Abstract
Valid screening devices are critical for an early diagnosis of dementia. The DemTect is such an internationally accepted tool. We aimed to characterize the neural networks associated with performance on the DemTect's subtests in two frequent dementia syndromes: early Alzheimer's disease (AD) and frontotemporal lobar degeneration (FTLD). Voxel-based group comparisons of cerebral glucose utilization (as measured by F-18-fluorodeoxyglucose positron emission tomography) and gray matter atrophy (as measured by structural magnetic resonance imaging) were performed on data from 48 subjects with AD (n = 21), FTLD (n = 14) or subjective cognitive impairment (n = 13) as a control group. We performed group comparisons and correlation analyses between multimodal imaging data and performance on the DemTect's subtests. Group comparisons showed regional patterns consistent with previous findings for AD and FTLD. Interestingly, atrophy dominated in FTLD, whereas hypometabolism in AD. Across diagnostic groups performance on the “wordlist” subtest was positively correlated with glucose metabolism in the left temporal lobe. The “number transcoding” subtest was significantly associated with glucose metabolism in both a predominantly left lateralized frontotemporal network and a parietooccipital network including parts of the basal ganglia. Moreover, this subtest was associated with gray matter density in an extensive network including frontal, temporal, parietal and occipital areas. No significant correlates were observed for the “supermarket task” subtest. Scores on the “digit span reverse” subtest correlated with glucose metabolism in the left frontal cortex, the bilateral putamen, the head of caudate nucleus and the anterior insula. Disease-specific correlation analyses could partly verify or extend the correlates shown in the analyses across diagnostic groups. Correlates of gray matter density were found in FTLD for the “number transcoding” subtest and the “digit span reverse” subtest. Correlates of glucose metabolism were found in AD for the “wordlist” subtest and in FTLD for the “digit span reverse” subtest. Our study contributes to the understanding of the neural correlates of cognitive deficits in AD and FTLD and supports an external validation of the DemTect providing preliminary conclusions about disease-specific correlates.
Abbreviations: AD, Alzheimer's disease; ANOVA, Analysis of variance; BA, Brodmann area; CDR, Clinical dementia rating scale; DARTEL, Diffeomorphic anatomical registration through exponentiated lie algebra; FDG-PET, F-18-fluorodeoxyglucose positron emission tomography; FTLD, Frontotemporal lobar degeneration; MMSE, Mini-Mental State Examination; MNI, Montreal Neurological Institute; MRI, Magnetic resonance imaging; PVE, Partial volume effects; SPM, Statistical parametric mapping
Keywords: Alzheimer's disease, DemTect, FDG-PET, Frontotemporal lobar degeneration, MRI, Voxel based morphometry
Highlights
-
•
The DemTect is a frequently used clinical instrument to assess dementia syndromes.
-
•
Neural correlates of the DemTect's subtests were examined with MRI & PET imaging data.
-
•
Atrophy dominates in early FTLD, whereas hypometabolism in early AD.
-
•
The DemTect's neural correlates were investigated in early AD and FTLD.
-
•
Study contributes to the validation of the DemTect as a dementia screening instrument.
1. Introduction
Today, dementia disorders are a major health problem — affecting about 35.6 million people worldwide in 2010 (World Health Organisation, 2012). An early diagnosis is crucial to identify dementia-related diseases and administer appropriate therapeutic interventions; valid clinical screening and treatment progression devices are necessary to investigate specifically impaired cognitive domains. One of the most important and frequently used clinical dementia screening devices is the DemTect (Kalbe et al., 2004), which has obtained international acceptance as a neuropsychological dementia screening test in recent years (Jacova et al., 2007). The DemTect consists of five subtests: learning of a ten item wordlist in two trials (“wordlist”), transcoding numbers in numerals and vice-versa (“number transcoding”), a semantic word fluency task (“supermarket task”), a task in which the patient has to repeat sequences of numbers in backward order (“digit span reverse”), and finally the wordlist's delayed recall (“wordlist, delayed recall”). In comparison to the more established Mini-Mental State Examination (MMSE) (Folstein et al., 1975), the DemTect has been shown to be superior in several studies (Kalbe et al., 2004; Perneczky, 2003), especially concerning the detection of mild dementia, which is a well-known weakness of the MMSE (Simard, 1998). Although the neural correlates of the MMSE have been investigated in studies with magnetic resonance imaging (MRI) (Apostolova et al., 2006; Baxter et al., 2006; Ferrarini et al., 2008; Jack et al., 2002; Nickl-Jockschat et al., 2011) and F-18-fluorodeoxyglucose positron emission tomography (FDG-PET) (Mielke et al., 1994), these studies described neural correlates of total MMSE scores only. In order to link dementia syndromes to underlying impairments in neural networks, investigation of subtests addressing specific cognitive domains is necessary.
The neural networks affected by neurodegenerative diseases, especially Alzheimer's disease (AD) (Barnes et al., 2007; Baron et al., 2001; Boxer et al., 2003; Buckner et al., 2005; Chetelat et al., 2008; Frisoni et al., 2002; Rabinovici et al., 2007; Schroeter et al., 2009; Seeley et al., 2009) and frontotemporal lobar degeneration (FTLD) (Barnes et al., 2007; Boxer et al., 2003; Chetelat et al., 2008; Desgranges et al., 2007; Ishii et al., 1998; Jeong et al., 2005; Mummery et al., 2000; Rabinovici et al., 2007; Rosen et al., 2002; Schroeter et al., 2008, 2011; Seeley et al., 2009), have been described thoroughly in recent years in several studies and meta-analyses (Schroeter et al., 2008; Schroeter et al., 2009). Underlying different etiologies and pathomechanisms (Finder, 2010; Rabinovici and Miller, 2010), AD and FTLD have been related to specific metabolic and atrophic brain changes. MRI provides information about gray matter atrophy, whereas glucose metabolism is investigated with FDG-PET. However, results of FDG-PET analyses may be biased by gray matter atrophy if they are not corrected for partial volume effects (PVE) (Rousset et al., 1998). Accordingly, the correction for partial volume effects is a state-of-the-art step in preprocessing of FDG-PET imaging data (see also Baete et al., 2004). Recently, PVE-correction has been successfully applied when comparing atrophy and hypometabolism in AD (Chetelat et al., 2008). Although one study has investigated glucose metabolism and amyloid plaque density in subjects with AD and semantic dementia (Drzezga et al., 2008), to date, no study using FDG-PET has integrated other FTLD subtypes into a PVE corrected group comparison.
Firstly, our study aimed at investigating differences in FDG metabolism and gray matter atrophy with regard to their localization, as well as their extent in subjects suffering from AD or FTLD, using data corrected for PVE. Second, we intended to contribute to the external validation of the DemTect as a diagnostic tool capable of detecting cognitive deficits and linking them to morphological and glucose metabolic changes in the brain. Although the DemTect has already been conceptually validated as a sensitive and specific screening tool for AD by the use of FDG-PET as an in-vivo reference method (Scheurich et al., 2005), and there are studies that have investigated the neural networks involved in cognitive paradigms similar to those used in the DemTect in healthy subjects and dementia patients (Andreasen et al., 1995; Awh et al., 1996; Cabeza et al., 2002; Demonet et al., 1992; Fiez et al., 1996; Grasby et al., 1993; Henson et al., 2000; Jonides et al., 1998; Peters et al., 2009; Sato et al., 1999; Schroeter et al., 2012; Smith and Jonides, 1997; Smith et al., 1996, 1998), no study has systematically examined the neural correlates of the subtests of the DemTect. Accordingly, our study aimed to investigate the DemTect in relation to two neurodegenerative diseases (AD and FTLD) using a multimodal imaging study including MRI and FDG-PET. We hypothesized that performance in the DemTect subtests is associated with temporoparietal regions in AD and frontotemporal regions in FTLD. After correlating DemTect scores with MRI and FDG-PET data in the whole cohort to isolate the neural correlates of this test per se, we combined results with group comparisons between AD or FTLD patients and the control cohort in a conjunction analysis, and calculated disease-specific correlation analyses to identify neural correlates of the DemTect for AD and FTLD.
2. Methods
2.1. Subjects
We included 48 right-handed subjects from age 40 to 74 (25 females, 23 males), who were admitted to the Clinic of Cognitive Neurology at the University of Leipzig (Table 1; (Dukart et al., 2011)). They had presented with complaints of cognitive and/or behavioral alterations, by their own account and/or by the account of caregivers. Upon admittance, subjects underwent a high-quality FDG-PET and structural MRI scan; a comprehensive neurological and psychiatric history and examination; neuropsychological rating of behavioral deficits (Hughes et al., 1982); and testing of memory, executive function, attention and language. Details of the test batteries involved in our assessment are described in Frisch et al. (2013) and Schroeter et al. (2011, 2012). Inclusion criteria were a diagnosis of either probable AD, according to the revised NINCDS–ADRDA criteria (McKhann et al., 1984), FTLD, in accordance to criteria proposed by Neary et al. (1998), or subjective cognitive impairment, characterized by complaints of cognitive impairment that could not be confirmed by neuropsychological testing. The last group was chosen as a control group (please see also Discussion). Patients were excluded if structural imaging revealed lesions due to stroke, traumatic head injury, brain tumor or inflammatory diseases. All data were acquired for diagnostic purposes. Within the whole group, 29 subjects from age 40 to 74 (16 females, 13 males) had valid psychometric data from the German version of the DemTect-A (Table 1). We obtained informed consent from all patients. The research protocol was approved by the local ethics committee and was in accordance with the Declaration of Helsinki.
Table 1.
Demographic and clinical characteristics of the patient groups.
| AD | FTLD | Control | Group difference | |
|---|---|---|---|---|
| Whole group | ||||
| N | 21 | 14 | 13 | – |
| Age | 61.1 ± 6.7 | 60.8 ± 6.4 | 53.9 ± 6.0 | 5.8, 2, 0.006a |
| Sex (f/m) | 12/9 | 7/7 | 6/7 | 0.4, 2, 0.809b |
| Education (years) | 10.7 ± 3.1 | 11.6 ± 3.8 | 12.3 ± 3.1 | 1.0, 2, 0.368a |
| CDR | 0.7 ± 0.3 | 0.8 ± 0.4 | 0.2 ± 0.3 | 13.9, 2, 0.000a |
| MMSEc | 23.2 ± 3.9 | 24.4 ± 4.2 | 28.8 ± 1.3 | 4.5, 2, 0.019a |
| DemTect group | ||||
| N | 14 | 9 | 6 | – |
| Age | 61.5 ± 7.6 | 59.3 ± 7.4 | 51.2 ± 7.6 | 3.9, 2, 0.032a |
| Sex (f/m) | 9/5 | 5/4 | 2/4 | 1.6, 2, 0.443b |
| Education (years) | 11.1 ± 3.2 | 10.9 ± 3.5 | 13.0 ± 3.3 | 0.9, 2, 0.427a |
| CDR | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.3 ± 0.3 | 10.3, 2, 0.001a |
| MMSEc | 24.3 ± 3.8 | 23.8 ± 4.3 | 28.0 ± 1.0 | 1.4, 2, 0.263 a |
| DemTect sum score | 7.9 ± 3.4 | 7.3 ± 5.8 | 15.5 ± 2.9 | 8.3, 2, 0.002a |
| DemTect subscoresd | ||||
| Wordlist | 1.43 ± 1.22 | 1.11 ± 1.05 | 2.67 ± 0.52 | 4.1, 2, 0.028a |
| Number transcoding | 1.64 ± 0.93 | 1.67 ± 1.32 | 2.50 ± 0.55 | 1.7, 2, 0.209a |
| Supermarket task | 1.71 ± 1.27 | 1.00 ± 1.41 | 4.00 ± 0.00 | 12.0, 2, 0.000a |
| Digit span reverse | 2.21 ± 0.98 | 1.89 ± 0.93 | 2.50 ± 0.84 | 0.8, 2, 0.462a |
| Wordlist (delayed recall) | 0.86 ± 0.77 | 1.67 ± 2.06 | 3.83 ± 1.84 | 8.3, 2, 0.002a |
Note. All values given in mean ± standard deviation. AD = Alzheimer's disease, CDR = clinical dementia rating scale (Hughes et al., 1982), f = female, FTLD = frontotemporal lobar degeneration, m = male, MMSE = Mini-Mental State Examination (Folstein et al., 1975), n = total number.
As tested with One-Way ANOVA: F, degrees of freedom (df), p.
As tested with two-tailed chi-square test: chi-square, df, p.
For twelve/four subjects MMSE was not available.
Transformed scores.
2.2. Acquisition of neuroimaging data
2.2.1. MRI scanning
For each patient, a high resolution T1-MRI dataset was recorded on a 3 T Siemens TRIO (Siemens, Erlangen, Germany) or a Bruker Medspec 30/100 (Bruker, Ettlingen, Germany) scanner. Therefore either a magnetization-prepared rapid gradient echo sequence or a modified driven equilibrium Fourier transform imaging technique was used, recording 128 anterior–posterior, commissure line adjusted partitions in sagittal orientation and providing a spatial resolution of 1 × 1 × 1.5 mm (TR = 1300 ms, TI = 650 ms, TE = 3.93 ms, TE = 10 ms; FOV 25 × 25 respectively 25 × 24 cm; matrix = 256 × 256 or 256 × 240 voxels).
2.2.2. PET scanning
We conducted MRI and FDG-PET imaging within a period of a few weeks from admittance. Patients fasted for one night before PET imaging and were injected with 370 MBq of the radiotracer 18F-FDG immediately before the scanning procedure, which was conducted in 2-dimensional mode using a Siemens ECAT EXACT HR + PET device (CTI/Siemens, Knoxville, TN, USA). A dynamic scan was performed for 60 min under standard resting-state conditions with eyes open, recording 63 transaxial slices simultaneously with an axial resolution of 5 mm full width at half maximum (FWHM) and an in-plane resolution of 4.6 mm. Each collected slice had a thickness of 2.45 mm and a matrix size of 128 × 128 voxels. After correction for attenuation, scatter, decay and scanner-specific dead-time, the PET data were reconstructed by filtered back-projection using a Hann filter (4.9 mm full width at half maximum).
2.3. Preprocessing of neuroimaging data
Preprocessing was performed using the Statistical Parametric Mapping software package (SPM5, www.fil.ion.ucl.ac.uk/spm/) run with Matlab 7.7 (The MathWorks Inc., Natick, MA). Data preprocessing consisted of co-registration of FDG-PET and MRI data, segmentation of MRI data into different tissue classes (only the gray matter tissue class is used for further analyses), convolution-based voxel-wise PVE correction of FDG-PET data using the modified Müller–Gärtner method (Müller-Gärtner et al., 1992; Rousset et al., 1998) implemented in the PVElab software package (Quarantelli et al., 2004) and spatial normalization using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL, Ashburner, 2007). The PVE procedure uses the segmented MR images to account for PVE and for potential atrophy effects in FDG-PET. The same deformation matrices used to normalize gray matter images were applied to FDG-PET images. Gray matter images were additionally modulated to preserve the total amount of signal from each region. As modulated gray matter maps identify differences in local volume they are commonly considered as measurements of atrophy (Mechelli et al., 2005). Non-gray matter voxels were masked before and after smoothing. Smoothing was performed using a Gaussian kernel of 12 mm FWHM. FDG-PET data were normalized to cerebellar mean uptake values (Dukart et al., 2010). Additionally, prior to smoothing, we normalized all obtained images to Montreal Neurological Institute (MNI) space using affine only transformations. We retrieved anatomical descriptions of correlating brain regions by consensus, supported by FSLView 3.1 software (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html). The obtained modulated and smoothed gray matter probability maps are further referred to as MRI data.
2.4. Statistical analysis
We performed group comparisons for demographic and clinical characteristics, as well as the transformed (age-adjusted) DemTect subscores, in SPSS 19.0 (http://www-01.ibm.com/software/de/analytics/spss/) by means of an analysis of variance (ANOVA) and two-tailed chi-square tests (p < 0.05). Post-hoc analyses were performed with two-tailed post-hoc t-tests (p < 0.05 Bonferroni-corrected for multiple comparisons).
A voxel-based whole brain analysis of all 48 subjects' FDG-PET and MRI data was applied for group comparisons of the AD and FTLD patients, and the control subjects. Age and sex were included as covariates in all analyses (Kalpouzos et al., 2009). MR scanner types and sequences were not included as covariates because their distribution of use was even across subjects and did not differ significantly between diagnostic groups (Dukart et al., 2011). Results were displayed as t-maps. The extension of hypometabolic and atrophic brain regions for different contrasts was calculated by the sum of all clusters (in voxels) exceeding an uncorrected threshold of p < 0.001 on the voxel level and p < 0.05 (corrected) on the cluster level.
After the group comparisons, we conducted whole brain correlation analyses between the neuroimaging data and the subscores of the DemTect in the 29 patients with neuropsychological test scores. We included patients with neurodegenerative dementia of different etiology (AD and FTLD), as well as control subjects, to gain a high variance in correlation analyses, as suggested in previous studies (Schroeter et al., 2011, 2012). In this subgroup, age, type of scanner, and protocol were added as covariates in the MRI analyses to correct for an unequal distribution between diagnostic groups. Results were displayed as t-maps. A threshold of p < 0.001 on voxel-level and of p < 0.05 (corrected) on cluster-level was administered.
Finally, we conducted a conjunction analysis relating the neural correlates of the DemTect subscores across the different diagnostic groups to the specifically impaired brain regions in AD and FTLD, for glucose utilization and gray matter density. Moreover, to draw further disease-specific conclusions we conducted a correlation analysis within the dementia subgroups under the same conditions mentioned for the correlation analysis across diagnostic groups.
3. Results
3.1. Analysis of demographic and clinical data
Demographic and clinical data for the whole group involved in the group comparison, and for the DemTect subgroup involved in the correlation analysis, are described in Table 1. If there were category-specific, significant, between-group effects in an ANOVA, post-hoc t-tests were carried out (Bonferroni-corrected for multiple comparisons, significance threshold p < 0.05, based on directed hypotheses one-tailed p for comparisons of dementia subgroups vs. control group for Clinical Dementia Rating (CDR)-score, DemTect scores and MMSE score, for age two-tailed p reported).
For the whole group, significant group differences in age could be shown between the AD and control subgroups, and between the FTLD and control subgroups, whereas there were no differences between the AD and FTLD subgroups (AD vs. control: p = 0.008, t = 3.181, df = 32; FTLD vs. control: p = 0.024, t = 2.861, df = 25; AD vs. FTLD: p = 1.000, t = − 0.157, df = 33). In the DemTect group, a significant difference in age could be shown between the AD and control subgroups, but not between the other subgroups (AD vs. control: p = 0.029, t = 2.767, df = 18; FTLD vs. control: p = 0.154, t = 2.064, df = 13; AD vs. FTLD: p = 1.000, t = − 0.669, df = 21). Accordingly, age was included as a covariate in the subsequent analyses of imaging data.
As expected, CDR-scores were lower in the control group than in both dementia subgroups for both, the whole group (AD vs. control: p < 0.001, t = 5.357, df = 32; FTLD vs. control: p < 0.001, t = 4.347, df = 25; AD vs. FTLD: p = 0.977, t = 0.942, df = 33) and for the DemTect group (AD vs. control: p = 0.001, t = 3.888, df = 18; FTLD vs. control: p < 0.001, t = 4.266, df = 13; AD vs. FTLD: p = 1.000, t = 0.762, df = 21). As expected, in the whole group MMSE was higher in controls than in both dementia subgroups; significant differences could be shown particularly between AD and control subgroups (AD vs. control: p = 0.008, t = − 3.143, df = 23; FTLD vs. control: p = 0.055, t = − 2.298, df = 14; AD vs. FTLD: p = 1.000, t = 0.809, df = 29). For the DemTect group, no significant MMSE differences were observed between subgroups (probably due to reductions in statistical power), however the mean values showed the same descriptive differences.
The DemTect sum score and subscores for the three cohorts are reported in Table 1. In accordance with the hypothesis, the DemTect sum score was higher in the control group than in the patients with dementia syndromes (AD vs. control: p = 0.002, t = − 4.831, df = 18; FTLD vs. control: p = 0.002, t = − 3.155, df = 13; AD vs. FTLD: p = 1.000, t = − 0.274, df = 21). Group comparisons in the DemTect subtests revealed lower scores in both dementia groups in comparison with the control subjects for the “wordlist” subtest with no differences between AD and FTLD (AD vs. control: p = 0.038, t = − 3.184, df = 18; FTLD vs. control: p = 0.016, t = − 3.328, df = 13; AD vs. FTLD: p = 1.000, t = − 0.640, df = 21). Significant differences between both dementia groups and control subjects were also observed for “wordlist, delayed recall” (AD vs. control: p < 0.001, t = − 3.831, df = 5.8; FTLD vs. control: p = 0.017, t = − 2.079, df = 13; AD vs. FTLD: p = 0.654, t = 1.128, df = 9.5) and for the “supermarket task” (AD vs. control: p = 0.001, t = − 6.752, df = 13; FTLD vs. control: p < 0.001, t = − 6.364, df = 8; AD vs. FTLD: p = 0.516, t = − 1.262, df = 21). There were no between-group effects for the subtests “number transcoding” and “digit span reverse”.
In summary, dementia cohorts were characterized by lower MMSE/DemTect sum scores and three of the five DemTect subtest scores, and higher CDR scores in comparison with control subjects. Remarkably, we did generally not find statistical significant differences between dementia cohorts and relatively low impairment in both dementia groups indicating comparable stages of early dementia.
3.2. Group comparison between AD, FTLD and control subjects
Results for the comparisons of glucose metabolism and gray matter density between groups are illustrated in Fig. 1. Voxel-based group comparisons showed bilateral parietotemporal, retrosplenial and left-hemispheric prefrontal hypometabolic and atrophic regions in AD compared to control subjects. FTLD subjects had a lower glucose metabolism than control subjects in the bilateral prefrontal, left temporal and parieto-occipito-temporal junction regions. In the same contrast for MRI, we further observed gray matter atrophy in a bilateral, mainly left-sided prefrontal and a large lefthemispherial temporal region. Subjects with AD showed reduced glucose metabolism in bilateral retrosplenial cortex compared to FTLD subjects.
Fig. 1.
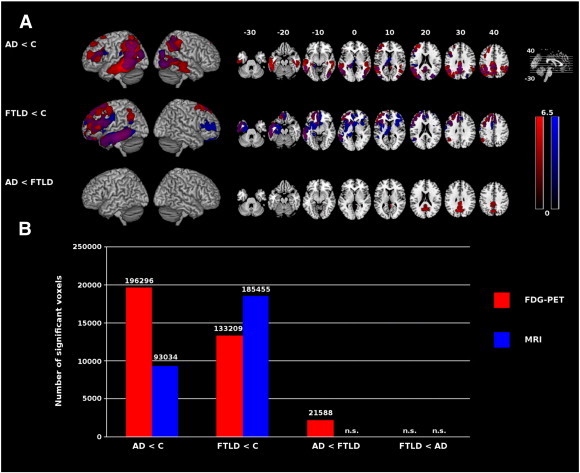
A: Reductions in glucose utilization (red) and gray matter density (blue) in dementia syndromes in comparison with control subjects and each other, rendered onto MNI template. Overlap is in purple. Only clusters with p < 0.05 (corrected) are displayed. Color spectrum represents t values. Left is left. B: The bar chart illustrates the extension of hypometabolic and atrophic brain regions (sum of all clusters in voxels exceeding an uncorrected threshold of p < 0.001 on the voxel level, and p < 0.05 corrected on the cluster level). AD = Alzheimer's disease, C = control subjects, FTLD = frontotemporal lobar degeneration, MNI = Montreal Neurological Institute, n.s. = not significant.
We consecutively calculated the sum of all significant clusters (in voxels) found in group comparisons (Fig. 1). We observed substantially more pronounced gray matter atrophy in FTLD than in AD patients when comparing them to control subjects. In contrast, hypometabolism was more pronounced in AD than in FTLD patients, both when comparing them to control subjects and when comparing them directly with each other. In the comparison AD vs. control subjects, hypometabolism extended gray matter atrophy. Gray matter atrophy extended hypometabolism if FTLD was compared with control subjects. In AD vs. FTLD we found no significant gray matter atrophy. No significant differences in the extent of gray matter atrophy or hypometabolism could be found in the contrast FTLD vs. AD.
3.3. Correlation of performance in DemTect subtests with glucose metabolism and gray matter density across diagnostic groups
Fig. 2 and Tables 2 & 3 show the results of the correlation analysis. In the following, we describe the results for each DemTect subtest score separately.
Fig. 2.
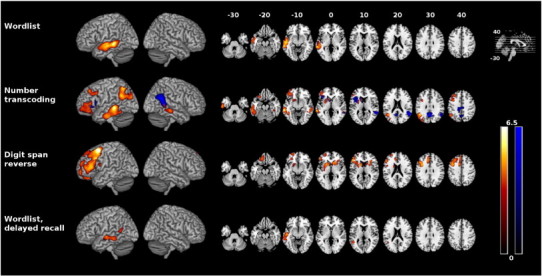
Correlation between DemTect raw scores and glucose utilization (red-yellow) and gray matter density (blue) rendered onto MNI template. Only clusters with p < 0.05 (corrected) are displayed. Color spectrum represents t values. Left is left. MNI = Montreal Neurological Institute.
Table 2.
Correlation between glucose metabolism and DemTect raw scores across diagnostic groups.
| Anatomical regions | Lat. | Coordinates |
T score | Z score | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Wordlist | |||||||
| Left superior and middle temporal gyrus (BA 21/22) | L L L |
− 62 − 57 − 48 |
− 34 − 15 − 27 |
− 4 − 10 0 |
6.14 5.32 4.94 |
4.79 4.34 4.11 |
16,396*** |
| Number transcoding | |||||||
| Left inferior and middle temporal gyrus (BA 20/21) | L L L |
− 65 − 59 − 49 |
− 35 − 17 − 48 |
− 4 − 30 − 13 |
6.03 4.55 4.54 |
4.73 3.87 3.86 |
14,356*** |
| Left posterior angular gyrus, BA 39, middle occipital gyrus (BA 19) | L L L |
− 43 − 38 − 44 |
− 51 − 57 − 61 |
31 22 51 |
5.80 5.29 5.25 |
4.60 4.32 4.30 |
11,135** |
| Left middle frontal gyrus (BA 9/46) | L L L |
− 33 − 33 − 42 |
12 35 26 |
37 43 34 |
4.93 4.33 3.96 |
4.11 3.72 3.47 |
4798* |
| Right middle temporal gyrus (BA 21) | R R R |
51 39 53 |
− 41 − 36 − 32 |
− 7 − 12 − 9 |
4.88 4.69 4.45 |
4.07 3.96 3.80 |
4844* |
| Left putamen and head of caudate nucleus, left inferior frontal gyrus, pars orbitalis and triangularis, anterior, posterior and lateral orbital gyrus (BA 11–13/45–47) | L L L |
− 21 − 36 − 43 |
11 37 35 |
7 − 6 3 |
4.60 4.57 4.39 |
3.90 3.88 3.76 |
17,307*** |
| Supermarket task | |||||||
| No significant suprathreshold clusters | |||||||
| Digit span reverse | |||||||
| Left superior, middle and inferior frontal gyrus (BA 20/21), frontomedian cortex (BA 9/10/32), anterior insula (BA 15–16), putamen and head of caudate nucleus | L L L |
− 34 − 43 − 55 |
9 19 33 |
58 35 9 |
7.26 5.56 5.08 |
5.32 4.47 4.20 |
45,506*** |
| Right putamen and head of caudate nucleus, anterior insula (BA 15–16) | R R R |
23 18 27 |
18 27 29 |
5 1 11 |
4.85 4.76 4.71 |
4.06 4.00 3.97 |
6717* |
| Left frontal pole (BA 10/11), left gyrus rectus, medial and anterior orbital gyrus | L L L |
− 28 − 16 − 28 |
63 37 57 |
− 5 − 22 20 |
4.27 4.22 4.19 |
3.68 3.65 3.63 |
7379** |
| Wordlist (delayed recall) | |||||||
| Left middle and superior temporal gyrus (BA 21/22), left posterior temporal sulcus | L L L |
− 53 − 46 − 60 |
− 14 − 48 − 33 |
− 7 8 − 10 |
4.92 4.17 4.12 |
4.10 3.61 3.58 |
6887* |
Note. Age included as covariate. Cluster size in voxels, voxel size = 1 × 1 × 1 mm. p (voxel-level uncorrected) < 0.001. *p < 0.05, **p < 0.01, ***p < 0.001, corrected for multiple comparisons on cluster-level. Cluster size threshold of 30 voxels. Coordinates in MNI space. BA = Brodmann area, Lat. = laterality, L = left, R = right.
Table 3.
Correlation between gray matter density and DemTect raw scores across diagnostic groups.
| Anatomical regions | Lat. | Coordinates |
T score | Z score | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Wordlist | |||||||
| No significant suprathreshold clusters | |||||||
| Supermarket task | |||||||
| No significant suprathreshold clusters | |||||||
| Number transcoding | |||||||
| Right angular gyrus (BA 39), posterior superior and middle temporal gyrus (BA 22/37) | R R R |
56 60 49 |
− 53 − 46 − 40 |
21 9 23 |
6.57 6.51 4.75 |
4.92 4.89 3.95 |
8931** |
| Bilateral posterior inferior precuneus (BA 7/31), dorsal posterior cingular cortex (BA 23, 31) and retrosplenial cortex (BA 30) | R L L |
8 − 11 − 2 |
− 57 − 56 − 59 |
32 26 33 |
6.23 5.65 5.45 |
4.76 4.46 4.35 |
10,168** |
| Left inferior frontal gyrus, pars opercularis (BA 44), triangularis (BA 45) and orbitalis (BA 47), pars ascendens and horicontalis of lateral sulcus, head of caudate nucleus, putamen, anterior insular cortex (BA 15/16), posterior orbital gyrus (BA 12, 47) | L L L |
− 42 − 12 − 28 |
11 3 4 |
13 7 12 |
4.48 4.44 4.43 |
3.78 3.75 3.75 |
11,967** |
| Digit span reverse | |||||||
| No significant suprathreshold clusters | |||||||
| Wordlist (delayed recall) | |||||||
| No significant suprathreshold clusters | |||||||
Note. Age, scanner type and protocol included as covariates. Cluster size in voxels, voxel size = 1 × 1 × 1 mm. p (voxel-level, uncorrected) < 0.001. **p < 0.01, corrected for multiple comparisons on cluster-level. Cluster size threshold of 30 voxels. Coordinates in MNI space. Lat. = laterality, L = left, R = right.
3.3.1. Wordlist
Impairment in the “wordlist” subtest (sum of both trials) was associated with lower glucose metabolism in the left superior and middle temporal gyrus. Evaluation of the first trial (not displayed) revealed a correlation in the same regions but spreading further to the left angular gyrus and the temporal pole. No results were obtained for correlation with gray matter density.
3.3.2. Number transcoding
Impairment in “number transcoding” subtest was significantly correlated with hypometabolism in a left lateralized network including the middle and inferior temporal gyri, the posterior angular gyrus, the middle occipital gyrus, parts of the middle and inferior frontal gyrus, the posterior and lateral orbital gyrus, the putamen and the head of caudate nucleus. There was also a significant correlation in the right middle temporal gyrus. Furthermore we could show a correlation with gray matter density in the right angular gyrus and adjacent posterior, superior and middle temporal gyri, bilaterally in the posterior inferior precuneus, the dorsal posterior cingulate, the retrosplenial cortex, the left inferior frontal gyrus, parts of the lateral sulcus, the head of caudate nucleus, the putamen and the anterior insular cortex.
3.3.3. Supermarket task
Impairments in the semantic word fluency subtest (“supermarket task”) did not correlate with either hypometabolism or gray matter density at the chosen significance threshold.
3.3.4. Digit span reverse
Impairments in the “digit span reverse” subtest correlated with hypometabolism in the left frontal cortex (including the superior, middle and inferior frontal gyri, the frontomedian cortex, the gyrus rectus and the medial and anterior orbital gyri), and bilaterally in the putamen, the heads of the caudate nucleus and the anterior insula. No significant results were obtained for correlation with gray matter density.
3.3.5. Wordlist, delayed recall
Impairments in the “wordlist delayed recall” subtest were associated with hypometabolism in the left middle and superior temporal gyri and posterior temporal sulcus. Again, no significant results were obtained for correlation with gray matter density.
3.4. Conjunction analysis
The first analysis compared glucose utilization and gray matter atrophy in the dementia syndromes AD and FTLD with the control group, whereas the second correlation analysis related test performance in the DemTect's subtests to both of the imaging measures taken, across the whole cohort. Finally, a conjunction analysis between both analyses aimed to relate results of the DemTect correlation to the dementia subgroups AD and FTLD. Fig. 3 shows almost similar results for glucose utilization, whereas gray matter atrophy was related to performance in the “number transcoding” subtest differently in AD and FTLD, in particular to gray matter atrophy in the retrosplenial/precuneal/posterior cingulate cortex and the right posterior middle temporal gyrus in AD, and to atrophy in the left anterior insula, the caudate head and the putamen in FTLD.
Fig. 3.
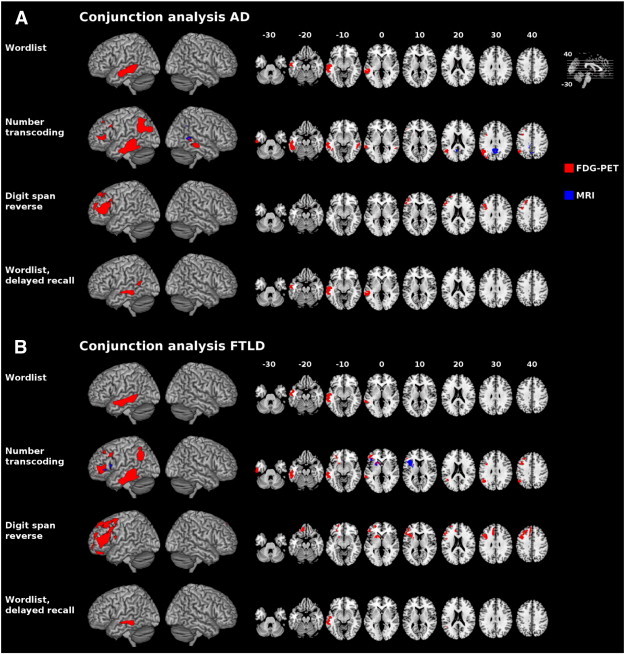
Conjunction analysis for group comparisons (patients with dementia syndromes vs. control subjects) and correlation analysis of the DemTect subscores rendered onto MNI template. A: Conjunction analysis for AD < control subjects. B: Conjunction analysis for FTLD < control subjects. Glucose utilization (red) and gray matter density (blue). Only clusters with p < 0.05 (corrected) are displayed. Left is left. AD = Alzheimer's disease, FDG-PET = F-18-fluorodeoxyglucose positron emission tomography, FTLD = frontotemporal lobar degeneration, MNI = Montreal Neurological Institute, MRI = magnetic resonance imaging.
3.5. Correlation of performance in DemTect subtests with glucose metabolism and gray matter density within the dementia subgroups
Fig. 4 and Tables 4 & 5 show the results of the correlation analysis within the two dementia subgroups. In the following, we describe the results for AD and FTLD separately.
Fig. 4.
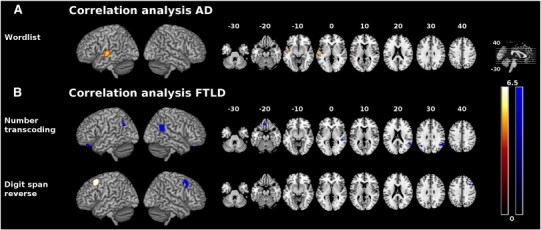
Correlation between glucose utilization (red-yellow)/gray matter density (blue) and DemTect raw scores within dementia subgroups rendered onto MNI template. Only clusters with p < 0.05 (corrected) are displayed. Color spectrum represents t values. Left is left. A: Correlation between DemTect raw scores and glucose utilization/gray matter density in AD. B: Correlation between DemTect raw scores and glucose utilization/gray matter density in FTLD. AD = Alzheimer's disease, FTLD = frontotemporal lobar degeneration, MNI = Montreal Neurological Institute.
Table 4.
Correlation between glucose metabolism and DemTect raw scores in dementia subgroups.
| Anatomical regions | Lat. | Coordinates |
T score | Z score | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| AD | |||||||
| Wordlist | |||||||
| Left superior temporal gyrus (BA 22) | L L |
− 55 − 46 |
− 14 − 7 |
− 1 − 13 |
5.49 4.97 |
3.73 3.53 |
3909* |
| Number transcoding | No significant suprathreshold clusters | ||||||
| Supermarket task | No significant suprathreshold clusters | ||||||
| Digit span reverse | No significant suprathreshold clusters | ||||||
| Wordlist (delayed recall) | No significant suprathreshold clusters | ||||||
| FTLD | |||||||
| Wordlist | No significant suprathreshold clusters | ||||||
| Number transcoding | No significant suprathreshold clusters | ||||||
| Supermarket task | No significant suprathreshold clusters | ||||||
| Digit span reverse | |||||||
| Left posterior middle frontal gyrus (BA 9) | L | − 35 | 18 | 56 | 12.77 | 4.34 | 2785** |
| Wordlist (delayed recall) | no significant suprathreshold clusters | ||||||
Note. Age included as covariate. Cluster size in voxels, voxel size = 1 × 1 × 1 mm. p (voxel-level uncorrected) < 0.001. *p < 0.05, **p < 0.01, corrected for multiple comparisons on cluster-level. Cluster size threshold of 30 voxels. Coordinates in MNI space. AD = Alzheimer's disease, BA = Brodmann area, FTLD = frontotemporal lobar degeneration, Lat. = laterality, L = left, R = right.
Table 5.
Correlation between gray matter density and DemTect raw scores in dementia subgroups.
| Anatomical regions | Lat. | Coordinates |
T score | Z score | Cluster size | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| AD | |||||||
| No significant suprathreshold clusters | |||||||
| FTLD | |||||||
| Wordlist | No significant suprathreshold clusters | ||||||
| Supermarket task | No significant suprathreshold clusters | ||||||
| Number transcoding | |||||||
| Right angular gyrus (BA 39) | R R R |
53 57 48 |
− 49 − 50 − 41 |
22 30 26 |
31.24 28.96 8.05 |
4.52 4.45 3.22 |
2486*** |
| Right superior temporal sulcus (BA 21/22) | R R R |
48 42 42 |
− 32 − 34 − 38 |
− 4 6 13 |
20.39 10.22 8.56 |
4.14 3.47 3.28 |
901** |
| Left angular gyrus (BA 39) | L L |
− 40 − 54 |
− 63 − 61 |
33 46 |
17.45 7.95 |
4.00 3.20 |
686* |
| Bilateral gyrus rectus (BA 11) | R L L |
1 − 12 − 4 |
33 40 47 |
− 26 − 25 − 19 |
16.47 13.76 7.77 |
3.95 3.77 3.18 |
1290** |
| Digit span reverse | |||||||
| Right posterior middle frontal gyrus (BA 9) | R R R |
37 41 41 |
18 11 19 |
41 58 54 |
135.22 | 5.63 | 1221** |
| Wordlist (delayed recall) | No significant suprathreshold clusters | ||||||
Note. Age, scanner type and protocol included as covariates. Cluster size in voxels, voxel size = 1 × 1 × 1 mm. p (voxel-level, uncorrected) < 0.001. *p < 0.05, **p < 0.01, ***p < 0.001, corrected for multiple comparisons on cluster-level. Cluster size threshold of 30 voxels. Coordinates in MNI space. AD = Alzheimer's disease, FTLD = frontotemporal lobar degeneration, Lat. = laterality, L = left, R = right.
3.5.1. AD
In the AD sample we detected correlates between performance in the wordlist task and glucose metabolism in the left superior temporal gyrus. No correlates concerning other tests or gray matter density could be shown.
3.5.2. FTLD
In the FTLD group, we detected correlates both concerning glucose utilization and atrophy. In the “number transcoding” subtest, performance was associated with gray matter density bilaterally in the angular gyrus and rectal gyrus, and in the right superior temporal sulcus. For the “digit span reverse” task we found neural correlates for glucose metabolism in the posterior left middle frontal gyrus and correlates for gray matter density in the posterior right middle frontal gyrus.
4. Discussion
In our study, we aimed to identify regional hypometabolism and gray matter atrophy in AD and FTLD patients using PVE corrected data. Based on these data, we intended to discover the neural correlates of performance in the DemTect with multimodal imaging. Note that both dementia cohorts involved clinically comparable early stages of the disease as shown by similar CDR, MMSE and DemTect scores.
Concerning the inclusion of subjects with subjective cognitive impairment as a control group in our study, one could raise the point that this potentially would not be a healthy population, as there are several studies in individuals with subjective memory impairment showing a progression to mild cognitive impairment or dementia (e.g. Heun et al., 2006; Luck et al., 2010; Scheef et al., 2012; van Harten et al., in press). Hence, such a control group with subjective cognitive impairment could potentially reduce the extent of conclusions that can be drawn from comparisons with AD and FTLD subjects. Nevertheless, we think that such a control group is justified and actually has several advantages in comparison with healthy subjects: First, memory or cognitive complaint is also known to occur in healthy aging as a discrepancy between demands and normal decline of cognitive abilities (see also Frisch et al., 2013). In recent studies, cognitive complaint could not differentiate between memory-declining and cognitively normal older adults and it could not be shown as a predictor for the development of dementia (Mol et al., 2006; Weaver Cargin et al., 2008). In our control subjects, mild cognitive impairment or dementia were excluded by thorough examination, neuropsychological testing and comprehensive multimodal neuroimaging with MRI and FDG-PET. These subjects with subjective cognitive impairment did not show abnormalities on relevant measures compared to age-matched healthy cohorts. Note that normal imaging findings in our control cohort with subjective cognitive impairment make beginning AD very unlikely, because a recent study has shown precuneal hypometabolism and hippocampal atrophy already in persons with subjective memory impairment (Scheef et al., 2012). Second, the differentiation between patients with subjective cognitive impairment and patients with dementia syndromes is most important in clinical practice (Dukart et al., 2010, 2011; Schroeter et al., 2011). Third, for ethical reasons (exposure to radiation) FDG-PET should not be applied without clinical justification. Fourth, we want to emphasize that our control cohort consisted of individuals with subjective cognitive impairment reporting complaints in one or more of all possible cognitive domains (memory, attention, executive functions) and not only memory such as in subjective memory complainers. Hence, our control cohort does not show a specific pattern of subjective complaints possibly indicating prestages of Alzheimer's disease and exhibits rather unspecific subjective complaints. Furthermore, note that in our group comparisons between FTLD or AD patients and control subjects the same control group was involved, making systematic biases very unlikely. We hold the view that – due to the mentioned reasons – in the context of our study sufficient conclusions can be drawn from subjects seeking for diagnostic evaluation of subjective cognitive complaint. Accordingly, we consider subjects with subjective cognitive impairment not only an acceptable, but also an ideal control group from a clinical point of view. There are several current studies including subjects with subjective cognitive impairment as a control group in the literature (e.g. Schroeter et al., 2012).
We also want to discuss an interesting point of our analysis of clinical data. As described we did generally not find significant differences between performances of AD and FTLD groups in the subtests of the DemTect. However, on a descriptive level the DemTect subscores show subtle differences between AD and FTLD, which are generally in accordance with the traditional concept of neuropsychological profiles of these diseases (see for example Thompson et al., 2005): AD subjects performed worse than FTLD subjects in delayed recall of the wordlist, a finding which would be predicted by several studies assuming a poorer memory consolidation in AD. In contrast, AD subjects performed better in the supermarket task and in digit span reverse, which could partly be related to frontal lobe functions which have been assumed to be more impaired in FTLD (at least FTD). We cannot exclude that the absence of significant subscore differences between the groups is due to the relatively small number of subjects and the heterogeneity of the FTLD cohort. Nevertheless, these findings are also in correspondence with recent data in the literature. These studies question the specificity of the neuropsychological profile for both neurodegenerative diseases, rather showing overlapping deficits for instance for memory and executive functions (see meta-analysis by Hutchinson and Mathis, 2007; and Frisch et al., 2013; Schroeter et al., 2012). This has been discussed in the context of large distributed networks contributing to the different cognitive functions which break down in dementing illnesses (see Sporns, 2010 among others for an overview).
4.1. Dissociation between atrophy and hypometabolism in FTLD and AD
In our study, we performed group comparisons between AD, FTLD and control subjects with subjective cognitive impairment using a morphometric approach. Results for the group comparisons were in agreement with previous studies, showing mainly impaired parietotemporal networks, including the posterior cingulate cortex for AD (Barnes et al., 2007; Baron et al., 2001; Boxer et al., 2003; Buckner et al., 2005; Chetelat et al., 2008; Frisoni et al., 2002; Rabinovici et al., 2007; Schroeter et al., 2009; Seeley et al., 2009) and alterations in mainly anterior frontotemporal regions, including the anterior cingulate cortex for FTLD, when compared to healthy subjects (Barnes et al., 2007; Boxer et al., 2003; Chetelat et al., 2008; Desgranges et al., 2007; Ishii et al., 1998; Jeong et al., 2005; Mummery et al., 2000; Rabinovici et al., 2007; Rosen et al., 2002; Schroeter et al., 2008, 2011; Seeley, 2010; Seeley et al., 2009). In both cases, alterations occurred predominantly in the left hemisphere and included parts of the basal ganglia. Most interestingly, metabolic decline appeared greater than gray matter loss in AD compared to control subjects, whereas the opposite was the case in the FTLD cohort (see Fig. 1).
For AD, this is consistent with previous findings (Chetelat et al., 2008) and attributable to the fact that the metabolic decline is known to antecede morphological decline in the course of AD (Jack et al., 2010). Apart from cytoplasmic inclusions of neurofibrillary tangles due to aggregation of hyperphosphorylated tau protein, the extracellular accumulation of beta-amyloid has been discussed to play an important role in initiating synaptic dysfunction, which may represent the main cause of hypometabolism in FDG-PET (Chetelat et al., 2008; Jack et al., 2010). The localization of these beta-amyloid deposits has been investigated in several studies and supports the correctness of hypometabolic regions in our AD cohort (Barthel et al., 2011; Forsberg et al., 2008; Kemppainen et al., 2006; Klunk et al., 2004; Li et al., 2008; Rowe et al., 2007). Histopathological correlates of FTLD differ from those of AD, including three different types of cytoplasmic inclusions – FTLD-tau, FTLD-TDP43 and FTLD-FUS due to a number of possible mutations, especially in the tau and the progranulin gene (Rabinovici and Miller, 2010). However, the exact succession of pathological changes has not yet been elucidated satisfactorily. Our results may hint on pathomechanisms in FTLD, with gray matter loss being less dependent on a wide-ranging decline of glucose metabolism. Multimodal follow-up studies investigating different stages of FTLD and its subtypes might provide further answers to this question.
Interestingly, we did not observe differences concerning atrophy in direct comparison of AD and FTLD in our study. This missing finding might be related to early disease stage for both dementia subgroups, the heterogeneity of the FTLD cohort and correction of imaging data for multiple comparisons, finally leading to insufficient statistical power. However, there are significant differences comparing AD and FTLD groups directly with respect to FDG metabolism in typical regions (precuneus) underlining the relevance of FDG-PET for early diagnosis and differential diagnosis of dementia syndromes with a higher reliability than MRI (Dukart et al., 2011). As PET data was corrected for partial volume effects, one would even expect larger clusters in uncorrected data.
4.2. Neural correlates of performance in DemTect subtests
The second focus of our study was the correlation between dementia specific networks and performance in the DemTect's subtests. Correlation analyses firstly were performed across diagnostic groups with early dementia syndromes and including control subjects to increase variances in exploring the neural correlates of the DemTect as a screening instrument in early dementia. The same approach was used likewise in several other studies to increase statistical power (Rankin et al., 2006; Rosen et al., 2005; Schroeter et al., 2012). The dilemma between disease specificity and statistical power could be solved in the future by recruiting very large patient groups, and investigating patients with a further progression of disease. Nevertheless, we also performed a correlation analysis within the relatively small dementia subgroups to draw further disease-specific conclusions.
In our correlation analyses across diagnostic groups, we could characterize functional neural correlates using FDG-PET in four out of the five subtests. Further, for one subtest, structural neural correlates were established. The DemTect's subtests examine the cognitive domains of verbal short term memory and learning, processing of language and numbers in combination with executive functions, semantic word fluency, verbal working memory and verbal long term memory. In the following we discuss results for each of the DemTect's subtests.
The “wordlist” paradigm with immediate recall (sum of two trials) is applied to detect impairments in auditory verbal short-term memory (Kessler et al., 2010) and learning. Impairments in explicit memory are known as a striking clinical sign of AD in particular, whereas impairment of language processing plays an important role in FTLD. In our groups of early AD and FTLD subjects, performance in this task was significantly impaired compared to healthy subjects. The correlation analysis with the scores of the wordlist subtest and imaging data identified metabolic correlates in the left medial and superior temporal gyri, including Wernicke's Region (BA 22). Concerning the first trial of this subtest (not displayed), these areas spread slightly to the angular gyrus and the temporal pole. These regions have been related to auditory, memory and language information processing (Sato et al., 1999) and our results support prior findings about the neural correlates of verbal span in short-term memory in AD subjects (Collette et al., 1997; Desgranges et al., 1998). Posterior parts of the predominantly left temporal lobe have been discussed to play a role in recoding visual into phonological verbal material (Henson et al., 2000), the left temporal pole, the posterior temporal lobe and the angular gyrus are mentioned in the context of semantic processing of verbal items (Price et al., 1997). Although there also are other regions known to be related to verbal recall in healthy subjects, such as the anterior cingulate cortex (BA 24, BA 32), the hippocampus, and the prefrontal cortex (BA 9, BA 10), in the case of supraspan tasks (Baron et al., 2001), and in AD for example parts of frontal and parietal lobes, putamen and posterior cingulate gyrus (Desgranges et al., 1998), an involvement of these regions could not be shown in our cohort. The “wordlist, delayed recall” subtest is designed to address auditory verbal long term memory (Kessler et al., 2010). Our correlates in regions similar to, but smaller than those in the immediate recall, support the assumption that short-term memory strongly limits long-term performance, as has been discussed in the literature for a long time (Atkinson and Shiffrin, 1968; Baddeley et al., 1988).
The cognitive functions needed for the “number transcoding” subtest are cognitive flexibility, reading, writing and number processing (Kessler et al., 2010), suggesting a large neural network. One region, which emerged with a correlation in gray matter loss, included the precuneus, the retrosplenial cortex and the posterior cingulate cortex. The precuneus, which fulfills different integrative functions, has been proposed to be divided into heterogeneous anatomical and functional subregions (Cavanna and Trimble, 2006; Margulies et al., 2009) — an anterior region is involved in sensorimotor processes, a central region is involved in cognitive processes and a posterior region is involved in visual integrative processes. Functional resting-state connectivity analyses (Margulies et al., 2009) have previously shown connections between the central (cognitive) parts of the precuneus and the angular gyrus, as well as dorsal prefrontal areas, a network that is identified also in our study. Moreover, the precuneus, concerted with the posterior cingulate/retrosplenial cortex, the predominantly left-lateralized lateral parietal cortex and a frontal region near premotor cortex, have been discussed as parts of the memory retrieval network (Buckner et al., 2005). As for the angular and supramarginal gyrus, their integrative involvement in reading, writing and mathematical processes has been known for long time (Seghier, 2013). Interestingly, we not only observed metabolic correlates in the left hemisphere, but also correlates with atrophy in the right hemisphere. We also found an involvement of the occipital cortex, which is explainable by the necessity of integration of visually presented material. Metabolic correlates in the temporal lobe account for verbal processing, as already discussed for the wordlist subtest. The involvement of a prefrontal dorsolateral network refers to the involvement of verbal working memory (Baddeley, 1986). This has also been observed in the “digit span reverse” subtest and is discussed in the following section, as well as correlates of the anterior insula, the putamen and the head of caudate nucleus.
“Digit span reverse” is a test which demands memorizing verbal presentations of numbers and manipulating them in their order. This is a typical function of working memory (Kessler et al., 2010) and according to Baddeley (1986), of the so called phonological loop and the central executive especially. As has been described by Smith and Jonides (1997), the dorsolateral prefrontal cortex is involved in the neural representation of verbal working memory. Moreover, this area has been suggested to participate in retrieval, together with the inferior frontal cortex, among other areas, and to play a role in storage of phonologically coded verbal information (Jonides et al., 1998). As was predictable for this task, we found metabolic correlates in the mentioned regions. We also found correlates bilaterally in the putamen and the head of caudate nucleus, in agreement with the proposed involvement of the basal ganglia in learning and memory (Packard and Knowlton, 2002). Furthermore, metabolic correlates were found in the left and right anterior insula, known to be involved in auditory and phonological processing (Bamiou et al., 2003).
The “supermarket task” subtest addresses cognitive flexibility, semantic word fluency, cognitive speed and imaginative abilities (Kessler et al., 2010). Recent studies have shown semantic or categorial word fluency to be related to hypometabolism in the left prefrontal cortex, in particular the inferior frontal junction (Raczka et al., 2010; Schroeter et al., 2012), the temporal and inferior frontal regions, the fusiform gyrus, the posterior cingulate cortex, the middle occipital gyrus and the precuneus (Collette et al., 1997; Melrose et al., 2009). Although dementia patients performed less efficiently in our cohort in comparison with control subjects (see Table 1), we did not detect associations of performance with regional glucose metabolism or gray matter atrophy on the chosen level of significance. This absence might be related to a very low variance in the control cohort, the diversity of cognitive domains involved in this task, and the overrepresentation of early AD subjects in comparison with FTLD subjects that are predominantly characterized by executive and language deficits (Raczka et al., 2010; Schroeter et al., 2012) finally leading to a low signal-to-noise ratio.
Focusing on the DemTect as a screening instrument for different diseases with dementia syndromes we may cautiously assume that neural correlates of the DemTect correspond to typically impaired regions both in AD and FTLD as illustrated by overlapping regions of correlations and group comparison in a descriptive conjunction analysis. In recent studies, the DemTect has been shown to be a highly sensitive diagnostic tool for mild cognitive impairment, early AD, dementia with Lewy bodies and vascular dementia (Kalbe et al., 2004; Perneczky, 2003; Scheurich et al., 2005). Our conjunction analysis shows wide overlaps with pathological patterns in both our AD and FTLD cohort, especially concerning glucose metabolism, suggesting the DemTect as an appropriate tool in the detection of both of these dementia types. Interestingly, the conjunction of contrasts suggests that hypometabolism in almost identical brain regions in both dementia syndromes was correlated with the performance in the DemTect's subtests, whereas for gray matter atrophy we observed a dissociation between AD and FTLD in case of the number transcoding subtest. As the shown conjunction of contrasts provides descriptive, though not statistical results, we may very carefully assume that there are specifically affected networks related to the DemTect performance in both diseases, AD and FTLD. Although an involvement of quite comparable neural networks in both diseases seems to be suggestive, a correlation analysis within diagnostic groups allowed further conclusions about disease-specific correlates and supports the hypothesis of different networks in both dementia cohorts driving the results of the analysis across groups. Of most interest we could verify the correlates of performance in the “wordlist” subtest from our approach across diagnostic groups in the AD cohort. In AD subjects, we would traditionally expect problems with learning a wordlist due to deficits in memory consolidation. Correlates of the “number transcoding” and the “digit span reverse” subtest could partly be verified in the FTLD cohort. In the “number transcoding” subtest, we again observed the correlation of performance with loss of gray matter density in the right angular gyrus. This was also the case in the left angular gyrus, where we could only show correlates with respect to glucose metabolism in the correlation analysis across diagnostic groups. The results of our disease-specific correlation analysis of the “digit span reverse” task verify the correlates of glucose metabolism from analyses across diagnostic groups in the left dorsolateral frontal lobe and additionally show correlates of gray matter density in the contralateral area. These correlates fit very well to the frontal lobe functions that are required to solve this subtest and that are impaired in FTLD.
4.3. Limitations
Although distinguishing between subjective cognitive impairment and AD respectively FTLD subjects is of most clinical interest, the inclusion of a subjective cognitive impairment cohort as a control group might have reduced effects observed in the group comparisons and could be seen as a weakness of this study with respect to recent literature discussing subjective cognitive impairment as a prodromal stage of mild cognitive impairment or dementia.
To perform a correlation analysis, it is important to investigate a group exhibiting a preferably high variance. Accordingly, we decided to involve patients with AD and FTLD, and control subjects and performed correlation analyses across diagnostic groups to increase statistical power as suggested previously (Rankin et al., 2006; Rosen et al., 2005; Schroeter et al., 2011, 2012). Conclusions about disease-specific correlates are limited by this approach, the illustration of overlapping regions of the group comparison and the correlation analysis across diagnostic groups in a conjunction analysis provides descriptive, but not statistical information. Hence, disease-specific conclusions should be drawn very cautiously at best. To further investigate different associated networks in the two dementia subgroups, we conducted a correlation analysis within the dementia subgroups. However, probably due to small sample sizes, we could not detect all disease-specific correlates. Accordingly, our findings should be seen as preliminary. Our study should be replicated within a larger cohort to provide more information about the subtests of the DemTect. We would also suggest correlation analyses within different FTLD subtypes and of other neurodegenerative diseases with dementia syndromes to further establish the DemTect as a screening instrument for these diseases. Furthermore, it would be an interesting approach in future studies to perform correlation analyses within diagnostic groups by inclusion of patients with different stages of dementia syndromes to investigate the DemTect in rating the progression of dementia syndromes.
5. Conclusion and perspectives
Using brain MRI and PVE-corrected brain FDG-PET data, our study shows structural and functional neural correlates of the subtests of the DemTect in two very frequent dementia syndromes — early AD and FTLD. Results support an external validation of this frequently used screening device. Interestingly, the DemTect's subtests did not appear to correlate exclusively for AD, but also showed correlations in regions known as typically afflicted in FTLD. Moreover, this study contributes to a better understanding of cognitive impairments in dementia on a neural level. In this sense, it may be conducive to facilitate a stronger integration of neuroimaging findings in dementia diagnostic criteria, as suggested previously. Finally we showed inversed relations between gray matter loss and hypometabolism in AD and FTLD, hinting at the pathomechanisms which are still not completely understood.
Author contributions
Conceived and designed the experiments: TBW JD SF HB OS KM MLS
Performed the experiments: TBW JD SF HB KM MLS
Analyzed the data: TBW JD SF KM MLS
Contributed reagents/materials/analysis tools: SF HB OS KM MLS
Wrote the paper: TBW JD SF HB OS KM MLS
Acknowledgment
Juergen Dukart, Henryk Barthel, Osama Sabri and Matthias L. Schroeter have been supported by LIFE — Leipzig Research Center for Civilization Diseases at the University of Leipzig. LIFE is funded by means of the European Union, by the European Regional Development Fund (ERFD) and by means of the Free State of Saxony within the framework of the excellence initiative. Matthias L. Schroeter has further been supported by the German Consortium for Frontotemporal Lobar Degeneration, funded by the German Federal Ministry of Education and Research, MaxNet Aging, and by the Parkinson's Disease Foundation (Grant No. PDF-IRG-1307) together with Karsten Mueller.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Andreasen N.C., O'Leary D.S., Arndt S., Cizadlo T., Hurtig R., Rezai K., Watkins G.L., Ponto L.L., Hichwa R.D. Short-term and long-term verbal memory: a positron emission tomography study. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova L.G., Lu P.H., Rogers S., Dutton R.A., Hayashi K.M., Toga A.W., Cummings J.L., Thompson P.M. 3D mapping of mini-mental state examination performance in clinical and preclinical Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20:224–231. doi: 10.1097/01.wad.0000213857.89613.10. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Atkinson R.C., Shiffrin R.M., editors. Academic Press; New York: 1968. Human Memory: A Proposed System and Its Control Processes. [Google Scholar]
- Awh E., Jonides J., Smith E.E., Schumacher E.H., Koeppe R.A., Katz S. Dissociation of storage and rehearsal in verbal working memory. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley A. Oxford University Press; Oxford: 1986. Working Memory. [Google Scholar]
- Baddeley A., Papagno C., Vallar G. When long-term learning depends on short-term storage. Journal of Memory and Language. 1988;27:586–595. [Google Scholar]
- Baete K., Nuyts J., Laere K.V., Van Paesschen W., Ceyssens S., De Ceuninck L., Gheysens O., Kelles A., Van den Eynden J., Suetens P., Dupont P. Evaluation of anatomy based reconstruction for partial volume correction in brain FDG-PET. NeuroImage. 2004;23:305–317. doi: 10.1016/j.neuroimage.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Bamiou D.E., Musiek F.E., Luxon L.M. The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Research. Brain Research Reviews. 2003;42:143–154. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Barnes J., Godbolt A.K., Frost C., Boyes R.G., Jones B.F., Scahill R.I., Rossor M.N., Fox N.C. Atrophy rates of the cingulate gyrus and hippocampus in AD and FTLD. Neurobiology of Aging. 2007;28:20–28. doi: 10.1016/j.neurobiolaging.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Baron J.C., Chetelat G., Desgranges B., Perchey G., Landeau B., de la Sayette V., Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. NeuroImage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Barthel H., Gertz H.J., Dresel S., Peters O., Bartenstein P., Buerger K., Hiemeyer F., Wittemer-Rump S.M., Seibyl J., Reininger C., Sabri O., Florbetaben Study G. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurology. 2011;10:424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- Baxter L.C., Sparks D.L., Johnson S.C., Lenoski B., Lopez J.E., Connor D.J., Sabbagh M.N. Relationship of cognitive measures and gray and white matter in Alzheimer's disease. Journal of Alzheimer's Disease. 2006;9:253–260. doi: 10.3233/jad-2006-9304. [DOI] [PubMed] [Google Scholar]
- Boxer A.L., Rankin K.P., Miller B.L., Schuff N., Weiner M., Gorno-Tempini M.L., Rosen H.J. Cinguloparietal atrophy distinguishes Alzheimer disease from semantic dementia. Archives of Neurology. 2003;60:949–956. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Snyder A.Z., Shannon B.J., LaRossa G., Sachs R., Fotenos A.F., Sheline Y.I., Klunk W.E., Mathis C.A., Morris J.C., Mintun M.A. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Dolcos F., Graham R., Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chetelat G., Desgranges B., Landeau B., Mezenge F., Poline J.B., de la Sayette V., Viader F., Eustache F., Baron J.C. Direct voxel-based comparison between grey matter hypometabolism and atrophy in Alzheimer's disease. Brain. 2008;131:60–71. doi: 10.1093/brain/awm288. [DOI] [PubMed] [Google Scholar]
- Collette F., Salmon E., Van der Linden M., Degueldre C., Franck G. Functional anatomy of verbal and visuospatial span tasks in Alzheimer's disease. Human Brain Mapping. 1997;5:110–118. doi: 10.1002/(sici)1097-0193(1997)5:2<110::aid-hbm4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Demonet J.F., Chollet F., Ramsay S., Cardebat D., Nespoulous J.L., Wise R., Rascol A., Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Desgranges B., Baron J.C., de la Sayette V., Petit-Taboue M.C., Benali K., Landeau B., Lechevalier B., Eustache F. The neural substrates of memory systems impairment in Alzheimer's disease. A PET study of resting brain glucose utilization. Brain. 1998;121:611–631. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- Desgranges B., Matuszewski V., Piolino P., Chetelat G., Mezenge F., Landeau B., de la Sayette V., Belliard S., Eustache F. Anatomical and functional alterations in semantic dementia: a voxel-based MRI and PET study. Neurobiology of Aging. 2007;28:1904–1913. doi: 10.1016/j.neurobiolaging.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Drzezga A., Grimmer T., Henriksen G., Stangier I., Perneczky R., Diehl-Schmid J., Mathis C.A., Klunk W.E., Price J., DeKosky S., Wester H.J., Schwaiger M., Kurz A. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. NeuroImage. 2008;39:619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Horstmann A., Vogt B., Frisch S., Barthel H., Becker G., Möller H.E., Villringer A., Sabri O., Schroeter M.L. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. NeuroImage. 2010;49:1490–1495. doi: 10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Horstmann A., Barthel H., Moller H.E., Villringer A., Sabri O., Schroeter M.L. Combined evaluation of FDG-PET and MRI improves detection and differentiation of dementia. PLoS One. 2011;6:e18111. doi: 10.1371/journal.pone.0018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini L., Palm W.M., Olofsen H., van der Landen R., Jan Blauw G., Westendorp R.G., Bollen E.L., Middelkoop H.A., Reiber J.H., van Buchem M.A., Admiraal-Behloul F. MMSE scores correlate with local ventricular enlargement in the spectrum from cognitively normal to Alzheimer disease. NeuroImage. 2008;39:1832–1838. doi: 10.1016/j.neuroimage.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Fiez J.A., Raife E.A., Balota D.A., Schwarz J.P., Raichle M.E., Petersen S.E. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finder V.H. Alzheimer's disease: a general introduction and pathomechanism. Journal of Alzheimer's Disease. 2010;22(Suppl. 3):5–19. doi: 10.3233/JAD-2010-100975. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Engler H., Almkvist O., Blomquist G., Hagman G., Wall A., Ringheim A., Langstrom B., Nordberg A. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiology of Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Frisch S., Dukart J., Vogt B., Horstmann A., Becker G., Villringer A., Barthel H., Sabri O., Müller K., Schroeter M.L. Dissociating memory networks in early Alzheimer's disease and frontotemporal lobar degeneration — a combined study of hypometabolism and atrophy. PLoS One. 2013;8:e55251. doi: 10.1371/journal.pone.0055251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni G.B., Testa C., Zorzan A., Sabattoli F., Beltramello A., Soininen H., Laakso M.P. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasby P.M., Frith C.D., Friston K.J., Bench C., Frackowiak R.S., Dolan R.J. Functional mapping of brain areas implicated in auditory-verbal memory function. Brain. 1993;116:1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Burgess N., Frith C.D. Recoding, storage, rehearsal and grouping in verbal short-term memory: an fMRI study. Neuropsychologia. 2000;38:426–440. doi: 10.1016/s0028-3932(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Heun R., Kölsch H., Jessen F. Risk factors and early signs of Alzheimer's disease in a family study sample. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:28–36. doi: 10.1007/s00406-005-0596-4. [DOI] [PubMed] [Google Scholar]
- Hughes C.P., Berg L., Danziger W.L., Coben L.A., Martin R.L. A new clinical scale for the staging of dementia. The British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hutchinson A.D., Mathis J.L. Neuropsychological deficits in frontotemporal dementia and Alzheimer's disease: a meta-analytic review. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78:917–928. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Sakamoto S., Sasaki M., Kitagaki H., Yamaji S., Hashimoto M., Imamura T., Shimomura T., Hirono N., Mori E. Cerebral glucose metabolism in patients with frontotemporal dementia. Journal of Nuclear Medicine. 1998;39:1875–1878. [PubMed] [Google Scholar]
- Jack C.R., Jr., Dickson D.W., Parisi J.E., Xu Y.C., Cha R.H., O'Brien P.C., Edland S.D., Smith G.E., Boeve B.F., Tangalos E.G., Kokmen E., Petersen R.C. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W., Petersen R.C., Trojanowski J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacova C., Kertesz A., Blair M., Fisk J.D., Feldman H.H. Neuropsychological testing and assessment for dementia. Alzheimer's & Dementia. 2007;3:299–317. doi: 10.1016/j.jalz.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Jeong Y., Cho S.S., Park J.M., Kang S.J., Lee J.S., Kang E., Na D.L., Kim S.E. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. Journal of Nuclear Medicine. 2005;46:233–239. [PubMed] [Google Scholar]
- Jonides J., Schumacher E.H., Smith E.E., Koeppe R.A., Awh E., Reuter-Lorenz P.A., Marshuetz C., Willis C.R. The role of parietal cortex in verbal working memory. Journal of Neuroscience. 1998;18:5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E., Kessler J., Calabrese P., Smith R., Passmore A.P., Brand M., Bullock R. DemTect: a new, sensitive cognitive screening test to support the diagnosis of mild cognitive impairment and early dementia. International Journal of Geriatric Psychiatry. 2004;19:136–143. doi: 10.1002/gps.1042. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G., Chetelat G., Baron J.C., Landeau B., Mevel K., Godeau C., Barre L., Constans J.M., Viader F., Eustache F., Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiology of Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kemppainen N.M., Aalto S., Wilson I.A., Nagren K., Helin S., Bruck A., Oikonen V., Kailajarvi M., Scheinin M., Viitanen M., Parkkola R., Rinne J.O. Voxel-based analysis of PET amyloid ligand [C-11]PIB uptake in Alzheimer disease. Neurology. 2006;67:1575–1580. doi: 10.1212/01.wnl.0000240117.55680.0a. [DOI] [PubMed] [Google Scholar]
- Kessler J., Calabrese P., Kalbe E. DemTect-B: a parallel test version to the cognitive screening instrument DemTect-A (R) Fortschritte der Neurologie-Psychiatrie. 2010;78:532–535. doi: 10.1055/s-0029-1245452. [DOI] [PubMed] [Google Scholar]
- Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P., Bergstrom M., Savitcheva I., Huang G.F., Estrada S., Ausen B., Debnath M.L., Barletta J., Price J.C., Sandell J., Lopresti B.J., Wall A., Koivisto P., Antoni G., Mathis C.A., Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Li Y., Rinne J.O., Mosconi L., Pirraglia E., Rusinek H., DeSanti S., Kemppainen N., Nagren K., Kim B.C., Tsui W., de Leon M.J. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck T., Riedel-Heller S.G., Luppa M., Wiese B., Wollny A., Wagner M., Bickel H., Weyerer S., Pentzek M., Haller F., Moesch E., Werle J., Eisele M., Maier W., van den Bussche H., Kaduszkiewicz H. Risk factors for incident mild cognitive impairment—results from the German study on ageing, cognition and dementia in primary care patients (AgeCoDe) Acta Psychiatrica Scandinavica. 2010;121:260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- Margulies D.S., Vincent J.L., Kelly C., Lohmann G., Uddin L.Q., Biswal B.B., Villringer A., Castellanos F.X., Milham M.P., Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mechelli A., Price C., Friston K., Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- Melrose R.J., Campa O.M., Harwood D.G., Osato S., Mandelkern M.A., Sultzer D.L. The neural correlates of naming and fluency deficits in Alzheimer's disease: an FDG-PET study. International Journal of Geriatric Psychiatry. 2009;24:885–893. doi: 10.1002/gps.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke R., Herholz K., Grond M., Kessler J., Heiss W.D. Clinical deterioration in probable Alzheimer's disease correlates with progressive metabolic impairment of association areas. Dementia. 1994;5:36–41. doi: 10.1159/000106692. [DOI] [PubMed] [Google Scholar]
- Mol M.E.M., van Boxtel M.P.J., Willems D., Jolles J. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging study. International Journal of Geriatric Psychiatry. 2006;21:432–441. doi: 10.1002/gps.1487. [DOI] [PubMed] [Google Scholar]
- Müller-Gärtner H.W., Links J.M., Prince J.L., Bryan R.N., McVeigh E., Leal J.P., Davatzikos C., Frost J.J. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. Journal of Cerebral Blood Flow and Metabolism. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- Mummery C.J., Patterson K., Price C.J., Ashburner J., Frackowiak R.S., Hodges J.R. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M., Boone K., Miller B.L., Cummings J., Benson D.F. Frontotemporal lobar degeneration — a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T., Kleiman A., Schulz J.B., Schneider F., Laird A., Fox P.T., Eickhoff S.B., Reetz K. Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: a meta-analysis. Brain Structure & Function. 2011;217:115–125. doi: 10.1007/s00429-011-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M.G., Knowlton B.J. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Perneczky R. Die Eignung einfacher klinischer Tests für die Erkennung der leichtgradigen kognitiven Störung und der leichtgradigen Demenz. Aktuelle Neurologie. 2003;30:114–117. [Google Scholar]
- Peters F., Collette F., Degueldre C., Sterpenich V., Majerus S., Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain. 2009;132:1833–1846. doi: 10.1093/brain/awp075. [DOI] [PubMed] [Google Scholar]
- Price C.J., Humphreys G.W., Wise R.J.S. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Quarantelli M., Berkouk K., Prinster A., Landeau B., Svarer C., Balkay L., Alfano B., Brunetti A., Baron J.C., Salvatore M. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. Journal of Nuclear Medicine. 2004;45:192–201. [PubMed] [Google Scholar]
- Rabinovici G.D., Miller B.L. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24:375–398. [Google Scholar]
- Rabinovici G.D., Seeley W.W., Kim E.J., Gorno-Tempini M.L., Rascovsky K., Pagliaro T.A., Allison S.C., Halabi C., Kramer J.H., Johnson J.K., Weiner M.W., Forman M.S., Trojanowski J.Q., Dearmond S.J., Miller B.L., Rosen H.J. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. American Journal of Alzheimer's Disease and Other Dementias. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczka K.A., Becker G., Seese A., Frisch S., Heiner S., Marschhauser A., Barthel H., Scheid R., Sabri O., Schroeter M.L. Executive and behavioral deficits share common neural substrates in frontotemporal lobar degeneration — a pilot FDG-PET study. Psychiatry Research. 2010;182:274–280. doi: 10.1016/j.pscychresns.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Gorno-Tempini M.L., Goldman W.P., Perry R.J., Schuff N., Weiner M., Feiwell R., Kramer J.H., Miller B.L. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen H.J., Allison S.C., Schauer G.F., Gorno-Tempini M.L., Weiner M.W., Miller B.L. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset O.G., Ma Y., Evans A.C. Correction for partial volume effects in PET: principle and validation. Journal of Nuclear Medicine. 1998;39:904–911. [PubMed] [Google Scholar]
- Rowe C.C., Ng S., Ackermann U., Gong S.J., Pike K., Savage G., Cowie T.F., Dickinson K.L., Maruff P., Darby D., Smith C., Woodward M., Merory J., Tochon-Danguy H., O'Keefe G., Klunk W.E., Mathis C.A., Price J.C., Masters C.L., Villemagne V.L. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Sato H., Takeuchi T., Sakai K.L. Temporal cortex activation during speech recognition: an optical topography study. Cognition. 1999;73:B55–B66. doi: 10.1016/s0010-0277(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Scheef L., Spottke A., Daerr M., Joe A., Striepens N., Kölsch H., Popp J., Daamen M., Gorris D., Heneka M.T., Boecker H., Biersack H.J., Maier W., Schild H.H., Wagner M., Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- Scheurich A., Muller M.J., Siessmeier T., Bartenstein P., Schmidt L.G., Fellgiebel A. Validating the DemTect with 18-fluoro-2-deoxy-glucose positron emission tomography as a sensitive neuropsychological screening test for early Alzheimer disease in patients of a memory clinic. Dementia and Geriatric Cognitive Disorders. 2005;20:271–277. doi: 10.1159/000088248. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Von Cramon D.Y. Neural networks in frontotemporal dementia — a meta-analysis. Neurobiology of Aging. 2008;29:418–426. doi: 10.1016/j.neurobiolaging.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Stein T., Maslowski N., Neumann J. Neural correlates of Alzheimer's disease and mild cognitive impairment: a systematic and quantitative meta-analysis involving 1351 patients. NeuroImage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Vogt B., Frisch S., Becker G., Seese A., Barthel H., Mueller K., Villringer A., Sabri O. Dissociating behavioral disorders in early dementia—an FDG-PET study. Psychiatry Research. 2011;194:235–244. doi: 10.1016/j.pscychresns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Vogt B., Frisch S., Becker G., Barthel H., Mueller K., Villringer A., Sabri O. Executive deficits are related to the inferior frontal junction in early dementia. Brain. 2012;135:201–215. doi: 10.1093/brain/awr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W. Anterior insula degeneration in frontotemporal dementia. Brain Structure & Function. 2010;214:465–475. doi: 10.1007/s00429-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. The angular gyrus: multiple functions and multiple subdivisions. The Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M. The Mini-Mental State Examination: strengths and weaknesses of a clinical instrument. The Canadian Alzheimer's Disease Review. 1998;2:10–12. [Google Scholar]
- Smith E.E., Jonides J. Working memory: a view from neuroimaging. Cognitive Psychology. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J., Koeppe R.A. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J., Marshuetz C., Koeppe R.A. Components of verbal working memory: evidence from neuroimaging. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. MIT Press; Cambridge: 2010. Networks of the Brain. [Google Scholar]
- Thompson J.C., Stopford C.L., Snowden J.S., Neary D. Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:920–927. doi: 10.1136/jnnp.2003.033779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten A.C., Visser P.J., Pijnenburg Y.A., Teunissen C.E., Blankenstein M.A., Scheltens P., van der Flier W.M. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimer's & Dementia. 2013 doi: 10.1016/j.jalz.2012.08.004. (in press, available online on 8 December 2012) [DOI] [PubMed] [Google Scholar]
- Weaver Cargin J., Collie A., Masters C., Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline. Journal of Clinical and Experimental Neuropsychology. 2008;30:245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- World Health Organisation . World Health Organisation; Geneva: 2012. Dementia: A Public Health Priority. [Google Scholar]


