Abstract
Adults with attention-deficit/hyperactivity disorder (ADHD) often display executive function impairments, particularly in inhibitory control. The antisaccade task, which measures inhibitory control, requires one to suppress an automatic prosaccade toward a salient visual stimulus and voluntarily make an antisaccade in the opposite direction. ADHD patients not only have longer saccadic reaction times, but also make more direction errors (i.e., a prosaccade was executed toward the stimulus) during antisaccade trials. These deficits may stem from pathology in several brain areas that are important for executive control. Using functional MRI with a rapid event-related design, adults with combined subtype of ADHD (coexistence of attention and hyperactivity problems), who abstained from taking stimulant medication 20 h prior to experiment onset, and age-match controls performed pro- and antisaccade trials that were interleaved with pro- and anti-catch trials (i.e., instruction was presented but no target appeared, requiring no response). This method allowed us to examine brain activation patterns when participants either prepared (during instruction) or executed (after target appearance) correct pro or antisaccades. Behaviorally, ADHD adults displayed several antisaccade deficits, including longer and more variable reaction times and more direction errors, but saccade metrics (i.e., duration, velocity, and amplitude) were normal. When preparing to execute an antisaccade, ADHD adults showed less activation in frontal, supplementary, and parietal eye fields, compared to controls. However, activation in these areas was normal in the ADHD group during the execution of a correct antisaccade. Interestingly, unlike controls, adults with ADHD produced greater activation than controls in dorsolateral prefrontal cortex during antisaccade execution, perhaps as part of compensatory mechanisms to optimize antisaccade production. Overall, these data suggest that the saccade deficits observed in adults with ADHD do not result from an inability to execute a correct antisaccade but rather the failure to properly prepare (i.e., form the appropriate task set) for the antisaccade trial. The data support the view that the executive impairments, including inhibitory control, in ADHD adults are related to poor response preparation.
Keywords: ADHD, Saccade, fMRI, Preparation, Inhibition
Highlights
► The neural correlates of inhibitory control in adults with ADHD were examined. ► We used an interleaved pro and antisaccade task simultaneously with functional MRI. ► This enabled the dissociation of automatic versus voluntary control. ► Patients had less activity in fronto-parietal areas during antisaccade preparation. ► Overall, antisaccade deficits in ADHD adults likely arise from poor preparation.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders of childhood. Approximately 50–60% of youths diagnosed with ADHD have symptoms that persist into adulthood (Barkley et al., 2002), and recent epidemiological data indicate that ADHD affects approximately 2–4% of adults (Kessler et al., 2006). ADHD is characterized by three core symptoms — inattention, hyperactivity, and impulsivity. These define three subtypes of ADHD: 1) inattentive; 2) hyperactive/impulsive; and 3) combined (symptoms of both inattention and hyperactivity/impulsivity), with the combined subtype being the most common (Wolraich et al., 1996; Hurtig et al., 2007).
Adults with ADHD often have difficulty performing a wide range of tasks that comprise a series of complex behaviors classified as ‘executive functions’ (Gallagher and Blader, 2001; Boonstra et al., 2005; Seidman, 2006). Executive functions include filtering out distracting stimuli, inhibiting automatic responses, working memory, and planning to carry out goal-directed behavior. Response inhibition deficits are particularly widespread in ADHD. Many behavioral and brain imaging studies have assessed inhibitory control in children with ADHD (see Doyle, 2006; Quay, 1997; Willcutt et al., 2005; for review); however, very few studies have been conducted examining the impact of inhibitory control deficits in adults with ADHD (see Ossmann and Mulligan, 2003; Schneider et al., 2006; Seidman, 2006).
Here, we used interleaved pro and antisaccade tasks (described below), combined with simultaneous eye-tracking and blood oxygen-level dependent (BOLD) functional magnetic resonance imaging (fMRI), to investigate specific inhibitory control deficits in adults with ADHD and their underlying neural correlates. The antisaccade task is a simple, yet elegant tool to measure inhibitory control because it requires participants to first inhibit an automatic, visually-guided eye movement toward a suddenly appearing target (a prosaccade), and instead produce a voluntary saccade in the opposite direction of the target (an antisaccade) (Hallet, 1978; Munoz and Everling, 2004). Therefore, to properly perform an antisaccade, participants are required not only to execute a saccade in the correct direction, but they must also sufficiently prepare themselves to inhibit the unwanted prosaccade during the instruction period (i.e., establish a task set), prior to target appearance. The antisaccade task therefore enables investigation of processes relating to response preparation and execution, and how they specifically contribute to deficits in inhibitory control.
The cortical and subcortical regions that are involved in the suppression and/or generation of saccadic eye movements include regions in the parietal and frontal cortices, basal ganglia, and superior colliculus (SC) based on research using monkey neurophysiology (Wurtz and Goldberg, 1989; Munoz and Everling, 2004; Leigh and Zee, 2006; Watanabe and Munoz, 2011a). Briefly, when a target appears in the visual field, many saccade-related neurons in both the frontal eye fields (FEF) and SC (on the contralateral side of the brain relative to target location) discharge a burst of action potentials (Mohler and Wurtz, 1976; Bruce and Goldberg, 1985). These same neurons then discharge a motor burst to drive the eyes to the visual target. During antisaccade trials, the excitability of these neurons must be suppressed, prior to target onset, so that the visual response does not trigger a saccade. Instead, saccade neurons in the FEF and SC on the other side of the brain, ipsilateral to the target, need to be activated to drive the voluntary antisaccade (Everling et al., 1999; Everling and Munoz, 2000). Accumulator models can be used to explain the neural contributions of the oculomotor network during antisaccade trials (Munoz and Everling, 2004; Munoz et al., 2007). During antisaccade trials (Fig. 1A), two processes race toward a threshold, and a saccade is triggered once neuronal activity surpasses this threshold. The first process is set into action by the appearance of the target, initiating a rise in activation (contralateral to the target) that is associated with a movement toward that target (i.e., an automatic prosaccade), while the other process is initiated (on the ipsilateral side of the brain) by the inversion of the target vector to initiate a voluntary antisaccade. To perform an antisaccade correctly, processes related to the initiation of the automatic prosaccade (Fig. 1A; dashed gray line) must be inhibited to allow time for the voluntary antisaccade response (Fig. 1A; solid red line) to accumulate toward the threshold. Therefore, saccade-related neurons that specifically code automatic prosaccades must be inhibited prior to target onset to prevent the initiation of direction errors. Mechanisms for this pre-target inhibition are present in several structures, including FEF (Everling and Munoz, 2000), supplementary eye fields (SEF; Amador et al., 2004; Schlag-Rey et al., 1997), dorsolateral prefrontal cortex (DLPFC; Johnston and Everling, 2006), and caudate nucleus (CN; Watanabe and Munoz, 2010a, 2011a) of the basal ganglia. The parietal eye fields (PEF) and anterior cingulate cortex (ACC) are also involved in antisaccade production, particularly in the transformation of sensory signals to motor signals and error detection and/or monitoring, respectively (Gottlieb and Goldberg, 1999; Zhang and Barash, 2000; Medendorp et al., 2005; Johnston et al., 2007; Nyffeler et al., 2007).
Fig. 1.
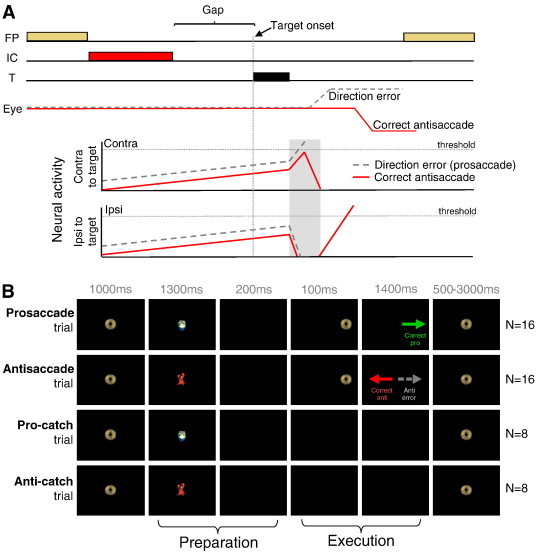
(A) Accumulator model of antisaccade task performance. When neural activity crosses the saccade threshold, a saccade is triggered. Neural activity of saccade-related neurons in superior colliculus on correct antisaccade trials (solid red line) and antisaccade direction error trials (dashed gray line). On correct trials, activity is reduced prior to target onset to prevent target-evoked activity from crossing the threshold. During error trials, this reduction is absent, resulting in target-evoked activity to cross the threshold. FP = fixation point. IC = instruction cue. T = target. (B) Illustration of stimuli and timing of events for the 4 trial types.
Studies examining antisaccade control in humans have reported differential activation of key oculomotor areas between antisaccade and prosaccade generation (Sweeney et al., 1996; Matsuda et al., 2004; Ettinger et al., 2008). Furthermore, recent event-related fMRI experiments have demonstrated increased BOLD activity during the preparatory, pre-target phase of the antisaccade task, as compared to the prosaccade task, in FEF, SEF, DLPFC, and ACC (Connolly et al., 2002, 2005; Ford et al., 2005; Brown et al., 2007), suggesting that components of the oculomotor network are recruited differently when preparing for either an antisaccade or prosaccade, with more preparatory activity requirements during antisaccade trials.
Adults with ADHD have difficulties exerting voluntary control over saccade generation: they not only have slower and more variable saccadic reaction times (SRTs), but also make more direction errors during antisaccade trials (i.e., a prosaccade was erroneously executed toward the target), more anticipatory eye movements (reaction times < 90 ms), and more express saccades (reaction times between 90 and 140 ms) (Munoz et al., 2003; Feifel et al., 2004; Carr et al., 2006). Yet saccade metrics (i.e., duration, peak velocity, and amplitude) in this group are typically normal to near-normal (Munoz et al., 2003; Feifel et al., 2004), suggesting that saccade deficits in ADHD participants stem from an inability to establish the appropriate task set rather than the execution of the saccadic motor response itself. This hypothesis has never been explicitly investigated and is the aim of the current study. We predict that antisaccade deficits produced by ADHD adults may arise from reduced activity of fronto-striatal areas that are responsible for suppressing saccade-related neurons prior to target onset.
2. Methods
All experiments were approved by the Research Ethics Board of Queen's University and were conducted in accordance with the principles of the Canadian Tri-council Policy Statement on Ethical Conduct for Research Involving Humans.
2.1. Participants
A total of 49 adult participants (28 ADHD and 21 controls) with normal or corrected-to-normal vision were recruited for this study via newspaper advertisements and posters displayed in doctor's offices. Twenty participants were subsequently excluded for several reasons. Twelve participants (8 ADHD and 4 control) were excluded because of excessive movement in the MRI scanner (> 3 mm movement in any of the 3 translational dimensions and/or > 3 deg movement in any of the 3 rotational dimensions), 6 participants (4 ADHD and 2 control) because the eye tracking was unable to reliably detect the pupil, one control participant because of an incidental finding, one ADHD participant because she took stimulant medication within 20 h of the MRI appointment, and one ADHD participant because of dental artifacts. Approximately 1.5 times more adults with ADHD were excluded due to motion artifacts compared to controls (30% of adults with ADHD and 19% of adult controls), which was expected, particularly when ADHD participants were off medication during the scanning session. The final group of participants included 14 adults diagnosed with the combined subtype of ADHD (9 males, mean age 29.5 ± 9.4 years) and 14 age- and gender-matched controls (mean age 29.6 years ± 9.6 years). Participants with ADHD provided documentation of a recent diagnosis (within 5 years) meeting DSM-IV criteria for ADHD, combined subtype. The documentation was provided by a licensed professional (either a psychologist or psychiatrist). Relying on ADHD diagnoses from community practitioners only is not ideal; however, we are confident that all participants in our ADHD group have been given an accurate diagnosis, given that the behavioral deficits exhibited by this group are in-line with those observed in other studies (Munoz et al., 2003; Feifel et al., 2004; Carr et al., 2006). Upon screening for neurological, developmental and current or past psychiatric disorders, two ADHD participants, who were included in the study, reported a current diagnosis of depression. ADHD participants were asked to abstain from taking stimulant medication for at least 20 h before the MRI session. Table 1 outlines the pharmacological treatments for each participant. Control subjects were screened (via telephone interview and a follow-up questionnaire) for absence of neurological, developmental, and previously diagnosed psychiatric disorders, and no family history of ADHD. All participants gave their written and informed consent and received free parking and $20/h as compensation for their participation.
Table 1.
Clinical information for ADHD participants.
| Patients | Sex | Age (years) | Medication |
|---|---|---|---|
| 1 | M | 39 | |
| 2a | F | 23 | |
| 3 | M | 38 | |
| 4a | F | 19 | Strattera, Doxepin |
| 5 | M | 27 | |
| 6 | M | 19 | Ritalin |
| 7 | M | 20 | Ritalin |
| 8 | M | 27 | |
| 9 | F | 19 | Concerta, Ritalin |
| 10 | M | 28 | Ritalin |
| 11 | M | 20 | |
| 12 | F | 32 | |
| 13 | M | 24 | Strattera |
| 14 | F | 32 | Ritalin |
M = male; F = female.
Patients with an additional diagnosis of depression.
2.2. Paradigm
An interleaved, rapid event-related design was employed for two reasons. First, trials were randomly interleaved to increase the difficulty of the task, eliciting higher antisaccade error rates. Secondly, trials were presented at a rapid rate to enable the presentation of different trial types in a reasonable time period. Two-thirds of all trials consisted of full pro or antisaccade trials aimed at examining both the preparatory and execution components of saccades (Fig. 1B, top 2 rows), and one-third of all trials consisted of ‘preparation’ only trials (i.e., catch trials) that exclusively measured preparatory actions (Fig. 1B, bottom 2 rows). Participants were asked to fixate on a central fixation stimulus (a ‘gold coin’) that appeared for 1000 ms at the center of the screen to start each trial. The symbol used as a fixation stimulus, and its color, was then changed to indicate either the instruction to make a pro or an antisaccade. The symbols used were colored cartoon images: a green turtle indicating that a prosaccade was required or a red crab indicating that an antisaccade was required. Colored cartoon symbols were chosen because the experiment was conducted across various patient groups that included child-aged participants, and this made the task easier for children to learn.
Following presentation of the instructional cue, which remained present for 1300 ms, a 200 ms gap period occurred in which the participant was presented with a black screen. The gap period was introduced to enable participants to generate more ‘automatic’ saccades, inducing shorter SRTs and more antisaccade direction errors and express prosaccades (Munoz and Corneil, 1995; Fischer and Weber, 1997; Munoz et al., 1998). On saccade trials, a peripheral target (gold coin) was then flashed for 100 ms to the left or right of central fixation, at eccentricities of either 6° or 7° in separate trials. Participants were instructed to execute a prosaccade (look toward the target location) or antisaccade (look away from the target in the opposite direction) based on the colored instruction cue. The central fixation stimulus (gold coin) reappeared 1400 ms later, and participants were required to re-establish central fixation to initiate the next trial. On catch trials, the instruction cue was presented and disappeared to initiate the gap period, but the peripheral target did not appear to signal a saccade; subjects were instead required to maintain central fixation for 1700 ms without generating a saccade response. Since participants did not know whether or not the peripheral target would appear, the instruction cue would always elicit preparation for a pro or antisaccade. Full saccade and catch trials were therefore both 4500 ms in length. The inter-trial interval was jittered, using fixation periods that spanned 1 repetition time (TR) (1.5 s; 8 times), 2 TR (3.0 s; 4 times) and 3 TR (4.5 s; 4 times) to increase the statistical efficiency and power in the rapid event-related design (Dale, 1999). Prior to commencing the task, participants were instructed to make a correction saccade if they generated errors.
Runs consisted of 48 trials that included 8 pro-catch trials, 8 anti-catch trials, 16 prosaccade trials, and 16 antisaccade trials (Fig. 1B). Each participant performed 5–9 runs (depending on eye tracking success), with each run lasting 277.5 s. Each run started with an additional fixation period of 3 s, while MR images were acquired, to allow the MR signal to reach a steady-state, and ended with a 16.5 s fixation period to allow the hemodynamic response to return toward baseline. Trial types were pseudorandomly interleaved, and pro and antisaccade trials were balanced for right and left presentation in each run. Both groups did not differ in terms of the number of completed runs: the mean number of runs administered to the control and ADHD groups was 6.5 (± 1.4) and 6.1 ± (1.4), respectively; groups did not differ in terms of the number of runs they completed (one-way ANOVA: F(1, 26) = 0.66, p = .42).
2.3. Visual display and eye tracking
Visual stimuli were generated and controlled using E-PRIME software (Psychology Software Tools Inc., Pittsburgh, PA, USA) on a personal computer. Images were back-projected onto a high-contrast rear projection screen (DA-LITE), positioned at the head end of the MRI system, using a NEC LT265 DLP video projector (Tokyo, Japan) with a refresh rate of 60 Hz and resolution of 1024 × 768. Participants viewed the screen via a mirror attached to the head coil (described below). Using DQW software v1.10X, the right eye was tracked using an ISCAN ETL-400 camera (Burlington, MA, USA) that sampled eye position at 120 Hz. To ensure synchronization, the MRI sequences directly triggered the E-PRIME software using a trigger signal from the scanner. An infrared fiber-optic illuminator, which was fixed to the head coil, was used to illuminate the right eye for tracking. After the anatomical scan, the eye tracker was calibrated using a nine-point array that covered most of the visual field. Analysis of the eye movement data was performed off-line using custom-made MATLAB programs.
2.4. Imaging protocol
All imaging data were acquired using a Siemens 3 T Magnetom Trio system (Erlangen, Germany) fitted with a 12-channel receive-only head coil located at the Queen's University MRI facility. High-resolution T1-weighted whole-brain structural scans were performed on each participant using a MPRAGE sequence (TR = 1760 ms, TE = 2.2 ms, flip angle = 9°, 256 × 256 mm field-of view, and 256 × 256 matrix size providing 1 mm isotropic voxels). Functional data were collected using a T2*-weighted EPI acquisition (TR = 1500 ms, TE = 30 ms, flip angle = 72°, 211 × 211 mm field-of-view, 64 × 64 matrix size, 3.3 mm isotropic voxel resolution, 185 volumes) for blood oxygenation-level dependent (BOLD)-based imaging (Ogawa et al., 1990). Twenty-four slices were acquired, positioned to include all regions of interest (ROI: FEF, SEF, PEF, DLPFC, ACC, and CN) extending from the top of the brain to the ventral striatum.
2.5. MRI pre-processing
All functional imaging runs were preprocessed using Brain Voyager 1.9 (Maastricht, the Netherlands). The first two volumes of each functional time series were removed before any pre-preprocessing to allow the MR signal to reach a steady state. To correct for between-scan movements, all volumes were realigned to the first volume of each functional run. Slice scan time correction was conducted to adjust for time differences due to multi-slice imaging acquisition using cubic spline interpolation based on the TR and order of slice scanning (ascending interleaved). 3D spatial smoothing was then performed using a 4 mm full-width at half-maximum Gaussian filter on the volumes, and each run was filtered to remove linear drift using a high-pass filter with the upper cut-off frequency corresponding to 3 cycles over the run's length. Finally, all functional data were superimposed onto 3D anatomical images, resampled into 3 × 3 × 3 mm cubic voxels, aligned to the anterior commissure–posterior commissure axis, and transformed into Talairach space (Talairach and Tournoux, 1988).
To ensure that there were no significant between-group movement differences, which may have led to apparent BOLD contrast differences, we compared the groups' average displacement from the mean head position in six dimensions (translation in x, y, and z, and rotation around the x, y, and z axes) for the 14 participants selected per group. The total absolute movement of the ADHD group was 0.104 mm, 0.096 mm, and 0.175 mm for translation in the x, y, and z axes, respectively and 0.119 deg, 0.050 deg, and 0.082 deg for rotation in the x, y, and z axes, respectively. For the control group, it was 0.074 mm, 0.088 mm, and 0.135 mm for translation in the x, y, and z axes, respectively and 0.097 deg, 0.039 deg, and 0.065 deg for rotation in the x, y, and z axes, respectively. Adults with ADHD did not significantly differ from control participants on any translational or rotational measure (t-test, p > .05 for all measures).
2.6. Statistics and data analyses
2.6.1. Behavioral analyses
Behavioral data were analyzed using custom-written scripts in MATLAB 7.4 (The MathWorks Inc., Natick, MA, USA). Saccadic reaction time (SRT) was measured as the first saccade away from fixation after stimulus onset, when the velocity exceeded the mean + 3 × the SD of the background velocity. The velocity had to remain above this threshold for 5 sample points for it to be classified as a saccade. Saccades with SRT < 110 ms were considered anticipatory and thus were excluded from analysis. This value was selected because it was the point at which errors in prosaccade trials were no longer executed at chance (50% correct: incorrect). Therefore, 110 ms was the earliest time at which detection of the visual target could influence behavior. Express saccades, which are the shortest visually-triggered saccades, have typically been calculated as saccades with SRTs between 90 and 135 ms (Fischer et al., 1993; Munoz et al., 1998); however, the boundaries of this epoch change according to the participant age and stimulus conditions (Bell et al., 2006; Peltsch et al., 2011). In the current study, the express saccade epoch was measured between 110 and 140 ms, where 140 ms was the latency at which both groups made more correct responses than errors during antisaccade trials (data not shown). Prosaccade direction errors corresponded to saccades executed away from the target in prosaccade trials; antisaccade direction errors were prosaccades executed toward the target location during antisaccade trials. ADHD participants made 12.03% prosaccade errors and 23.08% antisaccade errors, while control participants made 5.21% prosaccade errors and 6.25% antisaccade errors. To ensure that direction errors were attributable to insufficient inhibitory control, rather than to inattention, distraction, or guessing of the cue location, we only examined errors that were corrected by the participants. ADHD participants corrected 69.8% of prosaccade and 75.8% of antisaccade errors, while control participants corrected 80.3% of prosaccade and 90.6% of antisaccade errors; group differences in prosaccade (F(1, 26) = 0.75, p = .39, d = − 0.32) and antisaccade (F(1, 26) = 2.37, p = .14, d = − 0.56) error correction were not significant. Direction error rate was calculated by dividing the total number of errors by the total number of valid trials (error + correct). Intra-subject variability for SRT was calculated using the coefficient of variation (CV) for correct trials (SD / mean × 100).
Valid trials consisted of all trials except for those that included: 1) failure to fixate during fixation trials; 2) failure to fixate during the instruction period of a full pro or antisaccade trial; 3) failure to execute a saccade during the response period; 4) execution of multiple saccades during the response period; 5) saccades executed during catch trials; 6) antisaccades executed during prosaccade trials; 7) failure to correct an antisaccade direction error; and 8) trials in which eye-tracking was unsuccessful. These aforementioned excluded trials were modeled separately as ‘invalid trials’ in the functional MRI analysis described below. Importantly the proportion of valid trails that were analyzed was similar between the two groups. The range of valid trials for ADHD participants was 22–63 for anti-catch trials, 18–64 for pro-catch trials, 33–108 for correct antisaccade trials, 21–128 for correct prosaccade trials, and 5–47 for antisaccade error trials. For control participants, valid trials ranged from 22 to 64 for anti-catch trials, 31 to 64 for pro-catch trials, 38 to 122 for correct antisaccade trials, 56 to 125 for correct prosaccade trials, and 5 to 18 for antisaccade error trials.
Independent-measures ANOVAs were conducted to examine ‘preparatory’ differences in behavior between the control and ADHD groups for mean SRTs, SRT coefficient of variation, mean percentage of express saccades, and mean percentage of direction errors in anti and prosaccade trials. Group differences on saccade measures were not observed for leftward versus rightward saccades and 6° versus 7° eccentricities (p > .05); therefore, these responses were pooled together. Furthermore, one-way ANOVAs were also used to measure between-group differences of saccade metrics, including pro and antisaccade duration, amplitude, and velocity. Paired t-tests (non-directional) were conducted to compare behavior within each experimental group. The effect sizes for all comparisons were measured using Cohen's d.
2.6.2. fMRI analyses
In the rapid event-related design we employed, events occurred in rapid succession so that the BOLD signal recorded at any given point in time was the sum of the BOLD response from several preceding task events. Consequently, the measured BOLD time-series for each voxel was deconvolved with the canonical hemodynamic response function (HRF) in order to estimate the underlying time-course of neural activity. The HRF was modeled with a 13-point time-series with a temporal resolution of 1.54 s. The result of this process demonstrates the responses to each individual trial, without overlap in time. Events were modeled separately in the design matrix and pertained to trial type, including: 1) correct anti-catch trials; 2) correct pro-catch trials; 3) correct antisaccade trials; 4) correct prosaccade trials; 5) corrected antisaccade direction errors; and 6) invalid trials. Fixation trials were used as an implicit baseline.
Several statistical parametric maps were computed for each group, with the statistics reflecting the significance of the consistent response of each voxel to each trial type, as defined above. First, to ensure that the saccade task recruited all of our ROIs, we looked at full antisaccade trials and full prosaccade trials over time points 5–7 (7.7, 9.3, and 10.8 s from trial onset, Fig. 3B) that spanned the time interval from the presentation of the instruction cue to the execution of the saccade. Further, we computed contrast maps looking at both antisaccade preparatory (anti-catch minus fixation) and prosaccade preparatory (pro-catch minus fixation) activations (Fig. 4A), taken from time points 5 and 6 after trial onset (Fig. 5B). This interval was chosen as it corresponded to the peak of the hemodynamic response curve. To examine brain areas related to the execution of a saccade, contrasts examining antisaccade execution (full antisaccade minus anti-catch) and prosaccade execution (full prosaccade minus pro-catch) (Fig. 4B) were computed for the 6th and 7th time points after trial onset (Fig. 6B). Time points for the execution contrast were shifted by 1.5 s to include the 6th and 7th time points because the onset of the peripheral target occurred 1.5 s (one time point) after the appearance of the instruction cue. Finally, we computed contrast maps examining correct antisaccade trials (antisaccade minus fixation) versus erroneous antisaccade trials (corrected antisaccade direction errors minus fixation), taken from the 5th to the 7th time point after trial onset (Fig. 7). We were unable to examine differences in preparation or execution during erroneous saccades because there were no saccades on catch trials; thus, we were unable to separate catch trials into those that led to correct versus erroneous antisaccades.
Fig. 3.
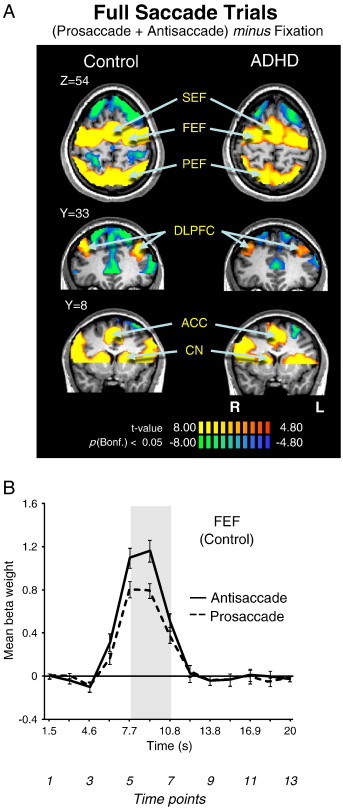
(A) Contrast map of correct prosaccade trials and antisaccade trials added together, cluster size corrected at p < .05 (9 contiguous voxels). Significant BOLD activations are observed in all ROIs (‘hot’ colors), which are labeled. Activations are overlaid on a representative control brain. Coordinate values of planes in Talairach space are given. (B) Representation of mean BOLD signal time course in FEF separately for pro and antisaccade trials in control participants. Shaded bar corresponds to a region of peak activation from trial onset. The three time points (5,6,7) under that shaded area were used to derive the voxels showing significantly greater activation on saccade trials in A. Error bars represent standard error of the mean (SE). R = right; L = left.
Fig. 4.
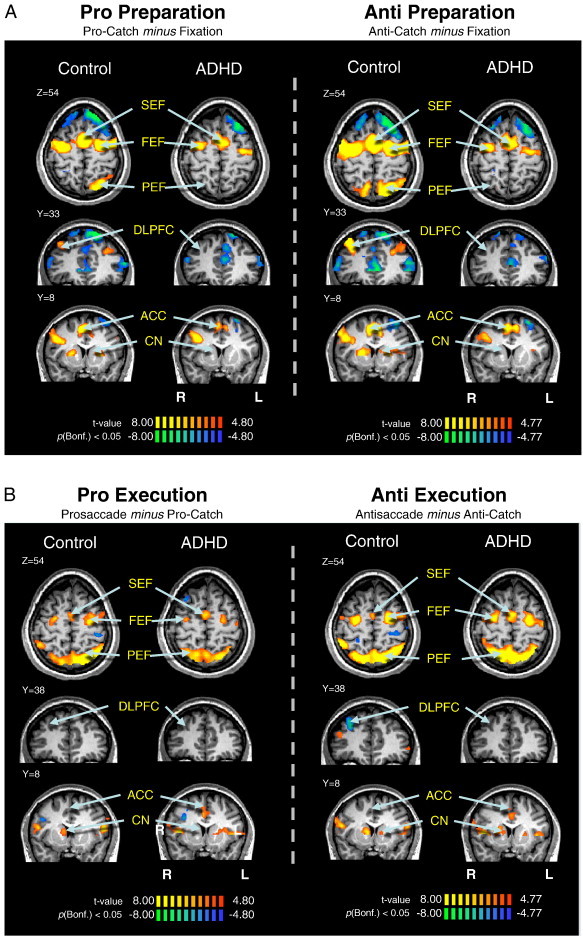
Saccade preparation (A) and saccade execution (B) contrast maps. (A) Contrast map of pro-catch trials or anti-catch trials subtracted from fixation trials, cluster size corrected at p < .05 (9 contiguous voxels). The 5th and 6th time points, relative to trial onset, were used in subtraction. Locations of oculomotor ROIs are identified. (B) Contrast map of full pro or antisaccade trials subtracted from pro-catch and anti-catch trials, respectively. The 6th and 7th time points were used in subtractions. Activations are overlaid on a representative control brain. R = right; L = left.
Fig. 5.
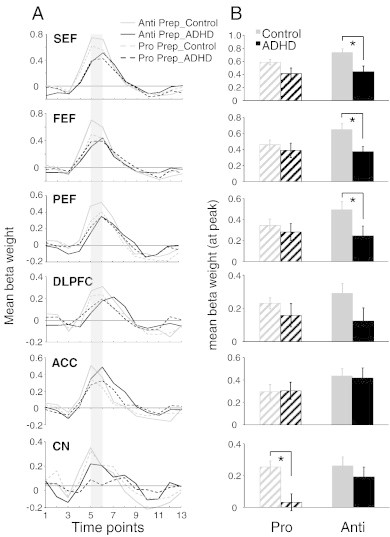
Region of interest (ROI) analysis for pro and antisaccade preparation. (A) Representation of mean BOLD signal time course for pro and anti prep for all ROIs derived from Fig. 4A. (B) Mean beta weight values for the 5th and 6th time points from the BOLD signal time course depicted in panel A (represented by the shaded bar in A). Left and right hemispheres were averaged. In ROIs where significant activation was not observed in one group, thresholds were lowered until beta weights could be obtained. Error bars represent standard error of the mean (SE). *p < .05.
Fig. 6.
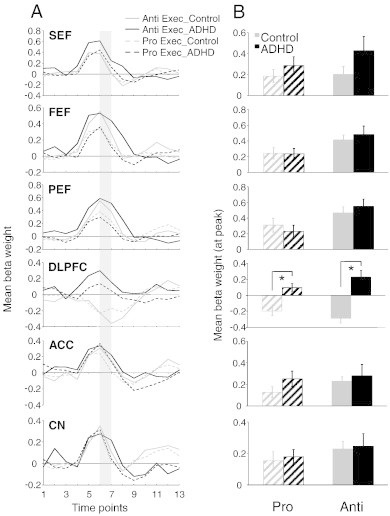
Region of interest (ROI) analysis for pro and antisaccade execution. (A) Representation of mean BOLD signal time course for prosaccade and antisaccade execution for all ROIs derived from Fig. 4B. (B) Mean beta weight values for the 6th and 7th time points from the BOLD signal time course in panel A (corresponding to shaded bar in A). Left and right hemispheres were averaged. In ROIs where significant activation was not observed in one group, thresholds were lowered until beta weights could be obtained. Error bars represent standard error of the mean (SE). *p < .05.
Fig. 7.
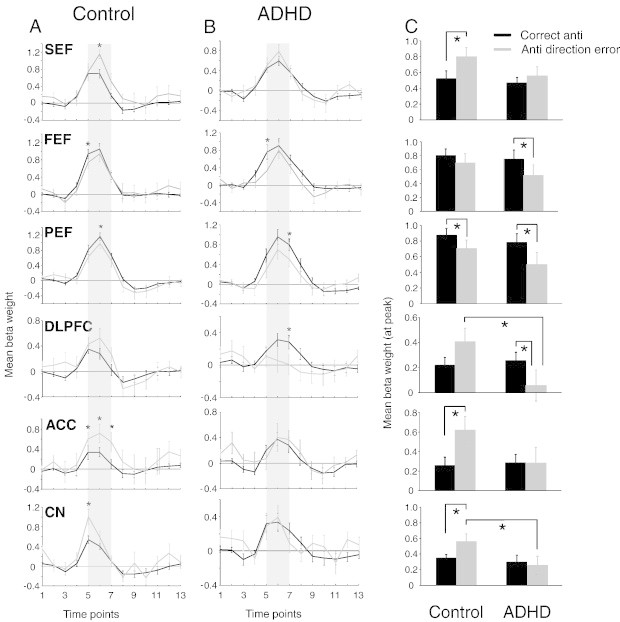
Region of interest (ROI) analysis for correct and erroneous antisaccade trials. (A–B) Representation of mean BOLD signal time course for correct and erroneous antisaccade trials for all ROIs in control (A) and ADHD (B) participants. (C) Mean beta weight values for the 5th, 6th and 7th time points (represented by the shaded bar in A and B) from fixed effects analysis of correct and erroneous antisaccade trials for 343 cubic voxels surrounding peak activations in regions showing greater activation on correct antisaccade trials compared to erroneous antisaccade trials. Left and right hemispheres were averaged. Error bars represent standard error of the mean (SE). *p < .05.
We used a mixed-effects analysis based on studies conducted by Brown et al. (2007). More precisely, group analysis was conducted using a fixed-effects general linear model (GLM) with separate subject predictors. This method was chosen (rather than constructing a single map for all participants using a random-effects analysis) as it ensures that every ROI was activated by both groups. Each statistical parametric map was Bonferroni corrected for multiple comparisons at p < .05 and cluster-size corrected at p < .05 (using a cluster threshold of nine contiguous voxels). Beta weight values (estimates of the BOLD signal change) were extracted from each individual after correcting for serial correlations, and then averaged across subjects in each ROI, defined as all the voxels within a 7 × 7 × 7 cubic cluster centered on the peak of activation, corrected for multiple comparisons. Beta weight values for bilateral structures (i.e., FEF, PEF, DLPFC, and CN) were extracted for both left and right locations and then averaged. ROIs were identified based on previous studies examining the neural correlates of saccades (Ford et al., 2005; Brown et al., 2007; Alvarez et al., 2010). One-way ANOVAs were then conducted between groups examining differences in mean beta weight values for all ROIs at each contrast described above.
3. Results
3.1. Behavior
Sample eye position traces recorded from a control participant, illustrating two correct antisaccades and one direction error that is corrected, are shown in Fig. 2A. Consistent with previous studies (Munoz et al., 2003; Feifel et al., 2004; Carr et al., 2006), adults with ADHD were slower to initiate correct antisaccades compared to controls (F (1, 26) = 9.58, p < .005, d = 1.20; Fig. 2B), produced more variable SRTs for both prosaccades (F (1, 26) = 7.39, p < .05, d = 1.05; Fig. 2C) and antisaccades (F (1, 26) = 4.97, p < .05, d = 0.84; Fig. 2C), and executed a higher percentage of direction errors on antisaccade trials than controls (F (1, 26) = 9.22, p < .005, d = 1.18; Fig. 2D). ADHD participants also made more direction errors than control participants on prosaccade trials, although the difference did not reach significance (F (1, 26) = 3.36, p = .08, d = 0.67). ADHD participants tended to make more express saccades during prosaccade trials than controls, although the difference did not reach significance (F (1, 26) = 2.66, p = .115, d = 0.59; Fig. 2E). Both groups did not differ in terms of correct prosaccade SRT (F (1, 26) = 0.31, p = .58, d = 0.22; Fig. 2B). Furthermore, to ensure that there were not any fatigue or time-on-task effects on task performance, we correlated the number of runs with several behavioral measures of saccade performance. There were no significant correlations between the number of runs and anti SRT (r(26) = − .21, p = .28), antisaccade direction errors (r(26) = .01, p = .95), pro SRT (r(26) = − .14, p = .49), prosaccade direction errors (r(26) = − .09, p = .64), or express prosaccades (r(26) = − .08, p = .68). Finally, group differences were not observed for saccade metrics, including prosaccade duration (F (1, 26) = 1.79, p = .28, d = 0.62), velocity (F (1, 26) = 0.74, p = .40, d = − 0.44), and amplitude (F (1, 26) = 0.14 p = .71, d = 0.52) or antisaccade duration (F (1, 26) = 0.96, p = .34, d = 0.68), velocity (F (1, 26) = 0.30, p = .59, d = − 0.46), and amplitude (F (1, 26) = 0.13, p = .72, d = 0.59). Overall, adults with ADHD showed several impairments in saccade control — they made more direction errors during antisaccade trials, produced longer mean antisaccade SRTs, and had more variable SRTs. These findings suggest that the saccade deficits produced by adults with ADHD were not based on a general inability to execute a saccade (because saccade metrics were normal), but rather arose from an inability to ‘preset’ the oculomotor network optimally prior to target onset (Fig. 1A), which subsequently led to higher variability in SRT and more direction errors.
Fig. 2.
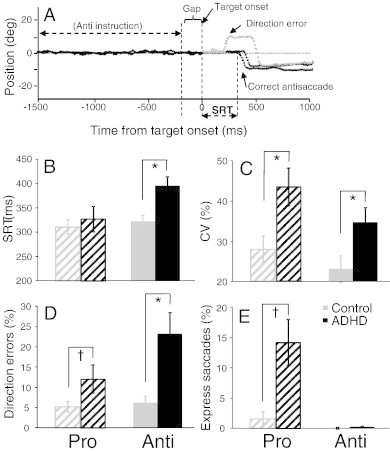
(A) Sample eye traces depicting correct antisaccade trials and an erroneous antisaccade trial (direction error) followed by a correction. (B) Mean saccadic reaction times (SRT) on correct trials. (C) Mean intra subject coefficient of variation (CV) for SRT. (D) Mean percentage of direction errors. (E) Mean percentage of express saccades (110–140 ms). Error bars represent standard error of the mean (SE); *p < .05, †p < .1.
3.2. fMRI
3.2.1. The saccade network
The behavioral deficits (Fig. 2B–E) may have occurred because ADHD participants did not recruit key areas of the oculomotor network optimally for saccade control, including SEF, FEF, PEF, DLPFC, ACC, and CN (Connolly et al., 2002, 2005; Ford et al., 2005; Brown et al., 2007). Fig. 3A depicts the most relevant slices for saccade (i.e., antisaccade plus prosaccade) generation, and Table 2 lists the Talairach locations of peak activation for all key regions of interest. We found that both groups recruited all pre-defined ROIs, suggesting that the observed behavioral deficits in the ADHD group likely were attributed to critical differences in sub-processes of pro and antisaccade control (i.e., saccade preparation or execution).
Table 2.
Talairach coordinates (X, Y, Z) of peak activation in GLM contrast maps for the antisaccade + prosaccade contrast (Fig. 3A).
| Group and region | Saccade: prosaccade plus antisaccade |
||||
|---|---|---|---|---|---|
| X | Y | Z | t | Vol | |
| ADHD | |||||
| SEF | − 3 | − 7 | 55 | 28.52 | 1460 |
| Right FEF | 27 | − 10 | 52 | 25.97 | 890 |
| Left FEF | − 27 | − 16 | 49 | 25.08 | 739 |
| Right PEF | 21 | − 64 | 49 | 29.75 | 1455 |
| Left PEF | − 24 | − 61 | 45 | 33.97 | 2177 |
| Right DLPFC | 30 | 40 | 34 | 13.40 | 241 |
| Left DLPFC | − 33 | 38 | 31 | 12.21 | 1459 |
| ACC | – | – | – | – | – |
| Right CN | 12 | 11 | 16 | 13.60 | 32 |
| Left CN | 10 | 5 | 14 | 10.41 | 19 |
| Control | |||||
| SEF | 0 | − 4 | 55 | 36.44 | 1575 |
| Right FEF | 24 | − 10 | 55 | 31.08 | 861 |
| Left FEF | − 27 | − 10 | 49 | 36.04 | 1296 |
| Right PEF | 15 | − 70 | 46 | 42.62 | 1758 |
| Left PEF | − 18 | − 67 | 46 | 49.44 | 2649 |
| Right DLPFC | 36 | 35 | 40 | 11.52 | 202 |
| Left DLPFC | − 36 | 32 | 34 | 11.45 | 184 |
| ACC | 1 | 18 | 30 | 14.52 | 394 |
| Right CN | 11 | 7 | 12 | 10.49 | 35 |
| Left CN | − 14 | 10 | 12 | 9.96 | 27 |
SEF, FEF, PEF, supplementary, frontal, parietal eye fields; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; CN, caudate nucleus. t = t-value; Vol = volume of cluster (voxel).
3.2.2. Preparatory findings
We hypothesized that increased direction errors during antisaccade trials in the ADHD group was attributed to insufficient preparation of the oculomotor network (i.e., inhibition of saccade-related neurons in FEF and SC before the peripheral target appeared; Everling et al., 1998; Everling and Munoz, 2000). For instance, the ADHD group could have made more direction errors in the antisaccade task because they were unable to prepare properly to suppress the automatic prosaccade during the pre-target period. Slices of the statistical map built from the preparation contrasts are displayed in Fig. 4A; ‘hot colored’ regions (i.e., orange/yellow) are those that produced a higher BOLD signal change for pro-catch or anti-catch trials, compared to fixation. Group comparisons of peak beta weight values at all regions of interest are shown in Fig. 5A–B and Talairach locations of the peak activation for each ROI are shown in Table 3. Critically, during the preparatory phase, ADHD adults exhibited less activation (hypoactivation) than controls in all ROIs analyzed. This was most pronounced during antisaccade preparation where significant group differences in SEF (F(1, 26) = 7.78, p < .01, d = − 1.11), FEF (F(1, 26) = 7.75, p < .01, d = − 1.01), and PEF (F(1, 26) = 4.30, p < .05, d = − 0.83) emerged, with the control group showing greater activation in these areas. Finally, compared to ADHD participants, controls displayed greater activation in CN during prosaccade preparation (F(1, 26) = 11.59, p < .01, d = − 1.39).
Table 3.
Talairach coordinates (X, Y, Z) of peak activation in GLM contrast maps for the prosaccade and antisaccade preparation contrasts (Fig. 4A).
| Group and region | Prosaccade preparation |
Antisaccade preparation |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | t | Vol | X | Y | Z | t | Vol | |
| ADHD | ||||||||||
| SEF | 6 | 2 | 52 | 11.49 | 330 | 6 | 2 | 52 | 13.65 | 464 |
| Right FEF | 27 | − 7 | 52 | 12.00 | 235 | 27 | − 7 | 52 | 12.44 | 386 |
| Left FEF | − 30 | − 16 | 49 | 11.44 | 149 | − 30 | − 16 | 49 | 12.17 | 187 |
| Right PEF | – | – | – | – | – | – | – | – | – | – |
| Left PEF | – | – | – | – | – | – | – | – | – | – |
| Right DLPFC | – | – | – | – | – | – | – | – | – | – |
| Left DLPFC | – | – | – | – | – | – | – | – | – | – |
| ACC | 3 | 14 | 34 | 6.55 | 14 | 6 | 14 | 40 | 9.01 | 12 |
| Right CN | – | – | – | – | – | – | – | – | – | – |
| Left CN | – | – | – | – | – | – | – | – | – | – |
| Control | ||||||||||
| SEF | − 3 | − 4 | 55 | 17.32 | 799 | − 3 | − 4 | 55 | 21.15 | 1115 |
| Right FEF | 36 | − 10 | 49 | 14.80 | 539 | 21 | − 7 | 55 | 18.61 | 767 |
| Left FEF | − 27 | − 10 | 49 | 15.61 | 580 | − 24 | − 10 | 49 | 19.73 | 637 |
| Right PEF | 27 | − 67 | 43 | 16.69 | 677 | 27 | − 67 | 43 | 19.33 | 980 |
| Left PEF | − 24 | − 64 | 46 | 21.10 | 823 | − 24 | − 64 | 46 | 24.70 | 1132 |
| Right DLPFC | 33 | 32 | 40 | 7.51 | 41 | 33 | 35 | 43 | 11.99 | 187 |
| Left DLPFC | − 27 | 38 | 28 | 5.61 | 50 | − 30 | 38 | 36 | 7.13 | 24 |
| ACC | − 3 | 16 | 41 | 10.94 | 215 | 4 | 14 | 41 | 12.22 | 193 |
| Right CN | – | – | – | – | – | 9 | 8 | 10 | 7.66 | 19 |
| Left CN | – | – | – | – | – | − 13 | 8 | 12 | 7.45 | 12 |
SEF, FEF, PEF, supplementary, frontal, parietal eye fields; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; CN, caudate nucleus. t = t-value; Vol = volume of cluster (voxel).
3.2.3. Execution findings
Although we hypothesized that antisaccade deficits in the ADHD group likely arise from poor preparation, it was necessary to also examine group differences in activity during saccade execution. Subsequent analyses explored activation patterns related only to the response components of pro or antisaccades. Slices of the statistical map built from the execution contrasts are depicted in Fig. 4B. ‘Hot colored’ areas are those that produced more BOLD activation during saccade execution than preparation. Group comparisons of peak beta weight values for each ROI are shown in Fig. 6A–B, and Talairach locations of the peak activation for each ROI are listed in Table 4. Overall, there were no differences in execution-related activation between ADHD and control participants. The only difference we observed was in the DLPFC, where ADHD participants exhibited significantly greater execution-related activation (hyperactivation) compared to controls for both prosaccades (F(1, 26) = 26.15, p < .001, d = 1.64) and antisaccades (F(1, 26) = 15.38, p < .001, d = 2.06). This enhanced DLPFC recruitment may serve as a compensatory mechanism to facilitate or enhance correct saccade execution, given the reduced activation during the preparatory phase.
Table 4.
Talairach coordinates (X, Y, Z) of peak activation in GLM contrast maps for the prosaccade and antisaccade execution contrasts (Fig. 4B).
| Group and region | Prosaccade execution |
Antisaccade execution |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | t | Vol | X | Y | Z | t | Vol | |
| ADHD | ||||||||||
| SEF | 3 | − 7 | 55 | 8.67 | 238 | − 3 | − 13 | 52 | 9.82 | 253 |
| Right FEF | 27 | − 13 | 52 | 7.67 | 40 | 27 | − 10 | 55 | 10.27 | 165 |
| Left FEF | − 24 | − 16 | 49 | 9.22 | 167 | − 24 | − 16 | 49 | 12.35 | 263 |
| Right PEF | 21 | − 67 | 49 | 8.85 | 117 | 21 | − 67 | 49 | 14.93 | 294 |
| Left PEF | − 18 | − 67 | 52 | 10.32 | 393 | − 21 | − 67 | 49 | 15.76 | 1189 |
| Right DLPFC | – | – | – | – | – | – | – | – | – | – |
| Left DLPFC | – | – | – | – | – | – | – | – | – | – |
| ACC | 3 | 8 | 34 | 6.99 | 57 | 0 | 14 | 31 | 7.39 | 85 |
| Right CN | – | – | – | – | – | 10 | 8 | 12 | 8.71 | 65 |
| Left CN | – | – | – | – | – | – | – | – | – | – |
| Control | ||||||||||
| SEF | 0 | − 10 | 55 | 7.80 | 61 | 0 | − 4 | 55 | 8.10 | 55 |
| Right FEF | 21 | − 10 | 55 | 8.45 | 79 | 24 | − 13 | 55 | 12.27 | 151 |
| Left FEF | − 30 | − 10 | 51 | 10.40 | 309 | − 24 | − 10 | 55 | 12.37 | 343 |
| Right PEF | 12 | − 73 | 46 | 12.49 | 309 | 15 | − 70 | 46 | 18.87 | 342 |
| Left PEF | − 9 | − 73 | 46 | 15.43 | 762 | − 18 | − 70 | 46 | 19.47 | 892 |
| Right DLPFC | – | – | – | – | – | 24 | 41 | 34 | − 9.10 | 86 |
| Left DLPFC | – | – | – | – | – | – | – | – | – | – |
| ACC | – | – | – | – | – | – | – | – | – | – |
| Right CN | 9 | 5 | 10 | 6.84 | 39 | 9 | 6 | 9 | 6.41 | 99 |
| Left CN | – | – | – | – | – | − 12 | − 4 | 19 | 7.23 | 67 |
SEF, FEF, PEF, supplementary, frontal, parietal eye fields; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; CN, caudate nucleus; negative t-value denotes catch > saccade. t = t-value; Vol = volume of cluster (voxel).
3.2.4. Correct versus incorrect antisaccade trials
Overall, the imaging data suggest that the biggest between-group differences at the contrast level for correct pro- and antisaccade processes occurred at the preparation level, particularly for the antisaccade task. Next, we investigated oculomotor function during trials in which an antisaccade direction error was produced. Based on accumulator models (Fig. 1A), we hypothesized that the increased antisaccade error rate produced by ADHD adults resulted from reduced activity of fronto-striatal areas that are responsible for suppressing saccade-related neurons, prior to target onset. Furthermore, inhibitory deficits in ADHD have been linked to deficient ACC activation (see Fassbender and Schweitzer, 2006 for review); therefore, we expected to see reduced ACC activity during error trials in this group. Group comparisons of peak beta weight values, for activation related to correct versus antisaccade direction error trials, are shown in Fig. 7, and Talairach locations of the peak activation for each ROI are shown in Table 5. Between-group differences were only observed in two ROIs: controls displayed higher activation in CN (F(1, 26) = 4.10, p < .05, d = − 0.81) and DLPFC (F(1, 26) = 4.57, p < .05, d = − 0.85) during antisaccade errors compared to ADHD participants. Furthermore, two-tailed paired-samples t-tests revealed that both control and ADHD participants showed greater activation in PEF (Control: t(13) = 2.30, p < .05, d = 0.51; ADHD: t(13) = 2.48, p < .05, d = 0.59) when performing correct antisaccades, while greater activity in FEF (t(13) = 2.94, p < .05) and DLPFC (t(13) = 2.18, p < .05, d = 0.46) during correct antisaccade trials was only observed in the ADHD group. Finally, control participants exhibited more activation in ACC (t(13) = − 2.77, p < .05, d = 0.88) and SEF (t(13) = − 2.13, p < .05, d = 0.75) during antisaccade error trials versus correct antisaccade trials. This enhanced activation on error trials was absent in ADHD subjects.
Table 5.
Talairach coordinates (X, Y, Z) of peak activation in GLM contrast maps for the correct and incorrect antisaccade generation contrasts.
| Group and region | Correct antisaccade |
Antisaccade direction error |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | t | Vol | X | Y | Z | t | Vol | |
| ADHD | ||||||||||
| SEF | − 3 | − 7 | 55 | 26.72 | 548 | 3 | 5 | 46 | 14.85 | 482 |
| Right FEF | 27 | − 10 | 55 | 26.84 | 1890 | 27 | − 10 | 52 | 14.88 | 2096 |
| Left FEF | − 27 | − 16 | 49 | 25.03 | 760 | − 30 | − 11 | 48 | 12.73 | 449 |
| Right PEF | 21 | − 64 | 49 | 30.81 | 1651 | 21 | − 64 | 49 | 15.01 | 747 |
| Left PEF | − 24 | − 61 | 43 | 31.71 | 2425 | − 24 | − 61 | 46 | 16.65 | 876 |
| Right DLPFC | 39 | 23 | 37 | 11.09 | 31 | 39 | 26 | 37 | 7.21 | 35 |
| Left DLPFC | − 33 | 38 | 31 | 12.61 | 175 | – | – | – | – | – |
| ACC | 2 | 15 | 41 | 12.01 | 267 | 1 | 7 | 43 | 9.31 | 275 |
| Right CN | 12 | 7 | 13 | 13.60 | 32 | 12 | 8 | 12 | 7.12 | 35 |
| Left CN | − 10 | 6 | 11 | 14.59 | 41 | − 11 | 6 | 10 | 7.39 | 48 |
| Control | ||||||||||
| SEF | 0 | − 4 | 55 | 34.16 | 1036 | − 3 | − 4 | 55 | 14.48 | 706 |
| Right FEF | 24 | 10 | 55 | 32.17 | 716 | 24 | − 10 | 48 | 10.00 | 84 |
| Left FEF | − 27 | − 10 | 49 | 35.27 | 1741 | − 21 | − 7 | 49 | 12.80 | 407 |
| Right PEF | 15 | − 70 | 46 | 45.18 | 1898 | 15 | − 70 | 46 | 13.50 | 596 |
| Left PEF | − 18 | − 67 | 46 | 50.14 | 2628 | − 18 | − 67 | 46 | 15.88 | 756 |
| Right DLPFC | 36 | 35 | 40 | 13.03 | 250 | 30 | 32 | 40 | 5.72 | 10 |
| Left DLPFC | − 39 | 32 | 34 | 10.45 | 161 | – | – | – | – | – |
| ACC | 2 | 16 | 27 | 13.54 | 271 | 4 | 19 | 25 | 6.14 | 23 |
| Right CN | 11 | 10 | 12 | 9.01 | 85 | 9 | 5 | 10 | 8.20 | 82 |
| Left CN | − 10 | 6 | 13 | 8.56 | 74 | − 12 | − 1 | 16 | 7.81 | 86 |
SEF, FEF, PEF, supplementary, frontal, parietal eye fields; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; CN, caudate nucleus. t = t-value; Vol = volume of cluster (voxel).
3.2.5. Correlations with antisaccade performance
To examine whether differences in the magnitude of antisaccade preparation activation across subjects correlated to antisaccade performance, post-hoc analyses were conducted to examine the relationship between behavior (anti SRT and percentage of direction errors) and activation in FEF, SEF, and PEF, areas that were hypoactive in ADHD adults during antisaccade preparation. Significant correlations were only observed when both groups' scores were combined. During antisaccade preparation, activity in FEF (r(26) = − .44, p < .05), SEF (r(26) = − .47, p < .05), and PEF (r(26) = − .38, p < .05) correlated negatively with antisaccade direction errors (Fig. 8A–C). Furthermore, anti SRT correlated negatively with activity in SEF (r(26) = − .44, p < .05) (Fig. 8D). Based on our findings that ADHD participants additionally recruited DLPFC during the antisaccade response epoch, we also correlated antisaccade execution activation with antisaccade direction errors and reaction times. In general, when both groups were combined, antisaccade response activation positively correlated with antisaccade direction errors (Fig. 8E; r(26) = .42, p < .05) and anti SRT (Fig. 8F; r(26) = .41, p < .05).
Fig. 8.
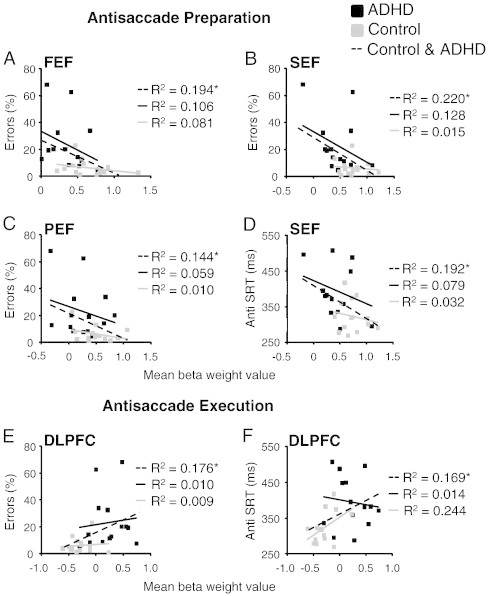
Correlations between antisaccade measures (direction errors and SRT) and BOLD activation during antisaccade preparation (A–D) and execution (E–F) in relevant ROIs. (A–D) Mean beta weight values were extracted from the 5th and 6th time points of the fixed effects analysis of anti preparation for 343 cubic voxels surrounding peak activations from ROIs in Fig. 5. (E–F) Mean beta weight values were taken from time points 6 and 7 from a fixed effects analysis of antisaccade execution for 343 cubic voxels surrounding peak activation from ROIs in Fig. 6. *p < .05.
4. Discussion
We demonstrated that inhibitory control deficits in adults with ADHD, as measured by the antisaccade task, likely arise from poor preparation rather than difficulties with saccadic execution. We used an interleaved pro and antisaccade task with simultaneous fMRI and eye-tracking to examine the neural substrates of inhibitory control in adults with ADHD. Behaviorally, ADHD adults showed impairments in inhibitory control — they had longer antisaccade SRTs, made more direction errors during antisaccade trials, and produced more variable SRTs for both pro and antisaccade trials (Fig. 2B–E), all of which are suggestive of poor preparation. Importantly, the ADHD adults did not differ from controls in terms of the execution of pro or antisaccades because group differences in saccade metrics were not significant. These results suggest that saccade impairments in the ADHD group likely arise from deficits in saccade preparation (i.e., an inability to establish the appropriate task set) rather than the execution of the saccadic motor response. The imaging data support this conclusion: adults with ADHD produced less activity in all ROIs (FEF, SEF, PEF, DLPFC, CN) when preparing a correct antisaccade. Importantly, this hypoactivation was not observed in ADHD adults during antisaccade execution. Instead, they produced greater activation than controls in DLPFC when executing correct antisaccades, perhaps as part of a compensatory mechanism to cope with the challenge produced in the antisaccade task with inadequate preparatory set. Our data suggest that the deficits observed in ADHD adults do not result from an inability to execute an antisaccade but rather stem from the failure to properly prepare for the antisaccade trial, which requires global top-down inhibition to suppress the automatic prosaccade (Munoz and Everling, 2004). Additionally, unlike control participants, adults with ADHD did not produced elevated ACC activation during antisaccade error trials, suggesting that they made more antisaccade errors because they were not efficiently recruiting the ACC, which is a key structure involved in processing and/or monitoring errors (Johnston et al., 2007). Taken together, our results suggest that the antisaccade deficits in ADHD adults stem from poor preparation for inhibiting unwanted responses and/or a reduced ability to adjust behavior based on previously made mistakes.
4.1. Behavioral deficits in ADHD
We first confirmed that adults with ADHD showed antisaccade impairments in the MRI environment. In particular, they had slower and more variable SRTs and made more direction errors, thereby replicating several behavioral studies measuring antisaccade performance in ADHD adults (Munoz et al., 2003; Feifel et al., 2004; Carr et al., 2006). Importantly, antisaccade deficits in adults with ADHD parallel those observed in other tasks measuring inhibitory control (Aron et al., 2003; Ossmann and Mulligan, 2003; Bekker et al., 2005; Lampe et al., 2007; Dibbets et al., 2009; McLoughlin et al., 2010; Schneider et al., 2010). For example, when required to inhibit an automatic response in a Go/NoGo task, ADHD adults not only produced more commission errors during trials in which no response was required, but also had longer and more variable reaction times during trials in which a response was required (Lampe et al., 2007; Dibbets et al., 2009). Given the extensive literature on inhibitory control deficits in ADHD, studies that link these deficits to brain function are important.
4.2. Hypoactivation of oculomotor network during preparation in ADHD
Both control and ADHD participants recruited a similar neural network during the saccade task (including FEF, SEF, PEF, ACC, DLPFC, and CN). Thus, the antisaccade impairments observed in ADHD adults are not due to an inability to recruit key frontostriatal areas of the oculomotor network, but instead an inability to employ these areas optimally for specific task processes (i.e., preparation versus execution of the saccade).
Successful antisaccade performance requires that recruitment of several cortical and sub-cortical brain regions, including DLPFC (Guitton et al., 1985; Pierrot-Deseilligny et al., 2003), FEF, PEF, and SEF (Connolly et al., 2002; Curtis and D'Esposito, 2003; DeSouza et al., 2003; Ford et al., 2005; Brown et al., 2007) and the basal ganglia (Ford and Everling, 2009; Watanabe and Munoz, 2009, 2010a, 2011a) be activated prior to the appearance of the peripheral target, which presets the motor system to execute the appropriate action (i.e., suppress prosaccade). Most importantly, we found that ADHD adults had less BOLD signal than controls in SEF, FEF, PEF, CN, and DLPFC during antisaccade preparation. Failure of the ADHD adults to activate these regions to the same degree as control subjects, particularly FEF, SEF and PEF where differences were statistically significant, likely indicates core deficits in the ability to preset frontoparietal circuits critical for optimal task performance.
Anatomical and functional imaging studies examining brain areas associated with inhibitory control in adults with ADHD are limited. There are reports of volume and cortical thickness reductions in several areas involved in inhibitory control, including frontal cortex and parietal lobes (Seidman et al., 2006; Makris et al., 2007). The hypoactivation of frontal and parietal areas in the ADHD group in the present study replicates and extends previous imaging studies that have found abnormal (usually less) activity in these brain regions in adults with ADHD when performing tasks that assess executive function (Hale et al., 2007; Cubillo et al., 2010; Karch et al., 2010; McLoughlin et al., 2010). Furthermore, the few studies that examined the neural correlates of inhibitory control in ADHD adults specifically have found hypoactivation in frontal and parietal areas (Cubillo et al., 2010; McLoughlin et al., 2010), yet hyperactivation in these brain regions has also been reported (Dibbets et al., 2009; Dillo et al., 2010). These conflicting results may be attributed to task difficulty, as studies that reported hyperactivation of frontal and parietal regions used a relatively easier task (Go/NoGo task) that involved selective attention, which is less impaired in ADHD patients than motor response inhibition (van Mourik et al., 2005). Although dysfunctional brain activation during preparation has been observed using event-related potentials (ERP; McLoughlin et al., 2010), we are the first to report, using fMRI, that inhibitory control deficits in adults with ADHD likely arise from hypoactivation of specific frontal and parietal structures during task preparation, namely, the FEF, SEF, and PEF. This result is not due to motion artifacts because: 1) both groups did not differ in terms of total motion during the MRI session; and 2) hypoactivity was not observed during the execution epoch.
The FEF and SEF are both involved in voluntary saccade generation. Humans with lesions to FEF have longer antisaccade reaction times (Gaymard et al., 1999), and human fMRI (Connolly et al., 2005) and monkey neurophysiology (Everling and Munoz, 2000) studies found that activity in FEF correlates with antisaccade reaction time. The SEF is involved in successful antisaccade performance; for example, Amador et al. (2004) reported that SEF neuronal activity predicted antisaccade success: monkeys generated greater activity in SEF during correct antisaccade trials compared to erroneous antisaccade trials. Interestingly, greater FEF and SEF BOLD signal associated with antisaccades, more so than prosaccades, is among the most consistent findings in the human functional neuroimaging literature on antisaccades (e.g., Curtis and D'Esposito, 2003; DeSouza et al., 2003; Ford et al., 2005; McDowell et al., 2005; Raemaekers et al., 2006; Ettinger et al., 2008). This increased activation was observed prior to saccade generation (Connolly et al., 2002; DeSouza et al., 2003; Ford et al., 2005; McDowell et al., 2005). It is important to note that human studies using fMRI report increased FEF activation during antisaccade preparation; conversely, saccade neurons in layer V of the FEF in monkeys are less activate during antisaccade preparation (Everling and Munoz, 2000). Recently however, Ford et al. (2009) showed that monkeys had increased BOLD-related activation for antisaccades, implying that the discrepancy with human FEF activation reports may be related to methodological differences between fMRI and extracellular single cell recording rather than differences between species.
Consistent with the accumulator model (Fig. 1A), the data indicate that the signal to generate a voluntary antisaccade (red solid line, Fig. 1A) races with the signal to generate the automatic prosaccade (gray dashed line, Fig. 1A), and that increased FEF and SEF BOLD signal prior to antisaccade execution offsets the tendency to produce an automatic prosaccade (i.e., direction error) toward the target. The increased occurrence of direction errors in ADHD adults likely results from compromised pre-target suppression signals in FEF and SEF, resulting in the unwanted automatic prosaccade command to reach threshold first. For correct antisaccade responses, the prosaccade signal must be sufficiently suppressed to allow the antisaccade signal time to reach threshold first. Overall, the reduced activity in FEF and SEF during antisaccade preparation may partially account for the longer anti SRTs and/or increased antisaccade error rate produced by ADHD adults, which is further supported by the correlation analyses (Fig. 8A–D).
Compared to controls, adults with ADHD produced hypoactivation in PEF during antisaccade preparation. PEF provides important visual input to the frontal cortical oculomotor areas (Ferraina et al., 2002), and also receives reciprocal inputs from these frontal areas (Lynch and Tian, 2006). Therefore, the reduced activity in PEF in the ADHD adults may have resulted from reduced activation of FEF and SEF. Furthermore, Matsuda et al. (2004) reported greater activity in inferior parietal cortex during antisaccade, compared to prosaccade trials, and a similar region showed activity during an inhibitory period preceding antisaccade generation (Ettinger et al., 2008), suggesting that the PEF may participate in presetting the oculomotor network for correct antisaccade generation. Evidence suggests that regions in the area of the intraparietal sulcus (within parietal cortex) may perform the vector inversion required to specify the correct antisaccade location (e.g., Zhang and Barash, 2000; Medendorp et al., 2005; Nyffeler et al., 2007). Therefore, a failure to activate the PEF optimally during antisaccade preparation may make it more difficult for ADHD adults to generate the voluntary saccade, resulting in more direction errors and/or increased SRTs during antisaccade trials.
Interestingly, control participants produced greater activation in CN during prosaccade preparation than ADHD participants. CN is involved in controlling prosaccade initiation and suppression (Ford and Everling, 2009; Watanabe and Munoz, 2010b, 2011b). For example, Watanabe and Munoz (2011b) reported that post-stimulus microstimulation of the monkey CN lengthened pro SRTs, while pre-target microstimulation shortened pro SRTs, possibly by altering excitability via different pathways through the basal ganglia. Therefore, insufficient recruitment of the CN during prosaccade preparation may have reduced the activation of these basal ganglia pathways, possibly explaining the increased variability of pro SRTs in the ADHD group. Furthermore, the CN is also involved in motivational aspects of prosaccade behavior (Watanabe and Hikosaka, 2005; Ding and Hikosaka, 2006; Hikosaka, 2007; Kobayashi et al., 2007). CN activity increased during prosaccade preparation when the expectancy of a reward was high (Watanabe and Hikosaka, 2005); moreover, injection of a dopamine antagonist into the CN greatly influenced the reward modulation of saccade behavior by reducing SRTs following a high-reward trial (Hikosaka, 2007). Several studies have linked the behavioral deficits of ADHD to a dopamine deficiency (see del Campo et al., 2011; Krause, 2008; Tripp and Wickens, 2009 for review). Therefore, the increased occurrence of prosaccade direction errors in the ADHD is perhaps related to poor motivation for the task, which may be explained by reduced activation in the CN during prosaccade preparation.
4.3. Adaptive mechanisms
Because ADHD adults exhibited normal saccade metrics, we did not expect to see group differences in activation during the saccade execution period of pro and antisaccade trials. Group differences during execution were only observed in DLPFC. When executing a correct pro or antisaccade, ADHD adults exhibited greater activation in DLPFC compared to controls. This may reflect some type of adaptive mechanism to deal with the antisaccade conflict, given the deficit during the preparatory epoch. The DLPFC may provide a supplementary, albeit less efficient (i.e., occurs post-target) signal to help reduce the production of the unwanted prosaccade during antisaccade trials.
Humans with lesions to the DLPFC produce more direction errors in the antisaccade task (Guitton et al., 1985; Pierrot-Deseilligny et al., 2003). Several functional neuroimaging studies have reported greater DLPFC activity during antisaccades compared to prosaccades (e.g., DeSouza et al., 2003; Matsuda et al., 2004; Ettinger et al., 2008) and this enhanced activation of DLPFC during antisaccade trials occurs during preparation, prior to antisaccade generation (DeSouza et al., 2003; Ford et al., 2005; McDowell et al., 2005; Brown et al., 2007). Together, these data suggest that the DLPFC is critically involved in suppressing reflexive saccades during the antisaccade task. However studies that used an interleaved, rather than a block, design did not see greater DLPFC in antisaccade trials compared to prosaccade trials (Raemaekers et al., 2002, 2006; O'Driscoll et al., 2005). McDowell et al. (2008) posit that in cases such as these, the DLPFC may instead produce tonic activity during the entire task due to the increased difficulty and more complex response selection requirements during the interleaved design. Based on this theory, we may not have seen differences in DLPFC activity between pro and antisaccades because we used an interleaved design.
The observation that adults with ADHD generated greater BOLD activity in DLPFC during saccade execution can be interpreted in two ways. First, ADHD participants recruited DLPFC for successful pro and antisaccade execution, perhaps as an ‘adaptive strategy’ to inhibit unwanted automatic saccades (i.e., direction errors during antisaccade trials or express saccades during prosaccade trials). In this case, their longer SRTs may reflect the additional processing time required to recruit the DLPFC. Our correlational analysis (Fig. 8F) supports this view as post-target activity in DLPFC correlated positively with anti SRT. Second, brain activation for pro and antisaccade execution was measured by subtracting activation occurring during the saccade preparation epoch from the saccade response epoch. Therefore, it is at least possible that control participants recruited the DLPFC more so during preparation, while ADHD participants recruited the DLPFC more so during execution. As such, saccade deficits in the ADHD group may result from a reduced suppression signal from the DLPFC during the preparation stage, leading to a reduced ability to inhibit prosaccades during antisaccade trials and more express saccades during prosaccade trials. This second suggestion was also supported by our data in that execution-related activity in DLPFC correlated positively with percentage of direction errors (Fig. 8E).
4.4. Saccade error monitoring
During error trials, participants generated two saccades (a direction error and a correction saccade), while during correct trials, only one saccade was generated. Therefore, differences in activation between correct and error trials may have arisen not only from the generation of the error itself, but also from differences in the motoric response (i.e., one versus two saccades). As such, we limit this discussion to findings in the ACC, an area that has been shown consistently to be involved in monitoring antisaccade performance (Polli et al., 2005) and signaling the likelihood and actual occurrence of antisaccade errors (Nieuwenhuis et al., 2001; Ford et al., 2005; Brown et al., 2006; Endrass et al., 2007). For example, Ford et al. (2005) found greater activation in ACC for correct antisaccades as opposed to incorrect antisaccades during antisaccade preparation; however, greater activation during incorrect antisaccade trials was observed during the execution period. These findings suggest that the ACC could act as some type of ‘learning tool’ or ‘performance evaluation’ of error responses that effect changes in future response selection based on feedback from previous responses. We found that controls had greater activation in ACC when generating incorrect antisaccades versus correct antisaccades, but this was not evident in ADHD adults (Fig. 7). This finding could not be attributed to group differences in the percentage of corrected antisaccade errors because this analysis only included trials in which antisaccade direction errors were corrected. Disturbed ACC functioning in adult ADHD is supported by a growing body of evidence. First, adults with ADHD have significantly smaller ACC volumes than normal adults (Seidman et al., 2006; Amico et al., 2011) and recently, decreased functional connectivity between the ACC and right inferior prefrontal cortex has been noted in ADHD (Cubillo et al., 2010). Second, studies using magnetic resonance spectroscopic imaging, reported abnormal metabolism in ACC in adults with ADHD (Colla et al., 2008; Kronenberg et al., 2008). Finally, reduced ACC activation during tasks that measure inhibitory control has been reported in adults with ADHD (Bush et al., 1999; Fallgatter et al., 2005; Cubillo et al., 2010; but see Dillo et al., 2010 for an alternative). Overall, the reduced activation in ACC during antisaccade errors in ADHD adults may indicate that they were not processing antisaccade errors, which thus led to increased direction errors.
One must be careful when interpreting these findings as activations relating to preparation versus execution during correct versus incorrect antisaccade trials were not separated. Therefore, to more aptly examine the neural correlates of antisaccade errors in adults with ADHD, future studies should use a design that can separate activity related to preparation versus execution of antisaccade errors, like that employed by Ford et al. (2005), who used an extended pre-target period that allowed the separation of pre- versus post-target activations during individual trials in which an antisaccade error was generated. However, such a design may not be feasible when studying ADHD because they have difficulties maintaining fixation for prolonged periods (Munoz et al., 2003).
4.5. Limitations of the study
It is important to address concerns that the behavioral and brain activation differences in the ADHD group may result from the use of stimulant medication or illicit drugs on the testing day. To ensure that this did not occur, we asked each participant of their drug use using several verbal interviews prior to and on the day of testing. Despite these precautions, future studies should administer toxicity-screening to all participants, ensuring that they have not taken any medication or illicit drugs that may affect eye movements or BOLD activity. Another limitation of this study concerns the low number of participants in each group (n = 14), which limited our analysis of the fMRI data to the use of a fixed-effects design rather than a random-effects design. Future studies will need to be conducted with more participants, allowing the findings to be generalized to a population.
4.6. Conclusions
We have shown that antisaccade deficits in ADHD persist into adulthood and that these impairments do not result from an inability to execute an antisaccade but rather stem from the failure to properly prepare for the antisaccade. This novel pathophysiological inference that inhibitory control deficits in adults ADHD may arise from an overall inability to ‘preset’ critical brain areas involved in inhibitory control needs to be examined further. Studies examining preparatory mechanisms in other tasks that assess inhibitory control or the effect of stimulant medications on preparatory actions would be beneficial.
5. Funding
This work was supported by an operating grant from the Canadian Institutes for Health Research (grant number MOP-97741) to DPM and PWS. RMHS is the recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship and NA is the recipient of a CIHR postdoctoral fellowship. DPM was supported by the Canada Research Chair Program.
Acknowledgments
We thank the participants for their time and the members of the Munoz lab for comments on an earlier version of the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Rebecca M. Hakvoort Schwerdtfeger, Email: rebecca.hakvoort@queensu.ca.
Nadia Alahyane, Email: nadia.alahyane@queensu.ca.
Donald C. Brien, Email: Donald@biomed.queensu.ca.
Brian C. Coe, Email: coe@queensu.ca.
Patrick W. Stroman, Email: stromanp@queensu.ca.
Douglas P. Munoz, Email: doug.munoz@queensu.ca.
References
- Alvarez T.L., Alkan Y., Gohel S., Ward B.D., Biswal B.B. Functional anatomy of predictive vergence and saccade eye movements in humans: a functional MRI investigation. Vision Research. 2010;50:2163–2175. doi: 10.1016/j.visres.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Amador N., Schlag-Rey M., Schlag J. Primate antisaccade II. Supplementary eye field neuronal activity predicts correct performance. Journal of Neurophysiology. 2004;91:1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- Amico F., Stauber J., Koutsouleris N., Frodl T. Anterior cingulate cortex gray matter abnormalities in adults with attention deficit hyperactivity disorder: a voxel-based morphometry study. Psychiatry Research: Neuroimaging. 2011;191:31–35. doi: 10.1016/j.pscychresns.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Dowson J.H., Sahakian B.J., Robbins T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Barkley R.A., Fischer M., Smallish L., Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology. 2002;2:279–289. [PubMed] [Google Scholar]
- Bekker E.M., Overtoom C.C., Kenemans J.L., Kooij J.J., De Noord I., Buitelaar J.K. Stopping and changing in adults with ADHD. Psychological Medicine. 2005;35:807–816. doi: 10.1017/s0033291704003459. [DOI] [PubMed] [Google Scholar]
- Bell A.H., Meredith M.A., Van Opstal A.J., Munoz D.P. Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Experimental Brain Research. 2006;174:53–59. doi: 10.1007/s00221-006-0420-z. [DOI] [PubMed] [Google Scholar]
- Boonstra A.M., Oosterlaan J., Sergeant J.A., Buitelaar J.K. Executive functioning in adult ADHD: a meta-analytic review. Psychological Medicine. 2005;35:1097–1108. doi: 10.1017/s003329170500499x. (Review) [DOI] [PubMed] [Google Scholar]
- Brown M.R., Goltz H.C., Vilis T., Ford K.A., Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Vilis T., Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bruce C.J., Goldberg M.E. Primate frontal eye fields. I. Single neurons discharging before saccades. Journal of Neurophysiology. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bush G., Frazier J.A., Rauch S.L., Seidman L.J., Whalen P.J., Jenike M.A. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Carr L.A., Nigg J.T., Henderson J.M. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Colla M., Ende G., Alm B., Deuschle M., Heuser I., Kronenberg G. Cognitive MR spectroscopy of anterior cingulate cortex in ADHD: elevated choline signal correlates with slowed hit reaction times. Journal of Psychiatric Research. 2008;42:587–595. doi: 10.1016/j.jpsychires.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Connolly J.D., Goodale M.A., Menon R.S., Munoz D.P. Human fMRI evidence for the neural correlates of preparatory set. Nature Neuroscience. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Connolly J.D., Goodale M.A., Goltz H.C., Munoz D.P. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. Journal of Neurophysiology. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Cubillo A., Halrai R., Ecker C., Giampietro V., Taylor E., Rubia K. Reduced activation in inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Curtis C.E., D'Esposito M. Success and failure suppressing reflexive behavior. Journal of Cognitive Neuroscience. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo N., Chamberlain S.R., Sahakian B.J., Robbins T.W. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;69:e145–e157. doi: 10.1016/j.biopsych.2011.02.036. [DOI] [PubMed] [Google Scholar]
- DeSouza J.F.X., Menon R.S., Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related fMRI. Journal of Neurophysiology. 2003;89:1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Dibbets P., Evers L., Hurks P., Marchetta N., Jolles J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain and Cognition. 2009;70:73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Dillo W., Goke A., Prox-Vagedes V., Szycik G.R., Roy M., Donnerstag F. Neuronal correlates of ADHD in adults with evidence for compensation strategies — a functional MRI study with a go/no-go paradigm. German Medical Science. 2010;8 doi: 10.3205/000098. (Doc09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. The Journal of Neuroscience. 2006;26:6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A.E. Executive functions in attention-deficit/hyperactivity disorder. The Journal of Clinical Psychiatry. 2006;67(Suppl. 8):21–26. (Review) [PubMed] [Google Scholar]
- Endrass T., Reuter B., Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an anti-saccade task. European Journal of Neuroscience. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Ettinger U., Ffytche D.H., Kumari V., Kathmann N., Reuter B., Zelaya F. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cerebral Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- Everling S., Munoz D.P. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. The Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S., Dorris M.C., Munoz D.P. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. Journal of Neurophysiology. 1998;80:1584–1589. doi: 10.1152/jn.1998.80.3.1584. [DOI] [PubMed] [Google Scholar]
- Everling S., Dorris M.C., Klein R.M., Munoz D.P. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. The Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgatter A.J., Ehlis A.C., Rosler M., Strik W.K., Blocher D., Herrmann J.M. Diminished prefrontal brain function in adults with psychopathology in childhood related to attention deficit hyperactivity disorder. Psychiatry Research. 2005;138:157–169. doi: 10.1016/j.pscychresns.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Fassbender C., Schweitzer J.B. Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clinical Psychology Review. 2006;26:445–465. doi: 10.1016/j.cpr.2006.01.003. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D., Farber R.H., Clementz B.A., Perry W., Anllo-Vento L. Inhibitory deficits in ocular motor behavior in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2004;56:333–339. doi: 10.1016/j.biopsych.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Ferraina S., Paré M., Wurtz R.H. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. Journal of Neurophysiology. 2002;87:845–858. doi: 10.1152/jn.00317.2001. [DOI] [PubMed] [Google Scholar]
- Fischer B., Weber H. Effects of stimulus conditions on the performance of antisaccades in man. Experimental Brain Research. 1997;116:191–200. doi: 10.1007/pl00005749. [DOI] [PubMed] [Google Scholar]
- Fischer B., Weber H., Biscaldi M., Aiple F., Otto P., Stuhr V. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Experimental Brain Research. 1993;92:528–541. doi: 10.1007/BF00229043. [DOI] [PubMed] [Google Scholar]
- Ford K.A., Everling S. Neural activity in primate caudate nucleus associated with pro- and antisaccades. Journal of Neurophysiology. 2009;102:2334–2341. doi: 10.1152/jn.00125.2009. [DOI] [PubMed] [Google Scholar]
- Ford K.A., Goltz H.C., Brown M.R., Everling S. Neural processes associated with antisaccade task performance investigated with event-related fMRI. Journal of Neurophysiology. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Ford K.A., Gati S., Menon R.S., Everling S. BOLD fMRI activation for anti-saccades in non-human primates. NeuroImage. 2009;45:470–476. doi: 10.1016/j.neuroimage.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Gallagher R., Blader J. The diagnosis and neuropsychological assessment of adult attention deficit/hyperactivity disorder. Scientific study and practical guidelines. Annals of the New York Academy of Sciences. 2001;931:148–171. doi: 10.1111/j.1749-6632.2001.tb05778.x. (Review) [DOI] [PubMed] [Google Scholar]
- Gaymard B., Ploner C.J., Rivaud-Pechoux S., Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Experimental Brain Research. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Gottlieb J., Goldberg M.E. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nature Neuroscience. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Guitton D., Buchtel H.A., Douglas R.M. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hale T.S., Bookheimer S., McGough J.J., Phillips J.M., McCracken J.T. Atypical brain activation during simple & complex levels of processing in adult ADHD. Journal of Attention Disorders. 2007;11:125–140. doi: 10.1177/1087054706294101. [DOI] [PubMed] [Google Scholar]
- Hallet P.E. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Annals of the New York Academy of Sciences. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Hurtig T., Ebeling H., Taanila A., Miettunen J., Smalley S.L., McGough J.J. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;45:1605–1613. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- Johnston K., Everling S. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. The Journal of Neuroscience. 2006;26:12471–12478. doi: 10.1523/JNEUROSCI.4101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K., Levin H.M., Koval M.J., Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Karch S., Thalmeier T., Lutz J., Cerovecki A., Opgen-Rhein M., Hock B. Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:427–440. doi: 10.1007/s00406-009-0089-y. [DOI] [PubMed] [Google Scholar]
- Kessler C.R., Adler L., Barkley R., Biederman J., Conners C.K., Demler O., Faraone S.V., Greenhill L.L. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. The American Journal of Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kawagoe R., Takikawa Y., Koizumi M., Sakagami M., Hikosaka O. Functional differences between macaque prefrontal cortex and caudate nucleus during eye movements with and without reward. Experimental Brain Research. 2007;176:341–355. doi: 10.1007/s00221-006-0622-4. [DOI] [PubMed] [Google Scholar]
- Krause J. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Review of Neurotherapeutics. 2008;8:611–625. doi: 10.1586/14737175.8.4.611. [DOI] [PubMed] [Google Scholar]
- Kronenberg G., Ende G., Alm B., Deuschle M., Heuser I., Colla M. Increased NAA and reduced choline levels in the anterior cingulum following chronic methyphenidate. A spectroscopic test-retest study in adult ADHD. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:446–450. doi: 10.1007/s00406-008-0810-2. [DOI] [PubMed] [Google Scholar]
- Lampe K., Konrad K., Kroener S., Fast K., Kunert H.J., Herpertz S.C. Neuropsychological and behavioural disinhibition in adult ADHD compared to borderline personality disorder. Psychological Medicine. 2007;27:1717–1729. doi: 10.1017/S0033291707000517. [DOI] [PubMed] [Google Scholar]
- Leigh R.J., Zee D.S. Davis Company; Philadelphia: 2006. The Neurology of Eye Movements. [Google Scholar]
- Lynch J.C., Tian J.-R. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Progress in Brain Research. 2006;151:461–501. doi: 10.1016/S0079-6123(05)51015-X. [DOI] [PubMed] [Google Scholar]
- Makris N., Biederman J., Valera E.M., Bush G., Kaiser J., Kennedy D.N. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cerebral Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Matsuura M., Ohkubo T., Ohkubo H., Matsushima E., Inoue K. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Research. 2004;13:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McDowell J.E., Kissler J.M., Berg P., Dyckman K.A., Gao Y., Rockstroh B. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. NeuroReport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- McDowell J.E., Dyckman K.A., Austin B.P., Clementz B.A. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain and Cognition. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G., Albrecht B., Banaschewski T., Rothenberger A., Brandeis D., Asherson P. Electrophysiological evidence for abnormal preparatory states and inhibitory processing in adult ADHD. Behavioral and Brain Functions. 2010;6:66–77. doi: 10.1186/1744-9081-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp W.P., Goltz H.C., Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. Journal of Neurophysiology. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Mohler C.W., Wurtz R.H. Organization of monkey superior colliculus: intermediate layer cells discharging before eye movements. Journal of Neurophysiology. 1976;39:722–744. doi: 10.1152/jn.1976.39.4.722. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Corneil B.D. Evidence for interactions between target selection and visual fixation for saccade generation in humans. Experimental Brain Research. 1995;103:168–173. doi: 10.1007/BF00241974. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews. Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. (Review) [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Broughton J.R., Goldring J.E., Armstrong I.T. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Armstrong I.T., Hampton K.A., Moore K.D. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. Journal of Neurophysiology. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Armstrong I.T., Coe B. Using eye movements to probe development and dysfunction. In: van Gompel R.P.G., Fischer M.H., Murray W.S., Hill R.L., editors. Eye Movements: A Window on Mind and Brain (Chapter 5) Elsevier; Oxford: 2007. [Google Scholar]
- Nieuwenhuis S., Ridderinkhof K.R., Blom J., Band G.P., Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an anti-saccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Nyffeler T., Rivaud-Pechoux S., Pierrot-Deseilligny C., Diallo R., Gaymard B. Visual vector inversion of the posterior parietal cortex. NeuroReport. 2007;18:917–920. doi: 10.1097/WNR.0b013e32811d6cf5. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G.A., Dépatie L., Holahan A.V., Savion-Lemieux T., Barr R.G., Jolicoeur C., Douglas V.I. Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1452–1460. doi: 10.1016/j.biopsych.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T.M., Kay A.R., Tank D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossmann J.M., Mulligan N.W. Inhibition and attention deficit hyperactivity disorder in adults. The American Journal of Psychology. 2003;116:35–50. [PubMed] [Google Scholar]
- Peltsch A., Hemraj A., Garcia A., Munoz D.P. Age-related trends in saccade characteristics among the elderly. Neurobiology of Aging. 2011;32:669–679. doi: 10.1016/j.neurobiolaging.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C., Muri R.M., Ploner C.J., Gaymard B., Demeret S., Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- Polli F.E., Barton J.J.S., Cain M.S., Thakkar K.N., Rauch S.L., Manoach D.S. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15700–15705. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay H.C. Inhibition and attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 1997;25:7–13. doi: 10.1023/a:1025799122529. (Review) [DOI] [PubMed] [Google Scholar]
- Raemaekers M., Jansma J.M., Cahn W., Van der Geest J.N., van der Linden J.A., Kahn R.S. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Archives of General Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- Raemaekers M., Vink M., van den Heuvel M.P., Kahn R.S., Ramsey N.F. Effects of aging on BOLD fMRI during prosaccades and antisaccades. Journal of Cognitive Neuroscience. 2006;18:594–603. doi: 10.1162/jocn.2006.18.4.594. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M., Amador N., Sanchez H., Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schneider M., Retz W., Coogan A., Thome J., Rösler M. Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD) — a neurological view. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:32–41. doi: 10.1007/s00406-006-1005-3. (Review) [DOI] [PubMed] [Google Scholar]
- Schneider M.F., Krick C.M., Retz W., Hengesch G., Retz-Junginger R., Reith W., Rösler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults — a functional magnetic resonance imaging (fMRI) study. Psychiatry Research: Neuroimaging. 2010;183:75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Seidman L.J. Neuropsychological functioning in people with ADHD across the lifespan. Clinical Psychology Review. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. (Review) [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Valera E.M., Makris N., Monuteaux M.C., Boriel D.L., Kelkar K. Dorsolateral prefrontal and anterior cingulated cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Sweeney J.A., Mintun M.A., Kwee S., Weisman M.B., Brown D.L., Rosenberg D.R. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–478. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publisher; Stuttgart: 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tripp G., Wickens J.R. Neurobiology of ADHD. Neuropharmacology. 2009;57:879–889. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- van Mourik R., Oosterlaan J., Sergeant J.A. The Stroop revisited: a meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Hikosaka O. Immediate changes in anticipatory activity of caudate neurons associated with reversal of position-reward contingency. Journal of Neurophysiology. 2005;94:1879–1887. doi: 10.1152/jn.00012.2005. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Munoz D.P. Neural correlates of conflict resolution between automatic and volitional actions by basal ganglia. The European Journal of Neuroscience. 2009;30:2165–2176. doi: 10.1111/j.1460-9568.2009.06998.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Munoz D.P. Presetting basal ganglia for volitional actions. The Journal of Neuroscience. 2010;30:10144–10157. doi: 10.1523/JNEUROSCI.1738-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Munoz D.P. Saccade suppression by electrical microstimulation in monkey caudate nucleus. The Journal of Neuroscience. 2010;30:2700–2709. doi: 10.1523/JNEUROSCI.5011-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Munoz D.P. Neural correlates of conflict resolution between automatic and volitional actions by basal ganglia. The European Journal of Neuroscience. 2011;30:2165–2176. doi: 10.1111/j.1460-9568.2009.06998.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Munoz D.P. Saccade reaction times are influenced by caudate microstimulation following and prior to visual stimulus appearance. Journal of Cognitive Neuroscience. 2011;23:1794–1807. doi: 10.1162/jocn.2010.21554. [DOI] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. (Review) [DOI] [PubMed] [Google Scholar]
- Wolraich M.L., Hannah J.N., Pinnock T.Y., Baumgaertal A., Brown J. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a country-wide sample. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:319–324. doi: 10.1097/00004583-199603000-00013. [DOI] [PubMed] [Google Scholar]
- Wurtz R.H., Goldberg M.E. Elsevier; Amsterdam: 1989. The Neurobiology of Saccadic Eye Movements. [Google Scholar]
- Zhang M., Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408:971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]


