Abstract
Herein we describe the development and implementation of a nanoporous cell-therapy device with controllable biodegradation. Dopamine-secreting PC12 cells were housed within newly formulated alginate-glutamine degradable polylysine (A-GD-PLL) microcapsules. The A-GD-PLL microcapsules provided a 3-D microenvironment for good spatial cell growth, viability and proliferation. The microcapsules were subsequently placed within a poly(ethylene glycol) (PEG)-coated poly(ε-caprolactone) (PCL) chamber covered with a PEG-grafted PCL nanoporous membrane formed by phase inversion. To enhance PC12 cell growth and to assist in controlled degradation of both the PC12 cells and the device construct, small PCL capsules containing neural growth factor (PCL-NGF) and a poly(lactic-co-glycolic acid) pellet containing glutamine (PLGA-GLN) were also placed within the PCL chamber. Release of NGF from the PCL-NGF capsules facilitated cell proliferation and viability, while the controlled release of GLN from the PLGA-GLN pellet resulted in A-GD-PLL microcapsule degradation and eventual PC12 cell death following a pre-specified period of time (4 weeks in this study). In vivo, our device was found to be well tolerated and we successfully demonstrated the controlled release of dopamine over a period of four weeks. This integrated biodegradable device holds great promise for the future treatment of a variety of diseases.
Keywords: Nanoporous capsules, Controllable biodegradation, Glutamine-degradable PLL microcapsules, Neural growth factor, Dopamine secretion
1. Introduction
Controlled drug delivery has seen rapid advances in many pharmaceutical applications [1]. Typical drug delivery carriers include biodegradable micro-/nanoparticles [2], hydrogel-based carriers [3], liposome, and polyplex nanoparticles [4], and micro/nanofabricated devices [5] including a number of silicon micro-reservoir designs capped with thin nanoporous membranes thereby allowing pulsatile delivery of therapeutics [6–8].
To solve the problem of low drug loading and the limited release time of many of the aforementioned drug delivery carriers, the controlled-release of therapeutic proteins from cell-based devices has drawn a great deal of interest. These devices are also referred to as immunoisolation systems, since immunoisolation is the most critical requirement of the cell-based devices. Importantly, there exist microencapsulated and macroencapsulated cell-based carriers. Microcapsules with diameters in the range of 0.3–1.5 mm are capable of providing long-term delivery of therapeutic drugs in addition to immunoprotection from the host immune system [9–11]. For instance, the classic alginate-polylysine (PLL) microcapsules have been successfully applied to a number of cell types [12]. However, issues with these devices remain. High molecular weight PLL microcapsules are associated with the development of severe inflammatory reactions in the host, and the instability of these microcapsules has thus far restricted their clinical application [13]. Another concern of alginate-PLL microcapsules is their uncontrolled biodegradation. PLL materials were easily degraded by hydrolytic enzymes or proteolytic enzymes [14]. However, the alginate-PLL membrane complex was almost totally inert to the hydrolytic enzymes [15,16]. Many strategies have been employed to circumvent these issues including the modulation of alginate cross-linking to eliminate the use of PLL [17,18]; replacing PLL with more biocompatible materials [19–21]; and modifying the microcapsule surface with peptide and protein molecules [22,23]. In spite of these efforts, microcapsules alone still cannot achieve high mechanical stability, high biocompatibility and controlled biodegradability.
Macro-sized cell capsules are capable of providing adequate immunoprotection since the development of nanofabricated pore arrays and semi-permeable membranes [24]. For example, Desai and Ferrari [25] developed a silicon-based biocapsule to house pancreatic islet cell transplants for insulin therapy. In addition, alumina nanopore membranes produced by anodization have been used for the immunoisolation of transplanted cells [26,27]. Given these developments, there have been efforts to combine the advantages of both microcapsules and macro-sized devices. British researchers reported a case of recovery of function in a man following a hemorrhagic stroke and subsequent surgical implantation of a permeable polymer-based device containing microcapsules in the fashion of a “tea-bag”[28]. Unfortunately, this patient required a second surgery for device retrieval to prevent any long-term side effects from the implanted device and transplanted cells. Recently, our group developed an implantable cell-based device consisting of a biodegradable poly(ε-caprolactone) (PCL) outer chamber and alginate-PLL microcapsules [29]. This device demonstrated controlled release of a reporter gene product and generated only a minimal inflammatory reaction by the host immune system. But the fate of cell containing microcapsules after PCL degradation is still a major concern. It would be highly desirable if the implanted foreign cells and devices can be completely degraded in vivo after a pre-specified therapeutic treatment time in a controllable manner.
In this study, we describe a new PLL microcapsule formulation by cross-linking low molecular weight PLL with dimethyl dithiobispropionimidate (DTBP) in combination with alginate. These new PLL microcapsules (A-GD-PLL) are degradable by manipulating the concentration of glutamine (GLN). Importantly, GLN has a secondary effect of increasing the buffer pH of the PCL device. Following microcapsule degradation by GLN, dopamine-secreting PC12 cells that were housed within the microcapusules are exposed to the alkaline buffer of the PCL device. Since cells are sensitive to pH changes in the buffer solution, increased buffer pH will eventually lead to cell apoptosis. By placing both cell-containing A-GD-PLL microcapsules and biodegradable PLGA-GLN pellets in the same PEG coated nanoporous PCL device, controlled degradation of microcapsules and the cells of interest can be easily achieved prior to degradation of the entire PEG-PCL outer chamber. PC12 cells that secrete dopamine were chosen to evaluate our device performance and may serve as a potential treatment of Parkinson’s disease. To promote cell growth and viability, small PCL capsules containing nerve growth factor (PCL-NGF) were also placed inside the PCL chamber. The release of dopamine by the encapsulated PC12 cells and the controllable degradation of the A-GD-PLL microcapsules and the outer PCL chamber were evaluated both in vitro and in vivo. The biocompatibility of the device by the host immune system was evaluated in vivo.
The transplantation of cells with the ability to produce dopamine locally is an alternative approach for patients with Parkinson’s disease [30]. Dopamine-secreting PC12 cells have been successfully encapsulated in a number of carriers [12,31]. However, long-term effects of the macro/microcapsules, the possibility of xenograft rejection, and the necessity for device retrieval are a few of the issues that need to be addressed. We hypothesize that the present integrative device can solve some of these problems.
Fig. 1 outlines the device schematic and the proposed device degradation process. The degradation takes place in two stages. Initially, GLN is released from a PLGA-GLN/Alkali pellet due to PLGA degradation after a specific application time. GLN release leads to the degradation of the A-GD-PLL microcapsules, with subsequent release of the encapsulated PC12 cells inside the PCL chamber. Importantly, alkali is also released from the PLGA-GLN pellets. This results in a rapid increase in the media pH, contributing to the fast decomposition of remaining microcapsules and eventual PC12 cell death. Moreover, the enhanced influx of immune biomolecules through the enlarged nanopores (due to the degradation of the PCL membrane) results in additional/complete foreign cell death. In the second stage, the whole PCL device would degrade and the remaining cell and microcapsule debris could be cleared by the host immune system.
Fig. 1.
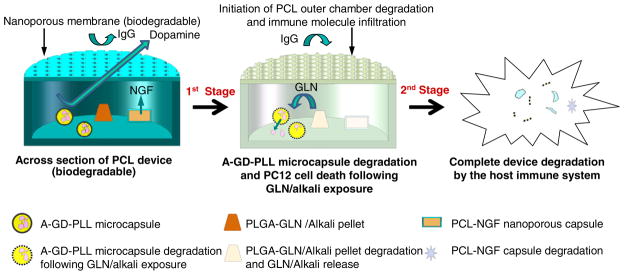
Design of a multifunctional and biodegradable cell-based device. A-GD-PLL microcapsules containing PC12 cells were enclosed within a large nanoporous PCL chamber and cultured with a small PCL-NGF chamber. Therapeutic levels of dopamine were produced and released from the integrated device. A-GD-PLL microcapsule degradation was initiated following exposure to GLN. After a designated time (varies based on PCL outer chamber degradation rate), the entire device degraded and PC12 cells were cleared by the host immune system.
2. Experimental
2.1. Chemicals and reagents
Alginate sodium (low viscosity), PLL (MW 500–2000), PCL (MW 14,000 and 70,000), polyethyleneglycol monoacrylate (PEGMA), dimethyl dithiobispropionimidate (DTBP), ethidium homodimer-1, calcein AM, gelatin, and monothioglycerol were purchased from Aldrich Chemicals (Milwaukee, WI). 1,4-dioxane was obtained from Mallinckrodt Chemicals (Philipsburg, NJ). 2-methoxyethanol (ACS reagent, ≥93% pure) and citrate sodium were purchased from Sigma-Aldrich (St. Louis, MO). Biodegradable PLGA was provided by Alkermes (Cambridge, MA). PC12 cells were purchased from ATCC (Manassas, VA). CellTiter 96® AQueous One Solution Reagent was purchased from Promega (Madison, WI). Phosphate Buffered Saline (PBS), Dulbecco’s Modified Eagle’s Medium (DMEM with 4.5 g/L D-glucose), horse serum, newborn calf serum, fetal bovine serum (FBS), penicillin and streptomycin, non-essential amino acids, L-glutamine, and sodium pyruvate were purchased from Invitrogen (Carlsbad, CA). The NGF reagent was purchased from BD Bioscience (Franklin Lakes, NJ). NGF levels were measured using an NGF ELISA kit (GenWay Biotech Inc., San Diego, CA) as described in the instructions manual. All reagents, unless specified, were used without further purification.
2.2. Cell culture
PC12 cells were grown in tissue culture flasks in maintenance medium consisting of DMEM/F12 supplemented with 10% (v/v) horse serum, 5% (v/v) new calf serum, 2 mM L-glutamine, and 1 mM sodium pyruvate at 37 °C in a humidified incubator with 5% CO2 and 95% air. Fresh medium was added to the PC12 cells each day.
2.3. Device design and fabrication
The design of the nanoporous PCL chamber has been previously described [32]. Briefly, the device consists of two parts: a nanoporous PCL (MW=70,000) membrane gate and a PCL (MW=70,000) chamber. PCL nanoporous membranes were prepared via the combination of thermally and non-solvent induced phase separations. The PCL chambers (8.0 mm in diameter and 5.0 mm in height) were fabricated by a hot-embossing technique. Following PEG-grafting using the plasma technique [33], the nanoporous membrane and PCL chambers were ready for further assembly.
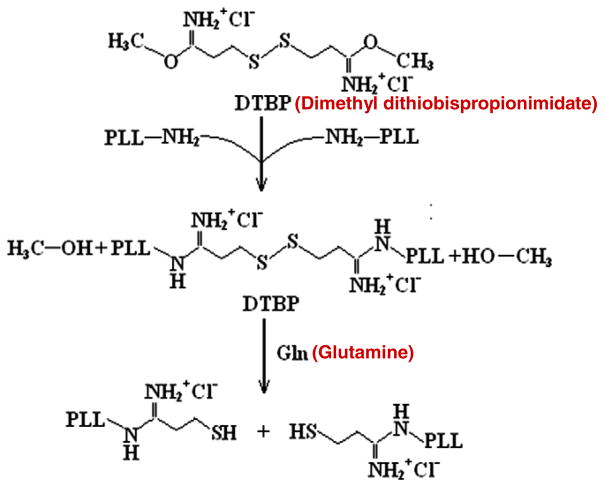
Microcapsules were generated as previously described [34] with an important modification. Briefly, PC12 cells (2×106 cells/mL) were suspended in 2% w/v sterilized sodium alginate and passed through a 27-gage needle into 100 mM CaCl2 using an electrostatic droplet generator (NISCO, Sweden) to form calcium alginate gel beads. The voltage used was 5.5 kV and the distance between the needle tip and the solution level was 1.6 cm. For the creation of glutamine-degradable microcapsules, dimethyl dithiobispropionimidate (DTBP) was cross-linked with a low molecular weight PLL (MW 500–2000) at a 2:1 molar ratio of DTBP to primary amine (i.e. lysine monomer for polylysine and histone). DTBP is a homobifunctional, membrane-permeable cross-linker that can be cleaved by exposure to glutamine molecules [35]. Fig. 2 outlines the cross-linking reaction involved in the synthesis of DTBP-PLL and the breakup by glutamine. Specifically, 20 mg PLL was added into 2 mL of Hepes buffer (25 mM, pH 8.0). 30 mg DTBP dry powder was then added to the solution and the mixture was incubated for 3 h at room temperature [36]. After 3 h, the mixture was filtrated through a 0.22 μm filter. Following filtration, the calcium alginate gel beads were incubated with purified DTBP-PLL for 30 min at room temperature, forming a GD-PLL membrane around the beads. The membrane-enclosed gel beads were further suspended in 55 mM sodium citrate to liquefy the alginate gel core. The resulting A-GD-PLL microcapsules were 300–400 μm in diameter. Microcapsules with encapsulated PC12 cells were cultured in a 24-well plate at 37 °C in 5% CO2 using PC12 maintenance medium.
Fig. 2.
Schematic diagram of DTBP-PLL synthesis and GLN cleavage. A-GD-PLL microcapsules were generated by cross-linking dimethyl dithiobispropionimidate (DTBP) with PLL at a 2:1 molar ratio of DTBP to primary amine. The addition of GLN results in DTBP-PLL cleavage.
The small PCL-NGF chamber (2.4 mm in diameter and 1.8 mm in height) was also fabricated using the hot-embossing technique using a low molecular weight PCL (MW 14,000). Following PEG surface modification, each reservoir was loaded with 7 μL NGF solution (0.1 μg/μL) and covered with a nanoporous membrane using the same PCL. The PLGA-GLN/alkali pellet (2.0 mm in diameter and 0.5 mm in height) was formed by mixing glutamine with sodium hydroxide powder (~1 mg), followed by encapsulation with a PLGA cover without pores.
The PEG-grafted PCL chambers and PEG-modified nanoporous membranes were sterilized overnight using a UV lamp. Microencapsulated cells (~1.5×105), one PLGA-GLN/alkali pellet and one small nanoporous PCL-NGF chamber were then seeded into each large nanoporous PCL chamber. A nanoporous PCL membrane was bonded to the large PCL chamber to form a laminated assembly using a NuSil bioadhesive. Each completely integrated device was then placed into one well of a 24-well plate with 500 μL medium for further use.
2.4. Morphology and viability of PC12 cells w/o nerve growth factor
Determination of cell viability of cultured PC12 cells was performed by light microscopy using live/dead staining. Staining solution was prepared by diluting 2 mM ethidium homodimer-1 (ED-1) in D-PBS, producing 4 μM ED-1 solution. The addition of 4 mM calcein AM stock solution resulted in 2 μM calcein AM. PC12 cells were removed from the devices at different times, washed once with PBS, and incubated with staining solution at 37 °C for 25 min in the dark. After washing three times with D-PBS, samples were observed under a fluorescence microscope (Nikon TS100, Japan). Live cells were labeled with calcein AM and produced green fluorescence at a wavelength of 485±10 nm, while dead cells were labeled with ED-1 and emitted red fluorescence at a wavelength of 530±12.5 nm.
2.5. Microcapsule degradation by GLN and alkali
To assess the ability of GLN and alkali to degrade A-GD-PLL microcapsules with or without PC12 cells, 20 mM GLN was added to a 24-well plate containing microcapsules in solution (PBS and culture medium) at 37 °C for 30 min. The pH of the solution was monitored using a pH monitor. Microcapsule morphology was observed using a fluorescence microscope (Nikon TS100) after 30 min and 24 h.
2.6. Viability of microencapsulated PC12 cells following exposure to IgG
A-GD-PLL microcapsules containing PC12 cells (~3.0×105 cells) were incubated with a GLN solution for either 30 min or 2 h at 37 °C. Following incubation, the GLN solution was replaced by fresh medium containing 1 mg/ml IgG. Cell proliferation was measured after 24 and 48 h using an MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-etrazolium] assay. 100 μl of CellTiter 96® AQueous One Solution was added to each well of a 24-well assay plate containing 500 μl of the aforementioned medium. The microtiter plate was incubated at 37 °C for 1.4 h in a humidified atmosphere containing 5% CO2. Concentration changes were identified by color changes in the medium and the absorbance at 492 nm was measured using a micro-plate reader (Tecan, GENois Pro, San Jose, CA).
2.7. Dopamine release and device biocompatibility
Dopamine secretion from PC12 cells contained within A-GD-PLL microcapsules alone and after incorporation of the microcapsules into PCL chambers was conducted in 24-well plates containing 500 μl medium. Dopamine levels were measured using a Dopamine ELISA kit according to the manufacturer’s instructions.
To assess in vivo PC12 cell dopamine release and biocompatibility of the PCL device, immunocompetent Balb/c mice (The Jackson Laboratory, Bar Harbor, ME) were implanted with PEGMA modified PCL chambers loaded with PBS, alginate-PLL microcapsules, or the newly developed A-GD-PLL microcapsules. All mice were maintained at the animal facilities of The Ohio State University, accredited by The Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Mice were housed under standard conditions with a 12-hour light/dark cycle. Both water and food were provided ad libitum. The animal protocol (2009A0179) was approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University prior to initiation of the research. The devices were implanted subcutaneously as previously described [37]. Weekly blood samples were obtained for measurements of C-reactive protein (CRP) and dopamine. On Day 30, the mice were euthanized and the tissue surrounding the implanted devices was harvested and subjected to H&E staining. The tissue specimens were analyzed by an expert pathologist for evidence of inflammation. Statistical analysis was performed using the Student’s t-test at a significance of p<0.005. All data reported are means±SD, unless otherwise noted.
3. Result and discussion
As a result of our sophisticated cell-based design, we first carried out a series of experiments in vitro to evaluate the function of each individual component. We subsequently evaluated device performance including in vitro and in vivo dopamine secretion. We also evaluated the tolerability of our device by the host immune system.
3.1. Component evaluation
3.1.1. Morphology and viability of PC12 cells within the PCL chamber
Nerve growth factor (NGF), a small secreted protein with a molecular weight around 26,000 Da, is critical for the growth, maintenance, and survival of a wide variety of target cells. Using the PCL membranes with a pore size of 20–30 nm, we were able to achieve a constant release of NGF through the PCL nanoporous gate (data not shown). Approximately 5.0 ng/ml of NGF was released every 48 h, which is capable of supporting PC12 cell viability.
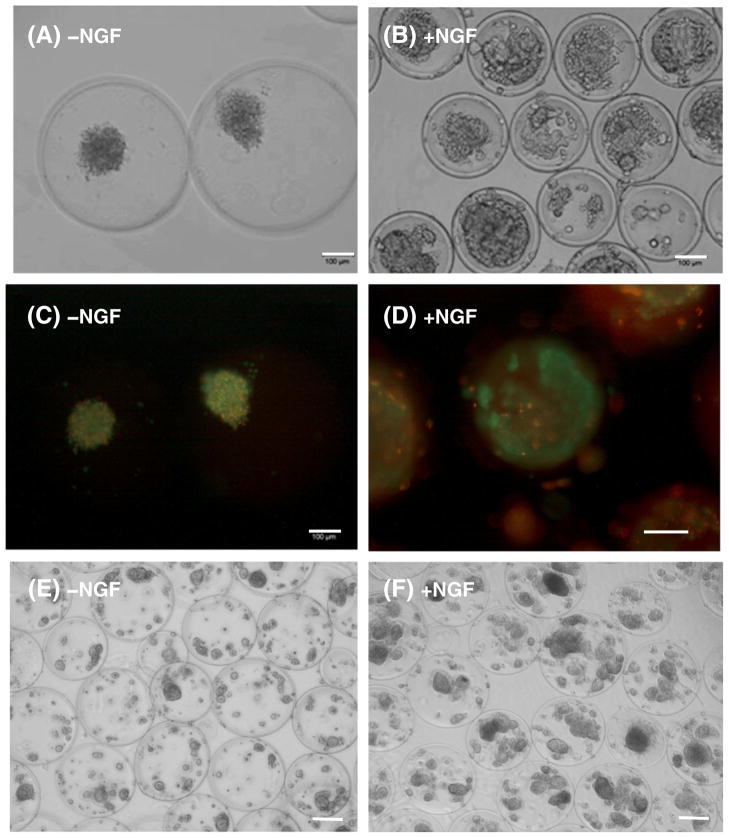
The effect of NGF supplementation on PC12 cell growth and viability in the device was examined in vitro (Fig. 3). A-GD-PLL microcapsules containing PC12 cells were cultured alone in 24-well plates (Fig. 3A & B). On Day 14, PC12 cells cultured in the absence of NGF grew in small clusters (<100 μm in diameter) (Fig. 3A). In contrast, NGF supplementation (50 ng/ml) stimulated PC12 cells to form large spheroids ~200 μm in diameter (Fig. 3B). Using immunofluorescence staining, cell death was found to be abundant in the clusters of PC12 cells that lacked NGF supplementation. On the other hand, the majority of PC12 cells supplemented with NGF were alive with only a few dead cells present on the inner membrane of the microcapsules (Fig. 3C & D). These findings indicate that the proliferation and growth of PC12 cells within A-GD-PLL microcapsules were significantly enhanced by supplementation with NGF.
Fig. 3.
A-GD-PLL microcapsules containing PC12 cells in a 24-well plate on Day 14: Phase contrast images of (A) PC12 cells grow to small clusters without NGF, (B) PC12 cells grow to large spheroids with NGF supplementation, and fluorescence images of (C) PC12 cells growth without NGF, and (D) PC12 cells growth with NGF. In images (C) & (D), the living cells showed the green color and the dead cells stained the red color. Phase contrast images of (E) PC12 cell growth within PCL devices without PCL-NGF capsules, (F) PC12 cell growth in the presence of PCL-NGF capsules. Scale bar: 100 μm.
A-GD-PLL microcapsules containing PC12 cells were also incorporated into a fully integrated PCL device (Fig. 3E & F). Lack of PCL-NGF capsules within the PCL chambers led to the generation of very small PC12 cell clusters (<50 μm in diameter) (Fig. 3E). In comparison, cell cluster formation within A-GD-PLL microcapsules ranged from 70 to 140 μm when a PCL-NGF capsule (5.0 ng/ml) was present within the PCL integrated device (Fig. 3F).
3.1.2. Microcapsule degradation and cell apoptosis upon exposure to GLN and alkali
Nanoporous membrane of microcapsules is formed by the complexion of alginate and polycationic polymers such as high molecular weight PLL or chitosan [38]. Formation of nanoporous membranes using low molecular weight PLL alone is not feasible due to insufficient cross-linking between –NH2 (in PLL) and –COOH (in alginate) groups. DTBP was therefore used to form stable cross-links with low molecular weight PLL. DTBP contains an amine-reactive imidoester that reacts with primary amines at a certain pH range (8–10) to form stable amidine bonds. The amidine is protonated and possesses a positive charge at physiological pH [39]. DTBP cross-linking can be easily reversed by the addition of reducing agents, such as glutamine.
Fig. 4 shows the degradation of A-GD-PLL microcapsules following exposure to GLN over time. Without GLN, A-GD-PLL microcapsules exhibit a spherical shape (Fig. 4A). When the microcapsules were immersed in culture medium supplemented with GLN, they began to degrade in a bivalve fashion along their equator after 30 min (Fig. 4B). Microcapsule degradation was nearly complete after 24 h (Fig. 4C). GLN addition triggered the cleavage of –S–S– bonds within DTBP-PLL, initiating microcapsule degradation. The addition of GLN also increased the pH of the culture medium to ~8.5 after 24 h. Complete microcapsule degradation was not observed until the pH was adjusted to ~10 by the addition of sodium hydroxide to the culture medium (Fig. 4D). This caused the dissociation of the polyelectrolyte complexation between alginate and polylysine, leading to the disappearance of the microcapsule membrane. It is well known that the medium pH influences cell viability, and most cells cannot grow in media with pH values >9. In this study, the addition of GLN only increased the media pH to ~8.5 and therefore some cells remained alive. As the media pH was adjusted to above 10 with sodium hydroxide, all cells underwent cell death.
Fig. 4.
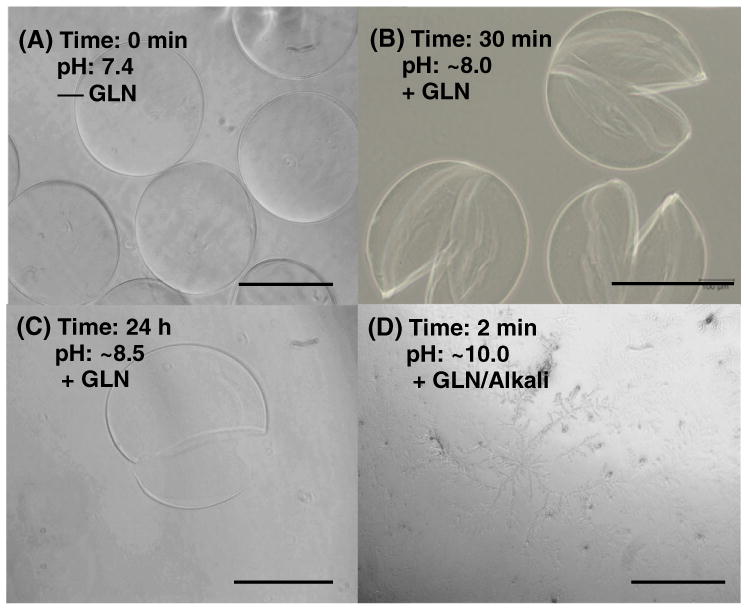
A-GD-PLL microcapsule degradation following GLN exposure. A-GD-PLL microcapsules were incubated in PC12 culture medium (A) without GLN, (B) with GLN for 30 min, (C) with GLN for 24 h, and (D) with GLN/Alkali. In C, the pH of the culture medium increased to 8.5 because of the presence of GLN. The pH value in D was manually adjusted by adding sodium hydroxide solution. Scale bar: 200 μm.
To achieve controlled degradation of our PCL device, both GLN and sodium hydroxide were loaded into a small PLGA pellet. The dissolution time of the A-GD-PLL microcapsules was found to be dependent on the degradation rate of the PLGA pellet. Once the PLGA cover degraded, GLN and sodium hydroxide were burst-released, leading to the dissolution of the microcapsules with eventual PC12 cell death. PLGA with a one month degradation rate was used in this study. For clinical applications, other biodegradable polymers such as PCL may be better suited because they will not affect the medium pH value within the PCL device. By manipulating the degradation rate of the polymer material used for GLN pellets we can control the burst degradation rate of the A-GD-PLL microcapsules.
Fig. 5 compares the cell viability of microencapsulated PC12 cells when the cell-containing A-GD-PLL microcapsules were incubated with or without a GLN solution (50 ng/ml) for 30 min, which was subsequently replaced by fresh medium containing 1 mg/ml IgG for 24 and 48 h. The viable cell number was linearly related to the optical density (OD) of MTS. The OD value PC12 cell viability was significantly lower in the presence of GLN at both 24 and 48 h. GLN led to A-GD-PLL microcapsule degradation enabling the immune molecule IgG to diffuse within the microcapsule and cause the cell death, which is in correspondence to a lower OD value. PC12 cell death was found to be a direct result of the immune molecule IgG in combination with the increased media pH by GLN.
Fig. 5.
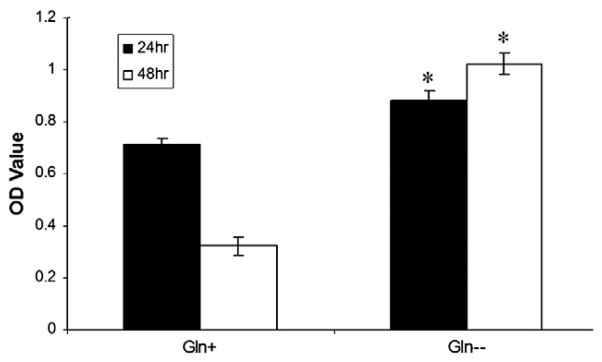
Microencapsulated PC12 cell viability in the presence of GLN. Comparison of microencapsulated PC12 cell viability following incubation with or without a GLN solution for 30 min which was subsequently replaced with fresh containing 1 mg/ml IgG for 24 and 48 h. Data were derived from n=3 samples. *p<0.005.
The aforementioned results demonstrate the efficient cell culture and immunoprotection, followed with controllable degradation of implanted cells. For applications where implanted cells need to stay alive and eventually be integrated with the surrounding tissue, one can reduce the GLN content and not placing any alkali materials in the system. Our biodegradable nanoporous device may provide certain ‘adjustment time’ to allow foreign cells to survive better in vivo.
3.2. Device evaluation
3.2.1. Release of PC12-derived dopamine in vitro and in vivo
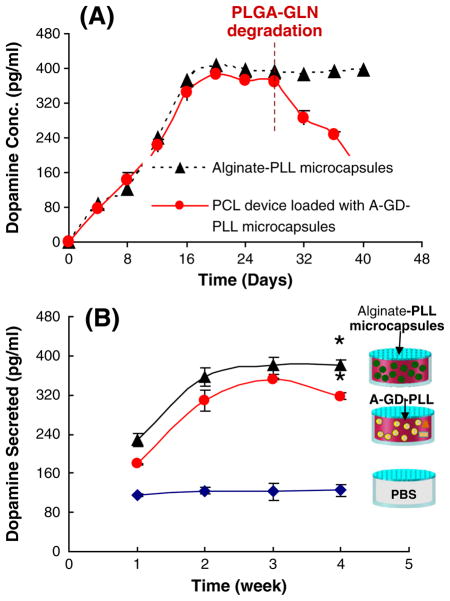
The amount of dopamine released from PC12 cells encapsulated within alginate-PLL microcapsules alone and after implantation into a fully integrated PCL device loaded with A-GD-PLL microcapsules was measured over the course of 40 days in vitro (Fig. 6A). For PC12 cells not incorporated into the PCL device, the secretion of dopamine increased daily, reaching a maximum value on Day 20. Dopamine secretion remained relatively constant over the course of 20–40 days. This correlates with PC12 cell growth patterns observed previously within alginate-PLL microcapsules. With regard to A-GD-PLL microcapsules incorporated within integrated PCL devices, dopamine release followed a similar trend, reaching a maximum concentration level on Day 20. Dopamine secretion notably decreased until Day 40 as a result of A-GD-PLL microcapsule degradation and PC12 cell death by GLN and alkali release as described previously.
Fig. 6.
(A) Comparison of dopamine release profiles in vitro. PC12-derived dopamine was measured following culture within alginate-PLL microcapsules or implantation in a fully integrated PCL device loaded with A-GD-PLL microcapsules. (B) Comparison of serum dopamine concentrations in mice implanted with PCL devices loaded with PBS, alginate-PLL microcapsules, or A-GD-PLL microcapsules. Data were derived from n=3 devices for each condition. *Statistically significant increase in serum dopamine concentration compared to PBS control device (p<0.005).
Serum dopamine levels from various groups of mice can be seen in Fig. 6B. PCL devices loaded with PBS produced baseline levels of dopamine. Integrated PCL devices containing alginate-PLL microcapsules or A-GD-PLL microcapsules secreted statistically significantly higher levels of dopamine over the course of 4 weeks as compared to control. Dopamine levels plateaued around week three likely because of PC12 cell population stabilization inside the microcapsules. A-GD-PLL microcapsule degradation secondary to GLN and subsequent PC12 cell death may have contributed to the slight decrease in dopamine observed in the PCL devices containing A-GD-PLL microcapsules. PLGA-GLN pellets and small PCL capsules containing nerve growth factor serve as examples to demonstrate the multiple functionality of this device. Depending on applications, fewer or more components can be placed in the chamber.
3.2.2. In vivo C-reactive protein testing and inflammation analysis
C-reactive protein (CRP) is an acute phase protein that can be measured in the serum of mice and is felt to be indicative of overall levels of inflammation. Fig. 7 summarizes the serum CRP concentrations of mice implanted with PCL devices with different contents. Control conditions consisted of untreated mice and mice injected with an alginate-PLL microcapsule solution without prior implantation into a PCL device. Untreated mice produced relatively low levels of serum CRP. Mice implanted with alginate-PLL microcapsules exhibited the highest level of serum CRP concentrations over the course of 4 weeks. However, similar levels of serum CRP concentrations were measured in mice implanted with a PCL device, irrespective of device contents, likely secondary to PEG-grafting. PEG-grafting improved PCL device biocompatibility and was the critical factor that resulted in a gradual decrease in serum CRP concentration over the course of 4 weeks.
Fig. 7.
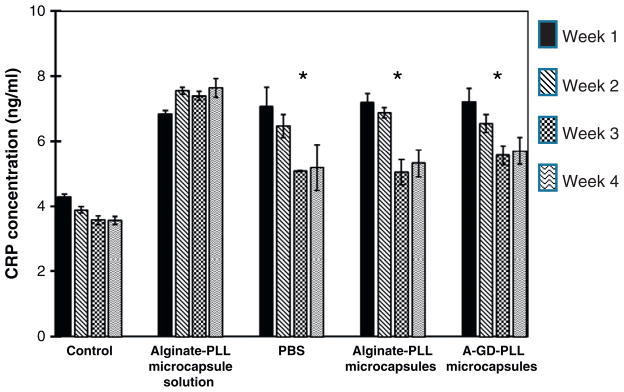
Comparison of CRP levels in the serum of mice implanted with various PCL devices. Control conditions consisted of untreated mice and mice implanted with alginate-PLL microcapsule alone. Data were derived from n=3 devices for each condition. * Statistically significant decrease in serum CRP concentration as compared to controls (p<0.005).
Athymic mice were surgically implanted with various PCL devices or alginate-PLL microcapsules alone (Fig. 8). After 30 days, mice were sacrificed and the remaining PCL devices were removed. Tissue samples from mice implanted with PCL devices containing PBS exhibited a mild mixed inflammatory infiltrate involving subcutaneous and perivascular tissues (Fig. 8A). Samples from mice implanted with alginate-PLL microcapsules and mice implanted with PCL chambers containing alginate-PLL microcapsules exhibited the most severe inflammatory reactions (Fig. 8B and C). Tissue from mice implanted with PCL devices containing A-GD-PLL microcapsules exhibited a spectrum of inflammation ranging from no inflammation to a mixed inflammatory response.
Fig. 8.
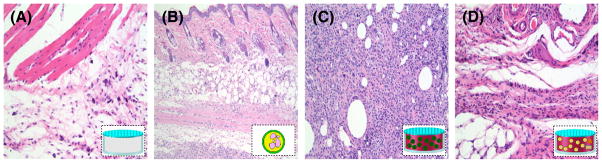
Microscopic images of the inflammatory reaction produced by (A) PBS loaded capsules, (B) alginate-PLL microcapsules, (C) capsules loaded with alginate-PLL microcapsules, and (D) capsules loaded with A-GD-PLL microcapsules in immunocompetent mice on Day 30. All images taken at 200×. A: Occasional mast cells and lymphocytes. B: Prominent chronic inflammation with a predominance of lymphocytes. C: Mixed inflammatory infiltrate with a predominance of neutrophils, occasional eosinophils and lymphocytes. D: Rare lymphocytes present (near normal histology).
4. Conclusion
In this study, we describe the design, fabrication and evaluation of a nanoporous, cell-based device with controllable degradation for long-term drug release. Because of the variable degradation rates and multifunctionality, this device possesses great possibility for use in clinical practice. Using PC12 cells as a model, we demonstrated that controlled release of NGF from a small nanoporous PCL capsule could facilitate cell growth and proliferation. In addition, GLN and alkali released from a PLGA-GLN/alkali pellet resulted in A-GD-PLL microcapsule degradation and eventual PC12 cell death. Our integrated device served as a diffusion barrier, limiting the ability of the immune system from penetrating the nanoporous membrane of both microcapsules and PCL chamber, enabling the release of cell-secreted products and the diffusion of nutrients. Following a pre-specified degradation time, our device design allowed for the safe clearance of implanted foreign cells and the remaining device by the host immune system. The current device appeared to be tolerated by the host even though some persistent inflammation was present at 30 days post-implantation.
The improved biocompatibility and the controlled biodegradability of the integrated device have the potential to reduce the problems seen previously with implanting living foreign cells and microcapsules in vivo. For further clinical applications, the antigenicity of therapeutic drugs via this integrated biodegradable device and a panel of toxicity studies will be conducted. In the future, one could utilize engineered cells derived from human stem cells or induced pluripotent stem cells (iPSCs) to deliver dopamine or other biologically active compounds useful in the treatment of clinical diseases. Integrated biodegradable devices hold promise for the future treatment of a variety of diseases.
Acknowledgments
This work is funded by the National Science Foundation sponsored Nanoscale Science and Engineering Center for Affordable Nanoengineering of Polymeric Biomedical Devices (NSEC-CANPBD) at The Ohio State University. This work was also supported by National Institutes of Health (NIH) Grants: P01 CA095426 and T32 CA090223 (EL) and T32 CA009338 (VG).
References
- 1.Wang NX, von Recum HA. Affinity-based drug delivery. Macromol Biosci. 2011;11:321–332. doi: 10.1002/mabi.201000206. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006. [Google Scholar]
- 3.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 4.Gregoiraidis G. Engineered liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13:527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 5.Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J Control Release. 2003;88:215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 6.Santini JT, Cima MJ, Langer RA. Controlled release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 7.Prescott JH, Lipka S, Baldwin S, Sheppard NF, Maloney JM, Coppeta J, Yomtov B, Staples MA, Santini JT. Chronic, programmed polypeptide delivery from an implanted, multireservoir microchip device. Nat Biotechnol. 2006;24:437–438. doi: 10.1038/nbt1199. [DOI] [PubMed] [Google Scholar]
- 8.Fine D, Grattoni A, Zabre E, Hussein F, Ferrariac M, Liu X. A low-voltage electro-kinetic nanochannel drug delivery system. Lab Chip. 2011;11:2526–2534. doi: 10.1039/c1lc00001b. [DOI] [PubMed] [Google Scholar]
- 9.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 10.Orive G, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Survival of different cell lines in alginate-agarose microcapsules. Eur J Pharm Sci. 2003;18:23–30. doi: 10.1016/s0928-0987(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 11.Jeon Y, Kwak K, Kim S, Kim Y, Lim J, Baek W. Intrathecal implants of microencapsulated xenogenic chromaffin cells provide a long-term source of analgesic substances. Transplant Proc. 2006;38:3061–3065. doi: 10.1016/j.transproceed.2006.08.098. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Liu X, Xie Y, Zhang H, Yu W, Xiong Y, Xie W, Ma X. Microencapsulation using natural polysaccharides for drug delivery and cell implantation. J Mater Chem. 2006;16:3252–3267. [Google Scholar]
- 13.Strand BL, Ryan TL, In’t Veld P, Kulseng B, Rokstad AM, Skjak-Brek G, Espevik T. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10:263–275. [PubMed] [Google Scholar]
- 14.Westwood M, Roberts D, Parker R. Enzymatic degradation of poly-L-lysine-polygalacturonic acid multilayers. Carbohydr Polym. 2011;84:960–969. [Google Scholar]
- 15.Quong D. Stability of chitosan and poly-L-lysine membranes coating DNA-alginate beads when exposed to hydrolytic enzymes. J Microencapsul. 1999;16:73–82. doi: 10.1080/026520499289329. [DOI] [PubMed] [Google Scholar]
- 16.Etrych T, Boustta M, Leclercq L, Vert M. Release of polyanions from polyelectrolyte complexes by selective degradation of the polycation. J Bioact Compat Polym. 2006;21:89–105. [Google Scholar]
- 17.Morch YA, Donati I, Strand BL, Skjak-Break G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules. 2006;7:1471–1480. doi: 10.1021/bm060010d. [DOI] [PubMed] [Google Scholar]
- 18.Dandoy P, Meunier CF, Michiels C, Su BL. Hybrid shell engineering of animal cells for immune protections and regulation of drug delivery: towards the design of “artificial organs”. PLoS One. 2011;6:e20983. doi: 10.1371/journal.pone.0020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen A, Chen M, Wang S, Huang X, Liu Y, Chen Z. Poly(L-histidine)-chitosan/alginate complex microcapsule as a novel drug delivery agent. J Appl Polym Sci. 2012;124:3728–3736. [Google Scholar]
- 20.Yu S, Han B, Peng C. A preliminary study of alginate, heparin-chitosan-alginate and heparin microencapsulated hepatocytes system. Hepatogastroenterology. 2012;59:1234–1240. doi: 10.5754/hge11638. [DOI] [PubMed] [Google Scholar]
- 21.Yan S, Rao S, Zhu J, Wang Z, Zhang Y, Duan Y, Chen X, Jin J. Nanoporous multilayer poly(L-glutamic acid)/chitosan microcapsules for drug delivery. Int J Pharm. 2012;427:443–451. doi: 10.1016/j.ijpharm.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 23.Shachar M, Tsur-Gang O, Dvir T, Leor J, Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2001;7:152–162. doi: 10.1016/j.actbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Algire GH, Weaver JM, Prehn RT. Growth of cells in vivo in diffusion chambers: I. survival of homografts in immunized mice. J Natl Cancer Inst. 1954;15:493–507. [PubMed] [Google Scholar]
- 25.Desai TA, Hansford DJ, Ferrari M. Micromachined interfaces: new approaches in cell immunoisolation and biomolecular separation. Biomol Eng. 2000;17:23–36. doi: 10.1016/s1389-0344(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 26.LaFlamme KE, Mor G, Gong D, LaTempa T, Fusaro VA, Grimes CA, Desai TA. Nanoporous alumina capsules for cellular macroencapsulation: transport and biocompatibility. Diabetes Technol Ther. 2005;7:684–694. doi: 10.1089/dia.2005.7.684. [DOI] [PubMed] [Google Scholar]
- 27.Gong D, Yadavalli V, Paulose M, Pishko M, Grimes CA. Controlled molecular release using nanoporous alumina capsules. Biomed Microdevices. 2003;5:75–80. [Google Scholar]
- 28.https://www.dailymail.co.uk/health/article-1091445/The-miracle-teabag-Stem-cells-pack-help-stroke-victim-talk-again.html.
- 29.Zhang X, He H, Yen C, Ho W, Lee LJ. A biodegradable, immunoprotective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29:4253–4259. doi: 10.1016/j.biomaterials.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Roy S, Ferrara LA, Fleischman AJ, Benzel EC. Microfabricated dermabraders for plastic surgical applications. In: Ferrari M, Desai TA, Bhatia SN, editors. BioMEMS Biomedical Nanotechnology. Springer; New York: 2006. pp. 95–123. [Google Scholar]
- 31.Aebischer P, Tresco PA, Winn SR, Greene LA, Jaeger CB. Long-term cross-species brain transplantation of a polymer-encapsulated dopamine-secreting cell line. Exp Neurol. 1991;111:269–275. doi: 10.1016/0014-4886(91)90093-r. [DOI] [PubMed] [Google Scholar]
- 32.Yen C, He H, Lee LJ, Ho W. Synthesis and characterization of polycaprolactone nanoporous membranes for biomedical applications. J Membr Sci. 2009;343:180–188. [Google Scholar]
- 33.Carlisle ES, Mariappan MR, Nelson KD, Thomes BE, Timmons RB, Constantinescu A. Enhancing hepatocyte adhesion by pulsed plasma deposition and polyethylene glycol coupling. Tissue Eng. 2000;6:45–52. doi: 10.1089/107632700320883. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Vacek I, Sun A. Generation of alginate-polylysine-alginate (APA) biomicrocapsules: the relationship between the membrane strength and the reaction conditions. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:43–69. doi: 10.3109/10731199409117399. [DOI] [PubMed] [Google Scholar]
- 35.http://www.piercenet.com/Objects/View.cfm?type=ProductFamily&ID=02030242.
- 36.Trubetskoy VS, Loomis A, Slattum PM, Hagstrom JE, Budker VG, Wolff JA. Caged DNA does not aggregate in high ionic strength solutions. Bioconjug Chem. 1999;10:624–628. doi: 10.1021/bc9801530. [DOI] [PubMed] [Google Scholar]
- 37.He H, Grignol V, Karpa V, Yen C, LaPerle K, Zhang X, Jones NB, Liang MI, Lesinski GB, Ho WS, Lee LJ. Use of a nanoporous biodegradable miniature device to regulate cytokine release for cancer treatment. J Control Release. 2011;151:239–245. doi: 10.1016/j.jconrel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev. 2008;60:1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Hand ES, Jencks WP. Mechanism of the reaction of imidoesters with amines. J Am Chem Soc. 1962;84:3505–3514. [Google Scholar]