Abstract
Background
Despite wide availability of treatment options for hepatocellular carcinoma (HCC), several studies have suggested underutilization in clinical practice.
Aims
To quantify utilization rates for HCC treatment among patients with HCC in the United States and summarize patterns of association between utilization rates and patient socio-demographic characteristics.
Methods
We performed a systematic literature review using the Medline database from January 1989 through March 2013. Two investigators independently extracted data on patient populations, study methods, and results using standardized forms. Pooled treatment rates for any treatment and curative treatment, with 95% confidence intervals, were calculated. Pre-specified subgroup analysis was performed to identify patient-level correlates of treatment utilization.
Results
We identified 24 studies that met inclusion criteria. The pooled rates of any treatment and curative treatment were 52.8% (95%CI 52.2-53.4%) and 21.8% (95%CI 21.4-22.1%) respectively. Among patients diagnosed at an early stage, the pooled curative treatment rate was 59.0% (95%CI 58.1-59.9%). Elderly, non-Caucasians and patients of low socioeconomic status had lower treatment rates than their counterparts.
Conclusions
Rates of HCC treatment in the United States, including curative treatment rates among patients detected at an early stage, are disappointingly low. Future efforts should focus on identifying appropriate intervention targets to increase treatment rates and reduce socio-demographic disparities.
Keywords: Hepatocellular carcinoma, treatment, socio-demographic disparities, United States
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and is one of the leading causes of death among patients with cirrhosis1. Its incidence in the United States is increasing due to the current epidemic of non-alcoholic fatty liver disease and hepatitis C virus (HCV) infection1. Prognosis for patients with HCC depends on tumor stage, degree of underlying liver dysfunction, and patient performance status, with curative therapies only available for patients detected at an early stage. Patients detected at an early stage can achieve 5-year survival rates of 70% with transplant or resection, whereas those with advanced HCC are only eligible for palliative treatments and have a median survival of less than one year2, 3.
HCC disproportionately affects disadvantaged populations, with the highest age-specific incidence occurring among minorities. HCC rates are two times higher in Asian Americans than African Americans, whose rates are two times higher than those in Caucasians1. Elderly, African Americans and patients of low socioeconomic status (SES) have poorer survival rates than their counterparts4, 5. The reasons for differences in survival are likely multi-factorial, involving a combination of medical, financial, and social factors. Several studies have reported lower rates of surveillance, whereas others have postulated biologic differences in tumor behavior, and others have reported differential rates of HCC treatment 4, 6-8. The aims of our study were to 1) quantify utilization rates for any treatment and curative treatment among patients with HCC in the United States and 2) to summarize patterns of association between utilization rates and patient socio-demographic characteristics.
METHODS
Literature Search
We conducted a computer-assisted search with the Ovid interface to Medline to identify relevant published articles. We searched the Medline database from January 1, 1989 through March 1, 2013 with the following keyword combinations: [treat$ OR therap$ OR transplant$ OR resect$ OR surg$ OR ablat$ OR RFA OR chemo$ OR emboliz$ OR TACE OR nexavar OR sorafenib] AND [hepatocellular ca$ OR liver ca$ or HCC]. Given our focus on current utilization of treatment within the United States, our search was limited to human studies published in English after 1989. Manual searches of references from relevant articles were performed to identify studies that were missed by our computer-assisted search. Finally, consultation with expert hepatologists was performed to identify additional references or unpublished data.
Study Selection
One investigator (D.T.) reviewed all publication titles of citations identified by the search strategy. Potentially relevant studies were retrieved, and selection criteria were applied. The articles were independently checked for inclusion (D.T. and A.S.) and disagreements were resolved through consensus. Inclusion criteria included: (i) cohort studies that described receipt of HCC treatment in patients with HCC, ii) studies from the United States after 1989 so as to be representative of current delivery of care, and (iii) available data regarding socio-demographic information for patients who did and did not receive treatment. We excluded: i) clinical trials with a protocol and/or extra nursing support as they do not evaluate delivery of care in a real-world clinical setting, ii) studies conducted outside the United States, and iii) survey studies because of high rates of over-reporting by physicians. Additional exclusion criteria included non-English language, non-human data, and lack of original data. If publications used the same patient cohort, data from the most recent manuscript were included.
Data Extraction
Two reviewers (D.T. and A.S.) independently extracted required information from eligible studies using standardized forms. A third investigator (A.Y.) was available to resolve any discrepancies. Data were collected on study design, geographic location and date of the study, number of patients with cirrhosis, number of HCC patients, and number of patients with early stage HCC in each study. We recorded definitions of any treatment, curative treatment, and early stage HCC for each study. Finally, data were collected on age, gender, race/ethnicity, and SES (insurance status and income) for those who received treatment and those who failed to receive treatment. Authors were contacted as necessary for missing information.
Clinical End Point and Statistical Analysis
Our primary study outcomes were rates of any treatment and rates of curative treatment among patients with HCC. Rates of any treatment included curative treatments (transplant, resection, or radiofrequency ablation) and non-curative treatments (chemoembolization, radiation-based therapy, or systemic therapy). Studies that only reported rates of transplantation, resection, and/or radiofrequency ablation were included in analyses for receipt of curative treatment but not those for receipt of any treatment.
The proportion of patients who received treatment was derived for each study, and 95% confidence intervals were calculated using the adjusted Wald method. A weighed pooled estimate of treatment rates was computed by multiplying the point estimate for each study by the proportion of individuals in that study relative to the number of individuals in all included studies. Sensitivity analyses were planned for the following predefined variables: 1) the study cohort (single-center vs. multi-center administrative database), 2) the proportion of patients with early stage HCC, 3) the definition of curative treatment, and 4) introduction of the Milan criteria for liver transplantation in 1996. All data analysis was performed using Stata 11 (StataCorp, College Station, TX).
RESULTS
Literature Search
The computer-assisted search yielded 22280 potentially relevant articles. After initial review, 264 titles were potentially appropriate, and these abstracts were reviewed. Fifty-seven publications underwent full-text review, and 34 were excluded. Sixteen of these articles were excluded as they were repeat analyses using the same cohort as other studies, eleven were not related to receipt of HCC treatment, four did not have extractable data, and three did not have any original data. One additional relevant article was identified through recursive literature searches. The remaining twenty-four studies met all inclusion criteria9-32 (Supplemental Figure, Table 1).
Table 1.
Characteristics of Studies Assessing Hepatocellular Carcinoma Treatment Utilization
| Author, Year | Study Setting | Mean Age (Years) | Gender (% Male) | Race (% Caucasian) | Cirrhosis (%) |
|---|---|---|---|---|---|
| Altekruse 20129 | SEER-Medicare Database | NR | 74 | 49 | NR |
| Cance 200010 | National Cancer Database | 60-69 | 71 | 60 | NR |
| Harrison 200411 | University of Medicine and Dentistry, New Jersey | 59 | 80 | 61 | 93 |
| Jan 201212 | Tulane University | 64 | 76 | 67 | 94 |
| Jou 201013 | Duke University | NR | 80 | 68 | 100 |
| Kanwal 201232 | Liver Cancer Research Network | 59 | 76 | 77 | 100 |
| Kemmer 200814 | University of Cincinnati | 57 | 80 | 64 | 100 |
| Kitisin 201115 | University of Pittsburgh | 62 | 75 | 87 | 84 |
| Kooby 200816 | Emory University | 60 | 72 | 71 | 82 |
| Kozyreva 201117 | Tufts University and Massachusetts General Hospital | 62 | 79 | 77 | 90 |
| Leykum 200718 | South Texas VA hospitals | 55 | 100 | 40 | 79 |
| Sanyal 201019 | Marketscan Claims Research Database | 63 | 66 | NR | NR |
| Sarkar 201220 | University of California, San Francisco | 50-64 | 78 | 0 | 100 |
| Schwartz 199521 | Mount Sinai Medical Center | NR | NR | NR | 100 |
| Shah 201122 | SEER-Medicare Database | 75 | 67 | 74 | 55 |
| Stravitz 200823 | Virginia Commonwealth University | 57 | 86 | 63 | 100 |
| Stuart 199624 | Deaconess Health System | 64 | 78 | NR | 68 |
| Theodoropoulos 201125 | Hahnemann University | 55 | 80 | 47 | 83 |
| Tong 201026 | University of California, Los Angeles | 62 | 78 | 0 | 73 |
| Wong 201227 | Hawaii Medical Center | 62 | 75 | 19 | 74 |
| Yang 201128 | Mayo Clinic | 62 | 72 | 83 | 83 |
| Yu 201029 | Columbia University | 60 | 80 | 40 | NR |
| Zak 201130 | California Cancer Registry | 55-64 | 71 | 37 | NR |
| Zaydfudim 201031 | Tennessee Cancer Registry | 61 | 74 | 78 | NR |
NR-Not Reported; SEER- Surveillance, Epidemiology, and End Results; VA-Veterans Administration
Treatment Utilization
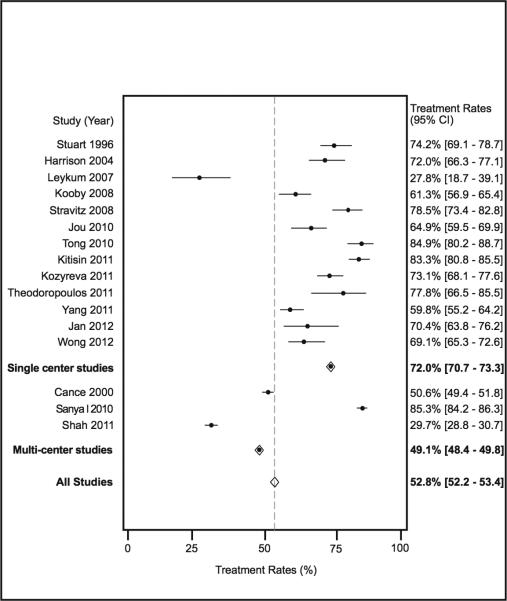
There were 16 studies, with a total of 24237 patients, which assessed receipt of any treatment, including both curative and non-curative treatments, among patients with HCC. Rates of treatment ranged from 28% to 85% among studies, with a pooled treatment rate of 52.8% (95%CI 52.2-53.4%) (Figure 1). We evaluated potential sources of heterogeneity through pre-planned subgroup analysis. The pooled treatment rate was 49.1% (95%CI 48.4 – 49.8%) among the 19489 patients in the three multi-center studies, which was significantly lower than the 72.0% (95%CI 70.7 – 73.3%) treatment rate among the 13 single-center studies, which contained a total of 4748 HCC patients (p<0.001). Among the multi-center studies, the study by Sanyal and colleagues reported substantially higher treatment rates than the other two studies. Given this study used insurance claims data, untreated patients who did not receive hospice may not have been fully captured. If this study was removed, the pooled treatment rate of the two remaining multi-center studies was only 38.5% (95%CI 37.7-39.3%).
Figure 1.
Treatment Rates for Hepatocellular Carcinoma
Utilization of Curative Treatment
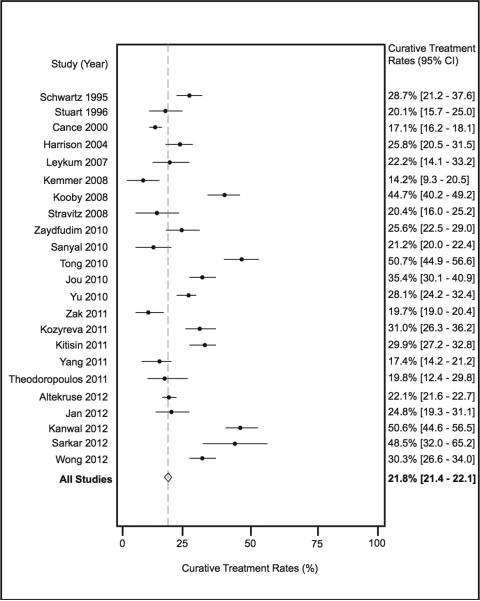
There were 23 studies, with a total of 50769 patients, which assessed receipt of curative treatment among patients with HCC. Rates of curative treatment ranged from 14% to 51% among studies, with a pooled treatment rate of 21.8% (95%CI 21.4-22.1%) (Figure 2). Once again, we found substantial heterogeneity between studies, which was explored though sensitivity and subgroup analyses. We first performed a sensitivity analysis based on introduction of the Milan criteria for liver transplantation. When excluding the studies by Stuart and Cance, which both exclusively included cohorts prior to 1996, the pooled treatment rate was 22.5% (95%CI 22.1-22.9%). We next explored heterogeneity through subgroup analyses. The pooled curative treatment rate was 20.8% (95%CI 20.5 – 21.2%) among 45244 patients in the six multi-center studies, which was significantly lower than the 29.4% (95%CI 28.2 – 30.7%) curative treatment rate among the 17 single-center studies, which contained a total of 5525 HCC patients (p<0.001). We also performed a subset analysis, based on the definition of curative treatment. Studies that included transplant, resection, and RFA as curative treatments had a pooled curative treatment rate of 22.2% (95%CI 21.8-22.6%) compared to a pooled rate of 19.8% (95%CI 19.0-20.6%) among studies that only included surgical treatment (liver transplantation and/or resection) (p<0.001).
Figure 2.
Rates of Curative Treatment for Hepatocellular Carcinoma
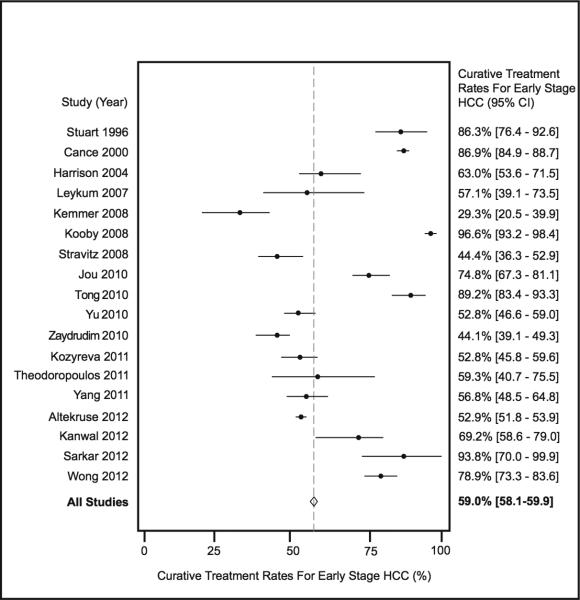
Eighteen of the studies reported the number of patients with early HCC. Of the 32884 HCC patients in these studies, 12455 (37.9%) had early stage HCC. The pooled curative treatment rate among patients with early stage HCC was 59.0% (95%CI 58.1-59.9%) (Figure 3). When excluding the two studies by Stuart and Cance, the pooled treatment rate was 54.9% (95%CI 54.0-55.9%). Only three studies defined early stage with the Barcelona Clinic Liver Cancer (BCLC) staging system, while an additional 8 studies used Milan criteria. The other seven studies used a variety of definitions including the American Joint Committee on Cancer (AJCC) or Tumor Node Metastases (TNM) staging systems. The pooled curative treatment rate among studies using the BCLC or Milan criteria was 72.4% (95%CI 69.9-74.8%), which was significantly higher than the pooled curative treatment rate of 56.7% (95%CI 55.8-57.6%) among studies using other definitions (p<0.001).
Figure 3.
Curative Treatment Rates among Patients with Early Stage Hepatocellular Carcinoma
Correlates of HCC Treatment
Several patient factors are associated with higher utilization rates for HCC treatment, but heterogeneity in the reporting of these associations precluded pooling of the data.
Age
Older age was a consistent negative predictor of HCC treatment, with five studies reporting higher treatment rates in younger patients17, 19, 22, 29, 30. Most studies lacked sufficient data to adjust for potential differences in tumor stage at presentation19, 22, 30. However, Kozyreva and colleagues found patients older than 70 years were significantly less likely to receive any treatment than younger patients (36.8% vs. 22.9%, p=0.01) despite having similar tumor stage (p=0.95) and liver function as younger patients (Child A 57.9% vs. 56.7%)17.
Gender
The majority of studies that evaluated the impact of gender found no difference in treatment rates between males and females16, 22, 29, 30. The study by Zaydfudim and colleagues was the only that suggested differential treatment rates by gender31. They found that females had a 1.78-odds (95%CI 1.15-2.76) of undergoing surgical treatment, after adjusting for age, race, insurance status, and tumor stage.
Race/Ethnicity
Five included studies demonstrated disparities in HCC treatment utilization, particularly that of curative treatments, according to race11, 22, 27, 29, 30. Studies by Zak and Harrison reported lower treatment rates among African American patients but lacked sufficient data to adjust for differences in tumor stage and/or liver function11, 30. Similarly, Shah and colleagues found higher treatment rates among Asian patients using the SEER-Medicare database, but it is unknown if this is related to differential rates of underlying cirrhosis22. Yu and colleagues found African Americans were significantly less likely to receive a transplant than Caucasian patients (OR 0.03, 95%CI 0.00 – 0.37) after adjusting for confounders including age, insurance status, and tumor stage but did not find a significant association with Hispanic ethnicity (OR 0.42, 95%CI 0.09 – 2.08)29. Similarly, Wong and colleagues found that Pacific Islanders and Filipinos who were detected at an early stage were significantly less likely to undergo liver transplant than Caucasians (10.0% vs. 38.0%)27.
Socioeconomic Status
The impact of SES on HCC treatment utilization has only been evaluated in four studies22, 29-31. Several other studies evaluated patients with insurance or easy access to health care and therefore were unable to determine the impact of SES on treatment utilization9, 18, 19. There was a consistent effect of higher treatment rates among patients with private insurance compared to patients without insurance or those with Medicare/Medicaid. In the Tennessee Cancer Registry, both uninsured patients (OR 0.05, 95%CI 0.01-0.37) and those with Medicaid (OR 0.32, 95%CI 0.15-0.69) were significantly less likely to receive surgical therapy than those with private insurance, after adjusting for age, gender, race, and tumor stage31. Yu and colleagues reported similar results, with privately insured patients significantly more likely to receive liver transplantation (OR 22.07, 95%CI 2.67-182.34), independent of tumor stage29.
DISCUSSION
Despite strong evidence demonstrating HCC treatment significantly improves survival, our meta-analysis highlights that many patients with HCC fail to receive treatment in clinical practice. We found less than one-fourth of patients with HCC undergo curative treatment, and nearly 50% do not receive any treatment. The low rates of curative treatment are in part related to diagnoses at an advanced stage; however, more than one-third of patients diagnosed at an early stage do not receive curative treatment. Our study also highlights the presence of significant socio-demographic disparities, with the lowest treatment rates among non-Caucasians and patients of low SES.
The transition from diagnosis to treatment is a complex process, involving several steps and interfaces with multiple new providers33. Providers must be aware of the cancer diagnosis, complete the staging work-up, determine the optimal treatment, and finally refer patients to the appropriate consultants34. The complex array of potential treatment options, each delivered by a different type of provider, may make this process even more difficult for HCC. These treatment decisions for HCC have become increasingly complex with the availability of novel therapies and the growing use of multimodal and multi-provider treatments. Patients may be asked to make multiple transitions between several providers as various treatment options are considered. A breakdown at any step can result in treatment underutilization and/or treatment delays. Even in the setting of optimal processes, patients may choose to forgo therapy given disinterest, other barriers to care, or perceived excess risk from the treatment. Current studies fail to provide an in-depth analysis to clarify which factors mediate or moderate underutilization of HCC treatment. A multidisciplinary approach involving a team of hepatologists, surgeons, interventional radiologists, radiation oncologists, medical oncologists, and radiologists may improve communication and allow better delivery of optimal treatment35. Further research is needed to evaluate the benefits of multidisciplinary care and identify other potential intervention strategies to increase appropriate HCC treatment36.
Therapeutic choices for HCC are dependent on tumor stage, liver function and performance status2. Although there is not one universally accepted staging system, the BCLC staging system has been incorporated into guidelines and is the most widely used, given that it combines all three features37, 38. However, it is important to note that most studies, particularly those from large administrative databases, provide limited data regarding liver function or patient performance status. This lack of data precludes an accurate assessment of the appropriateness of lack of treatment. For example, it would be appropriate to not treat a patient with poor functional status, but this would be regarded as treatment underutilization in several studies included in this meta-analysis. Automated data has been demonstrated to underestimate quality of care in other areas, such as HCV-related care, for similar reasons39. It is crucial that future studies provide data regarding liver function and patient performance status to better interpret treatment utilization rates.
The low curative treatment rates appear to be related to high rates of late stage diagnosis, as only 40% of patients are diagnosed at an early stage despite the availability of efficacious surveillance tools40. When examining the subgroup of patients detected at an early stage, curative treatment rates are closer to 57-73%, depending on the definition of early stage HCC. The low rates of early tumor detection are multi-factorial, with surveillance underuse and suboptimal effectiveness of surveillance tools in clinical practice both playing a large role41-44. Patients diagnosed incidentally or symptomatically are significantly more likely to be diagnosed at an advanced stage, when curative options are no longer an option23, 45. Interventions are needed to improve surveillance rates, which can increase early tumor detection, facilitate higher rates of curative treatment, and thereby improve overall survival36, 46.
Racial and socioeconomic disparities have been well described in the survival of patients with HCC4. Although prior studies have suggested difference in tumor biology and/or surveillance rates, our meta-analysis highlights the importance of socio-demographic disparities in treatment utilization. Patients who are elderly, non-Caucasian, and of low SES suffer from significantly lower HCC treatment rates than their counterparts. While current studies suggest an association between socio-demographic factors and HCC treatment, none have explored why treatment is not being performed in these subgroups. The roles of patient attitudes, co-morbid conditions, and barriers to accessing care have not been clearly evaluated. For example, elderly patients and patients of low SES may have lower treatment rates due to difficulty accessing medical care or a higher rate of co-morbid conditions. Similarly, race and SES are often highly correlated so independent causal effects can be difficult to identify.
The primary limitation of our meta-analysis was our inability to identify specific reasons for underutilization of HCC treatment. Current studies did not distinguish cases in which physicians failed to order treatment, cases in which treatment was not appropriate (e.g., patients with significant co-morbidities or those with Child C cirrhosis who were not transplant candidates), and those in which patients were non-adherent after treatment was recommended. Studies evaluating the reasons behind treatment under-utilization are necessary to identify intervention targets that can increase treatment rates.
Our meta-analysis was also limited by clinical heterogeneity among studies, such as the different operational definitions used for early stage disease and/or curative treatment. This variability in definitions makes it difficult to compare treatment rates across studies. Clear consistent definitions and measures are necessary to better quantify and interpret HCC treatment rates47. This clinical heterogeneity may also relate to other etiologies such as inter-center variation in treatment rates, similar to what has been reported for HCC surveillance43. Inter-center variation in treatment rates may be even larger given the selected availability of some treatments, such as liver transplantation. Another possible explanation for clinical heterogeneity is changes in treatment expertise over time; however, we did not see any evidence of a time trend on subgroup analysis.
In summary, HCC treatment is underutilized nationally, with nearly 50% failing to receive any treatment and less than 25% receiving curative treatment. Even among patients diagnosed at an early stage, more than 1 in 3 fail to receive curative treatment. There are also significant socio-demographic disparities with the lowest treatment rates in non-Caucasians and patients of low SES. Further studies are needed to explore reasons for the underutilization of treatment, particularly in these disadvantaged subgroups. These studies will be the first crucial step in identifying appropriate intervention targets to increase HCC treatment rates and reduce socio-demographic disparities.
Supplementary Material
Supplemental Figure: Flow Diagram of Included Articles
Table 2.
Treatment Utilization Rates for Hepatocellular Carcinoma
| Author, Year | Study Years | Number of HCC Patients | Rates of any HCC Treatment (%) |
|---|---|---|---|
| Altekruse 20129 | 1998 – 2008 | 21390 | NR |
| Cance 200010 | 1985 – 1996 | 6353 | 3213 (50.6%) |
| Harrison 200411 | 1997 – 2003 | 264 | 190 (72.0%) |
| Jan 201212 | 2003 – 2011 | 206 | 145 (70.4%) |
| Jou 201013 | 2002 – 2008 | 319 | 207 (64.9%) |
| Kanwal 201232 | 2001 – 2007 | 267 | NR |
| Kemmer 200814 | 2000 – 2005 | 169 | NR |
| Kitisin 201115 | 2000 – 2009 | 1010 | 841 (83.3%) |
| Kooby 200816 | 1990 – 2004 | 501 | 307 (61.3%) |
| Kozyreva 201117 | 1998 – 2008 | 335 | 245 (73.1%) |
| Leykum 200718 | 2000 – 2005 | 72 | 20 (27.8%) |
| Sanyal 201019 | 2002 – 2008 | 4406 | 3757 (85.3%) |
| Sarkar 201220 | 1997 – 2008 | 31* | NR |
| Schwartz 199521 | 1998 – 1994 | 115 | NR |
| Shah 201122 | 1991 – 2005 | 8730 | 2595 (29.7%) |
| Stravitz 200823 | 1997 – 2005 | 297 | 233 (78.5%) |
| Stuart 199624 | 1986 – 1995 | 314 | 233 (74.2%) |
| Theodoropoulos 201125 | 2001 – 2007 | 81 | 63 (77.8%) |
| Tong 201026 | 2000 – 2007 | 278 | 236 (84.9%) |
| Wong 201227 | 1992 – 2009 | 618 | 427 (69.1%) |
| Yang 201128 | 2007 – 2009 | 453 | 271 (55.2%) |
| Yu 201029 | 2002 – 2008 | 462 | NR |
| Zak 201130 | 1996 – 2006 | 12148 | NR |
| Zaydfudim 201031 | 2004 – 2006 | 680 | NR |
NR-Not Reported
Subset of total population with cirrhosis
Table 3.
Rates of Curative Treatment for Hepatocellular Carcinoma
| Author, Year | Number of Early HCC | Definition of Early HCC | Curative Treatment (%) | Definition Curative Treatment |
|---|---|---|---|---|
| Altekruse 20129 | 8940 (41.8%) | Localized | 4727 (22.1%) | Resection, OLT and RFA |
| Cance 200010 | 1252 (19.7%) | AJCC Stage I-II | 1088 (17.1%) | Surgery |
| Harrison 200411 | 108 (40.9%) | AJCC Stage I-II | 68 (25.8%) | Resection and OLT |
| Jan 201212 | NR | NR | 51 (24.8%) | OLT |
| Jou 201013 | 151 (47.3%) | BCLC Stage A | 113 (35.4%) | Resection, OLT and RFA |
| Kanwal 201232 | 76 (28.5%) | BCLC Stage A | 135 (50.6%) | Resection, OLT and RFA |
| Kemmer 200814 | 82 (48.5%) | Milan Criteria | 24 (14.2%) | OLT |
| Kitisin 201115 | NR | NR | 302 (29.9%) | Resection, OLT and RFA |
| Kooby 200816 | 232 (46.3%) | Milan Criteria | 224 (44.7%) | Resection, OLT and RFA |
| Kozyreva 201117 | 197 (58.8%) | CLIP Stage I-II | 104 (31.0%) | Resection and OLT |
| Leykum 200718 | 28 (38.9%) | Milan Criteria | 16 (22.2%) | Resection, OLT and RFA |
| Sanyal 201019 | NR | NR | 932 (21.2%) | Resection, OLT and RFA |
| Sarkar 201220 | 16 (51.6%) | Milan Criteria | 15 (48.5%) | Resection, OLT and RFA |
| Schwartz 199521 | NR | NR | 33 (28.7%) | Resection and OLT |
| Shah 201122 | 3197(36.6%) | AJCC Stage I-II | NR | Resection, OLT and RFA |
| Stravitz 200823 | 135 (45.5%) | Milan Criteria | 60 (20.4%) | OLT |
| Stuart 199624 | 73 (23.2%) | TNM Stage I-II | 63 (20.1%) | Resection and OLT |
| Theodoropoulos 201125 | 27 (33.3%) | BCLC Stage A | 16 (19.8%) | Resection and OLT |
| Tong 201026 | 158 (56.8%) | Milan Criteria | 141 (50.7%) | Resection, OLT and RFA |
| Wong 201227 | 237 (38.3%) | Milan Criteria | 187 (30.3%) | Resection and OLT |
| Yang 201128 | 139 (30.7%) | Milan Criteria | 79 (17.4%) | Resection, OLT and RFA |
| Yu 201029 | 246 (53.2%) | AJCC Stage I-II | 130 (28.1%) | OLT |
| Zak 201130 | NR | NR | 2390 (19.7%) | Resection, OLT and RFA |
| Zaydfudim 201031 | 358 (52.6%) | AJCC Stage I-II | 158 (23.2%) | Resection, OLT and RFA |
AJCC – American Joint Committee on Cancer; BCLC – Barcelona Clinic Liver Cancer; HCC – hepatocellular carcinoma; NR-Not Reported; OLT – Orthotopic liver transplantation; RFA – radiofrequency ablation; TNM – Tumor, Node, Metastases
Acknowledgments
Financial support: This work was conducted with support from Center for Translational Medicine, NIH/NCATS Grant Number KL2TR000453 and an ACG Junior Faculty Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, UT Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Footnotes
Author Contributions
Debra Tan involved in acquisition of data, interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Adam Yopp involved in study concept and design, interpretation of data, and critical revision of the manuscript for important intellectual content.
Muhammad Beg involved in critical revision of the manuscript for important intellectual content.
Purva Gopal involved in critical revision of the manuscript for important intellectual content.
Amit Singal involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and study supervision. Amit Singal is the guarantor of the article.
All authors approved the final version of the manuscript.
Conflicts of Interest: Amit Singal and Adam Yopp are on the Speaker Bureau for Bayer and Onyx Pharmaceuticals. None of the other authors have any conflicts of interest relevant to this manuscript to disclose.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–47. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 4.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD, Kim J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–77. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 5.Decker KM, Harrison M, Chateau D. Influence of direct referrals on time to diagnosis after an abnormal breast screening result. Cancer Detect Prev. 2004;28:361–7. doi: 10.1016/j.cdp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Sloane D, Chen H, Howell C. Racial disparity in primary hepatocellular carcinoma: tumor stage at presentation, surgical treatment and survival. J Natl Med Assoc. 2006;98:1934–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: a population-based study. Clin Gastroenterol Hepatol. 2006;4:104–10. quiz 4-5. [PubMed] [Google Scholar]
- 8.Siegel AB, McBride RB, El-Serag HB, Hershman DL, Brown RS, Jr., Renz JF, Emond J, Neugut AI. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998-2002. Am J Gastroenterol. 2008;103:120–7. doi: 10.1111/j.1572-0241.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 9.Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012;55:476–82. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cance WG, Stewart AK, Menck HR. The National Cancer Data Base Report on treatment patterns for hepatocellular carcinomas: improved survival of surgically resected patients, 1985-1996. Cancer. 2000;88:912–20. doi: 10.1002/(sici)1097-0142(20000215)88:4<912::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Harrison LE, Reichman T, Koneru B, Fisher A, Wilson D, Dela Torre A, Samanta A, Korogodsky M. Racial discrepancies in the outcome of patients with hepatocellular carcinoma. Arch Surg. 2004;139:992–6. doi: 10.1001/archsurg.139.9.992. [DOI] [PubMed] [Google Scholar]
- 12.Jan T, Medvedev S, Cannon RM, Saggi B, McGee J, Paramesh A, Killackey M, Shores NJ, Slakey DP, Balart L, Buell JF. Racial disparity and their impact on hepatocellular cancer outcomes in inner-city New Orleans. Surgery. 2012;152:661–6. doi: 10.1016/j.surg.2012.07.008. discussion 666-7. [DOI] [PubMed] [Google Scholar]
- 13.Jou JH, Chen PH, Jazwinski A, Bouneva I, Smith AD, Muir AJ. Rates of surveillance and management of hepatocellular carcinoma in patients evaluated at a liver transplant center. Dig Dis Sci. 2010;55:3591–6. doi: 10.1007/s10620-010-1366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemmer N, Neff G, Secic M, Zacharias V, Kaiser T, Buell J. Ethnic differences in hepatocellular carcinoma: implications for liver transplantation. Dig Dis Sci. 2008;53:551–5. doi: 10.1007/s10620-007-9872-7. [DOI] [PubMed] [Google Scholar]
- 15.Kitisin K, Packiam V, Steel J, Humar A, Gamblin TC, Geller DA, Marsh JW, Tsung A. Presentation and outcomes of hepatocellular carcinoma patients at a western centre. HPB (Oxford) 2011;13:712–22. doi: 10.1111/j.1477-2574.2011.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooby DA, Egnatashvili V, Graiser M, Delman KA, Kauh J, Wood WC, Staley Iii CA. Changing management and outcome of hepatocellular carcinoma: evaluation of 501 patients treated at a single comprehensive center. J Surg Oncol. 2008;98:81–8. doi: 10.1002/jso.21049. [DOI] [PubMed] [Google Scholar]
- 17.Kozyreva ON, Chi D, Clark JW, Wang H, Theall KP, Ryan DP, Zhu AX. A multicenter retrospective study on clinical characteristics, treatment patterns, and outcome in elderly patients with hepatocellular carcinoma. Oncologist. 2011;16:310–8. doi: 10.1634/theoncologist.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leykum LK, El-Serag HB, Cornell J, Papadopoulos KP. Screening for hepatocellular carcinoma among veterans with hepatitis C on disease stage, treatment received, and survival. Clin Gastroenterol Hepatol. 2007;5:508–12. doi: 10.1016/j.cgh.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–91. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar M, Stewart S, Yu A, Chen MS, Nguyen TT, Khalili M. Hepatocellular carcinoma screening practices and impact on survival among hepatitis B-infected Asian Americans. J Viral Hepat. 2012;19:594–600. doi: 10.1111/j.1365-2893.2011.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz ME, Sung M, Mor E, Fisher A, Popescu I, Fiel I, Sheiner P, Emre S, Guy S, Miller CM. A multidisciplinary approach to hepatocellular carcinoma in patients with cirrhosis. J Am Coll Surg. 1995;180:596–603. [PubMed] [Google Scholar]
- 22.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117:1019–26. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 23.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–26. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217–22. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2217::AID-CNCR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Theodoropoulos J, Brooks A. Inconsistency in the management of patients with hepatocellular carcinoma: the need for a strict protocol. Am Surg. 2011;77:207–14. [PubMed] [Google Scholar]
- 26.Tong MJ, Chavalitdhamrong D, Lu DS, Raman SS, Gomes A, Duffy JP, Hong JC, Busuttil RW. Survival in Asian Americans after treatments for hepatocellular carcinoma: a seven-year experience at UCLA. J Clin Gastroenterol. 2010;44:e63–70. doi: 10.1097/MCG.0b013e3181b4b68b. [DOI] [PubMed] [Google Scholar]
- 27.Wong LL, Hernandez B, Kwee S, Albright CL, Okimoto G, Tsai N. Healthcare disparities in Asians and Pacific Islanders with hepatocellular cancer. Am J Surg. 2012;203:726–32. doi: 10.1016/j.amjsurg.2011.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617–23. e1. doi: 10.1016/j.cgh.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, Lim E, Hershman DL, Brown RS, Jr., Siegel AB. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116:1801–9. doi: 10.1002/cncr.24936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778–84. doi: 10.1001/archsurg.2011.37. [DOI] [PubMed] [Google Scholar]
- 31.Zaydfudim V, Whiteside MA, Griffin MR, Feurer ID, Wright JK, Pinson CW. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann Surg Oncol. 2010;17:3104–11. doi: 10.1245/s10434-010-1181-2. [DOI] [PubMed] [Google Scholar]
- 32.Kanwal F, Befeler A, Chari RS, Marrero J, Kahn J, Afdhal N, Morgan T, Roberts L, Mohanty SR, Schwartz J, VanThiel D, Li J, Zeringue A, Di'Bisceglie A. Potentially curative treatment in patients with hepatocellular cancer--results from the liver cancer research network. Aliment Pharmacol Ther. 2012;36:257–65. doi: 10.1111/j.1365-2036.2012.05174.x. [DOI] [PubMed] [Google Scholar]
- 33.Taplin SH, Clauser S, Rodgers AB, Breslau E, Rayson D. Interfaces across the cancer continuum offer opportunities to improve the process of care. J Natl Cancer Inst Monogr. 2010;2010:104–10. doi: 10.1093/jncimonographs/lgq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sussman J, Baldwin LM. The interface of primary and oncology specialty care: from diagnosis through primary treatment. J Natl Cancer Inst Monogr. 2010;2010:18–24. doi: 10.1093/jncimonographs/lgq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, Nehra M, Lee WM, Marrero JA, Tiro JA. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camma C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N, Licata A, Latteri MA, Craxi A. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62–75. doi: 10.1111/j.1365-2036.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 38.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanwal F, Hoang T, Kramer J, Chrusciel T, El-Serag H, Dominitz JA, Asch SM. The performance of process measures in hepatitis C. Am J Gastroenterol. 2012;107:1512–21. doi: 10.1038/ajg.2012.201. [DOI] [PubMed] [Google Scholar]
- 40.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, Su GL, Lok AS, Marrero JA. Effectiveness of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, Lee WM. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425–32. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–7. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trevisani F, De NS, Rapaccini G, Farinati F, Benvegnu L, Zoli M, Grazi GL, Del PP, Di N, Bernardi M. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol. 2002;97:734–44. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 46.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11:472–7. doi: 10.1016/j.cgh.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vernon SW, Briss PA, Tiro JA, Warnecke RB. Some methodologic lessons learned from cancer screening research. Cancer. 2004;101:1131–45. doi: 10.1002/cncr.20513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Flow Diagram of Included Articles