effects. To answer this question, we compared the effects of the double inhibitor strategy on several parameters of lung injury in the current study with the results from previous experiments testing single inhibitor infusion in the same animal model (6, 11, 12). We found that either single iNOS (6) or nNOS inhibitor treatment (11, 12) partially mitigated the decline in PaO2/FiO2 ratio, an index of pulmonary oxygenation. While the values of PaO2/FiO2 ratio in untreated control animals decreased to approximately 100 mmHg at 48 hours post-injury (6, 11, 12), this index was about 200 mmHg in sheep treated with an iNOS inhibitor (6) and 250 mmHg when treated with a nNOS inhibitor (12). In contrast, the PaO2/FiO2 ratio in sheep treated with simultaneous blockade of both NOS isoforms was still at approximately 370 mmHg at 48 hours post-injury. Likewise, the injury-related increases in pulmonary shunt fraction and ventilatory pressures were only partially attenuated by either single iNOS or nNOS inhibition in previous experiments (6, 11, 12), but even fully prevented in sheep treated with concomitant inhibition of both NOS isoforms in the present study. Unfortunately, the effects of single nNOS inhibition after burn and smoke inhalation injury on bronchopulmonary lymph flux have not been tested before, but it has been reported that lymph flow can be decreased by single iNOS blockade (6). However, the lymph flow in injured and only iNOS inhibitor-treated sheep was still significantly elevated toward the sham-injured group (6), while the lymph flow was not significantly increased toward sham in sheep that were treated with simultaneous nNOS and iNOS blockade. Taken together, these data suggest that single blockade of either nNOS or iNOS attenuates the injury-related changes of several parameters of pulmonary function, but there are still indices of significant alterations in function and physiology compared to sham-injured animals. In contrast, the double inhibitor treatment in the present study more effectively prevents the injury-related changes, and in most parameters even no differences from the sham group are reported. Thus, simultaneous infusion of selective nNOS and iNOS blockers has synergistic effects as compared to single drug infusion on pulmonary alterations in sheep subjected to burn and smoke inhalation injury.
Furthermore, it is noteworthy that NOS inhibition may result in excessive systemic and microregional vasoconstriction. In this context, a preliminary report suggested potential hazardous effects of simultaneous NOS blockade on splanchnic perfusion (12). The authors of this previous experiment observed gut perforations in some animals concomitantly treated with the same doses of two NOS inhibitors that have been established as single-drug treatments (12). Thus, we tested the effects of simultaneous infusion of the selective iNOS inhibitor BBS-2 and the nNOS inhibitor 7-nitroindazole in reduced dose in the current study. Notably, this reduced doses proved safe with respect to systemic and pulmonary hemodynamic variables as well as surrogate parameters of hepatosphlanchnic and renal function. Furthermore, it is worth mentioning that arterial lactate concentrations, as possible indicator of splanchnic hypoperfusion, were not different among groups and no perforated or ischemia-suspicious regions in the gut were found at autopsy.
Some limitations of the current study need to be discussed. First, we did not include additional groups that were treated with sole iNOS and nNOS inhibitor treatment because these effects have previously been tested in the same animal model (6, 12). In addition, we did not conduct further dose response studies, but halved the established doses of NOS inhibitors. However, it is apparent that the reduced doses of these NOS inhibitors were safe and highly effective. In a previous clinical study, administration of high doses of the non-specific NOS inhibitor 546C88 has been reported to increase mortality in patients with septic shock (28), whereas lower doses of 546C88 exerted beneficial effects in previous studies (29). Thus, the results of previous and our studies suggest that either eNOS inhibition or simply excessive inhibition of all NOS isoforms may be detrimental. Future studies are warranted to explicitly test this hypothesis.
In conclusion, the current study demonstrates beneficial pulmonary effects of simultaneous pharmacological iNOS and nNOS blockade in sheep subjected to combined burn and smoke inhalation injury. Notably, the treatment proved more effective than sole iNOS or nNOS inhibition in previous experiments. Combined NOS blockade may represent a useful therapeutic adjunct for patients with these injuries.
Acknowledgments
This study was supported by grants from the National Institute of Health P012 GM066312, R01 GM060688, Shriners Burns Institute 85041, 84050, 84080, and GM60915.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmieri TL, Warner P, Mlcak RP, Sheridan R, Kagan RJ, Herndon DN, Tompkins R, Greenhalgh DG. Inhalation injury in children: a 10 year experience at Shriners Hospitals for Children. J Burn Care Res. 2009;30(1):206–208. doi: 10.1097/BCR.0b013e3181923ea4. [DOI] [PubMed] [Google Scholar]
- 2.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci. 2003;18:125–129. doi: 10.1152/nips.01427.2002. [DOI] [PubMed] [Google Scholar]
- 4.Soejima K, Schmalstieg FC, Sakurai H, Traber LD, Traber DL. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1233–1241. doi: 10.1152/ajplung.2001.280.6.L1233. [DOI] [PubMed] [Google Scholar]
- 5.Cox RA, Burke AS, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29(3):295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 6.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips GB, Parkinson JF, Cox R, Hawkins H, Herndon D, Traber D. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167(7):1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 7.Cox RA, Jacob S, Oliveras G, Murakami K, Enkhbaatar P, Traber L, Schmalstieg FC, Herndon DN, Traber DL, Hawkins HK. Pulmonary expression of nitric oxide synthase isoforms in sheep with smoke inhalation and burn injury. Exp Lung Res. 2009;35(2):104–118. doi: 10.1080/01902140802446832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips G, Parkinson J, Salsbury JR, Biondo N, Schmalstieg F, Burke A, Cox R, Hawkins H, Herndon D, Traber D. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am J Physiol Heart Circ Physiol. 2003;285(6):H2430–2436. doi: 10.1152/ajpheart.00055.2003. [DOI] [PubMed] [Google Scholar]
- 9.Soejima K, McGuire R, Snyder Nt, Uchida T, Szabo C, Salzman A, Traber LD, Traber DL. The effect of inducible nitric oxide synthase (iNOS) inhibition on smoke inhalation injury in sheep. Shock. 2000;13(4):261–266. doi: 10.1097/00024382-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Soejima K, Traber LD, Schmalstieg FC, Hawkins H, Jodoin JM, Szabo C, Szabo E, Virag L, Salzman A, Traber DL. Role of nitric oxide in vascular permeability after combined burns and smoke inhalation injury. Am J Respir Crit Care Med. 2001;163(3 Pt 1):745–752. doi: 10.1164/ajrccm.163.3.9912052. [DOI] [PubMed] [Google Scholar]
- 11.Saunders FD, Westphal M, Enkhbaatar P, Wang J, Pazdrak K, Nakano Y, Hamahata A, Jonkam CC, Lange M, Connelly RL, Kulp GA, Cox RA, Hawkins HK, Schmalstieg FC, Horvath E, Szabo C, Traber LD, Whorton E, Herndon DN, Traber DL. Molecular biological effects of selective neuronal nitric oxide synthase inhibition in ovine lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298(3):L427–436. doi: 10.1152/ajplung.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westphal M, Enkhbaatar P, Schmalstieg FC, Kulp GA, Traber LD, Morita N, Cox RA, Hawkins HK, Westphal-Varghese BB, Rudloff HE, Maybauer DM, Maybauer MO, Burke AS, Murakami K, Saunders F, Horvath EM, Szabo C, Traber DL. Neuronal nitric oxide synthase inhibition attenuates cardiopulmonary dysfunctions after combined burn and smoke inhalation injury in sheep. Crit Care Med. 2008;36(4):1196–1204. doi: 10.1097/CCM.0b013e31816a1a0c. [DOI] [PubMed] [Google Scholar]
- 13.Lange M, Enkhbaatar P, Traber DL, Cox RA, Jacob S, Mathew BP, Hamahata A, Traber LD, Herndon DN, Hawkins HK. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J Appl Physiol. 2009;107(1):176–184. doi: 10.1152/japplphysiol.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blasko E, Glaser CB, Devlin JJ, Xia W, Feldman RI, Polokoff MA, Phillips GB, Whitlow M, Auld DS, McMillan K, Ghosh S, Stuehr DJ, Parkinson JF. Mechanistic studies with potent and selective inducible nitric-oxide synthase dimerization inhibitors. J Biol Chem. 2002;277(1):295–302. doi: 10.1074/jbc.M105691200. [DOI] [PubMed] [Google Scholar]
- 15.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, McGuire R, Schmalstieg F, Cox R, Hawkins H, Jodoin J, Lee S, Traber L, Herndon D, Traber D. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R366–372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 16.Enkhbaatar P, Murakami K, Traber LD, Cox R, Parkinson JF, Westphal M, Esechie A, Morita N, Maybauer MO, Maybauer DM, Burke AS, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. The inhibition of inducible nitric oxide synthase in ovine sepsis model. Shock. 2006;25(5):522–527. doi: 10.1097/01.shk.0000209525.50990.28. [DOI] [PubMed] [Google Scholar]
- 17.Lange M, Connelly R, Traber DL, Hamahata A, Cox RA, Nakano Y, Bansal K, Esechie A, von Borzyskowski S, Jonkam C, Traber LD, Hawkins HK, Herndon DN, Enkhbaatar P. Combined neuronal and inducible nitric oxide synthase inhibition in ovine acute lung injury. Crit Care Med. 2009;37(1):223–229. doi: 10.1097/CCM.0b013e3181926104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal Melo MF, Harris RS, Layfield D, Musch G, Venegas JG. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology. 2002;97(3):671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Hinder F, Meyer J, Booke M, Ehardt JS, Salsbury JR, Traber LD, Traber DL. Endogenous nitric oxide and the pulmonary microvasculature in healthy sheep and during systemic inflammation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1542–1549. doi: 10.1164/ajrccm.157.5.9707161. [DOI] [PubMed] [Google Scholar]
- 20.Pearce ML, Yamashita J, Beazell J. Measurement of Pulmonary Edema. Circ Res. 1965;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 21.Nathan C, Xie QW. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 22.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411(2-3):437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 23.Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;278(5):H1480–1489. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- 24.McKinnon RL, Lidington D, Bolon M, Ouellette Y, Kidder GM, Tyml K. Reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle is mediated by nNOS-derived NO. Cardiovasc Res. 2006;69(1):236–244. doi: 10.1016/j.cardiores.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho P, Thompson WH, Charan NB. Comparative effects of alpha-receptor stimulation and nitrergic inhibition on bronchovascular tone. J Appl Physiol. 2000;88(5):1685–1689. doi: 10.1152/jappl.2000.88.5.1685. [DOI] [PubMed] [Google Scholar]
- 27.Westphal M, Cox RA, Traber LD, Morita N, Enkhbaatar P, Schmalstieg FC, Hawkins HK, Maybauer DM, Maybauer MO, Murakami K, Burke AS, Westphal-Varghese BB, Rudloff HE, Salsbury JR, Jodoin JM, Lee S, Traber DL. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med. 2006;34(5):1428–1436. doi: 10.1097/01.CCM.0000215828.00289.B9. [DOI] [PubMed] [Google Scholar]
- 28.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 29.Bakker J, Grover R, McLuckie A, Holzapfel L, Andersson J, Lodato R, Watson D, Grossman S, Donaldson J, Takala J. Administration of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002) Crit Care Med. 2004;32(1):1–12. doi: 10.1097/01.CCM.0000105118.66983.19. [DOI] [PubMed] [Google Scholar]


