Abstract
Background
Bipolar disorder (BD) is a chronic mental illness characterized by severe disruptions in mood and cognition. Diffusion tensor imaging (DTI) studies suggest that white matter (WM) tract abnormalities may contribute to the clinical hallmarks of the disorder. Using DTI and whole brain voxel-based analysis, we mapped the profile of WM anomalies in BD. All patients in our sample were euthymic and lithium free when scanned.
Methods
Diffusion-weighted and T1-weighted structural brain images were acquired from 23 lithium-free euthymic subjects with bipolar I disorder and 19 age- and sex-matched healthy control subjects on a 1.5 T MRI scanner. Scans were processed to provide measures of fractional anisotropy (FA) and mean and radial diffusivity (MD and RD) at each WM voxel, and processed scans were nonlinearly aligned to a customized brain imaging template for statistical group comparisons.
Results
Relative to controls, the bipolar group showed widespread regions of lower FA, including the corpus callosum, cortical and thalamic association fibers. MD and RD were abnormally elevated in patients in many of these same regions.
Conclusions
Our findings agree with prior reports of WM abnormalities in the corpus callosum and further link a bipolar diagnosis with structural abnormalities of the tapetum, fornix and stria terminalis. Future studies assessing the diagnostic specificity and prognostic implications of these abnormalities would be of interest.
Keywords: Bipolar disorder, White matter, Neuroimaging, DTI, Brain mapping, Fractional anisotropy
Highlights
-
•
Using DTI and whole brain voxel-based analysis, we mapped WM anomalies in BD.
-
•
Relative to controls, the bipolar group showed widespread regions of lower FA.
-
•
MD and RD were abnormally elevated in patients in many of these same regions.
1. Introduction
Bipolar disorder (BD) affects up to 3% of the adult population, and is a leading cause of functional impairment, morbidity and suicide (Merikangas et al., 2007). BD manifests clinically as a recurrent cycling between the emotional elevations of manic episodes and the dysphoria of depressive episodes, with intervening periods of euthymia, a mood state in which the range of emotions is neither depressed nor highly elevated. People with BD may also exhibit cognitive deficits, including impairments in attention, executive function, response inhibition, and short-term memory (Altshuler et al., 2005; Liu et al., 2002).
Histological studies show that BD is associated with a downregulation of key oligodendrocyte-related and myelin-related genes and their transcription factors, which are critical for axonal myelination and connectivity (Tkachev et al., 2003). Technological advances in neuroimaging techniques allow the investigation of white matter (WM) structure in vivo. While standard structural MRI provides limited contrast in the brain's WM, diffusion tensor imaging (DTI), a variant of MRI which measures water diffusion in living brain tissues (Beaulieu, 2002) is uniquely sensitive to WM microstructure, including axonal coherence, fiber density, and myelin integrity. One common measure of WM microstructure is fractional anisotropy (FA), an indirect scalar measure of the coordinated directionality and coherence of fibers within a WM bundle (Beaulieu, 2002; Mori and Zhang, 2006). Decreases in FA have been detected in disorders of central nervous system myelination (Harsan et al., 2006; Mori and Zhang, 2006), suggesting that it is a measure sensitive to myelination anomalies. Lower FA can also indicate a reduction in the density of WM fibers, a loss in axonal bundle coherence (loss of structural organization), or a variation in membrane permeability to water (Beaulieu and Allen, 1994). Also commonly included in WM assessments are radial diffusivity (RD), a measure of diffusion perpendicular to the axonal fibers, and mean diffusivity (MD), the average of the principal diffusivities in three diffusion directions, and an overall evaluation of the molecular motion in a voxel or region, characterizing the overall presence of obstacles to diffusion (Thomason and Thompson, 2011). MD and RD, which are unaffected by the loss of bundle coherence, often increase when myelin is damaged or fails to develop normally (Song et al., 2002; Song et al., 2005; Thomason and Thompson, 2011). A diffusivity-associated myelin reduction is consistent with post mortem studies that show an apparent loss of glia in patients with mood disorders (Ongur et al., 1998; Rajkowska et al., 1999), and a gene-expression study (Tkachev et al., 2003) in which bipolar brains showed a reduced expression of key oligodendrocyte and myelination genes.
Despite growing interest in impaired WM connectivity in BD, the DTI findings across studies have been inconsistent (Heng et al., 2010). Reports have found lower (Adler et al., 2004; Adler et al., 2006; Barnea-Goraly et al., 2009; Benedetti et al., 2011; Bruno et al., 2008; Chaddock et al., 2009; Chan et al., 2010; Chen et al., 2012; Frazier et al., 2007; Gonenc et al., 2010; Ha et al., 2011; Haller et al., 2011; James et al., 2011; Kafantaris et al., 2009; Lin et al., 2011; Macritchie et al., 2010; McIntosh et al., 2008; Pavuluri et al., 2009; Saxena et al., 2012; Sprooten et al., 2011; Sussmann et al., 2009; Wang et al., 2009; Zanetti et al., 2009), higher (Haznedar et al., 2005; Wessa et al., 2009; Yurgelun-Todd et al., 2007), or no difference (Beyer et al., 2005; Houenou et al., 2007) in FA between patients with BD and healthy volunteers. The most consistent findings have been of decreased FA and/or increased MD in limbic-striatal, callosal and prefrontal regions in adults (Adler et al., 2004; Benedetti et al., 2011; Beyer et al., 2005; Bruno et al., 2008; Chaddock et al., 2009; Chan et al., 2010; Chen et al., 2012; Ha et al., 2011; Lin et al., 2011; Macritchie et al., 2010; McIntosh et al., 2008; Sprooten et al., 2011; Sussmann et al., 2009; Wang et al., 2008a; Wang et al., 2008b; Zanetti et al., 2009), children (Frazier et al., 2007; Gonenc et al., 2010; James et al., 2011) and adolescents (Adler et al., 2006; Barnea-Goraly et al., 2009; Kafantaris et al., 2009; Pavuluri et al., 2009; Saxena et al., 2012) with BD. Such heterogeneity in findings may reflect DTI data acquisition differences and differences in patient samples, as well as the effects of lithium and mood state, which were not controlled for in many of these studies.
To better map the profile of these anomalies, we used a whole brain voxel-based analysis rather than TBSS (Smith et al., 2006). TBSS does pre-select voxels with high signal to noise ratio (SNR) (for FA) and these tend to be among the ones that show disease effects, as voxels with lower mean FA also tend to have lower SNR and disease effects are harder to detect. Even so, a great deal of the scan is discarded if one focuses only on the white matter skeleton (which is analyzed in TBSS). Since TBSS greatly reduces the data available and since we wanted to be sensitive to effects throughout the 3D extent of WM structures; throughout the imaged anatomy, we chose to use voxel-based analysis, as it allows for a more comprehensive survey. We realize this is a trade-off, in that the non-skeleton voxels actually deplete power if there is no disease effect there, as they cause a heavier multiple comparisons correction. But, in this case, there were effects throughout the WM and not on the skeleton, that TBSS would have missed. We also wanted to avoid the limitations of region of interest (ROI) based methods, which may focus on a limited number of structures. Whole brain statistical maps reveal spatial patterns of WM abnormalities occurring throughout the brain, and are well suited to studying psychiatric disease, where the location of neural abnormalities may not be known a priori.
An advantageous aspect of our study is that at scan time, all our patients were euthymic, and no patients were treated with lithium. This avoids the confounding effects of lithium on brain measures, which have been documented in studies of cortical thickness and subcortical gray matter volumes in BD patients (Brooks et al., 2011; Foland et al., 2008; Germana et al., 2010; Monkul et al., 2007; Sassi et al., 2002). Indeed, lithium-treated HIV-positive patients show diffusely increased FA and decreased MD (Schifitto et al., 2009), compared to lithium-free patients. Similarly, lithium-treated bipolar patients show higher FA in the WM tracts connecting the amygdala with the subgenual cingulate cortex than do BD individuals who are not treated with this medication (Benedetti et al., 2011). Additionally, due to prior reports of mood state effects on amygdala (Foland-Ross et al., 2012) and orbitofrontal cortex (OFC) gray matter volumes (Nery et al., 2009), as well as on other cortical and subcortical brain areas (Brooks et al., 2009; Brooks et al., 2011), and given that the impact of mood state on WM integrity is not yet understood, to avoid any confounding effects of mood state, we also took special precautions to control for mood state effects by sampling exclusively from euthymic individuals in our patient sample.
2. Materials and methods
2.1. Participants
Individuals with bipolar I disorder were recruited by the UCLA Mood Disorders Clinic, the Bipolar Disorders Clinic of the Veterans Affairs Greater Los Angeles Health Care System, and via newspaper advertising. Control subjects were recruited by advertisement in local newspapers and campus flyers. Patients and controls were both evaluated using the Structured Clinical Interview for DSM-IV (SCID) to confirm current mood state and an accurate diagnosis of BD or the absence thereof. Bipolar subjects with other active Axis I co-morbidities were excluded. Subjects were also excluded if they were taking lithium as a medication. Patients were considered lithium-free if they had not taken lithium medication for at least 1 month prior to the scan. Additional exclusion criteria included current manic or depressed mood state at scan time. Mood was assessed using the SCID, in combination with the Young Mania Rating Scale (YMRS; (Young et al., 1978)), and the 21-item Hamilton Depression Rating Scale (HAMD; (Hamilton, 1960)). A score of < 7 on the YMRS and HAM-D were required for patients included in the study. Information on patients' prior course of illness and prior and current medication use was obtained by self-report, from medical records when available, and by corroboration of family members or partners when available and permitted by patients. The study protocol was approved by the Institutional Review Board at UCLA and the VA Greater Los Angeles Healthcare System. Each participant provided written informed consent.
Healthy controls were excluded if they met SCID criteria for any current or past psychiatric diagnosis (including history of substance abuse or dependence) or were currently taking psychotropic medications. Additional exclusion criteria for all subjects included left-handedness, hypertension, neurological illness, metal implants, or a history of skull fracture or head trauma with loss of consciousness of more than 5 min.
2.2. Brain image acquisition
For all scans, the subject was scanned on a Siemens 1.5 T Sonata MRI scanner. Whole-brain T1-weighted structural images were acquired using a 3D magnetization-prepared rapid gradient echo (MP-RAGE) sequence (160 1-mm thick sagittal slices with a 256 × 256 acquisition matrix; field of view, 256 mm; TI/TR/TE = 1100/1900/4.38 ms; flip angle, 15°; scan time, 8.1 min). Diffusion-weighted images (DWIs) were acquired parallel to the anterior–posterior (AC–PC) commissural line using the following acquisition parameters: 55 2.5 mm thick axial slices (no gap); field of view, 240 mm, TR/TE = 6400/83 ms, with a 96 × 96 acquisition matrix, scan time: 3.9 min. Thirty-five DWIs were acquired per subject: 5 with no diffusion sensitization (i.e., T2-weighted b0 images) and 30 non-collinear DWIs (b = 1000 s/mm2) with gradient directions evenly distributed on the hemisphere.
2.3. Preprocessing
Images were visually inspected for artifacts that could interfere with image processing and analysis, and those that were not considered acceptable were excluded from the study. Extracerebral tissues were removed from each subject's T1-weighted MRI scan by manual editing. These whole-brain masks were also used to remove extracortical tissue from the DWIs after aligning the T1-weighted structural data to the non-diffusion weighted (b0) images via a rigid-body transform. All skull-stripped anatomical scans were aligned to a high-resolution single-subject average brain MRI scan, the Colin27 brain template (Holmes et al., 1998), in the ICBM standard stereotaxic space (Mazziotta et al., 2001) using a 9-parameter registration (allowing global scaling) via the FSL/FMRIB linear image registration tool (FLIRT) algorithm with default trilinear interpolation (Jenkinson and Smith, 2001).
Raw DWIs were corrected for eddy-current induced distortions using eddy_correct, from the FSL software package (FMRIB Analysis Group, 2012) and registered to standard space by elastically aligning subjects' averaged b0 images to their Colin27 aligned T1-weighted MP-RAGE structural scan using a mutual information cost function (Leow et al., 2005a) to account for EPI (echo planar imaging) induced distortions and susceptibility artifacts. Diffusion tensors were then computed from the 35-gradient diffusion weighted images using the FSL software's DTIFIT tool (FMRIB Analysis Group, 2012). Scalar maps of FA, RD and MD were computed for each subject from the resulting tensor eigenvalues (λ1, λ2, λ3) at each voxel (Basser et al., 1994). To improve inter-subject registration, and improve the power to detect group differences by reducing the macroscopic misregistration of WM, an FA-based minimal deformation template (MDT) image was used (Gutman et al., 2010). To do this, all individual FA maps were non-linearly registered to the affine average template using non-linear inverse-consistent elastic intensity-based registration (Leow et al., 2005b). The non-linear average template was computed as a voxel-wise average of the intensities of the FA maps that had been non-linearly registered to the affine average template. Finally, we created the MDT by applying inverse geometric centering of the displacement fields to the non-linear average (Jahanshad et al., 2010). Gutman et al. (2010) showed that using an MDT made in this way, versus an ICBM atlas or a single optimally chosen subject, leads to improved registration accuracy. To further ensure alignment of white matter tracts, FA maps were thresholded to include only regions where FA > 0.25 and re-registered to the thresholded MDT. The deformation fields from the second round of registration were then applied to the full FA map for each individual to avoid any missing data. Once the images were aligned they were then smoothed with a Gaussian filter with an 8 mm full-width-half-maximum (FWHM), to reduce noise and improve the sensitivity of group comparisons. The FA-based registrations were applied to the MD and RD maps to align them to the common template.
2.4. Statistical analyses
A general linear model was fitted at each voxel of the registered FA, MD and RD maps to identify WM regions showing a significant main effect of diagnosis. This model included age and sex as covariates. Sex differences were tested but were not significant for any map, and so, sex was dropped from the model. Supplementary analyses were performed adding HAM-D scores and educational level as additional covariates since many studies have shown higher educational levels may be neuroprotective (Coffey et al., 1999; Meguro et al., 2001) and since we controlled for the effects of mood state by recruiting only euthymic bipolar individuals. As these supplementary analyses found that both educational level and HAM-D scores were significant predictors of WM integrity, and because educational level was not collinear with age (r = 0.098, p = 0.54), the final model that was fit at each WM voxel included diagnosis, age, HAM-D, and educational level.
The anatomic location of each resulting significance cluster was determined with the help of a DTI WM fiber atlas (Mori et al., 2004). Since our voxel-wise approach involves running thousands of statistical tests (i.e., tests at many points in the brain), we controlled for false positives using the searchlight false discovery rate method (sFDR; (Langers et al., 2007)). All statistical maps in this study were thresholded at the appropriate corrected p-value of 0.05 after performing sFDR to show only regions of significance; uncorrected p-values are then shown only within these regions.
Post hoc exploratory analyses were performed to assess any relationships between diffusion characteristics (FA, MD, RD) and clinical measures in the patients, including psychotropic medication at scanning, history of psychosis, and duration of illness as a proportion of the patient's lifetime (R = 0.44 with age), where the start of illness was defined as the time of the first treatment. Psychotropic medication and history of psychosis were analyzed as yes/no variables. In addition, although all of our patients were lithium-free at scan time, seven had previous lithium exposure. For this reason, we also compared WM structure between previously exposed patients to those who were lithium naïve.
3. Results
3.1. Participants
34 euthymic subjects with BP I disorder (11f; 37.4 ± 12.0 years.) and 29 age- and sex-matched healthy control subjects (12f; 37.5 ± 13.3 years.) were initially scanned. 9 bipolar and 10 control subjects were excluded after visually assessing the DTI data, due to artifacts and signal dropouts; 2 bipolar subjects were excluded due to poor registration results in one subject and a lack of complete demographic information for the other subject. A total of 23 euthymic subjects with BP I disorder (7 women; mean age: 36.0 ± 12.5 SD years) and 19 age- and sex-matched healthy control subjects (6 women; mean age: 36.2 ± 12.4 SD years) were included in the analysis. Diagnostic groups did not differ in mean age (t = 0.03; p = 0.98), sex distribution (χ2 = 0.006; p = 0.94), or educational level (t = 1.76; p = 0.09).
Clinical information on bipolar subjects is presented in Table 1. Patients had a mean YMRS score of 1.7 ± 2.2 and a mean HAM-D score of 4.7 ± 2.2 on the day of the scan, and had been ill for 18.7 ± 12.1 years, on average. Seven of the 23 bipolar subjects (30.4%) were not on medications at the time of scanning. The remaining 16 subjects (69.6%) were currently taking one or more medications, including valproic acid (n = 4), lamotrigine (n = 4), aripiprazole (n = 10), olanzapine (n = 2), quetiapine (n = 3), paroxetine (n = 1), fluoxetine (n = 2), escitalopram (n = 1), bupropion (n = 3), oxcarbazepine (n = 1), trazodone (n = 1), citalopram (n = 1), and dextramphetamine–amphetamine (n = 1). Control subjects were not on medications at the time of scanning.
Table 1.
Subject demographics.
| Demographic variable | Patients with bipolar disorder (n = 23) | Normal controls (n = 19) | Group difference |
|---|---|---|---|
| Age (mean ± SD) | 36.0 ± 12.5 | 36.2 ± 12.4 | p = 0.98 |
| % female (n) | 30.4 (7) | 31.6 (6) | p = 0.94 |
| Years of education | 14.7 ± 1.8 | 15.6 ± 1.8 | p = 0.09 |
| % right handed | 100 (23) | 100 (19) | p = 1.00 |
| HAMDa | 4.7 ± 2.2 | 1.2 ± 1.4 | p < 0.001 |
| YMRSb | 1.7 ± 2.2 | 0.5 ± 1.5 | p = 0.04 |
| Duration of illness (years) | 18.7 ± 12.1 | – | – |
| Age at onset | 17.3 ± 7.6 | – | – |
| Prior manias | 13.0 ± 27.7 | – | – |
| Prior depressions | 10.8 ± 20.4 | – | – |
| Prior hospitalizations for mania | 1.6 ± 1.7 | – | – |
| Prior hospitalizations for depression | 0.8 ± 1.2 | – | – |
| Months euthymicc | 13.0 ± 16.0 | – | – |
All p-values show two-tailed significance levels.
Hamilton Depression Rating Scale.
Young Mania Rating Scale.
Indicates time euthymic prior to scanning.
Within the bipolar cohort, 12 subjects (52%) had a history of alcohol abuse or dependence, 8 subjects (35%) reported marijuana abuse or dependence, and 7 subjects (30%) reported cocaine abuse or dependence. There was also 1 case (4%) of opioid dependence, 4 (17%) of stimulant dependence, and 2 (9%) cases of abuse or dependence on another illicit substance. There was no history of hallucinogen abuse or dependence. Altogether, 7 (30%) subjects were not abusing or depending on illicit substances and 16 (70%) were. All patients needed to be substance free for six months or more to be enrolled in the study. None of the controls had abused or were dependent on illicit substances, as these were part of the exclusion criteria.
3.2. White matter
Compared to healthy individuals, the BD group had significantly lower FA in the corpus callosum (CC), as well as bilaterally in the inferior longitudinal and inferior fronto-occipital fasciculus (ILF and IFOF), the superior longitudinal fasciculus (SLF), and tapetum (Fig. 1). Axial sections also revealed FA deficits in patients in the anterior callosal genu. No significant FA differences were detected in the superior fronto-occipital fasciculus (SFOF). Within the limbic association fibers, the BD group showed lower FA in the left cingulum pertaining to the hippocampus, bilaterally in the fornix, and in a region including the right stria terminalis (ST). Within the projection fibers, patients had lower FA bilaterally in the posterior thalamic radiation (PTR) and the posterior corona radiata (PCR), along with the right anterior and superior corona radiata (ACR and SCR), and the left cerebral peduncle. No significant FA differences were detected in the internal capsule.
Fig. 1.
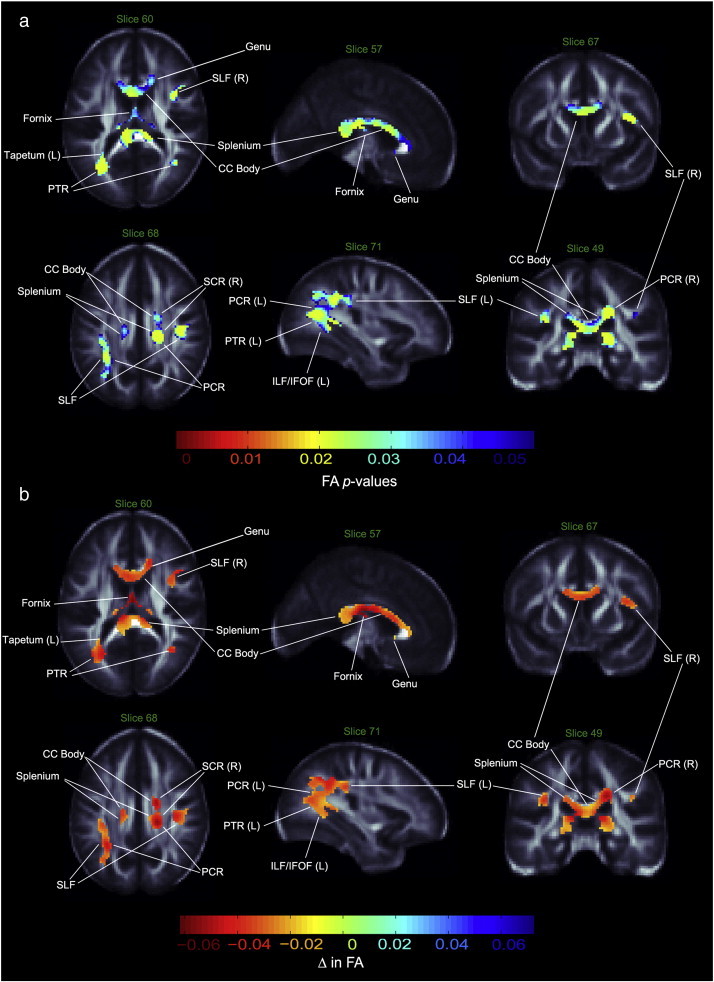
Fractional anisotropy differences in bipolar patients vs. controls. a. White matter regions where fractional anisotropy (FA) was significantly lower in bipolar patients compared to healthy controls. p-values have been corrected with searchlight FDR. b. Regression coefficients in white matter regions where voxels differed significantly between groups. Units are in FA unit difference between groups. PTR—posterior thalamic radiation; PCR—posterior corona radiata, SCR—superior corona radiata; SLF—superior longitudinal fasciculus; ILF—inferior longitudinal fasciculus; IFOF—inferior fronto-occipital fasciculus; CC—corpus callosum; L—left; R—right.
Maps of group difference in FA showed patterns similar to the radial and mean diffusivity maps. As predicted, RD and MD values were higher in bipolar patients than controls, with stronger effect sizes for RD and slightly weaker effect sizes for MD (Figs. 2, 3). In contrast to the FA maps, the genu did not show a significant group difference in diffusivity maps, while the bilateral SFOF demonstrated both higher RD and MD. Other dissimilarities between FA and RD/MD maps included a lack of detectable MD differences between groups in the tapetum or the corona radiata, an RD increase in the bilateral SCR and right PCR, a bilateral increase of MD and RD in the hippocampus, ST region, the anterior and posterior limbs of the internal capsule (ALIC and PLIC, respectively), a right-only increase in the retrolenticular limb of the internal capsule (RLIC), and no diffusivity differences in the ACR and cerebral peduncle. No regions with significantly higher FA or lower MD or RD were found in patients as compared to healthy controls. Table 2 summarizes our results. Exploratory analyses did not reveal any significant main or interactive effects of psychotropic medications, percent lifetime spent ill, prior exposure to lithium or history of psychosis on anisotropy or diffusivity measures.
Fig. 2.
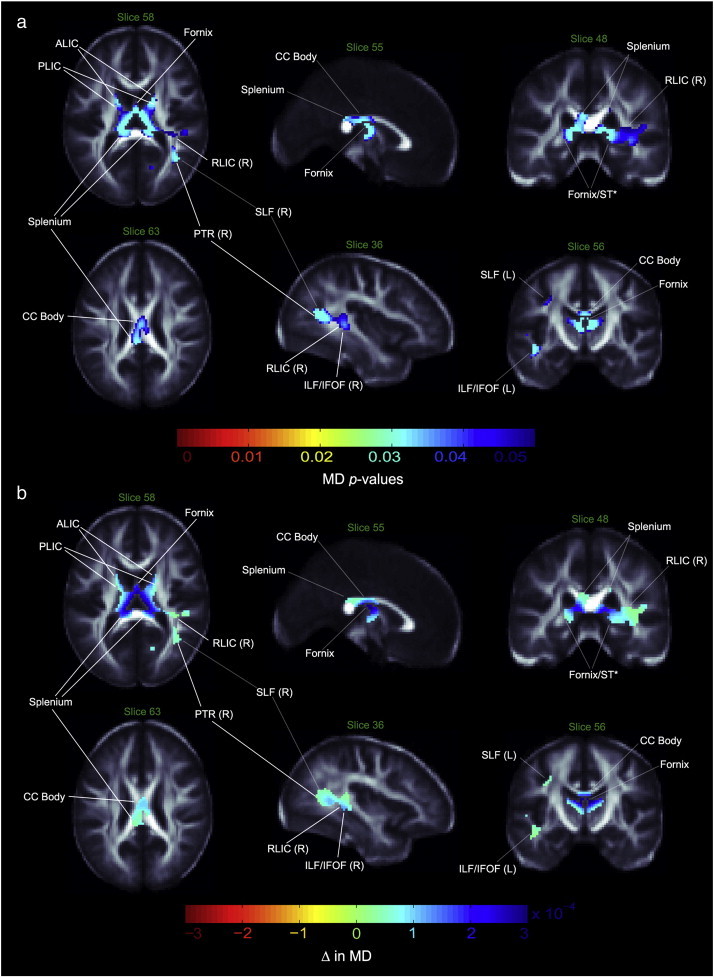
Mean diffusivity differences in bipolar patients vs. controls. a. White matter regions where mean diffusivity (MD) was significantly higher in bipolar patients compared to healthy controls. p-values have been corrected with searchlight FDR. b. Regression coefficients in white matter regions where voxels differed significantly between groups. Units are in MD unit difference between groups. ALIC—anterior limb of internal capsule; PLIC—posterior limb of internal capsule; RLIC—retrolenticular limb of internal capsule; PTR—posterior thalamic radiation; SLF—superior longitudinal fasciculus; ILF—inferior longitudinal fasciculus; IFOF—inferior fronto-occipital fasciculus; CC—corpus callosum; ST—stria terminalis; L—left; R—right; *—DTI resolution precludes resolving with anatomical certainty.
Fig. 3.
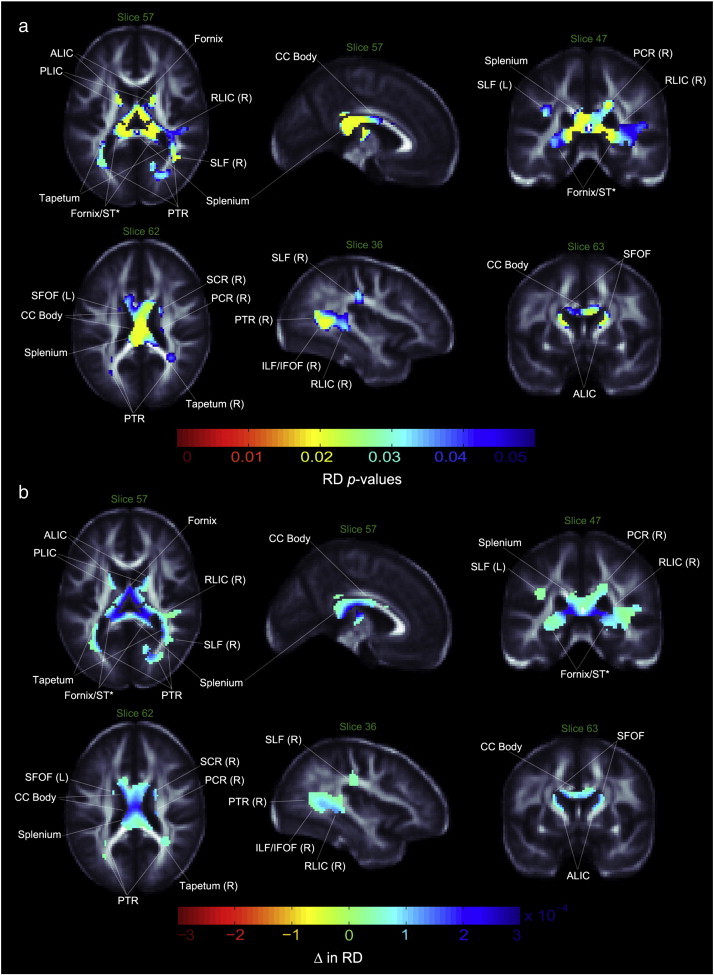
Radial diffusivity differences in bipolar patients vs. controls. a. White matter regions where radial diffusivity (RD) was significantly higher in bipolar patients compared to healthy controls. p-values have been corrected with searchlight FDR. b. Regression coefficients in white matter regions where voxels differed significantly between groups. Units are in RD unit difference between groups. ALIC—anterior limb of internal capsule; PLIC—posterior limb of internal capsule; RLIC—retrolenticular limb of internal capsule; PTR—posterior thalamic radiation; PCR—posterior corona radiata, SCR—superior corona radiata; SLF—superior longitudinal fasciculus; ILF—inferior longitudinal fasciculus; IFOF—inferior fronto-occipital fasciculus; SFOF—superior fronto-occipital fasciculus; CC—corpus callosum; ST—stria terminalis; L—left; R—right; *—DTI resolution precludes resolving with anatomical certainty.
Table 2.
Summary of findings.
| Areas of significant group differences | FA lower in BD |
MD higher in BD |
RD higher in BD |
|---|---|---|---|
| Corpus callosum—genu | Significant | No sig diff | No sig diff |
| Corpus callosum—body, splenium | Significant | Significant | Significant |
| Tapetum | Bilateral | No sig diff | Bilateral |
| Superior longitudinal fasciculus | Bilateral | Bilateral | Bilateral |
| Inferior longitudinal fasciculus | Bilateral | Bilateral | Bilateral |
| Inferior fronto-occipital fasciculus | Bilateral | Bilateral | Bilateral |
| Superior fronto-occipital fasciculus | No sig diff | Bilateral | Bilateral |
| Cingulum—hippocampus | Left | Bilateral | Bilateral |
| Fornix | Significant | Significant | Significant |
| Stria terminalis | Right | Bilateral | Bilateral |
| Posterior thalamic radiation/optic radiation | Bilateral | Bilateral | Bilateral |
| Corona radiata—superior | Right | No sig diff | Bilateral |
| Corona radiata—posterior | Bilateral | No sig diff | Right |
| Corona radiata—anterior | Right | No sig diff | No sig diff |
| Internal capsule—anterior | No sig diff | Bilateral | Bilateral |
| Internal capsule—posterior | No sig diff | Bilateral | Bilateral |
| Internal capsule—retrolenticular | No sig diff | Right | Right |
| Cerebral peduncle | Left | No sig diff | No sig diff |
4. Discussion
To our knowledge, this is one of the first studies to examine whole brain WM microstructure in bipolar adults who were all euthymic and lithium free at the time of the scan. Relative to healthy controls, bipolar patients showed pervasive aberrations in several major inter-and intra-hemispheric tracts, including the CC, frontal WM, limbic and projection fibers. These findings are consistent with a growing literature implicating WM abnormalities in BD (Leow et al., 2013; Lin et al., 2011; Sussmann et al., 2009; Zanetti et al., 2009) and in healthy individuals at high genetic risk for BD (Linke et al., 2012; Sprooten et al., 2011), suggesting that the WM pathology that we have observed here may precede the onset of BD. Our study builds upon prior research of microstructural WM deficits in BD, through demonstrating patterns of WM abnormalities in a BD sample that was carefully recruited to control for mood state and current lithium treatment. Moreover, our study suggests WM alterations in regions not previously reported as having associations with the disease (e.g., the fornix and ST), as well as a region with only one previous report of involvement (e.g., the tapetum).
A unique finding in our study was that of increased RD and MD in patients relative to healthy controls in the bilateral fornix and ST, and of decreased FA in the right ST. The fornix is central to the limbic system and contains pathways to the hypothalamus, thalamus, hippocampus, and nucleus accumbens, regions important for memory, emotional regulation and reward processing, which have been implicated in BD (Ahn et al., 2007; Frey et al., 2007). The ST connects the amygdala and hippocampus (Mendoza and Foundas, 2007), which plays an important role in episodic representations of emotionally significant memories (Phelps, 2004). There is only one DTI study that, consistent with our results, found significantly lower FA in the fornix in adolescent BD (Barnea-Goraly et al., 2009), but these findings did not extend to diffusivity measures. The fornix and ST are very thin structures, and we cannot rule out partial volume effects accounting for some of these findings, despite correcting for multiple comparisons. As a result, our findings in these regions should be interpreted with caution and warrant further study at higher spatial resolution.
Our findings of reduced FA and increased MD and RD of the CC in BD support the notion that inter-hemispheric communications have a key role in BD pathophysiology. This is consistent with several DTI studies of patients with or at high genetic risk of BD, including one recent study from our group of a non-overlapping bipolar cohort (Barnea-Goraly et al., 2009; Chaddock et al., 2009; Chan et al., 2010; Frazier et al., 2007; Ha et al., 2011; Haller et al., 2011; James et al., 2011; Leow et al., 2013; Pavuluri et al., 2009; Saxena et al., 2012; Sprooten et al., 2011; Wang et al., 2008a; Wang et al., 2008b), but are in contrast with Yurgelun-Todd et al. (2007), who found higher FA in the genu of BD patients relative to healthy controls. The CC, which interconnects the hemispheres, is the largest commissural fiber bundle of the brain, and is critically involved in the integration of emotional, attentional, perceptual and cognitive functions that are frequently compromised in BD (Schmahmann and Caplan, 2006). Further, we extend previous CC findings by showing significantly decreased FA and increased MD and RD in the tapetum, a callosal tract that projects from the splenium to the temporal lobes, which has only once been previously implicated in BD, among first episode patients experiencing psychosis (Lu et al., 2011).
The patterns of lower FA and higher MD and RD in association fibers that we observed in BD patients are consistent with a number of previous BD studies (Bruno et al., 2008; Chaddock et al., 2009; Chan et al., 2010; Frazier et al., 2007; Ha et al., 2011; Haznedar et al., 2005; Lin et al., 2011; Sprooten et al., 2011; Zanetti et al., 2009). Associative tracts implicated in our study are the SFOF, which is important for spatial awareness and symmetrical processing (Schmahmann and Pandya, 2007), the IFOF, which connects the orbito-frontal cortex with the occipital lobe (Wakana et al., 2004), the SLF, which connects the frontal lobe with parieto-temporal regions essential for working memory processing (Edin et al., 2009; Makris et al., 2005; Thomason and Thompson, 2011; Urbanski et al., 2008) and the ILF, which connects the anterior temporal lobe and amygdala with the occipital cortex, and supports visual processing of emotionally salient information (Catani et al., 2002; Versace et al., 2010). Disrupted WM microarchitecture in these regions may relate to disturbances in executive function observed in individuals with BD (Adler et al., 2006; Strakowski et al., 2005), such as poor judgment and impaired decision-making capacity (Kafantaris et al., 2009; Kawasaki et al., 2001). Further, in addition to the cognitive functions subserved by the SLF and ILF mentioned above, these tracts also work to integrate auditory and speech areas of the brain (Catani et al., 2002; Lin et al., 2011); disruptions in the WM microstructure of these areas may thus contribute to speech-related symptoms that are observed in BD (Lin et al., 2011). The ILF and IFOF have been implicated elsewhere as critical to the processing of facial emotion (Philippi et al., 2009). Difficulties in the ability to correctly identify facial emotions have been proposed as a potential endophenotype for BD (Bozikas et al., 2006; Brotman et al., 2008); structural anomalies in the ILF and IFOF of BD individuals may contribute to such a deficit. Additionally, decreased WM density in the bilateral SLF has been shown to be associated with the genetic risk for developing BD (van der Schot et al., 2010). Our findings of disrupted WM microstructure in the SLF may contribute to the further study of whether genetic risk for developing BD could be associated with some of the reported brain abnormalities in this illness.
Lower FA and increased MD and RD were also found in patients relative to healthy controls in the cingulum bundle, specifically in fibers connecting to the hippocampus. This finding is consistent with many bipolar DTI studies of the cingulum (Barnea-Goraly et al., 2009; Chan et al., 2010; Gonenc et al., 2010; Lin et al., 2011; Wang et al., 2008a). As the hippocampus is implicated in memory consolidation and spatial navigation (Lavenex and Amaral, 2000; Mega et al., 1997), disruptions in the WM tracts that connect this region to other parts of the brain may contribute to declarative memory impairments (Bearden et al., 2006; Deckersbach et al., 2004; Glahn et al., 2005), or to impairments in episodic memory and spatial span performance (Frey et al., 2007) in BD.
Finally, we observed group differences in WM structure within the projection fibers of the corona radiata, internal capsule, cerebral peduncle and the PTR. The corona radiata is a band of fibers projecting to and from the cerebral cortex that converges near the brainstem to form the internal capsule, and inferiorly, the cerebral peduncles. The ALIC contains the thalamic radiations, while the PLIC and cerebral peduncles encompass the corticobulbar, corticopontine, and corticospinal tracts, which are responsible for initiating and controlling movements. The PTR includes the optic radiation, which connects the thalamus and the occipital lobe; disruption to this tract may affect visual processing, which was found to be impaired in some BD patients in a study by MacQueen et al. (2001). While diffusivity measures were increased in the internal capsule, there were no accompanying FA differences. In contrast, FA was lower in the left cerebral peduncle, with no detected differences in diffusivity. Similarly, while FA was lower in the corona radiata, there were no MD differences. The PTR showed both decreased FA and increased RD and MD, which could be representative of compromised myelin and axonal integrity. Our findings in these areas are consistent with prior studies which have found reduced FA in the projection fibers of bipolar patients (Barnea-Goraly et al., 2009; Chaddock et al., 2009; Chen et al., 2012; Haznedar et al., 2005; McIntosh et al., 2008; Pavuluri et al., 2009; Sussmann et al., 2009) and those at high genetic risk for BD (Linke et al., 2012; Sprooten et al., 2011) compared to controls, and may relate to deficits in motor inhibition and cognitive function in BD (Dickstein et al., 2005; Heng et al., 2010), resulting in inappropriate modulation of emotional responses, execution of tasks with disregard to past experiences, and an inability to predict the outcomes of their actions (Haznedar et al., 2005; Price, 1999).
A recent study evaluating a non-overlapping cohort, with unrelated scan acquisition parameters (Torgerson et al., 2012) examined the fiber density of various tracts using tractography. In that study, significant differences between bipolar and control subjects were found in tract length and tract density, but no FA differences were detected in some of the regions reported here, except for a FA difference in the cerebrospinal tract. Cohort differences may account for this, as the samples were non-overlapping and were scanned at different field strengths. Also, both cohorts are relatively small and are only powered to find moderate but not small effect sizes. Thus, small effects may have been present but not detected in both studies. Further, our current voxel-based analyses survey the whole WM and map based methods do pick up patterns that ROI based methods may not, and vice versa. This also may affect the sensitivity to pick up group differences throughout the brain. As many studies are barely powered to detect subtle and distributed disease effects, we are making a serious effort to harmonize future neuroimaging studies of bipolar disorder to avoid this dilemma where differences in results may reflect cohort differences or protocol differences. Our ENIGMA bipolar working group (Hibar et al., 2013) pools MRI data from 9 sites (with more being added), allowing us to use meta-analysis to home in on the most consistent results.
Our findings should be interpreted with several limitations in mind. First, while we have noted some drawbacks of ROI and TBSS based methods, voxel based approaches also have some limitations. For example, the smoothing kernel size can influence study findings (Jones et al., 2005), and it needs to be set in advance, often without a priori information about the spatial extent of the effects of interest, which also may vary from one brain region to another. When voxel-based maps are smoothed, images are blurred to maximize the power to detect spatially coherent effects, and to minimize the impact of misalignment error and anatomical differences across subjects, which inevitably leads to some loss of spatial resolution and decreased sensitivity for detecting abnormalities in small structures or in areas of high anatomic variability. Even so, these are also limitations for the ROI method, which is effectively the same as applying a large uniform filter to a selected region. In voxel-based methods, some findings close to CSF borders can reflect structural differences rather than purely microstructural changes, but most of the voxels we report in this study were far from CSF. It can also be challenging to ensure that each voxel contains only data from the same part of the same WM tract in all subjects due to alignment inaccuracies (Smith et al., 2006). Voxel based methods have been criticized as being susceptible to ‘edge effects’ caused by misregistration of tracts (Kanaan et al., 2006), especially in regions where the FA changes sharply at an interface. As with any voxelwise analysis there is an increased risk of losing power by making a heavy correction for multiple comparisons, although we decreased this risk by restricting the analysis to WM only with a WM mask. Second, while we avoided the confounding effects of lithium in our WM measurements by recruiting only lithium-free patients, 16 bipolar subjects were taking one or more other psychotropic medications, whose effects on WM structure are unknown. However, exploratory analyses did not reveal significant effects of psychotropic medications on anisotropy or diffusivity measures, and previous studies conducted in bipolar (Frazier et al., 2007; Versace et al., 2008) and schizophrenic (Foong et al., 2000; Lim et al., 1999) populations failed to find significant effects of medication on diffusion measures as well. Third, there is some concern that substance use may have contributed to the detected effects on the brain. We have explored this further by calculating and plotting the mean FA values of the bipolar and control groups in the CC. The mean FA values for the bipolar subjects with a history of drug abuse or dependence were found to be distributed fairly evenly among the group's values, with no obvious pattern between subjects who were substance naïve versus those who had previous exposure. This is shown in Fig. 4, where the FA values (in red) belonging to the bipolar subjects who had a history of drug abuse or dependence are indicated with a purple border, for easier visualization. Fourth, the sample size, while consistent with other published studies, was limited. Our future studies should be able to increase the number of subjects by establishing consortium efforts (Jahanshad et al., 2013; Hibar et al., 2013; Turner et al., 2013) to investigate larger groups of patients with and without lithium exposure. Lastly, the current study used 30-direction DWI, which is common in clinical settings, but does not have high angular resolution. This number of directions does not allow us to determine whether the FA, MD and RD differences observed here were the result of a group difference in the number or relative density of crossing fibers. Higher spatial resolution may also allow us to better distinguish smaller brain structures such as the stria terminalis and fornix. Future studies with more diffusion-weighted gradients could assess this, at the expense of longer scan times. Future studies may also benefit from the use of DTI tractography, which can reconstruct white matter pathways in vivo, offering information on anatomical connectivity between distant brain areas (Leow et al., 2013).
Fig. 4.
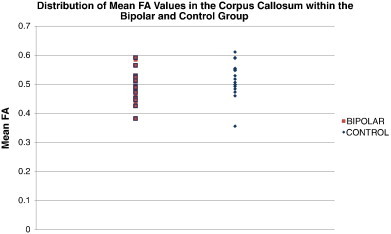
Mean FA values in the corpus callosum, for each subject, are plotted by diagnostic group. Control FA values are shown in blue and bipolar FA values are shown in red. Values for subjects who had a history of drug abuse or dependence are outlined in purple.
In conclusion, in a sample of euthymic, lithium-free BD patients, we found widespread abnormalities in the integrity of WM tracts connecting brain regions critically involved in regulating behavior, cognition and emotion. Future studies that examine the direct association between WM abnormalities and behavior in BD are of interest, and may assist in understanding symptoms and prognosis for patients with BD.
Financial disclosures
Unrelated to this study, Dr. Altshuler has received past funding from Forest Laboratories (advisory board honoraria, March 2008); Sepracor (advisory board honoraria, Jan 2010) and Eli Lilly (consultant, September 2010). All other authors have no commercial interests related to this study.
Acknowledgments
This study was funded by the National Institute of Mental Health (K24 MH001848, R21 MH075944, R01 MH084955, 5F31MH078556), the National Institute for Biological Imaging and Bioengineering (R01 EB008432, R01 EB007813, P41 RR013642) and the National Center for Research Resources (RR12169, RR13642, RR00865). For generous support, we thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Adler C.M., Holland S.K., Schmithorst V., Wilke M., Weiss K.L., Pan H., Strakowski S.M. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disorders. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Adler C.M., Adams J., DelBello M.P., Holland S.K., Schmithorst V., Levine A., Jarvis K., Strakowski S.M. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. The American Journal of Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- Ahn M.S., Breeze J.L., Makris N., Kennedy D.N., Hodge S.M., Herbert M.R., Seidman L.J., Biederman J., Caviness V.S., Frazier J.A. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. Journal of Affective Disorders. 2007;104:147–154. doi: 10.1016/j.jad.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Altshuler L.L., Bookheimer S.Y., Townsend J., Proenza M.A., Eisenberger N., Sabb F., Mintz J., Cohen M.S. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- FMRIB Analysis Group . The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Department of Clinical Neurology, University of Oxford; Oxford, UK: 2012. FMRIB Software Library. [Google Scholar]
- Barnea-Goraly N., Chang K.D., Karchemskiy A., Howe M.E., Reiss A.L. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological Psychiatry. 2009;66:238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden C.E., Glahn D.C., Monkul E.S., Barrett J., Najt P., Kaur S., Sanches M., Villarreal V., Bowden C., Soares J.C. Sources of declarative memory impairment in bipolar disorder: mnemonic processes and clinical features. Journal of Psychiatric Research. 2006;40:47–58. doi: 10.1016/j.jpsychires.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Allen P.S. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MRI of the nervous system. Magnetic Resonance in Medicine. 1994;32:579–583. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Absinta M., Rocca M.A., Radaelli D., Poletti S., Bernasconi A., Dallaspezia S., Pagani E., Falini A., Copetti M., Colombo C., Comi G., Smeraldi E., Filippi M. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disorders. 2011;13:414–424. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- Beyer J.L., Taylor W.D., MacFall J.R., Kuchibhatla M., Payne M.E., Provenzale J.M., Cassidy F., Krishnan K.R. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- Bozikas V.P., Tonia T., Fokas K., Karavatos A., Kosmidis M.H. Impaired emotion processing in remitted patients with bipolar disorder. Journal of Affective Disorders. 2006;91:53–56. doi: 10.1016/j.jad.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Brooks J.O., III, Bonner J.C., Rosen A.C., Wang P.W., Hoblyn J.C., Hill S.J., Ketter T.A. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Research. 2009;172:200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J.O., III, Foland-Ross L.C., Thompson P.M., Altshuler L.L. Preliminary evidence of within-subject changes in gray matter density associated with remission of bipolar depression. Psychiatry Research. 2011;193:53–55. doi: 10.1016/j.pscychresns.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman M.A., Guyer A.E., Lawson E.S., Horsey S.E., Rich B.A., Dickstein D.P., Pine D.S., Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. The American Journal of Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Bruno S., Cercignani M., Ron M.A. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disorders. 2008;10:460–468. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chaddock C.A., Barker G.J., Marshall N., Schulze K., Hall M.H., Fern A., Walshe M., Bramon E., Chitnis X.A., Murray R., McDonald C. White matter microstructural impairments and genetic liability to familial bipolar I disorder. The British Journal of Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Chan W.Y., Yang G.L., Chia M.Y., Woon P.S., Lee J., Keefe R., Sitoh Y.Y., Nowinski W.L., Sim K. Cortical and subcortical white matter abnormalities in adults with remitted first-episode mania revealed by Tract-Based Spatial Statistics. Bipolar Disorders. 2010;12:383–389. doi: 10.1111/j.1399-5618.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Cui L., Li M., Jiang L., Deng W., Ma X., Wang Q., Huang C., Wang Y., Collier D.A., Gong Q., Li T. Voxel based morphometric and diffusion tensor imaging analysis in male bipolar patients with first-episode mania. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36:231–238. doi: 10.1016/j.pnpbp.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Coffey C.E., Saxton J.A., Ratcliff G., Bryan R.N., Lucke J.F. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- Deckersbach T., Savage C.R., Reilly-Harrington N., Clark L., Sachs G., Rauch S.L. Episodic memory impairment in bipolar disorder and obsessive–compulsive disorder: the role of memory strategies. Bipolar Disorders. 2004;6:233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- Dickstein D.P., Garvey M., Pradella A.G., Greenstein D.K., Sharp W.S., Castellanos F.X., Pine D.S., Leibenluft E. Neurologic examination abnormalities in children with bipolar disorder or attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;58:517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Edin F., Klingberg T., Johansson P., McNab F., Tegner J., Compte A. Mechanism for top-down control of working memory capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland L.C., Altshuler L.L., Sugar C.A., Lee A.D., Leow A.D., Townsend J., Narr K.L., Asuncion D.M., Toga A.W., Thompson P.M. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Brooks J.O., III, Mintz J., Bartzokis G., Townsend J., Thompson P.M., Altshuler L.L. Mood-state effects on amygdala volume in bipolar disorder. Journal of Affective Disorders. 2012;139:298–301. doi: 10.1016/j.jad.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong J., Maier M., Clark C.A., Barker G.J., Miller D.H., Ron M.A. Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;68:242–244. doi: 10.1136/jnnp.68.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier J.A., Breeze J.L., Papadimitriou G., Kennedy D.N., Hodge S.M., Moore C.M., Howard J.D., Rohan M.P., Caviness V.S., Makris N. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disorders. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- Frey B.N., Andreazza A.C., Nery F.G., Martins M.R., Quevedo J., Soares J.C., Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behavioural Pharmacology. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- Germana C., Kempton M.J., Sarnicola A., Christodoulou T., Haldane M., Hadjulis M., Girardi P., Tatarelli R., Frangou S. The effects of lithium and anticonvulsants on brain structure in bipolar disorder. Acta Psychiatrica Scandinavica. 2010;122:481–487. doi: 10.1111/j.1600-0447.2010.01582.x. [DOI] [PubMed] [Google Scholar]
- Glahn D.C., Bearden C.E., Caetano S., Fonseca M., Najt P., Hunter K., Pliszka S.R., Olvera R.L., Soares J.C. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disorders. 2005;7:546–554. doi: 10.1111/j.1399-5618.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Gonenc A., Frazier J.A., Crowley D.J., Moore C.M. Combined diffusion tensor imaging and transverse relaxometry in early-onset bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1260–1268. doi: 10.1016/j.jaac.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman B., Svarer C., Leow A.D., Yanovsky I., Toga A.W., Thompson P.M. 16th Annual Meeting of the Organization for Human Brain Mapping. 2010. Creating unbiased minimal deformation templates for brain volume registration. (Barcelona, Spain) [Google Scholar]
- Ha T.H., Her J.Y., Kim J.H., Chang J.S., Cho H.S., Ha K. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neuroscience Letters. 2011;505:150–154. doi: 10.1016/j.neulet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Haller S., Xekardaki A., Delaloye C., Canuto A., Lovblad K.O., Gold G., Giannakopoulos P. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. Journal of Psychiatry & Neuroscience. 2011;36:391–401. doi: 10.1503/jpn.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsan L.A., Poulet P., Guignard B., Steibel J., Parizel N., de Sousa P.L., Boehm N., Grucker D., Ghandour M.S. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. Journal of Neuroscience Research. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Haznedar M.M., Roversi F., Pallanti S., Baldini-Rossi N., Schnur D.B., Licalzi E.M., Tang C., Hof P.R., Hollander E., Buchsbaum M.S. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biological Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Heng S., Song A.W., Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. Journal of Neural Transmission. 2010;117:639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- Hibar D.P., van Erp T.G., Rasmussen J., Turner J.A., Haukvik U.K., Agartz I., Gruber O., Krämer B., Lindberg B., Ekman C.J., Landen M., Nugent A., Laje G., McMahon F., Fears S., Bearden C., Freimer N., Glahn D., McDonald C., Cannon D., Phillips M., Strakowski S., Alder C., Frangou S., Thompson P.M., Andreassen O.A. 19th Annual Meeting of the Organization for Human Brain Mapping. Seattle. 2013. Meta-analysis of structural brain differences in bipolar disorder: the ENIGMA-Bipolar Disorder Project. (WA, USA) [Google Scholar]
- Holmes C.J., Hoge R., Collins L., Woods R., Toga A.W., Evans A.C. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Houenou J., Wessa M., Douaud G., Leboyer M., Chanraud S., Perrin M., Poupon C., Martinot J.L., Paillere-Martinot M.L. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Molecular Psychiatry. 2007;12:1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- Jahanshad N., Lee A.D., Barysheva M., McMahon K.L., de Zubicaray G.I., Martin N.G., Wright M.J., Toga A.W., Thompson P.M. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. NeuroImage. 2010;52:455–469. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N., Kochunov P.V., Sprooten E., Mandl R.C., Nichols T.E., Almasy L., Blangero J., Brouwer R.M., Curran J.E., de Zubicaray G.I., Duggirala R., Fox P.T., Hong L.E., Landman B.A., Martin N.G., McMahon K.L., Medland S.E., Mitchell B.D., Olvera R.L., Peterson C.P., Starr J.M., Sussmann J.E., Toga A.W., Wardlaw J.M., Wright M.J., Hulshoff Pol H.E., Bastin M.E., McIntosh A.M., Deary I.J., Thompson P.M., Glahn D.C. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA–DTI working group. Neuroimage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A., Hough M., James S., Burge L., Winmill L., Nijhawan S., Matthews P.M., Zarei M. Structural brain and neuropsychometric changes associated with pediatric bipolar disorder with psychosis. Bipolar Disorders. 2011;13:16–27. doi: 10.1111/j.1399-5618.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S.M. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Symms M.R., Cercignani M., Howard R.J. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kafantaris V., Kingsley P., Ardekani B., Saito E., Lencz T., Lim K., Szeszko P. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R.A., Shergill S.S., Barker G.J., Catani M., Ng V.W., Howard R., McGuire P.K., Jones D.K. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Research. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Kaufman O., Damasio H., Damasio A.R., Granner M., Bakken H., Hori T., Howard M.A., III, Adolphs R. Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience. 2001;4:15–16. doi: 10.1038/82850. [DOI] [PubMed] [Google Scholar]
- Langers D.R., Jansen J.F., Backes W.H. Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. NeuroImage. 2007;38:43–56. doi: 10.1016/j.neuroimage.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Lavenex P., Amaral D.G. Hippocampal–neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Leow A., Huang S.C., Geng A., Becker J., Davis S., Toga A., Thompson P. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. Information Processing in Medical Imaging. 2005;19:493–503. doi: 10.1007/11505730_41. [DOI] [PubMed] [Google Scholar]
- Leow A., Huang S.-C., Geng A., Becker J., Davis S., Toga A., Thompson P. Inverse consistent mapping in 3D deformable image registration: its construction and statistical properties. In: Christensen G.E., Sonka M., editors. Information Processing in Medical Imaging. Springer Berlin; Heidelberg: 2005. pp. 493–503. [DOI] [PubMed] [Google Scholar]
- Leow A., Ajilore O., Zhan L., Arienzo D., GadElkarim J., Zhang A., Moody T., Van Horn J., Feusner J., Kumar A., Thompson P., Altshuler L. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biological Psychiatry. 2013;73:183–193. doi: 10.1016/j.biopsych.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.O., Hedehus M., Moseley M., de Crespigny A., Sullivan E.V., Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Archives of General Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- Lin F., Weng S., Xie B., Wu G., Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. Journal of Affective Disorders. 2011;131:299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Linke J., Witt S.H., King A.V., Nieratschker V., Poupon C., Gass A., Hennerici M.G., Rietschel M., Wessa M. Genome-wide supported risk variant for bipolar disorder alters anatomical connectivity in the human brain. NeuroImage. 2012;59:3288–3296. doi: 10.1016/j.neuroimage.2011.10.083. [DOI] [PubMed] [Google Scholar]
- Liu S.K., Chiu C.H., Chang C.J., Hwang T.J., Hwu H.G., Chen W.J. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. The American Journal of Psychiatry. 2002;159:975–982. doi: 10.1176/appi.ajp.159.6.975. [DOI] [PubMed] [Google Scholar]
- Lu L.H., Zhou X.J., Keedy S.K., Reilly J.L., Sweeney J.A. White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disorders. 2011;13:604–613. doi: 10.1111/j.1399-5618.2011.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G.M., Young L.T., Galway T.M., Joffe R.T. Backward masking task performance in stable, euthymic out-patients with bipolar disorder. Psychological Medicine. 2001;31:1269–1277. doi: 10.1017/s0033291701004597. [DOI] [PubMed] [Google Scholar]
- Macritchie K.A., Lloyd A.J., Bastin M.E., Vasudev K., Gallagher P., Eyre R., Marshall I., Wardlaw J.M., Ferrier I.N., Moore P.B., Young A.H. White matter microstructural abnormalities in euthymic bipolar disorder. The British Journal of Psychiatry. 2010;196:52–58. doi: 10.1192/bjp.bp.108.058586. [DOI] [PubMed] [Google Scholar]
- Makris N., Kennedy D.N., McInerney S., Sorensen A.G., Wang R., Caviness V.S., Jr., Pandya D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K., Woods R., Paus T., Simpson G., Pike B., Holmes C., Collins L., Thompson P.M., MacDonald D., Iacoboni M., Schormann T., Amunts K., Palomero-Gallagher N., Geyer S., Parsons L., Narr K., Kabani N., Le Goualher G., Boomsma D., Cannon T., Kawashima R., Mazoyer B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.M., Moorhead T.W., Job D., Lymer G.K., Munoz Maniega S., McKirdy J., Sussmann J.E., Baig B.J., Bastin M.E., Porteous D., Evans K.L., Johnstone E.C., Lawrie S.M., Hall J. The effects of a neuregulin 1 variant on white matter density and integrity. Molecular Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Mega M.S., Cummings J.L., Salloway S., Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. The Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- Meguro K., Shimada M., Yamaguchi S., Ishizaki J., Ishii H., Shimada Y., Sato M., Yamadori A., Sekita Y. Cognitive function and frontal lobe atrophy in normal elderly adults: implications for dementia not as aging-related disorders and the reserve hypothesis. Psychiatry and Clinical Neurosciences. 2001;55:565–572. doi: 10.1046/j.1440-1819.2001.00907.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J.E., Foundas A.L. Springer; New York; London: 2007. Clinical Neuroanatomy: a Neurobehavioral Approach. [Google Scholar]
- Merikangas K.R., Akiskal H.S., Angst J., Greenberg P.E., Hirschfeld R.M., Petukhova M., Kessler R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkul E.S., Matsuo K., Nicoletti M.A., Dierschke N., Hatch J.P., Dalwani M., Brambilla P., Caetano S., Sassi R.B., Mallinger A.G., Soares J.C. Prefrontal gray matter increases in healthy individuals after lithium treatment: a voxel-based morphometry study. Neuroscience Letters. 2007;429:7–11. doi: 10.1016/j.neulet.2007.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C.M. 1st ed. Elsevier; Amsterdam, The Netherlands; San Diego, CA: 2004. MRI Atlas of Human White Matter. [Google Scholar]
- Nery F.G., Chen H.H., Hatch J.P., Nicoletti M.A., Brambilla P., Sassi R.B., Mallinger A.G., Keshavan M.S., Soares J.C. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disorders. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Ongur D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri M.N., Yang S., Kamineni K., Passarotti A.M., Srinivasan G., Harral E.M., Sweeney J.A., Zhou X.J. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Mehta S., Grabowski T., Adolphs R., Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J., Wei J., Dilley G., Pittman S.D., Meltzer H.Y., Overholser J.C., Roth B.L., Stockmeier C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Sassi R.B., Nicoletti M., Brambilla P., Mallinger A.G., Frank E., Kupfer D.J., Keshavan M.S., Soares J.C. Increased gray matter volume in lithium-treated bipolar disorder patients. Neuroscience Letters. 2002;329:243–245. doi: 10.1016/s0304-3940(02)00615-8. [DOI] [PubMed] [Google Scholar]
- Saxena K., Tamm L., Walley A., Simmons A., Rollins N., Chia J., Soares J.C., Emslie G.J., Fan X., Huang H. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. Journal of Child and Adolescent Psychopharmacology. 2012;22:112–119. doi: 10.1089/cap.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G., Zhong J., Gill D., Peterson D.R., Gaugh M.D., Zhu T., Tivarus M., Cruttenden K., Maggirwar S.B., Gendelman H.E., Dewhurst S., Gelbard H.A. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. Journal of Neurovirology. 2009;15:176–186. doi: 10.1080/13550280902758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. The complex history of the fronto-occipital fasciculus. Journal of the History of the Neurosciences. 2007;16:362–377. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sprooten E., Sussmann J.E., Clugston A., Peel A., McKirdy J., Moorhead T.W., Anderson S., Shand A.J., Giles S., Bastin M.E., Hall J., Johnstone E.C., Lawrie S.M., McIntosh A.M. White matter integrity in individuals at high genetic risk of bipolar disorder. Biological Psychiatry. 2011;70:350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Strakowski S.M., Adler C.M., Holland S.K., Mills N.P., DelBello M.P., Eliassen J.C. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. The American Journal of Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Sussmann J.E., Lymer G.K., McKirdy J., Moorhead T.W., Munoz Maniega S., Job D., Hall J., Bastin M.E., Johnstone E.C., Lawrie S.M., McIntosh A.M. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Thompson P.M. Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Tkachev D., Mimmack M.L., Ryan M.M., Wayland M., Freeman T., Jones P.B., Starkey M., Webster M.J., Yolken R.H., Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Torgerson C.M., Irimia A., Leow A.D., Bartzokis G., Moody T.D., Jennings R.G., Alger J.R., Van Horn J.D., Altshuler L.L. DTI tractography and white matter fiber tract characteristics in euthymic bipolar I patients and healthy control subjects. Brain Imaging and Behavior. 2012 doi: 10.1007/s11682-012-9202-3. (Oct 16 [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.A., Hibar D.P., Rasmussen J., Andreassen O., Haukvik U.K., Agartz I., Potkin S.G., Ophoff R., Hulshoff-Pol H., van Haren N.E., Gruber O., Krämer B., Erlich S., Hass J., Wang L., Alpert K., Glahn D.C., Thompson P.M., van Erp T.G. 19th Annual Meeting of the Organization for Human Brain Mapping. 2013. A Prospective Meta-Analysis of Subcortical Brain Volumes in Schizophrenia via the ENIGMA Consortium. (Seattle, WA, USA) [Google Scholar]
- Urbanski M., Thiebaut de Schotten M., Rodrigo S., Catani M., Oppenheim C., Touze E., Chokron S., Meder J.F., Levy R., Dubois B., Bartolomeo P. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schot A.C., Vonk R., Brouwer R.M., van Baal G.C., Brans R.G., van Haren N.E., Schnack H.G., Boomsma D.I., Nolen W.A., Hulshoff Pol H.E., Kahn R.S. Genetic and environmental influences on focal brain density in bipolar disorder. Brain. 2010;133:3080–3092. doi: 10.1093/brain/awq236. [DOI] [PubMed] [Google Scholar]
- Versace A., Almeida J.R., Hassel S., Walsh N.D., Novelli M., Klein C.R., Kupfer D.J., Phillips M.L. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Archives of General Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A., Ladouceur C.D., Romero S., Birmaher B., Axelson D.A., Kupfer D.J., Phillips M.L. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:1249–1259. doi: 10.1016/j.jaac.2010.09.007. (1259 e1241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Jiang H., Nagae-Poetscher L.M., van Zijl P.C., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wang F., Jackowski M., Kalmar J.H., Chepenik L.G., Tie K., Qiu M., Gong G., Pittman B.P., Jones M.M., Shah M.P., Spencer L., Papademetris X., Constable R.T., Blumberg H.P. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. The British Journal of Psychiatry. 2008;193:126–129. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kalmar J.H., Edmiston E., Chepenik L.G., Bhagwagar Z., Spencer L., Pittman B., Jackowski M., Papademetris X., Constable R.T., Blumberg H.P. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biological Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Kalmar J.H., He Y., Jackowski M., Chepenik L.G., Edmiston E.E., Tie K., Gong G., Shah M.P., Jones M., Uderman J., Constable R.T., Blumberg H.P. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M., Houenou J., Leboyer M., Chanraud S., Poupon C., Martinot J.L., Paillere-Martinot M.L. Microstructural white matter changes in euthymic bipolar patients: a whole-brain diffusion tensor imaging study. Bipolar Disorders. 2009;11:504–514. doi: 10.1111/j.1399-5618.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Silveri M.M., Gruber S.A., Rohan M.L., Pimentel P.J. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disorders. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M.V., Jackowski M.P., Versace A., Almeida J.R., Hassel S., Duran F.L., Busatto G.F., Kupfer D.J., Phillips M.L. State-dependent microstructural white matter changes in bipolar I depression. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


