Abstract
Objective
To examine whether phosphorylated vascular endothelial growth factor (VEGF) receptors shed into the vitreous reflect the ongoing retinal and choroidal signal pathway activity in wet age-related macular degeneration (AMD).
Methods
Vitreous samples obtained immediately prior to anti-VEGF injection from 11 patients with choroidal neovascularization were analyzed using reverse-phase microarrays. Two patients had samples collected at the time of injection and 1 month later. Samples from 5 patients were collected prior to vitrectomy for macular hole, epiretinal membrane, or retinal detachment.
Results
Phosphorylated forms of VEGF receptor (VEGFR Y996 and Y1175), platelet-derived growth factor receptor β (PDGFRβ Y716 and Y751), and c-KIT (Y703) were present in the vitreous. A significant difference in PDGFRβ Y751 (P <.002), VEGFR Y996 (P <.04), and VEGFR Y1175 (P <.006), but not c-KIT Y703 (P <.05) or PDGFRβ Y716 (P <.96), was noted for the responders to treatment (n=5) compared with nonresponders (n=6) and controls (n=5).
Conclusions
Vitreous levels of activated receptors constitute a new class of biomarkers. Activated forms of VEGF and PDGF receptors, previously not known to exist in the vitreous, correlate with response to anti-VEGF therapy. These findings could provide the basis for the development of individualized treatment and discovery of new therapeutic targets.
Age-related macular de-generation (AMD) is the leading cause of vision loss and blindness in individuals older than 60 years in the developed world.1,2 Treatments of wet AMD are rapidly evolving and some now target specific biochemical events such as angiogenesis. The use of pharmacotherapy targeting vascular endothelial growth factor (VEGF) has been shown to slow vision loss and even lead to vision improvement in some patients with wet macular degeneration. Currently, the 3 anti-VEGF therapies are pegaptanib, ranibizumab, and bevacizumab.3-9
The current experience with these inhibitors reveals that although these therapies are successful in most patients, the response to anti-VEGF therapy varies greatly and it is unknown what biological parameters play a role in determining response. Despite the increasingly sophisticated imaging technology and treatment modalities available, there is a tremendous unmet need for technologies that can (1) guide initiation of treatment at the earliest possible time, preferably prior to the loss of vision, (2) predict and guide the need for retreatment before recurrence of the exudative process occurs because many current treatment protocols rely on the recurrence of damaging leakage and edema to trigger retreatment, and (3) understand the other factors involved in patients who have suboptimal response to treatment or are recalcitrant to treatment, with the goal of developing new targets for drug development.
Growth factors such as VEGF interact with their target cells, most likely vascular endothelial cells in the case of wet AMD, by activating cellular receptors. When receptors are activated they become phosphorylated. Knowing that a receptor is phosphorylated gives a direct read-out indicating that the associated biochemical pathway is active and playing a role in biological behavior. If we could detect phosphorylated growth factor receptors in microliter samples of fluid vitreous from eyes with wet AMD, we might get new insights into the disease state of a given eye. Phosphorylated or activated growth factor receptors have not been previously described in the vitreous. In the present study, we tested the novel hypothesis that phosphorylated or activated forms of receptors associated with neovascularization could be found in the vitreous. In addition, we asked whether the levels of these receptors might be different in eyes that responded to anti-VEGF treatment compared with those that did not respond.
Levels of phosphorylated VEGF receptor were measured in fine-needle aspirates of fluid vitreous from eyes with wet AMD immediately prior to undergoing treatment with intravitreal bevacizumab and control eyes with macular hole, epiretinal membrane, and/or retinal detachment. The use of a novel technique using reverse-phase protein microarrays provided a method to measure levels of phosphorylated VEGF receptor as well as other phosphorylated receptors in volumes as small as 50 μL. The acquisition of these extremely small samples did not result in any complications during this study. Previous studies of protein levels in vitreous fluid have required larger sample sizes, thereby resulting in the need for vitrectomy.10-12 Techniques described in the current study allow fine needle aspirates to be acquired as a low-risk office procedure. We were able to detect levels of phosphorylated VEGF receptor in the vitreous of eyes with wet AMD that differed significantly from levels in eyes with other diseases. Furthermore, the level of activated VEGF receptor in eyes with wet AMD was correlated with response to anti-VEGF therapy. In 2 of the eyes, we measured VEGF receptor levels just prior to and 1 month following intravitreal injection of bevacizumab.
These findings introduce the potential for an unanticipated and novel diagnostic tool that could be instrumental in guiding and monitoring treatment of many blinding ocular diseases.
Methods
Specimens and Patient Data
Vitreous samples were collected from 16 patients after informed consent was obtained, following an institutional review board-approved protocol (Western IRB, Olympia, Washington) and adhering to the tenets of the Declaration of Helsinki. Control samples were collected from surgical patients immediately prior to pars plana vitrectomy (n=5) for macular hole, epiretinal membrane, or retinal detachment. Samples were collected from 10 patients with wet AMD and 1 patient with idiopathic choroidal neovascularization, all of whom required treatment with intravitreal bevacizumab. Patients underwent vitreous sampling in the office prior to intravitreal injection. Two patients had vitreous samples taken immediately prior to intravitreal injection and 1 month later, prior to reinjection.
In each case, a topical anesthetic followed by additional anesthetic was applied to the ocular surface via a cotton pledget. A sterile eyelid speculum was placed between the lids. Betadine, 5%, was applied to the ocular surface and fornix to achieve sterility. A 1-mL syringe with a 25-gauge needle was used to obtain a small quantity (0.05-0.2 mL) of liquid vitreous through the pars plana, 4 mm posterior to the limbus. Care was taken to avoid aspiration of any subconjunctival or surface fluid while withdrawing the needle from the eye. All specimens were frozen at −20°C for storage until subsequent analysis by reverse-phase protein microarrays.
Patients were characterized as responders vs nonresponders based on their response to bevacizumab treatment 1 month after the intravitreal injection. A patient was considered a responder if they met one of the following criteria: (1) improvement in visual acuity of 10 letters or more by Early Treatment Diabetic Retinopathy Study visual acuity, (2) decrease in central 1-mm retinal thickness by 40 μm using optical coherence tomography (OCT), (3) decreased area of leakage on fluorescein angiography by 50% (leakage area measured on Heidelberg between subsequent visits by a masked independent observer [G.D. ]), or (4) closure of retinal angiomatous proliferation lesion.
Patients were monitored for complications including endophthalmitis, vitreous hemorrhage, retinal detachment, and any unanticipated loss of vision during the 4 months following vitreous aspiration.
Reverse-Phase Protein Microarrays
Protein Microarray Construction
Total protein content of the vitreous samples was measured spectrophotometrically (Bradford method). The samples were diluted in extraction buffer (T-PER [Pierce, Indianapolis, Indiana], 2-mercaptoethanol [Sigma, St Louis, Missouri], and 2X sodium dodecyl sulfate Tris-glycine loading buffer [Invitrogen, Carlsbad, California]) and denatured by heating for 8 minutes at 100°C prior to dilution in the microtiter plate. Briefly, the lysates were printed on glass-backed nitrocellulose array slides (FAST Slides; Whatman, Florham Park, New Jersey) using an Aushon 2470 arrayer (Aushon BioSystems, Burlington, Massachusetts) equipped with 350-μm pins. Each lysate was printed in a dilution curve representing undiluted and 1:2, 1:4, 1:8, and 1:16 dilutions. The slides were stored with desiccant (Drierite; WA Hammond, Xenia, Ohio) at −20°C prior to immunostaining.13
Control Microarrays
Cellular lysates prepared from A431 ± EGF (epidermal growth factor), HeLa ± pervanadate, human endothelial ± pervana-date (Becton Dickinson, Franklin Lakes, New Jersey), and CHO-T (Chinese hamster ovary T cells) ± insulin (Biosource/Invitrogen, Carlsbad, California) were printed on each array for quality control assessments. Human endothelial ± pervana-date cellular lysates were printed on arrays for sensitivity and precision comparisons.
Protein Microarray Immunostaining
Immunostaining was performed on an automated slide stainer per manufacturer's instructions (Autostainer CSA kit; Dako, Carpinteria, California). Each slide was incubated with a single primary antibody at room temperature for 30 minutes. Polyclonal primary antibodies were VEGF receptor (VEGFR) Y996, VEGFRY1175, platelet-derived growth factor receptor β (PDGFRβ) Y716, PDGFRβ Y751, and c-KIT Y703 (Cell Signaling Technology, Danvers, Massachusetts). A negative control slide was incubated with antibody diluent. Secondary antibody was goat antirabbit IgG H+L (1:5000) (Vector Laboratories, Burlingame, California). Total protein per microarray spot was determined with a Sypro Ruby protein stain (Invitrogen/Molecular Probes, Eugene, Oregon) per manufacturer's directions and imaged with a charged couped device (CCD) camera (Alpha Innotech, San Leandro, California).
Bioinformatics Method for Microarray Analysis
Each array was scanned, spot-intensity analyzed, and data normalized, and a standardized single data value was generated for each sample on the array (Image Quant v5.2; GE Healthcare, Piscataway, New Jersey). Spot intensity was integrated over a fixed area. Local area background intensity was calculated for each spot with the unprinted adjacent slide background. This resulted in a single data point for each sample for comparison with every other spot on the array. Each sample was printed in duplicate in a miniature dilution curve. All of the data was analyzed to derive a concentration value averaged between the replicates and within the linear range of the dilution curve.
Statistics
Multiple means comparison (Kruskal-Wallis Test) was used to compare values among 3 groups. P < .05 was considered significant.
Results
Study Patients
Vitreous samples were collected from 16 patients. Five control samples were collected prior to pars plana vitrectomy from patients with macular hole (n=1), epiretinal membrane (n=1), or retinal detachment (n=3). Eleven patients received treatment with intravitreal bevacizumab. The number of intravitreal injections prior to vitreous sampling ranged from 0 to 5. All samples were obtained at least 4 weeks after prior treatment. Six patients were characterized as nonresponders and 5 as responders. Two patients (patients 2 and 7) had vitreous samples taken immediately prior to intravitreal injection and 1 month later, prior to reinjection. Patient 2, defined as a responder, was naive to treatment and received feeder vessel treatment and intravitreal bevacizumab. Patient 7, a nonresponder, had received 2 intravitreal bevacizumab injections prior to the first vitreous sample. The last injection was done 4 weeks before the first sample was obtained (Table 1 and Table 2).
Table 1. Patient Characteristics for Phosphoproteomic Vitreous Analysis.
| Characteristic | Patients, No. (%) (n=16) |
|---|---|
| Age, y | |
| 51-60 | 1 (6.3) |
| 61-70 | 4 (25.0) |
| 71-80 | 4 (25.0) |
| 81-90 | 6 (37.5) |
| >90 | 1 (6.3) |
| Sex | |
| Male | 6 (37.5) |
| Men with AMD | 1 |
| Female | 10 (62.5) |
| Women with AMD | 9 |
| Diagnosis | |
| Wet AMD | 10 (62.5) |
| Idiopathic CNV | 1 (6.3) |
| MH | 1 (6.3) |
| ERM | 1 (6.3) |
| Retinal detachment | 3 (18.7) |
Abbreviations: AMD, age-related macular degeneration; CNV, choroidal neovascularization; ERM, epiretinal membrane; MH, macular hole.
Table 2. Retinal Disease and Treatment Response Characteristics.
| Sample ID | Diagnosis | Vascularization Category | Vitreous Sample Collection Class | Antiangiogenesis Treatment Response |
|---|---|---|---|---|
| 1 | Wet AMD | Neovascularization | Nonresponder | |
| 3a | Idiopathic CNV | Neovascularization | Pretreatment | NA |
| 3b | Idiopathic CNV | Neovascularization | Posttreatment | Responder |
| 4 | Wet AMD | Neovascularization | Nonresponder | |
| 5 | Wet AMD | Neovascularization | Responder | |
| 8 | Wet AMD | Neovascularization | Responder | |
| 9 | Wet AMD | Neovascularization | Responder | |
| 10a | Wet AMD | Neovascularization | Pretreatment | NA |
| 10b | Wet AMD | Neovascularization | Posttreatment | Nonresponder |
| 11 | Wet AMD | Neovascularization | Responder | |
| 12 | Wet AMD | Neovascularization | Nonresponder | |
| 13 | Wet AMD | Neovascularization | Nonresponder | |
| 14 | Wet AMD | Neovascularization | Nonresponder | |
| 16 | Epiretinal membrane | Nonneovascularization | Control | |
| 18 | MH | Nonneovascularization | Control | |
| 21 | Retinal detachment/vitreous membrane | Nonneovascularization | Control | |
| 22 | Retinal detachment (1 defect) | Nonneovascularization | Control | |
| 23 | Retinal detachment | Nonneovascularization | Control |
Abbreviations: AMD, age-related macular degeneration; CNV, choroidal neovascularization; MH, macular hole; NA, not applicable.
There were no complications including endophthalmitis, vitreous hemorrhage, retinal detachment, or any unanticipated loss of vision during the 4 months following vitreous aspiration.
Reverse-Phase Protein Microarray Sensitivity and Precision
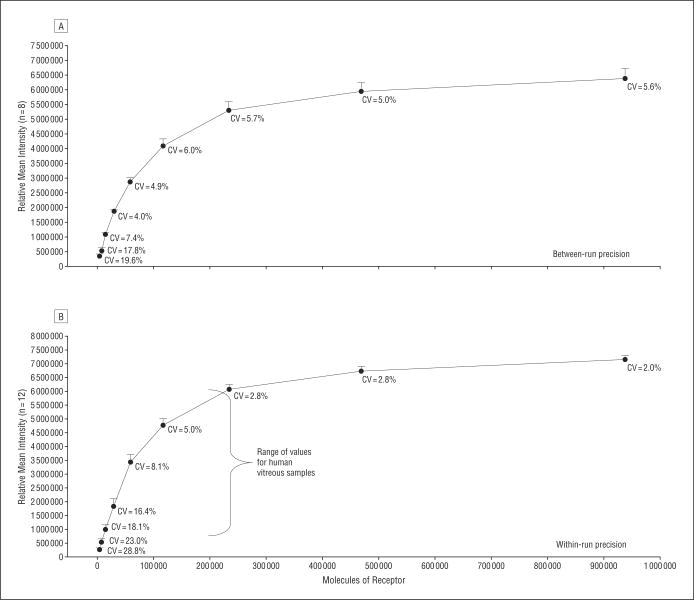
Sensitivity and precision of the reverse-phase protein microarrays using recombinant molecules, microdissected tissue samples, and fine-needle aspirates has been previously reported.13-16 Variations in robotic printing devices will affect microarray spot size, protein loading, and sensitivity; therefore, human endothelial cell lysates treated with pervanadate were used as a model of phosphorylated VEGFR sensitivity and precision (Figure 1). Human endothelial cells are known to express approximately 100 000 VEGFR per cell. Sensitivity of the arrays to VEGFR Y951 was found to be 3660 receptor molecules. To determine interslide precision, human endothelial cells treated with pervanadate were printed in duplicate on 8 slides and immunostained with anti-VEGFR Y951. Excellent dose-response curves were observed between arrays (coefficient of variation [CV], 5.0% to 17.8%; n = 8) (Figure 2A). Within-run variation (n=12) was found to be within 2.0% to 18.1% for the human endothelial + pervandate cell lysate, with good linearity (R2=0.9693) (Figure 2B).
Figure 1.
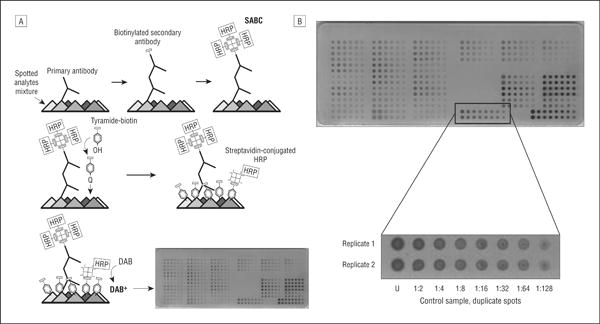
Reverse-phase protein microarray technology for quantitative analysis of human vitreous samples. A, Multiple vitreous samples and controls are immobilized on a nitrocellulose-coated slide and probed with a primary and secondary antibody, and the signal is amplified via horseradish peroxidase (HRP)–mediated deposition of biotinyl tyramide. B, The samples and controls are prepared in a 2-fold dilution series, allowing analysis within the linear dynamic range for each sample-antibody pair. DAB indicates diaminobenzidine complex; SABC, streptavidin-biotin; U, undiluted.
Figure 2.
Sensitivity and precision characteristics for vascular endothelial growth factor receptor (VEGFR) Y951, a model analyte. A lysate prepared with within run precision from human endothelial cells treated with pervanadate was printed on reverse-phase protein microarrays (RPA). A, Between-run precision (n=8) for VEGFR Y951 was between 5.6% (most concentrated sample) and 19.6% (most dilute sample). The sensitivity of the RPA for this model analyte was 3660 receptors. B, With-in run precision (n=12; R2=0.9693) ranged from 2.0% (most concentrated spot) to 28.8% (most dilute spot). CV indicates coefficient of variation.
Activated VEGF Receptor Levels Found in the Vitreous are Associated with Disease Response
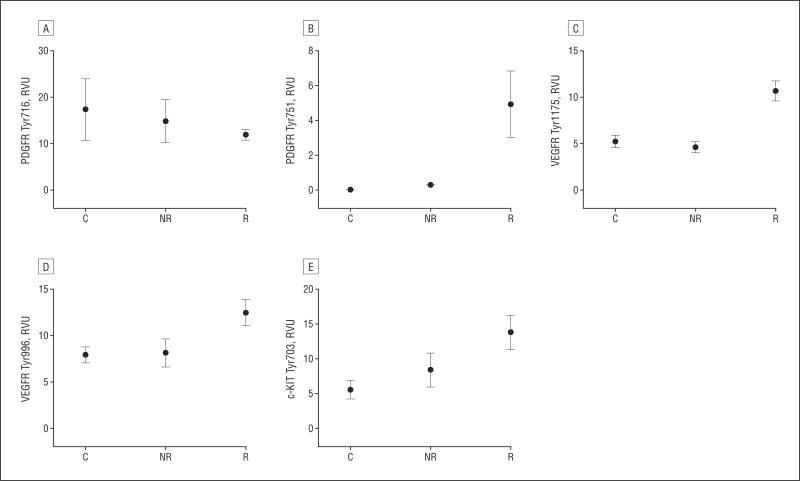
The phosphorylated form of VEGFR Y1175 was detectable in the vitreous, as were other angiogenesis-related receptors and proteins (PDGFRβ Y716, PDGFRβ. Y751, VEGFR Y996, and c-KIT Y703). There was a statistically significant difference (P <.006) in VEGFR Y1175 between the responders (mean [SEM], 10.70 [1.07] relative-value units [RVU]) and nonresponders (mean [SEM], 4.63 [0.55] RVU) and between responders and controls (mean [SEM], 5.24 [0.66] RVU) (Figure 3). There was no statistical difference between the nonresponders and controls. In addition, there was a statistically significant difference in VEGFR Y996 (P < .04) and PDGFRβ Y751 (P < .002) for the responder group compared with the controls and nonresponders. Levels of PDGFRβ Y716 (P <.96) and c-KIT Y703 (P < .05) were not found to be significantly associated with treatment response categories.
Figure 3.
Clinical response is associated with angiogenesis protein signaling. Mean levels of 5 angiogenesis proteins, platelet-derived growth factor receptor β (PDGFRβ) Tyr716 (A), PDGFRβ Tyr751 (B), vascular endothelial growth factor receptor (VEGFR) Tyr1175 (C), VEGFR Tyr996 (D), and c-KIT Tyr703 (E) were compared across groups. Levels of VEGFR Y1175 (responder n=5; mean [SEM], 10.70 [1.07] relative-value units [RVU]; P <.006), VEGFR Y996 (responder n=5; mean [SEM], 12.50 [1.42] RVU; P <.04), and PDGFRβ Y751 (responder n=5; mean [SEM], 4.94 [1.91] RVU; P < .002) were significantly different in the responder cohort compared with the nonresponder and control groups (VEGFR Y1175 nonresponder n=6; mean [SEM], 4.63 [0.55] RVU; VEGFR Y996 nonresponder n=6; mean [SEM], 8.16 [1.52] RVU; PDGFRβ Y751 nonresponder n=6; mean [SEM], 0.30 [0.30] RVU; VEGFR Y1175 control n=5; mean [SEM], 1.48 [0.66] RVU; VEGFR Y996 control n=5; mean [SEM], 7.96 [0.87] RVU; PDGFRβ Y751 control n=5; mean [SEM], 0.0 [0.0] RVU). Levels of c-KIT Y703 (P < .05) and PDGFRβ Y716 (P < .96) were not statistically significantly different between the 3 groups. C indicates control; NR, nonresponder; R, responder.
Phosphorylated VEGF Receptor as a Marker of Antiangiogenic Therapy
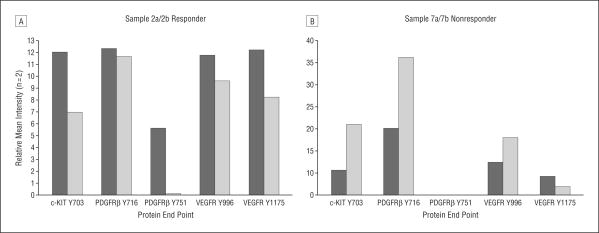
In 2 eyes (patients 2 and 7), VEGFR-Y1175 was measured from vitreous samples taken just prior to and 1 month following intravitreal injection of bevacizumab. Patient 2, characterized as a responder, showed a 50% decrease in area of leakage on fluorescein angiography and decreased retinal thickness from 348 to 307 μm on OCT. The patient's visual acuity improved from 20/80 to 20/60. This patient had an initial high level of VEGFR Y1175 prior to treatment, similar to others in the responder group. There was a 32% decrease in VEGFR-Y1175 levels after treatment with bevacizumab (Figure 4A).
Figure 4.
Phosphorylated angiogenesis-related receptors and signal proteins in human vitreous before and after treatment with bevacizumab. A, Patient 2, classified as a responder, exhibited a 32% decrease in vascular endothelial growth factor receptor (VEGFR) Y1175 1 month after bevacizumab treatment in addition to decreases in c-KIT Y703, platelet-derived growth factor receptor β (PDGFRβ Y716, PDGFRβ Y751, and VEGFR Y996. B, In contrast, patient 7, classified as a nonresponder, exhibited a 56% decrease in VEGFR Y1175, but showed concomitant increases in c-KIT Y703, PDGFRβ Y716, and VEGFR Y996 after bevacizumab treatment. Levels of PDGFRβ Y751 were not above background for this patient.
Patient 7, characterized as a nonresponder, showed no decrease in area of leakage on fluorescein angiography, increased central 1-mm retinal thickness on OCT from 361 to 422 μm, and no improvement in vision. This patient had a low level of VEGFR-Y1175 prior to treatment, similar to levels found in control and nonresponder eyes. This patient exhibited a concomitant increase in VEGFR Y996, c-KIT Y703, and PDGFRβ Y716 after treatment. This is in direct contrast to patient 2, who responded to treatment and exhibited reductions in these same proteins after treatment (Figure 4B). Although there were reductions in the target protein (VEGFR Y1175) in both of these patients, their disparate treatment response may be attributed to the activity of other angiogenic related receptors that appeared to increase after treatment in patient 7.
Comment
The presence of cell receptors and their activated phosphorylated forms in the fluid vitreous of human eyes supports the hypothesis of our study. In addition, the data show that levels of activated receptors can be measured in small samples that can be acquired as the result of an office procedure with seemingly minimal risk and that the activated receptor levels in this initial set of patients correlate with treatment response. These novel findings, as usual, require further study to determine the significance of this initial work. However, our current findings have the potential to serve as the basis for new diagnostic and research opportunities that may assist in advancing the treatment of AMD and many other ocular diseases.
The findings of this pilot study might not have been expected, based on our current understanding of the vitreous proteome.17,18 The vitreous is constantly bathing the retina and its supporting tissues, therefore vitreous fluid has the potential to accumulate by-products that could serve as indicators of cellular activity in diseases such as AMD.12,19 However, there have been no reports, to our knowledge, of activated growth factor receptors found in the vitreous of patients with AMD or other ocular disorders. Furthermore, even if cell receptors are released into surrounding fluids, they are larger than most molecules previously targeted for detection and there are significant barriers blocking transit of such large molecules from subretinal tissues and retina into the vitreous.20-23 Therefore, it is not clear whether these large cell receptor molecules could reach the vitreous. Even if they do, they may be cleared so rapidly that detection is prevented. Furthermore, if only the fluid portion of the vitreous is aspirated, the molecules of interest may be bound to the formed portions of the vitreous and remain undetected.17
Despite the aforementioned considerations, such activated receptors and signal pathway proteins known to be associated with neovascularization were indeed detected in the present vitreous study. Consequently, these findings may provide important insights into retinal tissue permeability for macromolecules and active pathways in disease pathogenesis.
While there have been reports of growth factor receptor shedding into serum (eg, Her2/neu receptor fragments), thus supporting the concept of receptor shedding into body fluids, the existence of growth factor receptors and, more importantly, phosphorylated receptors has, to our knowledge, never been reported in the vitreous.24 Here we have shown that phosphorylated forms of receptors and cell signaling proteins are shed or secreted and retained in the closed vitreous chamber (Figure 3). Past attempts to identify protein biomarkers in human vitreous fluid focused on a small number of soluble cytokines, but did not previously consider the soluble or shed forms of receptors or signal pathway proteins and did not previously consider the phosphorylated or posttranslational modification forms of proteins.17,25 These molecules potentially constitute a recording of the health or disease state of the retina. Just as a blood sample may contain markers for a heart attack, markers in the vitreous could provide information on the biology of wet AMD, diabetic retinopathy, venous occlusive disease, macular edema, and a host of other diseases, yielding unprecedented information about pathogenesis and treatment opportunities. Although the most likely source of activated VEGFR in the vitreous of patients with AMD is vascular endothelial cells, we need to consider other cellular origins in future studies.
The specific receptors that we focused on in the current study are associated with neovascularization and vascular permeability. We chose these receptors because neovascularization and increased vascular permeability in diseases such as AMD are controlled by VEGF and there are specific pharmaceutical agents that can block the effects of VEGF in these diseases.26-28 Growth factors such as VEGF interact with their target cells, most likely vascular endothelial cells in the case of wet AMD, by activating cellular receptors. When receptors are activated they become phosphorylated. Measuring levels of phosphorylated receptors may serve as an indication of growth factor and cellular activity in diseases such as AMD.
We showed that vitreous levels of phosphorylated VEGFR Y1175 in patients with wet AMD were significantly higher than levels found in control eyes with non-neovascular diseases such as epiretinal membrane, macular hole, and retinal detachment. Furthermore, the vitreous levels of phosphorylated VEGFR Y1175 in patients with wet AMD appear to correlate with the response to bevacizumab therapy. The 10 patients who received intravitreal bevacizumab were defined as either responders or nonresponders based on clinical parameters. The non-responder samples revealed VEGFR Y1175 levels similar to the 5 control samples, with no angiogenic activity (Figure 3B and Figure 4B). The nonresponder and control groups had lower mean levels of VEGFR Y1175 compared with the responder group, suggesting that the signature of changes in phosphorylated protein receptors in the fluid vitreous may reflect the active state of phosphorylation events in the eye.
Two patients had vitreous samples taken immediately prior to intravitreal injection and 1 month later. Patient 2, characterized as a responder, had a good clinical response, with decreased area of leakage on fluorescein angiography and decreased retinal thickness on OCT. The patient's visual acuity improved from 20/80 to 20/60. This patient had initial high levels of VEGFR Y1175 prior to treatment, similar to others in the responder group. There was a 32% drop in VEGFR Y1175 levels after treatment with bevacizumab. Patient 7, characterized as a nonresponder, showed no decrease in area of leakage on fluorescein angiography, increased central 1-mm retinal thickness on OCT, and no improvement in vision. This patient had a low level of VEGFRY1175 prior to treatment. There was some decrease in the already low levels of VEGFR Y1175 after treatment, but these levels were in the range of controls and nonresponders (Figure 4B). Interestingly, this patient exhibited a concomitant increase in VEGFR Y996, c-KIT Y703, and PDGFRβ Y716 after treatment, whereas patient 2 had a decrease in these activated receptor levels in the vitreous (Figure 4A). Specific phosphorylation sites on the VEGF receptor are associated with different functions. For example, phosphorylation at tyrosine 1175 activated receptor binding to downstream proteins that ultimately regulate cell growth.29 On the other hand, phosphorylation at tyrosine 996 causes receptor internalization.30 These data suggest that patients who have minimal or no response to anti-VEGF therapies may have other pathways responsible for neovascularization and exudation. With this new technology, we can probe for alternative growth factors and cell signaling proteins that may be responsible for disease activity in recalcitrant patients.
A limitation of the study is the small number of patients studied. It remains to be seen if the differences in VEGFR between responders and nonresponders will be observed with increased study sample size. Also, with the exception of one patient, all of our patients had previously received intravitreal bevacizumab. Now that we have demonstrated a technique to detect phosphorylated or activated receptors in the vitreous, we can further evaluate the interactions between growth factor inhibitors such as bevacizumab and receptor activation. For instance, we can examine patients naive to treatment and evaluate samples longitudinally to better determine how bevacizumab treatment alters growth factor cell signaling protein levels long-term. We can also ask if activated receptor levels can predict disease recurrence prior to the onset of damaging leakage and edema, which has to occur prior to detection with current modalities such as OCT and angiography.
In our study, response to therapy was defined as (1) improvement in visual acuity of 10 letters or more, (2) decrease in the height of subretinal fluid or decrease in central 1-mm retinal thickness by 40 μm on OCT, (3) decreased area of leakage on fluorescein angiography by 50%, and (4) closure of retinal angiomatous proliferation lesion. The criteria used to define response to therapy were based on clinical observations and a modification of criteria from the recently published Prospective Optical Coherence Tomography Imaging of Patients With Neovascular AMD Treated With Intraocular Ranizumab (PrONTO) study.31 We chose parameters that were clinically relevant, but as we were looking for a biologic, not treatment, effect, the parameters were necessarily somewhat different than those used in clinical trials designed to determine if treatment was of adequate functional consequence.
From a clinical point of view, a method to detect the activation of the VEGF pathway early in the process could result in a powerful diagnostic tool to predict and monitor the development and recurrence of wet AMD prior to the occurrence of any tissue damage. To be useful, this technique would need to be low-risk and have the ability to be repeated at multiple time points during the disease process. Without the knowledge brought to light by our study that activated receptors are shed and can be detected in the vitreous of patients, tissue biopsy would have been needed to measure receptor levels. Repeated tissue biopsy in cases of AMD is not practical owing to the inherent risks to the delicate and highly vascular structures involved.
Prior attempts to use vitreous samples to monitor a disease process have been hindered by the requirement of existing assays for large amounts of material. Therefore, the only way to obtain samples of sufficient size has been during vitrectomy. In addition, needle aspiration of vitreous fluid has been considered to carry risks that preclude its routine use, thereby relegating vitreous sampling to patients undergoing vitrectomy. However, vitrectomy is rarely performed in eyes with AMD and, in fact, is rarely performed in eyes with other sight-threatening diseases such as diabetic retinopathy or venous occlusive disease. These past concerns are being obviated by the advent of intraocular injection therapy and new highly sensitive technologies requiring small sample volumes.
Vitreous samples in our study were obtained in the office prior to intravitreal treatment with bevacizumab. Previous studies have obtained vitreous samples by pars plana vitrectomy, which requires the patient to undergo a surgical procedure. Our methods were designed so sufficient vitreous volume (50-200 μL) can be obtained by a simple in-office procedure. Though there may be differences in the composition of different areas of the vitreous, we have shown that sampling of the fluid vitreous via fine-needle aspiration yields significant differences in activated receptor levels among patients with AMD and that difference correlates with the response to anti- VEGF treatment. In this way, we have a potential diagnostic tool that can be used in an office setting that avoids the previous requirement for vitreous sampling as part of vitrectomy, a procedure rarely performed in patients with AMD.
There have been no complications due to fine-needle aspiration of fluid vitreous in this series. However, a relatively small number of patients were studied; therefore, further studies are necessary to ensure safety. The potential for safety of this diagnostic procedure is improved by the ability of our novel techniques to analyze samples as small as 50 μL.
At any point in time, the phosphorylated state of a cellular protein is the result of the balance between ongoing kinase and phosphatase activity. Consequently, phosphorylated proteins shed into the vitreous may provide a real-time recording or snapshot of the ongoing active state of signal pathway cascades. Such molecules may be a reflection of the ocular disease process. The new class of molecular targeted inhibitors such as pegaptanib, ranibizumab, and bevacizumab block the activity of kinase signal pathways. Bevacizumab binds and inhibits all forms of VEGF-A.6 Successful blockade of VEGF will suppress the phosphorylated state of the VEGF receptor. Thus if the treatment is suppressing the targeted pathway, it would be expected to alter or block the phosphorylation of the receptor and, in turn, block the phosphorylation of proteins downstream of the receptor. In the case of anti-VEGF therapy, efficacy will be reflected in the suppression of the phosphorylation of the VEGF receptor, which is normally stimulated by VEGF. In this way the phosphorylated form of the receptor may provide a functional read-out of whether the therapy is working and also may show how effectively it is working. Measuring the vitreous levels of phosphorylated receptors at any given time in the course of therapy may also provide a method of determining timing of retreatment. This could have major significance if the need for retreatment could be predicted prior to the recurrence of edema, hemorrhage, and tissue damage, however indolent, that is associated with these events.
Our data have revealed the previously unknown existence of phosphorylated (activated) forms of VEGF receptors, PDGF receptors, and c-KIT in human vitreous obtained by fine-needle aspiration of 0.05 mL or more of fluid vitreous during an office procedure. These activated signal pathway proteins presumably originate from retinal or subretinal cells. We showed significant differences in the levels of these biomarkers between neovascular AMD disease and nonneovascular disease. In a subset of patients for whom vitreous samples were available before and after treatment, the difference in receptor levels was associated with response to therapy with bevacizumab. Measurement of specific phosphorylation patterns of targets such as VEGFR Y1175 and other signal pathway proteins in the vitreous could become the basis for early detection, prognostic determinations, and individualized timing of therapy for patients with AMD as well as offering the potential to identify other therapeutic targets. This study warrants further exploration of the vitreous phosphoproteome.
Acknowledgments
Funding/Support: This study was supported in part by George Mason University.
Footnotes
Author Contributions: Drs Davuluri and Glaser had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, et al. Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291(15):1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 4.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration: emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakri SJ, Snyder MR, Pulido JS, McCannel CA, Weiss WT, Singh RJ. Six-month stability of bevacizumab (Avastin) binding to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina. 2006;26(5):519–522. doi: 10.1097/01.iae.0000225354.92444.7a. [DOI] [PubMed] [Google Scholar]
- 6.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Gerber HP, Wu X, Yu L, et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci U S A. 2007;104(9):3478–3483. doi: 10.1073/pnas.0611492104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shojaei F, Ferrara N. Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest. 2007;87(3):227–230. doi: 10.1038/labinvest.3700526. [DOI] [PubMed] [Google Scholar]
- 9.Takeda AL, Colquitt JL, Clegg AJ, Jones J. Pegaptanib and ranibizumab for neovascular age-related macular degeneration: a systematic review. Br J Ophthalmol. 2007;91(9):1177–1182. doi: 10.1136/bjo.2007.118562. published online ahead of print May 2, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizaki E, Takai S, Ueki M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141(1):129–134. doi: 10.1016/j.ajo.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49(2):106–108. doi: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 12.Katsura Y, Okano T, Matsuno K, et al. Erythropoietin is highly elevated in vitreous fluid of patients with proliferative diabetic retinopathy. Diabetes Care. 2005;28(9):2252–2254. doi: 10.2337/diacare.28.9.2252. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan KM, Calvert VS, Kay EW, et al. Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol Cell Proteomics. 2005;4(4):346–355. doi: 10.1074/mcp.T500003-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Liotta LA, Espina V, Mehta AI, et al. Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell. 2003;3(4):317–325. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 15.Paweletz CP, Charboneau L, Bichsel VE, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20(16):1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 16.Rapkiewicz A, Espina V, Zujewski JA, et al. The needle in the haystack: application of breast fine-needle aspirate samples to quantitative protein microarray technology. Cancer. 2007;111(3):173–184. doi: 10.1002/cncr.22686. [DOI] [PubMed] [Google Scholar]
- 17.Funatsu H, Yamashita T, Yamashita H. Vitreous fluid biomarkers. Adv Clin Chem. 2006;42:111–166. doi: 10.1016/s0065-2423(06)42004-7. [DOI] [PubMed] [Google Scholar]
- 18.Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 19.Gariano RF, Nath AK, D'Amico DJ, Lee T, Sierra-Honigmann MR. Elevation of vitreous leptin in diabetic retinopathy and retinal detachment. Invest Ophthalmol Vis Sci. 2000;41(11):3576–3581. [PubMed] [Google Scholar]
- 20.Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood- retinal barrier in diabetes. Br J Ophthalmol. 1975;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14(4):240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 23.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5(7):1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carney WP, Leitzel K, Ali S, Neumann R, Lipton A. HER-2/neu diagnostics in breast cancer. Breast Cancer Res. 2007;9(3):207. doi: 10.1186/bcr1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kauffmann DJ, van Meurs JC, Mertens DA, Peperkamp E, Master C, Gerritsen ME. Cytokines in vitreous humor: interleukin-6 is elevated in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1994;35(3):900–906. [PubMed] [Google Scholar]
- 26.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37(9):1929–1934. [PubMed] [Google Scholar]
- 27.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47(11):5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20(11):2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18(8):1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 31.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography- guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]