Abstract
The effect of ultrasound on the permeability of blood vessels to nanoemulsion droplets was investigated using excised mouse carotid arteries as model blood vessels. Perfluorocarbon nanodroplets were formed by perfluoro-15-crown-5-ether (PFCE) and stabilized by poly(ethylene oxide)-co-poly(D,L-lactide) (PEG-PDLA) block copolymer shells. Nanodroplet fluorescence was imparted by interaction with FITC-dextran (molecular weight of 70,000 Da). The permeability of carotid arteries to nanodroplets was studied in the presence or absence of continuous wave (CW) or pulsed therapeutic 1-MHz ultrasound. The data showed that the application of ultrasound resulted in permeabilization of the vascular wall to nanodroplets. The effect of CW ultrasound was substantially stronger than that of pulsed ultrasound of the same total energy. No effect of blood vessel pre-treatment with ultrasound was observed.
Keywords: Nanoemulsions, Nanodroplets, Perfluorocarbon, Ultrasound, Permeabilization, Extravasation
INTRODUCTION
On the way to its intracellular target, a drug has to overcome a series of biological barriers. After the intravenous injection, the first physical barrier is formed by the endothelial lining of blood vessels. Overcoming this barrier is essential for effective drug delivery to tumors and is vital for drug delivery to the brain. During the last decade, significant effort by a number of groups was dedicated to enhancing drug extravasation to tumor tissue (Rapoport et al. 2007; Gao et al. 2008; Rapoport et al. 2009a; Hitchcock et al. 2010; Rapoport et al. 2011b; Miller et al. 2012; Rapoport 2012) or penetration through blood-brain barrier (BBB) (Vykhodtseva et al. 2006; McDannold et al. 2007; Treat et al. 2007; McDannold et al. 2008; Vykhodtseva et al. 2008; Curiel et al. 2009; Chopra et al. 2010; McDannold et al. 2011; McDannold et al. 2012; Park et al. 2012; Treat et al. 2012) using ultrasound with or without contrast agents.
Tumor blood vessels are characterized by poorly organized vascular architecture and reduced lymphatic drainage; tumor vasculature is known to be more permeable than blood vessels in normal tissues. Leaky blood vessels and the lack of a lymphatic system result in an increased interstitial fluid pressure, which hinders convectional transport of macromolecular drugs or nanoparticle drug carriers across blood vessel walls. Nevertheless, nanoparticles of appropriate size may accumulate in tumor tissue via the enhanced permeability and retention (EPR) effect based on defective tumor microvasculature (Iyer et al. 2006). A characteristic pore cutoff size range between 380 and 780 nm has been shown in a variety of tumors (Hobbs et al. 1998). This allows extravasation of drug-loaded nanoparticles through large inter-endothelial gaps, while the poor lymphatic drainage of tumors results in longer retention of extravasated particles in tumor tissue.
After a drug carrier penetrates through the vessel wall into tumor tissue and overcomes problems associated with heterogeneous diffusion gradients, elevated interstitial pressure, clearance mechanisms, etc., cell plasma membranes create the next physical barriers. In addition, tumors are heterogeneous matrices with some components of tumor tissue creating physical barriers for a drug carrier or drug diffusion throughout tumor tissue, which creates drug gradients that may be responsible for inducing drug resistance. These issues impair the activity of anticancer drugs. This paper addresses an approach to overcoming the first barrier created by the vessel wall using ultrasound.
Ultrasound combined with nanodroplets has been shown to improve therapeutic outcome in preclinical experiments on ultrasound-mediated drug delivery in vitro and in vivo (Rapoport et al. 2009a; Fabiilli et al. 2010a; Fabiilli et al. 2010b; Rapoport et al. 2010b; Rapoport et al. 2011b); drug concentration and distribution throughout the tumor volume was enhanced by ultrasound (Gao et al. 2005). The anticipated mechanism of these effects was based on the droplet-to-bubble transition under ultrasound (acoustic droplet vaporization, or ADV)(Giesecke and Hynynen 2003; Kripfgans et al. 2004; Lo et al. 2007; Zhang et al. 2011; Samuel et al. 2012; Sheeran and Dayton 2012). The cavitation of bubbles formed has been anticipated to enhance nanoparticle and drug extravasation. However, many aspects of the mechanisms involved in the ultrasound action in drug delivery have remained obscure. In ultrasound-mediated drug delivery, ultrasound may affect both the drug carrier and biological tissue. Ultrasound modes of action may include thermal and cavitation mechanisms, and also radiation force as shortly discussed below.
After nanodroplet conversion into microbubbles, the latter can grow in an ultrasound field by rectified diffusion and collapse in a process called inertial cavitation. Inertial cavitation of microbubbles creates microjets and shock waves that can create holes in blood vessel walls and cell membranes, thus increasing their permeability for drugs, genes, and their carriers (Ohl et al. 2006; van Wamel et al. 2006a; van Wamel et al. 2006b; Hallow et al. 2007; Tran et al. 2008; Juffermans et al. 2009; Deckers and Moonen 2010; Deckers et al. 2011; Eker et al. 2011; Yudina et al. 2011). Inertial cavitation of microbubbles can also induce alternating invagination and distention of blood vessel walls, which can cause damage of the endothelial lining and temporarily increase vessel permeability (Chen et al. 2010; Gaitan et al. 2010; Chen et al. 2011; Matula and Guan 2011). At ultrasound energies that don’t induce inertial cavitation, microbubbles stably oscillate in the ultrasound field (this process is called stable cavitation).
Mechanical action of ultrasound in the absence of cavitation may also have consequences for drug transport in tissues. The most frequently discussed non-thermal and non-cavitation mechanisms are related to acoustic streaming and ultrasound radiation forces (Dayton et al. 1999; Dayton et al. 2006). Acoustic streaming and radiation force each produce particle translation in the acoustic field, and their effects can be combined. It has been demonstrated that acoustic streaming and/or radiation force presents a means to localize and concentrate droplets and bubbles near a vessel wall, which may assist the delivery of targeted agents. The application of radiation force pulses can bring the delivery vehicle into proximity with the cell for successful adhesion of the vehicle or its fragments to cell membranes (Shortencarier et al. 2004). Which mode of action is predominantly involved at a particular stage of drug delivery to its target remains to be studied.
In what follows, we report on the effect of ultrasound on the first stage of drug delivery to tumor cells, which involves the penetration of drug carrier or drug through vascular walls. It has been shown recently that perfluorocarbon nanodroplets may serve as effective ultrasound-activated drug carriers (Rapoport et al. 2011a; Rapoport 2012). Here we studied the effect of ultrasound on the penetration of phase-shift perfluorocarbon nanodroplets carrying a model macromolecular drug FITC-dextran (molecular weight of 70,000 Da) through the walls of excised mouse carotid arteries. Perfluorocarbon nanodroplets with various boiling temperatures of perfluorocarbon compounds were shown earlier to convert into microbubbles under the action of therapeutic ultrasound (Giesecke and Hynynen 2003; Rapoport et al. 2007; Rapoport et al. 2009b; Rapoport et al. 2009a; Rapoport et al. 2010a; Rapoport et al. 2011a). Even perfluoro-15-crown-5 ether (boiling temperature of 146 C) used in this work converted into microbubbles under the action of ultrasound, presumably via the evolution of the dissolved oxygen.
MATERIALS AND METHODS
Poly(ethylene oxide)-co-poly(D,L-lactide) (PEG-PDLA) block copolymer with 2000 Da PEG block and 2000 Da P(D,L)LA blocks was obtained from Akina, Inc. (West Lafayette, IN). Micellar solutions of the block copolymers were prepared by dissolution of the block copolymer (5% w/v) in PBS. To prepare the nanoemulsion, 1% (v/v) perfluorocarbon (perfluoro-15-crown-5-ether, PFCE) was pipetted into a micellar solution and sonicated by 20-kHz ultrasound (VCX500, Sonics and Materials, Inc., CT, USA) in ice-cold water until all perfluorocarbon was emulsified; the time required for emulsification depended on the applied ultrasound energy (usually 40% of the instrument power) and for the described formulation did not exceed 2 min (Rapoport, 2007). Nanoemulsions stored in the refrigerator were stable (i.e. did not change average particle sizes) for weeks.
Nanoparticle size distribution
Size distribution of nanoparticles was measured in non-diluted emulsions by dynamic light scattering at an angle of 165° using a DelsaNano S instrument (Beckman Coulter, Osaka, Japan) equipped with a 658 nm laser and a temperature controller. Size distribution was analyzed using the Non-Negative Least Squares (NNLS) method. Intensity-averaged particle sizes are presented here.
Sonicator
Unfocused 1-MHz ultrasound was generated by an Omnisound 3000 instrument (Accelerated Care Plus Inc, Sparks, NV) equipped with a 5 cm2 transducer head. Ultrasound pressure was measured using a needle hydrophone (Onda HNR-0500) placed 7 mm in front of the probe. To match the experimental setup, a separated section of polystyrene cuvette wall (see below) was placed between the ultrasound probe and the hydrophone to account for the ultrasound absorption by the plastic. Either continuous wave (CW) or pulsed ultrasound (with a pulse length of 1.2 ms and duty cycle of 33%) were used. Measured power density varied between 50 and 60 W/cm2. The absorption by the cuvette was found to be negligible, indicating that there was minimal absorption and reflection from the cuvette walls. During experiments, the possible presence of standing waves could not be completely excluded, but all possible precautions were taken. The cuvette was surrounded by water and a sound absorber was placed behind the cuvette. However it should be noted that sonication proceeded in the near field and, therefore, the beam pattern was highly variable.
Sonication of excised carotid arteries
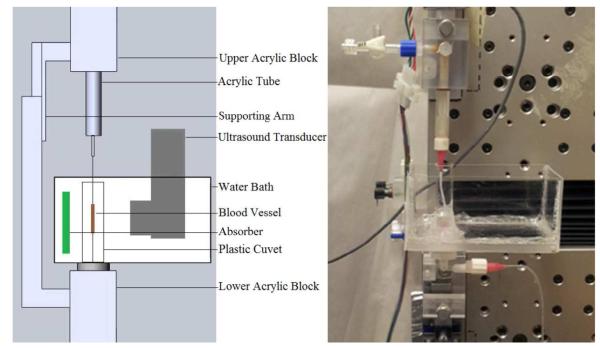
Carotid arteries were promptly excised from euthanized Swiss Webster white mice, in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health. The ends of the freshly excised arteries were tied to blunt-tipped, luer-hub, 30 gauge needles using 6-0 suture. The needles were attached to acrylic blocks whose relative position was controlled using a linear actuator (Parker Automation; Figure 1). The mounted vessel was closely surrounded by a small, plastic cuvette filled with degassed phosphate buffered saline (PBS). A larger bath was designed to fit around the cuvette to allow immersion of the ultrasound probe in degassed water. During testing, the probe was positioned approximately 7 mm away from the sample. An ultrasound absorber was placed on the opposite side of the cuvette. Vessels were stretched to their approximate in vivo length and pressurized to 13 kPa during testing, as described elsewhere (Bell et al. 2013). Luminal pressure was monitored using a transducer (Honeywell, MicroSwitch 26PCDFM6G) connected to the lower acrylic block. Axial force was measured with an 11 N load cell (Transducer Techniques, MDB 2.5).
Figure 1.
Schematic representation (left) and a photograph (right) of the experimental setup in the experiments on the ultrasound-modulated permeability of the carotid arteries. In the photograph on the right, the artery is replaced with a thin plastic tube.
In the experiments, 0.05% lysine fixable FITC-dextran solution, either mixed or not mixed with an equal volume of 1% PFCE/5% PEG-PDLA nanodroplets, was gently injected into the blood vessel through the lower fixture and needle; the valve in the upper block was opened to ensure that luminal pressure did not increase during injection. The sample was then either exposed or not exposed to ultrasound and allowed to incubate under room temperature conditions for 10 minutes. Unfocused 1-MHz ultrasound was applied at a nominal power density of 3.0 W/cm2. Continuous wave (CW) ultrasound was applied for 1 min; pulsed ultrasound was applied for 3 min, which produced equal total ultrasound energy for both CW and pulsed ultrasound. Temperature measurements with a needle thermocouple positioned next to a sham vessel during the specified sonication protocols showed temperature increases from 28.5 to 35.2 °C and from 28.4 to 33.3 °C for CW and pulsed sonication, respectively.
Following the incubation period, the FITC-dextran formulation inside the vessel was replaced with a 4% paraformaldehyde solution for artery fixation. The blood vessel was then removed from the needles and bathed in 4% paraformaldehyde where it was kept until being sliced into 200 μm sections for analysis of permeability by confocal imaging (Olympus FV1000).
Imaging
Images were obtained at the University of Utah School of Medicine Cell Imaging Facility at a resolution of 600 nm/pixel. To evaluate permeability, fifteen adjacent confocal sections (4.89 μm thick) were collected from each sample and collapsed into a composite image using ImageJ (NIH). It was determined that the autofluorescence of collagen and elastin fibers in the blood vessel wall could be suppressed by adjusting the detector voltage setting. All images were taken with the 20X objective using imaging parameters that suppressed fiber autofluorescence.
Experimental groups
Six groups were used in this study: three different control groups and three experimental groups (Table 1). In Groups 3-5, ultrasound (3 W/cm2 nominal intensity) was applied beginning at one minute after injection of the described formulation. In Group 6, the formulation was injected 3 minutes after ultrasound (3 W/cm2 nominal intensity). All ultrasound exposure durations were 1 min (CW) or 3 minutes (pulsed ultrasound with a 33% duty cycle).
Table 1.
Description of experimental groups; Groups 1-3 are controls.
| Group | FITC-dextran | Nanodroplets | Ultrasound | Number of Specimens |
|---|---|---|---|---|
| 1 | + | − | − | 2 |
| 2 | + | + | − | 1 |
| 3 | + | − | CW | 2 |
| 4 | + | + | CW | 4 |
| 5 | + | + | Pulsed | 5 |
| 6 | +* | +* | CW | 2 |
FITC-dextran + nanodroplets added 3 min after ultrasound exposure
RESULTS
1.Nanodroplet interaction with FITC-dextran
In preliminary experiments, we observed a phenomenon that dramatically benefited our research and made possible the current study. Upon mixing equal volumes of 1% PFC/5% PEG-PDLA nanodroplet suspension and 0.05% FITC-dextran solution, nanodroplets acquired strong and stable fluorescence (Figure 2), which allowed the monitoring of nanodroplet penetration and intravascular distribution using laser confocal microscopy. Mean nanodroplet size did not noticeably change upon interaction with FITC-dextran and remained about 300 nm. We hypothesize that the nanodroplet/dextran interaction is based on the collective formation of hydrogen bonds between the outer PEG block of the nanodroplet shell and dextran, which provides for strong physical bonding.
Figure 2.
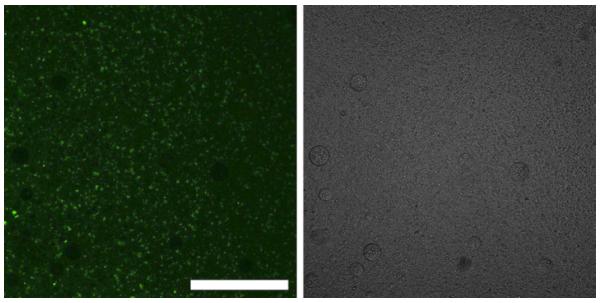
Fluorescence (left) and light (right) microscopy of nano- and micro-droplets and their conglomerates in physiologic saline after centrifugation from fluorescein isothiocyanate-dextran solution; note that nano-droplets agglomerate and coalesce during centrifugation, which allows their optical visualization; large non-fluorescent circles are air bubbles. Scale bar = 0.2 mm.
2. Effect of ultrasound on nanodroplet penetration through arterial walls
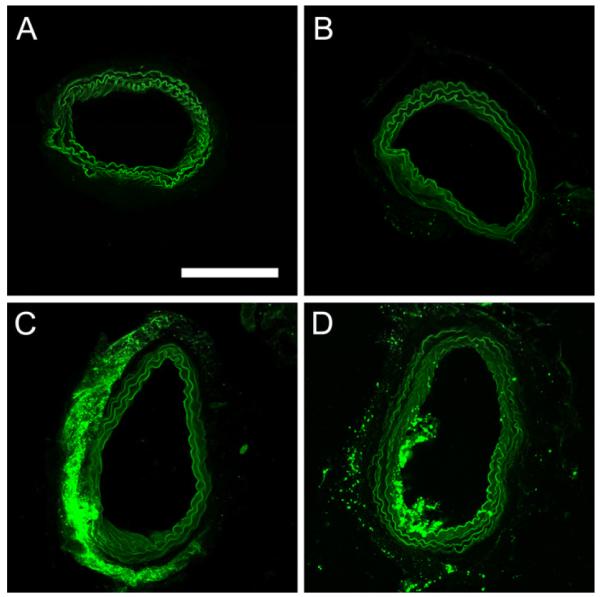
Incubation of arteries with FITC-dextran (control group 1) somewhat increased fluorescence of arterial walls (Figure 3A). However, the effect could not be quantified because, due to high aqueous solubility, FITC-dextran was partly lost at the arterial fixation stage with 4% formalin.
Figure 3.
Fluorescence images of representative slices from arteries incubated with A - FITC-dextran alone, no ultrasound; B, C, D - FITC-dextran with nanodroplets. B - no ultrasound (group 2); C – CW ultrasound (group 4); and D – pulsed ultrasound (group 5). Scale bar (0.2 mm) applies to all images.
No obvious effect of ultrasound without nanodroplets (data not shown) or nanodroplets without ultrasound (compare Figure 3B with Figure 3A) on vessel fluorescence was observed. Likewise, no droplet penetration through the arterial wall was observed without ultrasound (Figure 3B). The application of CW ultrasound noticeably enhanced penetration of nanodroplets through the arterial wall (Figure 3C); droplet conglomerates, and possibly individual nanodroplets, were seen inside the arterial wall at a higher magnification (Figure 4).
Figure 4.
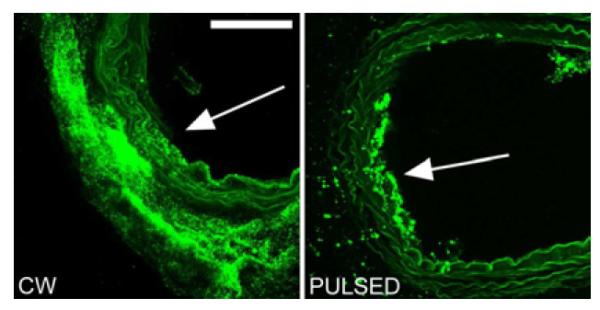
Close-up images of sections from vessels injected with nanodroplets and FITC-dextran and exposed to CW (left) or pulsed ultrasound (right). Arrows show anticipated direction of the ultrasound beam. Scale bar (0.1 mm) applies to both images.
At the same total energy, the effect of pulsed ultrasound was much weaker than that of CW ultrasound; most droplets were localized in some particular location on the luminal surface of the vessel but did not penetrate into or through the vessel wall (Figure 3D and 4B). If the penetration did occur, it was much more localized than that under CW ultrasound
No noticeable effect of sonication was observed when ultrasound was applied before the infusion of the droplets (data not shown).
In some samples, separation of the intima and adventitia from the arterial wall was observed (Figure 5). Though this disruption could have occurred during the cross-section cutting, it is noteworthy that no intima separation was observed in non-sonicated samples. Dramatic enhancement of the nanodroplet uptake was observed after the intima separation.
Figure 5.

Mechanical disruption (intima and adventitia separation) on the vessel wall of the sample exposed to CW ultrasound. Scale bar is 0.1 mm.
It is noteworthy that the effect of ultrasound on nanodroplet penetration was always directional, with the enhancement being observed in some focal region of the vessel wall. Because our experimental approach did not allow correlation of the direction of the beam with the location of the highest droplet penetration in the imaging, we can only hypothesize that the penetration occurred at a site opposite to the ultrasound probe, suggesting involvement of radiation force in the increased droplet extravasation.
DISCUSSION
The objective of this research was to investigate the effect of ultrasound on the penetration of phase-shift perfluorocarbon nanodroplets carrying a model macromolecular drug FITC-dextran (molecular weight 70,000 Da) through the walls of excised mouse carotid arteries. Data obtained showed that (1) without ultrasound, there was no penetration of nanodroplets through blood vessel walls; (2) penetration of nanoparticles was triggered by ultrasound; (3) at the same delivered total energy, the effect of CW ultrasound was much stronger than that of pulsed ultrasound; and (4) the effect of ultrasound was directional.
The mechanism of the increase of vessel permeability to nanodroplets and FITC-dextran is not evident. The directionality of the ultrasound action implies that radiation force played an important role in enhancing nanodroplet penetration through the arterial wall, as was shown earlier for microbubbles by Ferarra’s group (Dayton et al. 1999; Dayton et al. 2006; Ferrara et al. 2007; Ferrara 2008). Note that PFCE nanodroplets convert into microbubbles under the action of ultrasound (Rapoport et al. 2007; Rapoport et al. 2009b; Rapoport et al. 2009a; Rapoport et al. 2011a). Microbubbles “pushing” against, and oscillations in the close vicinity of, a vessel wall could be responsible for the opening of inter-endothelial gaps, thus enhancing vessel permeability (Gaitan et al. 2010; Matula and Guan 2011). This effect has been used for opening of the blood-brain barrier in works by Hynynen and McDannold (Hynynen et al. 2005; Hynynen 2009; McDannold et al. 2012).
Dramatic differences between the effects of pulsed and CW ultrasound of the same delivered energy cannot be attributed to thermal effects since the temperature rise was minimal, never reaching physiological range, and final temperatures achieved under CW and pulsed ultrasound were similar. As discussed above, during the ultrasound pulse, nanodroplets converted into microbubbles and may have been pushed against the vessel wall by the radiation force. However a pulse length of 20 ms generated by our transducer was apparently too short to ensure nanodroplet penetration into the wall; during the inter-pulse intervals (40 ms) microbubbles converted back into nanodroplets (Rapoport et al. 2007; Rapoport et al. 2009b; Rapoport et al. 2009a; Rapoport et al. 2011a). Based on the Stokes-Einstein equation, the diffusion coefficient of a nanodroplet of 300 nm diameter in water is 8.06×10−13 m2/s; during the inter-pulse interval of 40 ms, the nanodroplet could not travel a longer distance than 0.18 μm and therefore remained on the luminal vessel surface or in the close proximity before the arrival of the next ultrasound pulse.
Summarizing, the model experiments presented above visualize the triggering effect of ultrasound on the permeability of blood vessel walls to nanoemulsion droplets, which serves as a basis for using nanoemulsion as drug carriers for ultrasound-mediated drug delivery.
ACKNOWLEDGMENTS
Images were obtained at the University of Utah School of Medicine Cell Imaging Facility. This work was supported by NIH grants R01EB1033 (N.R.) and K25HD048643 (K.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bell ED, Kunjir RS, Monson KL. Biaxial and failure properties of passive rat middle cerebral arteries. J Biomech. 2013;46:91–6. doi: 10.1016/j.jbiomech.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Brayman AA, Bailey MR, Matula TJ. Blood vessel rupture by cavitation. Urol Res. 2010;38:321–6. doi: 10.1007/s00240-010-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett. 2011;106:034301. doi: 10.1103/PhysRevLett.106.034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra R, Vykhodtseva N, Hynynen K. Influence of exposure time and pressure amplitude on blood-brain-barrier opening using transcranial ultrasound exposures. ACS Chem Neurosci. 2010;1:391–8. doi: 10.1021/cn9000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel L, Huang Y, Vykhodtseva N, Hynynen K. Focused ultrasound treatment of VX2 tumors controlled by local harmonic motion. Phys Med Biol. 2009;54:3405–19. doi: 10.1088/0031-9155/54/11/009. [DOI] [PubMed] [Google Scholar]
- Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Dayton PA, Zhao S, Bloch SH, Schumann P, Penrose K, Matsunaga TO, Zutshi R, Doinikov A, Ferrara KW. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol Imaging. 2006;5:160–74. [PMC free article] [PubMed] [Google Scholar]
- Deckers R, Moonen CT. Ultrasound triggered, image guided, local drug delivery. J Control Release. 2010;148:25–33. doi: 10.1016/j.jconrel.2010.07.117. [DOI] [PubMed] [Google Scholar]
- Deckers R, Yudina A, Cardoit LC, Moonen CT. A fluorescent chromophore TOTO-3 as a ‘smart probe’ for the assessment of ultrasound-mediated local drug delivery in vivo. Contrast Media Mol Imaging. 2011;6:267–74. doi: 10.1002/cmmi.426. [DOI] [PubMed] [Google Scholar]
- Eker OF, Quesson B, Rome C, Arsaut J, Deminiere C, Moonen CT, Grenier N, Couillaud F. Combination of cell delivery and thermoinducible transcription for in vivo spatiotemporal control of gene expression: a feasibility study. Radiology. 2011;258:496–504. doi: 10.1148/radiol.10100767. [DOI] [PubMed] [Google Scholar]
- Fabiilli ML, Haworth KJ, Sebastian IE, Kripfgans OD, Carson PL, Fowlkes JB. Delivery of chlorambucil using an acoustically-triggered perfluoropentane emulsion. Ultrasound Med Biol. 2010a;36:1364–75. doi: 10.1016/j.ultrasmedbio.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiilli ML, Lee JA, Kripfgans OD, Carson PL, Fowlkes JB. Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions. Pharmaceutical research. 2010b;27:2753–65. doi: 10.1007/s11095-010-0277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–47. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- Ferrara KW. Driving delivery vehicles with ultrasound. Adv Drug Deliv Rev. 2008;60:1097–102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitan DF, Tessien RA, Hiller RA, Gutierrez J, Scott C, Tardif H, Callahan B, Matula TJ, Crum LA, Holt RG, Church CC, Raymond JL. Transient cavitation in high-quality-factor resonators at high static pressures. J Acoust Soc Am. 2010;127:3456–65. doi: 10.1121/1.3377062. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48:260–70. doi: 10.1016/j.ultras.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203–22. doi: 10.1016/j.jconrel.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Hynynen K. Ultrasound-mediated cavitation thresholds of liquid perfluorocarbon droplets in vitro. Ultrasound Med Biol. 2003;29:1359–65. doi: 10.1016/s0301-5629(03)00980-3. [DOI] [PubMed] [Google Scholar]
- Hallow DM, Mahajan AD, Prausnitz MR. Ultrasonically targeted delivery into endothelial and smooth muscle cells in ex vivo arteries. J Control Release. 2007;118:285–93. doi: 10.1016/j.jconrel.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock KE, Caudell DN, Sutton JT, Klegerman ME, Vela D, Pyne-Geithman GJ, Abruzzo T, Cyr PE, Geng YJ, McPherson DD, Holland CK. Ultrasound-enhanced delivery of targeted echogenic liposomes in a novel ex vivo mouse aorta model. J Control Release. 2010;144:288–95. doi: 10.1016/j.jconrel.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–12. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov NA, Jolesz FA, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2009;50:221–9. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Juffermans LJ, van Dijk A, Jongenelen CA, Drukarch B, Reijerkerk A, de Vries HE, Kamp O, Musters RJ. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol. 2009;35:1917–27. doi: 10.1016/j.ultrasmedbio.2009.06.1091. [DOI] [PubMed] [Google Scholar]
- Kripfgans OD, Fabiilli ML, Carson PL, Fowlkes JB. On the acoustic vaporization of micrometer-sized droplets. J Acoust Soc Am. 2004;116:272–81. doi: 10.1121/1.1755236. [DOI] [PubMed] [Google Scholar]
- Lo AH, Kripfgans OD, Carson PL, Rothman ED, Fowlkes JB. Acoustic droplet vaporization threshold: effects of pulse duration and contrast agent. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:933–46. doi: 10.1109/tuffc.2007.339. [DOI] [PubMed] [Google Scholar]
- Matula T, Guan J. Using optical scattering to measure properties of ultrasound contrast agent shells. J Acoust Soc Am. 2011;129:1675. [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol. 2007;33:584–90. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008;34:834–40. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Zhang Y, Vykhodtseva N. Blood-brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol. Ultrasound Med Biol. 2011;37:1259–70. doi: 10.1016/j.ultrasmedbio.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–63. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623–34. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl CD, Arora M, Ikink R, de Jong N, Versluis M, Delius M, Lohse D. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91:4285–95. doi: 10.1529/biophysj.105.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162:134–42. doi: 10.1016/j.jconrel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99:1095–106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- Rapoport N, Christensen DA, Kennedy AM, Nam KH. Cavitation properties of block copolymer stabilized phase-shift nanoemulsions used as drug carriers. Ultrasound Med Biol. 2010a;36:419–29. doi: 10.1016/j.ultrasmedbio.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Kennedy AM, Shea JE, Scaife CL, Nam KH. Ultrasonic nanotherapy of pancreatic cancer: lessons from ultrasound imaging. Mol Pharm. 2010b;7:22–31. doi: 10.1021/mp900128x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Nam KH, Gupta R, Gao Z, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Parker DL, Jeong EK, Kennedy AM. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release. 2011a;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N, Nam K-H, Gupta R, Gao Z, Mohan P, Payne A, Todd N, Liu X, Kim T, Shea J, Scaife C, Kennedy AM, Parker DL, Jeong E-K. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J Control Release. 2011b;153:4–15. doi: 10.1016/j.jconrel.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport N. Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:492–510. doi: 10.1002/wnan.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport NY, Kennedy AM, Shea JE, Scaife CL, Nam K-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009a;138:268–76. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bub Sci Eng Tech. 2009b;1:31–9. doi: 10.1179/175889709X446516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Duprey A, Fabiilli ML, Bull JL, Fowlkes JB. In vivo microscopy of targeted vessel occlusion employing acoustic droplet vaporization. Microcirculation. 2012;19:501–9. doi: 10.1111/j.1549-8719.2012.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeran PS, Dayton PA. Phase-change contrast agents for imaging and therapy. Curr Pharm Des. 2012;18:2152–65. doi: 10.2174/138161212800099883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:822–31. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- Tran TA, Le Guennec JY, Bougnoux P, Tranquart F, Bouakaz A. Characterization of cell membrane response to ultrasound activated microbubbles. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:43–9. doi: 10.1109/TUFFC.2008.615. [DOI] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121:901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved Anti-Tumor Effect of Liposomal Doxorubicin after Targeted Blood-Brain Barrier Disruption by Mri-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med Biol. 2012;38:1716–25. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wamel A, Kooiman K, Emmer M, ten Cate FJ, Versluis M, de Jong N. Ultrasound microbubble induced endothelial cell permeability. J Control Release. 2006a;116:e100–2. doi: 10.1016/j.jconrel.2006.09.071. [DOI] [PubMed] [Google Scholar]
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, de Jong N. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006b;112:149–55. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Vykhodtseva N, McDannold N, Hynynen K. Induction of apoptosis in vivo in the rabbit brain with focused ultrasound and Optison. Ultrasound Med Biol. 2006;32:1923–9. doi: 10.1016/j.ultrasmedbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–96. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudina A, Lepetit-Coiffe M, Moonen CT. Evaluation of the temporal window for drug delivery following ultrasound-mediated membrane permeability enhancement. Mol Imaging Biol. 2011;13:239–49. doi: 10.1007/s11307-010-0346-5. [DOI] [PubMed] [Google Scholar]
- Zhang M, Fabiilli ML, Haworth KJ, Padilla F, Swanson SD, Kripfgans OD, Carson PL, Fowlkes JB. Acoustic droplet vaporization for enhancement of thermal ablation by high intensity focused ultrasound. Academic radiology. 2011;18:1123–32. doi: 10.1016/j.acra.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]