Abstract
Context
The role of diet composition in response to overeating and energy dissipation in humans is unclear.
Objective
To evaluate the effects of overconsumption of low, normal, and high protein diets on weight gain, energy expenditure, and body composition.
Design, Setting, and Participants
A single-blind, randomized controlled trial of 25 US healthy, weight-stable male and female volunteers, aged 18 to 35 years with a body mass index between 19 and 30. The first participant was admitted to the inpatient metabolic unit in June 2005 and the last in October 2007.
Intervention
After consuming a weight-stabilizing diet for 13 to 25 days, participants were randomized to diets containing 5% of energy from protein (low protein), 15% (normal protein), or 25% (high protein), which they were overfed during the last 8 weeks of their 10- to 12-week stay in the inpatient metabolic unit. Compared with energy intake during the weight stabilization period, the protein diets provided approximately 40% more energy intake, which corresponds to 954 kcal/d (95% CI, 884–1022 kcal/d).
Main Outcome Measures
Body composition was measured by dual-energy x-ray absorptiometry biweekly, resting energy expenditure was measured weekly by ventilated hood, and total energy expenditure by doubly labeled water prior to the overeating and weight stabilization periods and at weeks 7 to 8.
Results
Overeating produced significantly less weight gain in the low protein diet group (3.16 kg; 95% CI, 1.88–4.44 kg) compared with the normal protein diet group (6.05 kg; 95% CI, 4.84–7.26 kg) or the high protein diet group (6.51 kg; 95% CI, 5.23–7.79 kg) (P=.002). Body fat increased similarly in all 3 protein diet groups and represented 50% to more than 90% of the excess stored calories. Resting energy expenditure, total energy expenditure, and body protein did not increase during overfeeding with the low protein diet. In contrast, resting energy expenditure (normal protein diet: 160 kcal/d [95% CI, 102–218 kcal/d]; high protein diet: 227 kcal/d [95% CI, 165–289 kcal/d]) and body protein (lean body mass) (normal protein diet: 2.87 kg [95% CI, 2.11–3.62 kg]; high protein diet: 3.18 kg [95% CI, 2.37–3.98 kg]) increased significantly with the normal and high protein diets.
Conclusions
Among persons living in a controlled setting, calories alone account for the increase in fat; protein affected energy expenditure and storage of lean body mass, but not body fat storage.
Obesity has become a major public health concern with more than 60% of adults in the United States categorized as overweight and more than 30% as obese.1,2 People who become obese have been in a positive energy balance for an extended period. Swinburn et al3 have argued that this reflects an increase in food intake, but Church et al4 have presented data showing that reduced occupational activity might account for the positive energy balance. Although a majority of people in the United States are overweight or obese, there is a significant number of people with normal weights who do not become overweight or obese. As obesity develops, a number of metabolic changes occur, which may not completely reverse when weight is lost.5 These differences may reflect differences in the way individuals handle the food they eat each day both during weight gain and weight loss.
The concept that when people overeat the amount of weight gain is highly individual has intrigued medical science for a century.6–9 In a critical review of macronutrient composition and response to overfeeding, Stock10 cites 12 studies in human beings to support the view that when people overeat a diet that contains either high or low protein, they are less “metabolically efficient” than diets of average protein intake.10–19 This concept is appealing from an evolutionary perspective because the ability to waste “excess” calories when eating an unbalanced diet would ensure an adequate supply of nutrients while avoiding risks to survival as a result of excess weight gain.20,21 In Stock's analysis,10 the greatest metabolic efficiency of weight gain during overfeeding was found when protein intake was 10% to 15% of the energy consumed. Conversely, the metabolic inefficiency or “wasting” of calories during overfeeding appeared when diets contained low or high amounts of energy from protein.22 Overeating a diet low or high in dietary protein may maintain body weight through metabolic inefficiency because of the energy cost involved in sparing lean body mass with a low protein diet but expanding lean body mass with a high protein diet.20
This study was designed to determine whether the level of dietary protein differentially affected body composition, weight gain, or energy expenditure under tightly controlled conditions in a randomized controlled trial.
METHODS
Participants
Twenty-five healthy, weight-stable males (n=16) and females (n=9) aged 18 to 35 years, with a body mass index (calculated as weight in kilograms divided by height in meters squared) between 19 and 30, were recruited from the Baton Rouge, Louisiana, community using newspaper advertisements approved by an institutional review board. Details about the recruitment, randomization, and follow-up of this study appear in Figure 1. Participants were compensated based on their length of time spent in the metabolic unit. Race was self-reported (7 non-Hispanic whites, 16 blacks, and 2 Asians). All food was provided and participants resided in a metabolic unit for 10 to 12 weeks with no prescribed or regular exercise program. Alcohol and caffeine were prohibited throughout the study and smokers were excluded.
Figure 1.
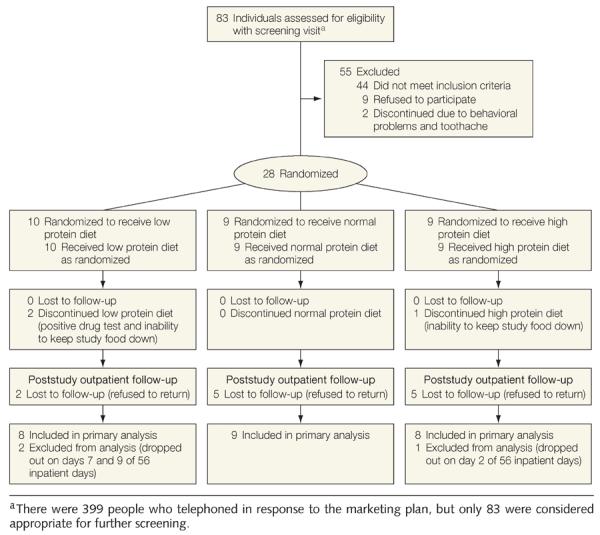
Flow Diagram Describing Recruitment, Study Flow, and Follow-up of the Participants
The study was reviewed prior to approval by a 3-member external advisory committee, reviewed after 1 year by another external advisory committee, and approved by the Pennington Biomedical Research Center institutional review board and the US Department of Agriculture. Safety was monitored by Donna Ryan, MD (associate director of clinical research, Pennington Biomedical Research Center, Louisiana State University). Participants provided written informed consent. The first participant was admitted in June 2005 and the last in October 2007. Additional information is provided in the eSupplement at http://www.jama.com.
Protocol
This was a randomized, parallel-group, inpatient study with the participants living in the metabolic unit from the beginning of the run-in period through the end of the overeating period (Figure 2). Neither participants nor inpatient staff was told the protein diet assignment, which was known only to the kitchen staff who supervised participants while they were eating. Participants were randomized to diets containing 5% of energy from protein (low protein), 15% (normal protein), or 25% (high protein), which they were overfed during the last 8 weeks of their 10- to 12-week stay in the inpatient unit. Compared with energy intake during the weight stabilization period, the diets provided 39.4% (95% CI, 37.3%–41.5%) more intake, which corresponds to 954 kcal/d (95% CI, 884–1022 kcal/d). Vital signs and body weights were measured daily. After the final day of overfeeding, participants remained in the unit for 1 day during which their diets were returned to baseline energy levels and diet compositions (15% from protein, 25% from fat, and 60% from carbohydrates).
Figure 2.
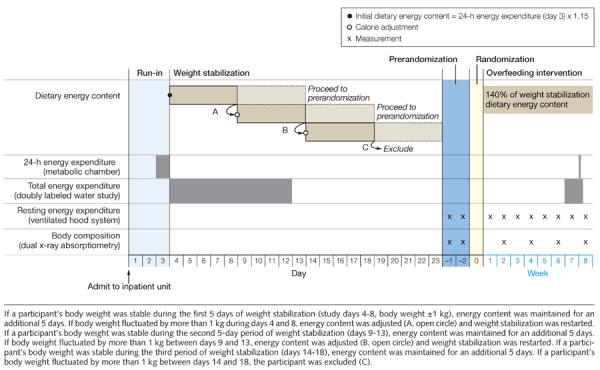
Study Design Showing Study Days Before Overfeeding and Weekly Events Thereafter
Baseline Weight Stabilization
Energy requirements for weight maintenance were established over 13 to 25 days while consuming an isocaloric diet with 15% of energy from protein, 25% from fat, and 60% from carbohydrates (Figure 2). The initial energy content of the weight stabilizing diet was determined from 24-hour energy expenditure measured in the metabolic chamber on day 3 of the run-in multiplied by an activity factor of 1.15.18 On day 4, participants commenced a 9-day doubly labeled water study as well as weight stabilization. Weight stability was a change in body weight of less than 1 kg during a 10-day period without changes in energy intake (Figure 2).
Diets
Diets were prepared by the metabolic kitchen and meals were provided in a 5-day rotation with overfeeding calories in proportion to run-in energy requirements. A 5-day diet for each participant was prepared in duplicate, composited, and frozen before shipping to Covance Laboratories for analysis of fat, protein, and carbohydrate. Absolute carbohydrate intake was kept constant throughout the study. The experimental diets varied by chemical analysis in the ratio of protein to fat. The low protein diet had 6% of energy from protein, 52% from fat, and 42% from carbohydrates. The normal protein diet had 15% of energy from protein, 44% from fat, and 41% from carbohydrates. The high protein diet had 26% of energy from protein, 33% from fat, and 41% from carbohydrates. There was 47 g/d (95% CI, 42.7–50.5 g/d) of protein in the in the low protein diet group, 139 g/d (95% CI, 117–162 g/d) in the normal protein diet group, and 228 g/d (95% CI, 188–268 g/d) in the high protein diet group compared with 90.2 g/d (95% CI, 83.6–96.9 g/d) during the weight stabilization period. Meal times were supervised by the dietary staff to ensure that all foods were eaten. The foods included in the 5-day dietary rotation and the quantity of food for each protein diet group at the level of calorie intake are listed in the eTable at http://www.jama.com
Resting Energy Expenditure
Resting energy expenditure was measured for 30 minutes each week with a ventilated hood system (Deltatrac II Metabolic Monitor, Datex-Ohmeda).23
Total Daily Energy Expenditure by Doubly Labeled Water
Total daily energy expenditure was measured during baseline and weeks 7 to 8 by the doubly labeled water method.24 After 2 baseline urine samples were collected, participants drank a solution with 0.2 g/kg of total body water of 1H218O (Cambridge Isotopes) and 0.115 g/kg of total body water of 2H2O (Isotec Inc). After the initial void, 2 urine samples were obtained after 4.5 hours and 6 hours and then daily for the next 9 days. The 2H and 18O isotope elimination rates (kH and kO) were calculated using linear regression based on the isotopic enrichment. The rate of carbon dioxide production was calculated from the equations created by Schoeller,25 as modified by Racette et al.26 Total energy expenditure was calculated by multiplying the rate of carbon dioxide by the energy equivalent of carbon dioxide based on the measured respiratory quotient in the metabolic chamber at baseline and week 8 (BOX).
Body Composition
Body composition was measured at baseline and then biweekly during overfeeding using dual x-ray absorptiometry (Hologics QDR 4500A whole-body scanner). The scans were analyzed with QDR software version 11.1 (Hologics).
Statistical Analysis
Randomization was performed by Project Statistician, using the minimization allocation method,27 and included stratification for sex and body mass index (dichotomized as ≤19-<26, and ≤26-≤30).28 When a participant was to be randomized, the statistician or associate was contacted for the participant's diet assignment. The randomization included a planned interim analysis after 30 participants had been enrolled.
Based on the point estimates of Stock,10 at the end of the overfeeding period, the normal protein group will increase their body weight by 6.6 kg, the high protein group by 4.2 kg, and the low protein group by 3.0 kg. A common dispersion estimate for weight gain (σd=3.01 kg) was used. A type I error rate (α level) of .05 was used with a 1-tailed alternative hypothesis that weight gain for the normal protein diet group exceeds that of the high protein diet group by 2.4 kg (6.6 kg–4.2 kg). A single interim analysis was taken into consideration.
An initial power analysis testing for a 2.4 kg difference in weight gain between the low protein (5% of energy) and high protein (25% of energy) diets suggested that 20 participants in each treatment group (total of 60 participants) would provide 80% power to reject the null hypothesis. The difference in weight of 2.89 kg between the 5% and 15% protein groups and 3.35 kg between the 5% and 25% protein groups (P=.002) when the interim analysis was completed (after 28 patients were randomized and 25 completed the study) exceeded our predetermined end point, and the study was terminated by agreement between the statistician and the investigators. A retrospective power analysis with the 25 participants who completed the study and the observed weight changes provided a β of 0.87.
Baseline data are expressed as mean (standard deviation). One-way analysis of variance was used to compare baseline data. Changes from baseline are expressed as mean (95% confidence interval) and are adjusted for the corresponding baseline covariates, age, and sex. Differences between groups were compared using the Tukey-Kramer method on the least squares means.
Predicted resting and total energy expenditures at 8 weeks were calculated from the baseline formula relating energy expenditure to lean body mass and body fat and inserting 8-week values for lean body mass and fat mass.29 The measured (observed) minus predicted values were then tested against the null hypothesis that the observed minus predicted value was 0 to determine whether the observed values differed significantly from the predicted ones. Energy stored in protein was calculated as 1000 kcal/kg and energy in fat as 9300 kcal/kg.25 Effects of treatment on change in body composition and energy expenditure were evaluated using simple regression analysis and again after adjusting for baseline covariates, age, and sex. The α level was set at .05 or less and statistical tests were 2-tailed. Analyses were performed using the JMP-7 statistics package (SAS Institute Inc).
RESULTS
Baseline Characteristics
Twenty-seven participants were randomized, but 2 dropped out before the final measures of energy expenditure were obtained and are not included in the analysis. Sex was balanced across groups but blacks predominated in the normal protein diet group (Table). The mean (SD) body mass index was 25.1 (3.0) and ranged from 19.7 to 29.5. Body weight, lean body mass, fat mass, total daily energy expenditure, and resting metabolic rate at baseline were not significantly different between the 3 protein diet groups (Table).
Table.
Body Composition and Energy Metabolism at Baseline and Changes During 8 Weeks of Overfeeding
| Baseline |
Change from Baselinea |
|||||||
|---|---|---|---|---|---|---|---|---|
| Protein Diet Group, Mean (SD)b |
P Valuec | Protein Diet Group, Mean (95% CI) |
P Valuec | |||||
| Low: 5% | Normal: 15% | High: 25% | Low: 5% | Normal: 15% | High: 25% | |||
| Age, y | 22.9 (2.75) | 22.9 (5.49) | 26.8 (1.98) | .08 | ||||
|
| ||||||||
| Sex, No. | ||||||||
| Male | 5 | 6 | 5 | |||||
|
| ||||||||
| Female | 3 | 3 | 3 | |||||
|
| ||||||||
| Race, No. | ||||||||
| White | 4 | 0 | 3 | |||||
|
| ||||||||
| Black | 3 | 8 | 5 | |||||
|
| ||||||||
| Asian | 1 | 1 | 0 | |||||
|
| ||||||||
| Weight, kg | 69.1 (11.6) | 77.6 (13.0) | 76.0 (15.4) | .40 | 3.16 (1.88 to 4.44) | 6.05 (4.84 to 7.26) | 6.51 (5.23 to 7.79) | .002 |
|
| ||||||||
| Fat mass, kg | 16.6 (5.07) | 18.3 (7.40) | 19.6 (6.38) | .66 | 3.66 (2.82 to 4.49) | 3.45 (2.67 to 4.24) | 3.44 (2.60 to 4.27) | .91 |
|
| ||||||||
| Lean body mass, kg | 53.2 (10.3) | 59.9 (12.5) | 57.1 (13.5) | .53 | −0.70 (−1.50 to 0.10) | 2.87 (2.11 to 3.62) | 3.18 (2.37 to 3.98) | <.001 |
|
| ||||||||
| Energy expenditure, kJ/d | ||||||||
| Total | 9334 (1505) | 9096 (2207) | 8736 (1893) | .82 | 176 (−740 to 1095) | 2186 (1321 to 3051) | 1898 (974 to 2813) | .007 |
|
| ||||||||
| Resting | 6475 (1413) | 6207 (1069) | 6153 (1187) | .85 | −86.1 (−343 to 171) | 669 (426 to 911) | 949 (690 to 1204) | <.001 |
|
| ||||||||
| Physical activity leveld | 1.48 (0.31) | 1.48 (0.29) | 1.43 (0.26) | .94 | 0.021 (−0.13 to 0.18) | 0.16 (0.019 to 0.31) | 0.079 (−0.078 to 0.23) | .38 |
|
| ||||||||
| Non–resting energy expenditure, kJ/d | 1924 (1473) | 1979 (1761) | 1706 (1558) | .12 | 245 (−668 to 1158) | 1296 (435 to 2157) | 756 (−156 to 1669) | .24 |
|
| ||||||||
| Protein, g/d | 85.2 (9.2) | 92.0 (18.0) | 93.3 (19.7) | .57 | −38.6 (−51.9 to −25.2) | 47.6 (34.9 to 60.2) | 135 (121 to 148) | <.001 |
|
| ||||||||
| Energy intake, kJ/d | 9457 (1047) | 10357 (2139) | 10395 (2194) | .53 | 3866 (209) | 4088 (209) | 3783 (222) | .13 |
Data for change from baseline were adjusted for age, sex, and baseline values.
Unless otherwise indicated.
Calculated using analysis of variance.
Calculated as total energy expenditure divided by resting energy expenditure.
Energy intake required for weight maintenance during the 10-day weight stabilization period that followed the 3-day run-in period was not different between the 3 diet groups (P = .57; Table), and was slightly but not significantly higher than baseline total energy expenditure (125 kcal/d [95% CI, 63–312 kcal/d]; P=.18). The target for excess energy was 40% above weight maintenance energy requirements and was chosen to make this study similar to other studies in the literature.10–19 The actual excess energy reached 40% (95% CI, 36%–44%) based on nutrient tables and 40% (95% CI, 32%–48%) based on chemical analysis of the study menus. This provided an extra 954 kcal/d (95% CI, 885–1022 kcal/d) and was at the lower end of the range (890–2000 kcal/d) used in the studies reported by Stock.10–19
Changes in Body Composition
During the 56 days of overeating, the participants consumed an excess of 50 729 kcal/d (95% CI, 47 065–54 393 kcal; P=.13 between groups); they all gained weight and there were no differences by sex (P=.12) or by race (P=.19; Figure 3). The weight gain in the low protein diet group was 3.16 kg (95% CI, 1.88–4.44 kg), about half that of the other 2 groups (normal protein diet: 6.05 kg [95% CI, 4.84–7.26 kg]; high protein diet: 6.51 kg [95% CI, 5.23–7.79 kg]; P=.002) (Table). The rate of weight gain in the low protein diet group was significantly less than in the other 2 groups (P<.001). The failure to increase lean body mass in the low protein group accounted for their smaller weight gain.
Figure 3.
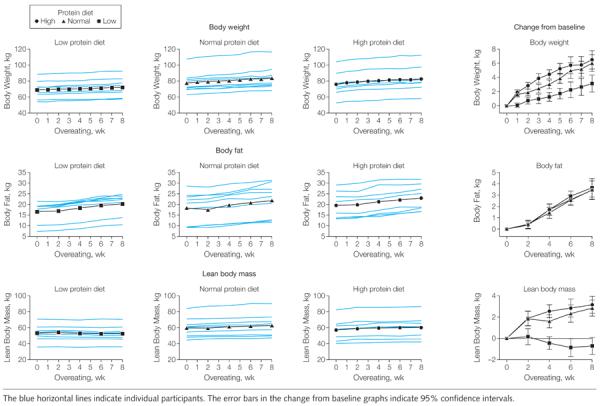
Changes in Body Weight, Body Fat, and Lean Body Mass During 8 Weeks of Overeating
Lean body mass decreased during the overeating period by −0.70 kg (95% CI, −1.50 to 0.10 kg) in the low protein diet group compared with a gain of 2.87 kg (95% CI, 2.11 to 3.62 kg) in the normal protein diet group and 3.18 kg (95% CI, 2.37 to 3.98 kg) in the high protein diet group (P<.001). The overall increase in fat mass for all 3 groups was 3.51 kg (95% CI, 3.06 to 3.96 kg) from baseline and was not significantly different between the 3 groups (P = .89), although the low protein group added on average more than 200 g of fat (about 2000 kcal).
Changes in Energy Expenditure
Overeating led to a significant increase in resting energy expenditure in both the normal and high protein groups. This increase occurred mainly in the first 2 to 4 weeks and the slopes of the regression lines were not significantly different from each other (Figure 4). In contrast, resting energy expenditure in the low protein group did not change significantly with overfeeding, and the slope of the regression line was not different from 0, but was significantly less than the other 2 groups (P<.001; Figure 4). Resting energy expenditure in the low protein group was significantly lower during the 8 weeks of overeating than the other 2 protein groups (P<.001), which were not different from each other (Figure 4). This increase in resting energy expenditure was strongly related to protein intake (r=0.75, P<.001).
Figure 4.
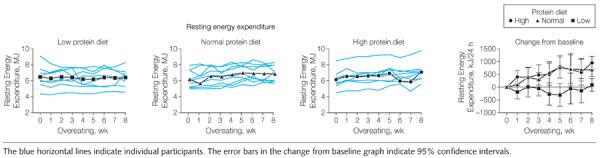
Changes in Resting Energy Expenditure During 8 Weeks of Overeating
In response to overfeeding, total energy expenditure (measured by doubly labeled water) increased significantly in the normal and high protein groups compared with the low protein group (P=.007), which was not significantly different from baseline (171 kcal [95% CI, −178 to 262 kcal]; P = .15; Figure 5). The change in total energy expenditure in the low protein group was significantly less than in the normal protein group (Tukey-Kramer P < .05; Figure 5). There also was a positive correlation between the change in total energy expenditure and protein intake (r = 0.56, P = .004). Baseline physical activity level (calculated as total energy expenditure divided by resting energy expenditure) was low (mean [SD], 1.46 [0.28]) and did not change during the overeating period (P = .38; Figure 5). Non–resting energy expenditure was about one-third of the total energy expenditure at baseline and did not change significantly with overeating.
Figure 5.
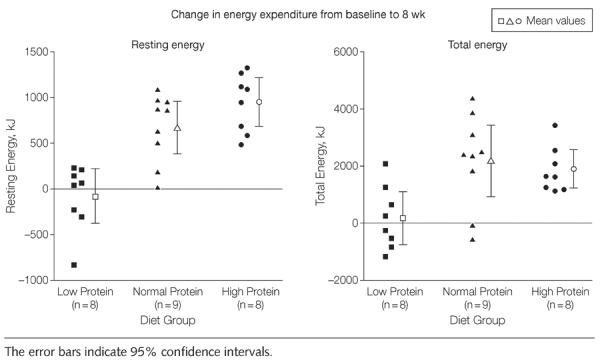
Changes in Resting and Total Energy Expenditure
Relation of Body Composition to Changes in Energy Expenditure
The metabolic efficiency of weight gain (defined as the excess energy intake divided by weight gain5) was significantly higher in the low protein group (75.1 MJ/kg [95% CI, 54.1–96.0 MJ/kg]) than in the high protein group (38.0 MJ/kg [95% CI, 18.6–60.5 MJ/kg]; P=.04). The metabolic efficiency of weight gain in the normal protein group was 45.5 MJ/kg (95% CI, 25.5–65.0 MJ/kg). When adjusted for age, sex, and baseline weight, there was no longer a treatment effect (P=.15). The limitations of this approach are clear when changes in body composition are taken into consideration. Extra energy intake predicted both the increase in lean body mass and body fat (Figure 6). In contrast, protein intake predicted the increase in lean body mass, but not the change in fat storage.
Figure 6.
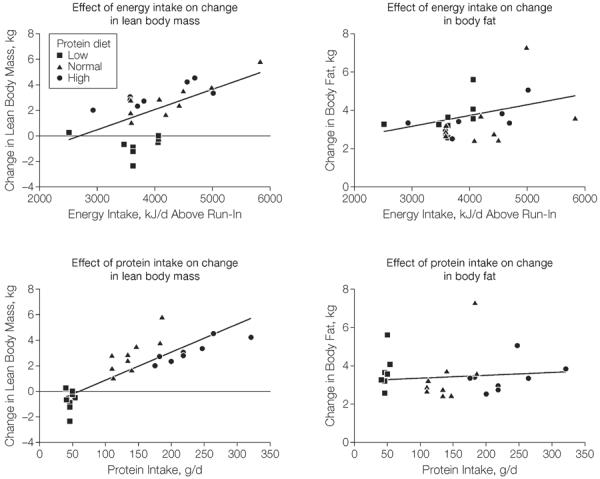
Relationship of Energy Intake and Protein Intake With Change in Body Fat and Change in Lean Body Mass
To examine further whether the change in energy expenditure could be explained by changes in body composition, we predicted 8-week values for energy expenditure from the baseline relation of energy expenditure to lean body mass and fat mass. The measured minus predicted energy expenditure for the low protein group was not significantly different from zero, indicating that the changes in body composition provided an adequate explanation for the changes in energy expenditure. For the normal and high protein groups, the measured energy expenditure after 8 weeks of overeating was significantly higher than predicted from the changes in body composition. From the regression line of protein intake and change in lean body mass, the protein intake required to prevent loss of lean body mass was 77.8 g/d (95% CI, 54.4–101.2 g/d), which is 30 g/d higher than the protein level that was provided by the low protein diet (47.0 g/d; 95% CI, 42.7–50.4 g/d).
COMMENT
The key finding of this study is that calories are more important than protein while consuming excess amounts of energy with respect to increases in body fat. This study examined the hypothesis proposed by Stock10 that overeating a low or high protein diet would produce less weight gain than overeating a normal protein diet. The extra energy provided was high relative to the usual life excesses of caloric intake, but matched other studies on overfeeding in the scientific literature.10–19 The low protein diet group gained less weight than the normal or high protein groups when extra calories were eaten. When the data are expressed as the energy cost of weight gain, our data (collected under rigorous experimental conditions in a metabolic ward) are consistent with those of Stock10 and imply that a diet providing only 5% of energy from protein was metabolically different with a higher energy cost of weight gain compared with diets that contained 15% and 25% of energy from protein. Using the energy cost of weight gain, Joosen and Westerterp30 concluded that 5 of 14 studies showed metabolic inefficiency (ie, a higher value for energy cost of storage than predicted).
The limits of this approach are evident in our study when changes in body composition and energy expenditure are included in the analysis. There were no significant differences between energy intake and energy expenditure between the 3 diets. We can account for all excess energy consumed through energy stored in fat and in protein or expended in higher total energy. With the low protein diet, more than 90% of the extra energy was stored as fat. Because there was no change in lean body mass, the 6.6% increase in total energy expenditure reflects the energy cost of storing fat and is close to the estimate of 4% to 8% for fat storage derived by Flatt.31 With the normal and high protein diets, only about 50% of the excess energy was stored as fat with most of the rest consumed (thermogenesis). The high total energy expenditure probably reflects the higher cost of protein turnover and storage.
Resting energy expenditure responded differently to low vs high protein intake. Neither resting energy expenditure, nor lean body mass increased in the low protein group. In contrast, the accretion of lean body mass in the normal and high protein groups was the principal contributor to the increase in resting energy expenditure. Much of the increase in resting energy expenditure occurred within the first 2 weeks in the groups with 15% (normal) and 25% (high) of energy from protein. Over the remaining 6 weeks, there was a further small increase in the normal protein diet group, but little change in the high protein diet group. Harris et al32 also noted that resting energy expenditure increased early during overfeeding, suggesting that it is responding to the increased thermic effect of feeding more than the increase in body mass. The extra calories in our study were fed as fat, as in several other studies,33,34 and were stored as fat with a lower percentage of the excess calories appearing as fat in the high (25%) protein diet group. The higher fat intake in the low protein group probably reduced nutrient absorption (metabolizable energy) relative to the other groups and this would have brought the intake and expenditure closer together in this group.
This study has several strengths. First, only a few studies of overfeeding had 25 or more participants. Second, only 4 other studies fed volunteers for 8 weeks or longer. Third, we measured both resting energy expenditure and total energy expenditure with the doubly labeled water method. To our knowledge, our study is the only one to examine a range of protein intake levels. In our study, the caloric requirement determined from at least 2 weeks of weight stabilization overestimated the energy expenditure that was estimated by doubly labeled water. This suggests that studies using stable body weight may underestimate actual energy requirements in human beings. One limitation of this study is that a majority of the participants were male and black. However, neither sex nor race significantly affected the weight gain produced by overfeeding, suggesting that these data may be generalizable.
In summary, weight gain when eating a low protein diet (5% of energy from protein) was blunted compared with weight gain when eating a normal protein diet (15% of energy from protein) with the same number of extra calories. Calories alone, however, contributed to the increase in body fat. In contrast, protein contributed to the changes in energy expenditure and lean body mass, but not to the increase in body fat.
Supplementary Material
Box. Equations Used in Protein Diet Study.
Total energy expenditure (kcal/d)=22.4 rate of carbon dioxide (3.9/respiratory quotient+1.10)
Physical activity level=total energy expenditure/resting energy expenditure
Non–resting energy expenditure=total energy expenditure–(resting energy expenditure+0.1×total energy expenditure)
Acknowledgments
Trial Registration clinicaltrials.gov Identifier: NCT00565149
Funding/Support: This study was supported in part by the US Department of Agriculture grant 2010-34323-21052 and by funding from Louisiana State University.
Role of the Sponsors: The US Department of Agriculture and Louisiana State University had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Additional Contributions: We thank all of the volunteers without whose active participation there would not have been a study. The diligence of the clinical and dietary staff were essential for the smooth functioning of this study, and we appreciate their work. We thank Eric Ravussin, PhD, Claude Bouchard, PhD, and Donna Ryan, MD (all with Pennington Biomedical Research Center, Louisiana State University) for their helpful comments during the preparation of the manuscript. None of these persons were compensated for their contributions.
Footnotes
Author Contributions: Drs Bray and Smith had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Bray and Smith contributed equally to this work.
Study concept and design: Bray, Smith, de Jonge, Rood, Most.
Acquisition of data: Bray, Smith, de Jonge, Rood, Martin, Most, Brock, Mancuso.
Analysis and interpretation of data: Bray, Smith, de Jonge, Xie, Rood, Martin, Redman.
Drafting of the manuscript: Bray, Smith, de Jonge, Xie, Brock, Mancuso, Redman.
Critical revision of the manuscript for important intellectual content: Bray, Rood, Martin, Most, Redman.
Statistical analysis: Bray, Smith, Xie, Redman.
Obtained funding: Bray.
Administrative, technical or material support: Bray, Smith, de Jonge, Rood, Martin, Most.
Study supervision: Bray, Smith, de Jonge.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bray reported that he has been a consultant to Abbott Laboratories and Takeda Global Research Institute; is an advisor to Medifast, Herbalife, and Global Direction in Medicine; and has received royalties for the Handbook of Obesity. Dr Martin reported that he is a consultant to Bristol-Myers Squibb, Eli Lilly, Elcelyx, Merck, and Philips; has received compensation from International Life Sciences Institute for manuscript preparation; and has been reimbursed for travel expenses from Catapult Health, Domain & Associates, and the University of Tennessee. No other author reported disclosures.
Online-Only Material: The eSupplement, eReferences, eTable, and Author Video Interview are available at http://www.jama.com.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Swinburn B, Sacks G, Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009;90(6):1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 4.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in US occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 6.Neumann RO. Experimentelle Beitrage zur Lehre von dem taglichen Nahrungsbedarf des Menschen unter besonderer Berucksichtigung der notwendigen Eiweissmenge. Arch Hyg. 1902;45:1–87. [Google Scholar]
- 7.Grafe E, Graham D. Uber die Anpassungsfahigkeit des tierischen Organismus an uiberreichliche Nahrungszufuhr. Ztsch f physiol Chem. 1911;73:1. [Google Scholar]
- 8.Gulick A. A study of weight regulation in the adult human body during overnutriton. Am J Physiol. 1922;60:371–395. [Google Scholar]
- 9.Wiley FH, Newburgh LH. The doubtful nature of “luxuskonsumption.”. J Clin Invest. 1931;10(4):733–744. doi: 10.1172/JCI100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord. 1999;23(11):1105–1117. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- 11.Miller DS, Mumford P, Stock MJ. Gluttony, 2: thermogenesis in overeating man. Am J Clin Nutr. 1967;20(11):1223–1229. doi: 10.1093/ajcn/20.11.1223. [DOI] [PubMed] [Google Scholar]
- 12.Miller DS, Mumford P. Gluttony, 1: an experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967;20(11):1212–1222. doi: 10.1093/ajcn/20.11.1212. [DOI] [PubMed] [Google Scholar]
- 13.Goldman R, Haisman M, Bynum G, Horton E, Sims E. Experimental obesity in man: metabolic rate in relation to dietary intake. In: Bray GA, editor. Obesity in Perspective: A Conference. US Government Printing Office; Washington, DC: 1975. pp. 165–186. [Google Scholar]
- 14.Norgan NG, Durnin JV. The effect of 6 weeks of overfeeding on the body weight, body composition, and energy metabolism of young men. Am J Clin Nutr. 1980;33(5):978–988. doi: 10.1093/ajcn/33.5.978. [DOI] [PubMed] [Google Scholar]
- 15.Webb P, Annis JF. Adaptation to overeating in lean and overweight men and women. Hum Nutr Clin Nutr. 1983;37(2):117–131. [PubMed] [Google Scholar]
- 16.Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr. 1986;56(1):1–9. doi: 10.1079/bjn19860080. [DOI] [PubMed] [Google Scholar]
- 17.Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. 1992;56(4):641–655. doi: 10.1093/ajcn/56.4.641. [DOI] [PubMed] [Google Scholar]
- 18.Dériaz O, Tremblay A, Bouchard C. Non linear weight gain with long term overfeeding in man. Obes Res. 1993;1(3):179–185. doi: 10.1002/j.1550-8528.1993.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 19.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 20.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23(5):373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 21.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 22.Dulloo AG, Jacquet J. Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Int J Obes Relat Metab Disord. 1999;23(11):1118–1121. doi: 10.1038/sj.ijo.0801110. [DOI] [PubMed] [Google Scholar]
- 23.Martin CK, Heilbronn LK, de Jonge L, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 24.Redman LM, Heilbronn LK, Martin CK, et al. Pennington CALERIE Team Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4(2):e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118(11):1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 26.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267(4 pt 1):E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 27.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 28.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 29.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49((5)(suppl)):968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 30.Joosen AM, Westerterp KR. Energy expenditure during overfeeding. Nutr Metab (Lond) 2006;3:25. doi: 10.1186/1743-7075-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flatt JP. The biochemistry of energy expenditure. In: Bray GA, editor. Recent Advances in Obesity Research: II. Proceedings of the 2nd International Congress on Obesity. Newman Publishing; London, England: 1978. pp. 211–228. [Google Scholar]
- 32.Harris AM, Jensen MD, Levine JA. Weekly changes in basal metabolic rate with eight weeks of overfeeding. Obesity (Silver Spring) 2006;14(4):690–695. doi: 10.1038/oby.2006.78. [DOI] [PubMed] [Google Scholar]
- 33.Dallosso HM, James WP. Whole-body calorimetry studies in adult men, 1: the effect of fat overfeeding on 24 h energy expenditure. Br J Nutr. 1984;52(1):49–64. doi: 10.1079/bjn19840070. [DOI] [PubMed] [Google Scholar]
- 34.Lammert O, Grunnet N, Faber P, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84(2):233–245. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


