Abstract
Background
The calcium-activated potassium channel KCa3.1 is critically involved in T cell activation, as well as in the proliferation of smooth muscle cells and fibroblasts. We sought to investigate whether KCa3.1 contributes to the pathogenesis of obliterative airway disease (OAD) and whether knockout or pharmacological blockade would prevent the development of OAD.
Methods
Tracheas from CBA donors were heterotopically transplanted into the omentum of C57Bl/6J wild-type or KCa3.1−/− mice. C57Bl/6J recipients were either left untreated or received the KCa3.1 blocker TRAM-34 (120mg/kg/d). Histopathology and immunological assays were performed on postoperative days (POD) 5 or 28.
Results
Subepithelial T cell and macrophage infiltration on POD 5, as seen in untreated allografts, was significantly reduced in the KCa3.1−/− and TRAM-34 groups. Also, systemic Th1 activation was significantly, and Th2 mildly reduced by KCa3.1 knockout or blockade. After 28 days, luminal obliteration of tracheal allografts was reduced from 89±21% in untreated recipients to 53±26% (p=0.010) and 59±33% (p=0.032) in KCa3.1−/− and TRAM-34-treated animals, respectively. The airway epithelium was mostly preserved in syngeneic grafts, mostly destroyed in the KCa3.1−/− and TRAM-34 groups, and absent in untreated allografts. Allografts triggered an antibody response in untreated recipients, which was significantly reduced in KCa3.1−/− animals. KCa3.1 was detected in T cells, airway epithelial cells and myofibroblasts. TRAM-34 dose-dependently suppressed proliferation of wild-type C57B/6J splenocytes, but did not show any effect on KCa3.1−/− splenocytes.
Conclusions
Our findings suggest that KCa3.1 channels are involved in the pathogenesis of OAD and that KCa3.1 blockade holds promise to reduce OAD development.
Keywords: KCa3.1, Obliterative airway disease, TRAM-34, Chronic rejection, Heterotopic tracheal transplantation
Introduction
Obliterative airway disease (OAD) remains the most common chronic complication and major obstacle to the long-term graft survival in lung transplant recipients (1, 2). Alloimmunogeneic T cell activation drives the development of fibroproliferative lesions, but the detailed pathogenesis of OAD remains incompletely understood (3–7). No specific or effective treatments have been developed yet.
Recent studies have shown that the intermediate-conductance Ca2+-activated potassium channel KCa3.1 plays an important role in Ca2+-signaling and T cell activation (8, 9). The KCa3.1 channel is composed of four α-subunits each containing 6 trans-membrane segments with calmodulin complexed to its C-terminus as calcium sensor (10). Opening of this channel due to elevated intracellular Ca2+ leads to K+ efflux, membrane hyperpolarization, and increases the driving force for store-operated Ca2+-entry through calcium-release activated calcium (CRAC) channels (11). The resulting increase in cytosolic Ca2+ turns on the calcineurin pathway and induces T cell activation‥ Interestingly, the expression of KCa3.1 increases from 5–35 channels per cell in resting T cells to 500 channels per cell in activated naïve and memory T cells (12), suggesting that activated T cells might be more sensitive to selective KCa3.1 blockade.
Pharmacological KCa3.1 blockade depolarizes T cells, reduces Ca2+-influx, and inhibits T cell proliferation and cytokine production in vitro (13–15), while in vivo studies have demonstrated that KCa3.1 blockers can prevent experimental autoimmune encephalomyelitis and anti-collagen antibody-induced arthritis in mice and contribute to the prevention of kidney graft rejection in rats (16, 17). Based on the additional involvement of KCa3.1 in smooth muscle cell and fibroblast proliferation and the efficacy of the KCa3.1 blocker TRAM-34 in models of restenosis (18, 19), atherosclerosis (20), and kidney fibrosis (21), KCa3.1 has also been proposed as a possible therapeutic target for cardiovascular diseases. However, whether the KCa3.1 channel could be considered as a novel therapeutic target for the prevention of OAD has not been investigated before.
Results
Tracheas from CBA donors were heterotopically transplanted into the greater omentum of C57Bl/6J mice. Recipients in the treatment group received TRAM-34 (120mg/kg/d, i.p.) for 5 days or 28 days. KCa3.1−/− mice receiving grafts from CBA donors and C57Bl/6J receiving syngeneic grafts were used as control (see table 1).
Table 1. Study groups.
Grafts were recovered on POD5 in groups 1–4 to investigate acute rejection and immune activation, or after 28 days in groups 5–8 to assess OAD development
| Study | No. | Group | Donor | Recipients | Treatment |
|---|---|---|---|---|---|
| Grafts recovered on POD5 | |||||
| 1 | no medication | CBA | C57Bl6 | - | |
| 2 | syngeneic | C57Bl6 | C57Bl6 | - | |
| 3 | knockout | CBA | KCa3.1 −/− | - | |
| 4 | TRAM-34 | CBA | C57Bl6 | TRAM-34 | |
| Grafts recovered on POD28 | |||||
| 5 | no medication | CBA | C57Bl6 | - | |
| 6 | syngeneic | C57Bl6 | C57Bl6 | - | |
| 7 | knockout | CBA | KCa3.1 −/− | - | |
| 8 | TRAM-34 | CBA | C57Bl6 | TRAM-34 | |
5-day study
Inflammatory Cell Infiltration
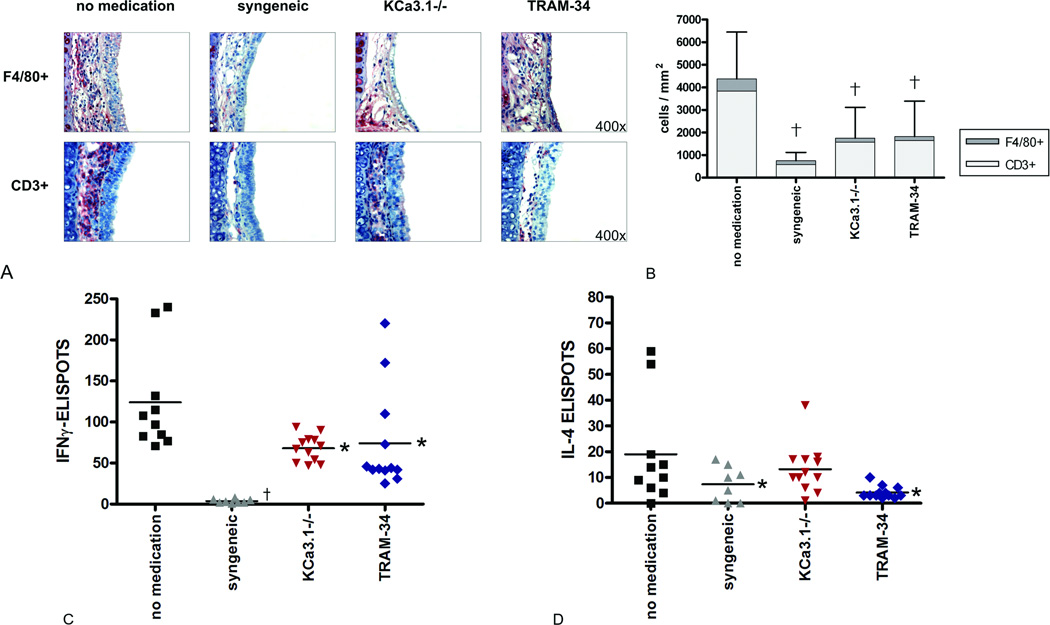
In untreated heterotopic tracheal allografts harvested on POD5, massive F4/80+ macrophage and CD3+ T lymphocyte infiltration occurred in the subepithelial area (Fig. 1A). The degree of infiltration was two- to three-fold higher than in the KCa3.1−/− and TRAM-34 groups (p<0.001 for both CD3+ and F4/80+ cells, Fig. 1B). Only a few infiltrating cells were found in syngeneic grafts and the differences to the KCa3.1−/− and TRAM-34 groups did not reach statistical significance.
Figure 1. Graft infiltrating cells and systemic cellular immune response.
Graft infiltration of F4/80+ macrophages and CD3+ lymphocytes within the subepithelial area on POD5 is shown by immunohistochemistry (A; magnification 400×). Mean numbers of F4/80+ macrophages (N=5 for no medication, N=6 for syngeneic, N=6 for KCa3.1−/−, N=5 for TRAM-34) and CD3+ T cells (N=4 for all groups) are expressed as cells/mm2 (B; †p<0.001 vs. no medication). On POD5, Elispot assays revealed attenuated systemic responses of IFN-γ producing Th1 cells (C) and IL-4 producing Th2 cells (D) in the KCa3.1 −/− (N=4) and TRAM-34 (N=5) groups (*p<0.05 vs. no medication; †p<0.001 vs. no medication (N=3), N=3 for syngeneic).
Elispot
Elispot assays on POD 5 revealed that knockout (KCa3.1−/−) or pharmacological blockade (TRAM-34) of the KCa3.1 channel resulted in decreased cellular immune activation. Spot frequencies for IFN-γ in the no medication group were significantly higher than those in the KCa3.1−/− (p=0.007) and TRAM-34 groups (p=0.015; Fig. 1C). Spots were lowest in the syngeneic group (p=0.004 vs. KCa3.1−/−, p=0.002 vs. TRAM-34, p<0.001 vs. no medication). The IL-4 spot frequencies, representing the Th2 response, were significantly lower in the TRAM-34 than the no medication group (p=0.005; Fig. 1D).
28-day study
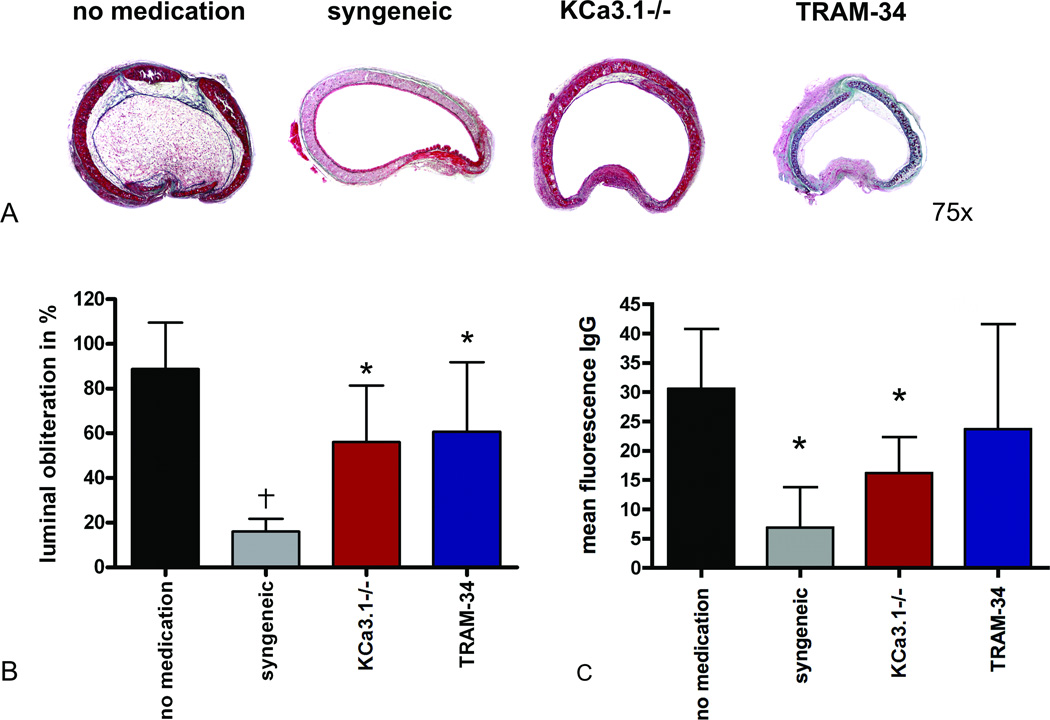
Luminal obliteration
In the 28-day study, we observed myoproliferative tissue of high cellularity in the allogeneic no medication group causing luminal obliteration of 88.7±20.9% (Fig. 2A). Tracheal grafts in the TRAM-34 and KCa3.1−/− groups showed significantly reduced luminal obliteration (p=0.032, p=0.010; Fig. 2B). However, KCa3.1 blockade or knockout did not completely prevent obliteration (p=0.014 and p=0.007, respectively vs. the syngeneic group). Only syngeneic grafts presented without fibrotic tissue growth in the epithelial or subepithelial areas.
Figure 2. Histopathology, luminal obliteration, and donor-reactive antibodies.
Representative cross sections of tracheal grafts on POD 28 stained with Masson-Goldner Trichrome at a magnification 75× are depicted (A). The average percent luminal obliteration is shown (B; *p<0.05 vs. no medication; †p<0.001 vs. no medication; N=7 for no medication and TRAM-34, N=5 for syngeneic, N=8 for KCa3.1 −/−). Mean fluorescence of IgG demonstrates a significant reduction in DSAs in the syngeneic (N=5) and KCa3.1 −/− (N=9) groups (C; p=0.001 and p=0.018, respectively, vs. no medication (n=7); N=7 for TRAM-34).
Donor-Specific Antibody (DSA) Assay
DSAs were evaluated on POD 28. The mean value of donor-reactive IgG in the no medication group was significantly higher than that of the syngeneic group (p=0.001). DSAs of the KCa3.1−/− group (p=0.018) and the TRAM-34 group (p=ns) were lower than those of the no medication group (Fig. 2C).
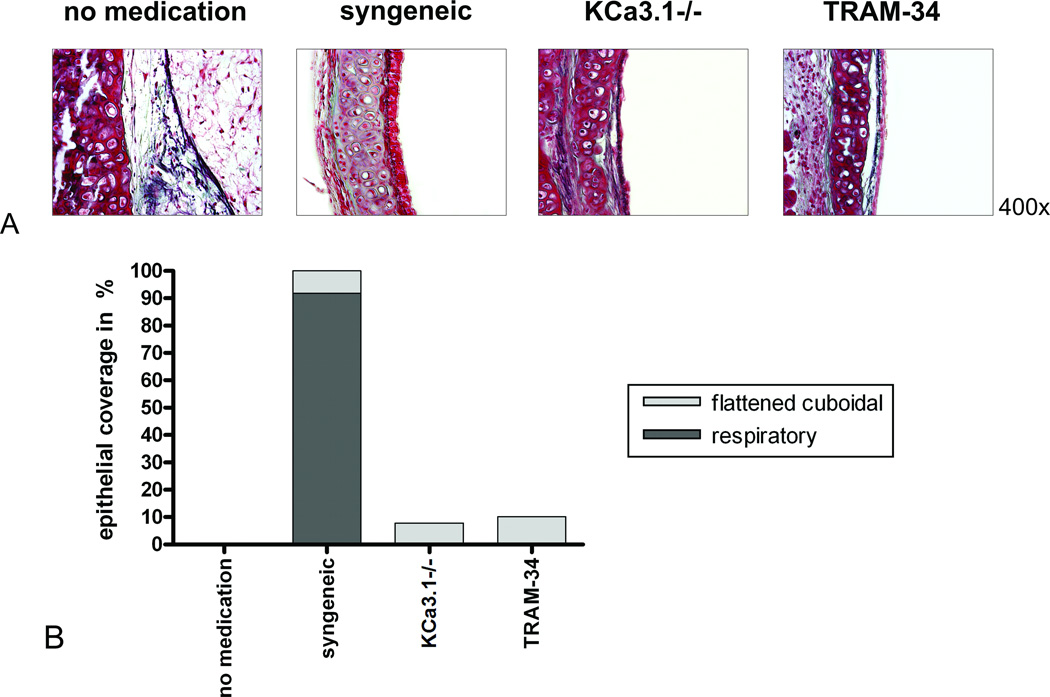
Epithelial coverage
Tracheal allografts of the no medication group showed a complete loss of epithelial coverage. In contrast, the epithelium in the syngeneic group was well preserved for most of the luminal circumference, with 91.8% respiratory morphology (p<0.001 vs. no medication). Syngeneic transplants using transgenic donors expressing firefly luciferase showed that the epithelium was donor-derived and not recipient-type ‘neo-epithelium’ (SDC, Fig. 1). In the KCa3.1−/− and TRAM-34 groups, scattered areas of flattened cuboidal epithelia (7.8% and 10.1%, respectively) remained (p=0.453 and p=0.356, vs. no medication, respectively), but we did not observe any epithelium of respiratory type (p<0.001 for both vs. no medication; Fig. 3).
Figure 3. Airway epithelium.
Representative sections of the tracheal epithelia on POD 28, stained with Masson-Goldner trichrome, are shown at a magnification of 400× (A). The respiratory epithelium of the syngeneic group (N=5) is widely preserved (B). The KCa3.1−/− (N=10) and TRAM-34 (N=8) groups show cuboidal or flattened epithelia, whereas the epithelium in the no medication group (N=8) is totally destroyed.
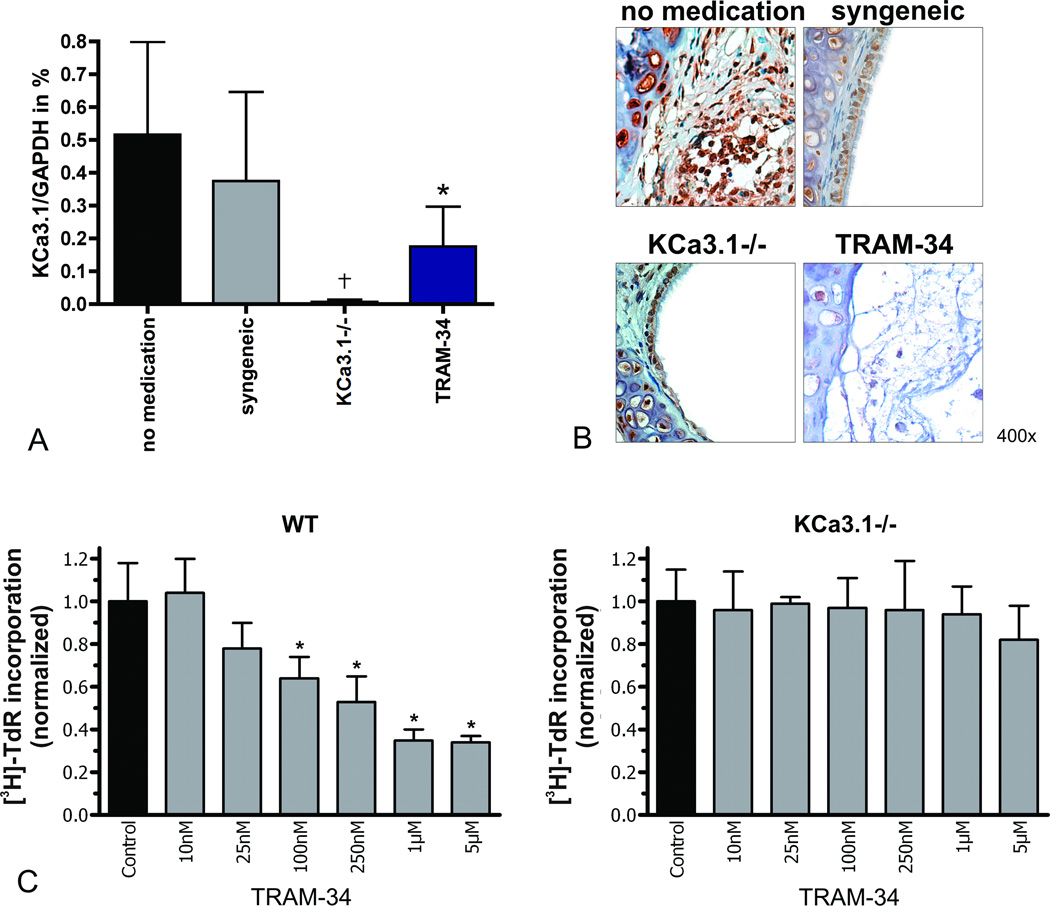
mRNA-Expression of KCa3.1 in Tracheal Grafts
Semi-quantitative RT-PCR on POD 28 revealed similar amounts of KCa3.1 mRNA in whole grafts from the allogeneic no medication group and the syngeneic group (Fig. 4A), while KCa3.1 mRNA was hardly detectable in allografts of KCa3.1−/− recipients (p<0.001 vs. no medication; p=0.005 vs. syngeneic). KCa3.1 mRNA amounts were significantly lower in the TRAM-34 group than in the no medication group (p=0.008), which most likely reflected lower numbers of infiltrating KCa3.1-expressing mononuclear cells, as well as reduced luminal obliteration by KCa3.1-expressing fibroblasts.
Figure 4. KCa3.1 expression and in vitro proliferation assay.
KCa3.1 mRNA-expression in tracheal grafts was analyzed by semi-quantitative RT-PCR (A; *p<0.008; †p<0.001 vs. no medication (N=6), N=4 for syngeneic, N=8 for KCa3.1−/−, N=5 for TRAM-34). Representative stainings for the KCa3.1 channel in tracheal graft are shown at a magnification of 400× (B). The results of the in vitro proliferation assay for WT or KCa3.1−/− splenocytes are shown as [3H]-TdR incorporation normalized to the ConA-stimulated controls (C; *p<0.05 vs.controls).
KCa3.1 Protein Expression in Tracheal Grafts
In the no medication group, intense KCa3.1 staining was found in the subepithelial area, which was confined mostly to immune cell infiltrates. Within the luminal granulation tissue, we observed very intense staining of fibroblast-like cells as well as T lymphocytes and macrophages. In the syngeneic group, KCa3.1-staining was most intense within the intact respiratory epithelium, which is in line with the reported physiological expression of the channel in this tissue (Fig. 4B). Significantly less KCa3.1 staining was observed in the KCa3.1−/− and TRAM-34 groups, which showed mostly destroyed epithelium, little myoproliferation, and only a few infiltrating cells.
Side Effects
Mice of all groups recovered well from surgery and there were no significant differences in body weight over the study period (data not shown). The mice did not show any obvious signs of discomfort, or side effects arising from TRAM-34 treatment or KCa3.1 knockout. Complete blood counts and blood biochemistry (AST, ALT, creatinine, and BUN) were in the normal range in all groups (data not shown). To screen for epithelial toxicity of TRAM-34, native C57B/6J mice and C57B/6J recipients of syngeneic tracheal grafts were treated for 28 days with TRAM-34 (SDC, Fig. 2). We did not observe any epithelial damage in the native lung or GI tract, nor in syngeneic tracheal grafts demonstrating that TRAM-34 does not exhibit any epithelial toxicity despite KCa3.1 being expressed in epithelia
Proliferation Assay in vitro
In vitro proliferation of ConA-stimulated splenocytes from C57B/6J wild-type (WT) or KCa3.1−/− mice under increasing concentrations of TRAM-34 is shown in Fig. 4C. In WT splenocytes, TRAM-34 dose-dependently suppressed proliferation (p=0.007 for 100nM p=0.006 for 250nM, p=0.0007 for 1µM, and p=0.0006 for 5µM). However, the same TRAM-34 concentrations did not affect the proliferation of ConA-stimulated KCa3.1−/− splenocytes, confirming that the TRAM-34 effect was mediated through inhibition of KCa3.1 and not through an unspecific off-target effect.
KCa3.1 in human OAD
To assess the relevance of the KCa3.1 channel in human disease, tissue specimens retrieved from lung transplant patients with OAD were studied (SDC, Fig. 3). KCa3.1 staining was abundant in human lung tissue, most prominent in the epithelium and myoproliferative areas.
Discussion
Based on previous studies showing that KCa3.1 is involved in the activation and proliferation of inflammatory cells (14, 22) the channel has been proposed as a novel target for imunomudulation (8). In this study, we demonstrate that KCa3.1 is also involved in the pathogenesis of OAD, and that KCa3.1 blockade or knockout slows disease progression.
KCa3.1 protein expression was observed in different cell populations of tracheal grafts, mainly infiltrating mononuclear cells, proliferating myofibroblasts, and respiratory epithelium. Semi-quantitative RT-PCR showed only negligible amounts of KCa3.1-mRNA in the KCa3.1−/− recipients. In keeping with the previously reported increased expression of KCa3.1 in activataed T cells (23), we observedhigh amounts of KCa3.1-mRNA and intensive KCa3.1 protein staining on inflammatory cells in the no medication group demonstrating marked KCa3.1 channel up-regulation in this allogeneic transplant setting. Compared to the no medication group, KCa3.1-mRNA expression was significantly lower in the TRAM-34 group, most likely due to lower numbers of KCa3.1-expressing inflammatory cells (14), destruction of the epithelium, and reduced myoproliferation.
OAD development has been shown to be mediated by alloimmune-activated T cells (24–26). Macrophages play a role in further recruiting inflammatory cells (27, 28) and producing pro-proliferative cytokines and growth factors (29, 30). We observed dense CD3+ T cell and F4/80+ macrophage infiltration in the subepithelial areas of untreated allografts, which were significantly reduced in both the KCa3.1−/− and TRAM-34 groups. Also, IFN-γ Elispot frequencies, reflecting the degree of cellular immune activation, were significantly reduced in both groups. IFN-γ increases the expressions of MHC-I and -II, adhesion molecules, and co-stimulatory ligands on APCs after lung transplantation (31, 32), and is considered a central cytokine in cellular rejection. Pharmacologic blockade or knockout of KCa3.1 alone was sufficient to mitigate allo-immune Th1 activation. However, it was reported that the functions of Th17 and regulatory T cells in KCa3.1−/− mice seemed unchanged (33). Furthermore, while Ca2+ influx and IL-2 production following TCR ligation was reduced in KCa3.1−/− T cells, the absolute numbers of peripheral T cells and the CD4/CD8 ratio, as well as the macroscopic appearance of all lymphoid organs was normal. KCa3.1-deficiency may either be compensated for by the up-regulation of other channels or KCa3.1 may not be crucial for maintaining T cell numbers. The specificity of TRAM-34 for KCa3.1 was confirmed in vitro. Proliferation assays showed that TRAM-34 dose-dependently reduced cell proliferation in WT, but not KCa3.1−/− splenocytes.
The DSA assays revealed reduced donor-specific IgG production in the KCa3.1−/− group compared to untreated animals. Cumulating evidence suggests that allogeneic antibody responses play an important role in acute lung graft rejection as well as in the development of OAD. Lung transplant recipients with pre-existing anti-HLA antibodies show a significantly higher risk for early graft dysfunction and have a poor prognosis (26, 34). The reduced antibody response of the KCa3.1−/− group may result from impaired T cell-mediated B cell activation.
The donor airway epithelium is considered to be the primary target for the allogeneic immune response (35), and epithelial injury plays a pivotal role in triggering rejection and OAD formation (36, 37). Preserved epithelial coverage of the airway lumen was found to slow the progression of OAD (7, 38). In our study, no airway epithelial was found in untreated animals after 28 days. In KCa3.1−/− and TRAM-34-treated animals, there were some scattered, flattened cuboidal epithelial cells visible, which occupied less than 10% of the whole circumference, while the syngeneic group exhibited well preserved ciliated epithelium. It therefore seems that neither KCa3.1 channel knockout nor blockade protect the epithelium from being destroyed or facilitate epithelial recovery. Because this is a non-ventilated model, the airway epithelium is more prone to injury and destruction and our results may exaggerate the epithelial damage with KCa3.1 blockade. In the clinical setting and ventilated trachea transplant models, the airway epithelium is preserved despite OAD development (39).
TRAM-34 treatment induced no significant side-effects in keeping with previous studies (18–20, 40). We also did not observe any differences between the KCa3.1−/− mice and the WT animals before or after transplantation and the KCa3.1−/− mouse strain is viable and fertile (41). Senicapoc, another potent and selective KCa3.1 blocker, already passed through clinical phase I to III trials for sickle cell disease and proved efficacy in its biological endpoint of reducing hemolysis (42, 43). Although, senicapoc did not prevent clinically-relevant vaso-occlusive pain crises, these studies showed that KCa3.1 blockade was safe and well-tolerated in humans (44).
Human OAD is a form of chronic lung allograft dysfunction from allo- and innate immune injury, autoimmunity, environmental pathogens, and contributing conditions like acid reflux disease. Its aggressiveness and clinical course are likely related to the extent of immunologic and non-immunologic insults (45). It is pathohistologically characterized by collagen-rich myoproliferative tissue that progressively obliterates the small airway lumen in the terminal and respiratory bronchioles (46, 47). Current treatment strategies involve switching to other calcineurin (48) or mTOR inhibitors (49), the addition of azithromycin (50) or statins (51), and extracorporeal photopheresis (52). Some patients with more active immune responses seem to be better amenable to the treatment of OAD, while the disease progresses steadily despite aggressive therapy in others. Although the heterotopic murine tracheal transplant model may not well represent human OAD in its whole complexity, it nicely mimics immune activation, graft infiltration, epithelial damage, and mice also mount a DSA response. The lack of environmental and viral challenges as well as the lack of airway clearance from secretions, however, somewhat restrict the transferability of results to the lung transplant setting.
To link our results to the human disease, we demonstrated similar KCa3.1 channel expression in mouse and human lung tissue. In accordance with the murine distribution, human KCa3.1 expression was most prominent in epithelial and myofibrotic cells. KCa3.1 blockade might therefore also benefit patients with OAD in the clinic Because KCa3.1 channel blockers are mild immunosuppressants that double up as anti-proliferative agents, they are unlikely to replace current immunosuppressive agents, but may be useful additions for long-term maintenance therapy.
In conclusion, the present study demonstrates that KCa3.1 blockade or knockout significantly decreases T cell activation and reduces the development of OAD suggesting KCa3.1 blockade as an additional maintenance strategy for patients after lung transplantation.
Materials and Methods
Animals
Male CBA mice were used as allogeneic and C57Bl/6J mice as syngeneic trachea donors, and male C57Bl/6J mice, or KCa3.1−/− mice on C57Bl/6J background, were used as recipients. Mice weighing 25 to 35 g were purchased from Charles River Laboratories (Sulzfeld, Germany) and KCa3.1−/− mice derived from our own breeding colonies were genotyped as described previously (53). All animals received humane care in compliance with the guide for the principles of laboratory animals, prepared by the Institute of Laboratory Animal Resources, and published by the National Institutes of Health. The animals were maintained in the animal care facilities of the University Hospital Hamburg-Eppendorf.
Heterotopic Tracheal Transplantations, Graft Recovery, and Tissue Processing
The heterotopic tracheal transplant model in mice was chosen because of its reliability in presenting the characteristics of OAD pathogenesis and its high reproducibility (54, 55). Transplantations were performed as previously described (55). For analysis, tracheal grafts were recovered from the greater omentum and cut into two segments. One segment was fixed in 4% paraformaldehyde solution, dehydrated, and embedded in paraffin. The second segment was snap-frozen in liquid nitrogen and stored at -80°C.
Experimental Groups
Eight groups were involved in this study (see table 1). All animals were randomly assigned to one of the groups prior to tracheal transplantation and there were no animal deaths due to technical failures. Mice in groups 4 and 8 were treated with 120mg/kg TRAM-34 intra peritoneally (18, 56) once daily. TRAM-34 was freshly dissolved in Miglyol 812 (Pharmacy of University Hospital Hamburg-Eppendorf) prior to use. Grafts were recovered 5 days after transplantation to investigate cellular rejection and immune activation (groups 1–4) or 28 days after transplantation for the study of OAD (groups 5–8).
Side Effect Screening
Blood was drawn during graft recovery for complete blood count, liver, and kidney toxicity. All recipients were examined daily for signs of discomfort or diarrhea.
Histology
General Histology
Sections of 5µm were cut and stained with hematoxylin and eosin (H&E) or Masson-Goldner trichrome. Histologic analyses were done using image-processing software (Leica, Bensheim, Germany). Luminal obliteration was quantified as previously described (57). Since the tissue inside the tracheal cartilage contains submucosal and epithelial tissue, the value for luminal obliteration in native tracheas is approximately 10% (58). The airway epithelium was classified as intact respiratory epithelium or flattened cuboidal epithelium. By computerized morphometry, the length of the respective epithelium along the whole luminal circumference was measured and calculated in percent. Mononuclear infiltrating cells were identified using monoclonal antibodies against CD3 (DakoCytomation, Glostrup, Denmark) and the macrophage marker F4/80 (Clone BM8, BMA Biomedicals, Augst, Switzerland). Antigens were retrieved in epitope-retrieval solution (Dako, pH 9) by the heating method in a steamer, or by trypsin digestion (Dako), respectively. After overnight incubation with the primary antibody at 4°C, the biotinylated rabbit-anti-rat secondary antibody (Dako) was used if necessary. These antibodies were further bound with alkaline phosphatase conjugated enzyme polymer (AP Polymer Kit, Zytomed Systems, Berlin, Germany) and finally visualized by New Fuchsin Substrate System (Dako). Sections were counterstained with hematoxylin. Three high power fields were analyzed per animal to quantify cell infiltration (expressed as cells/mm2).
KCa3.1 channel expression
After antigen retrieval in sodium citrate (10mM, pH 6), KCa3.1 channel proteins were identified by an anti-KCNN4 antibody (Sigma-Aldrich, St. Louis, MO). A biotinylated secondary antibody was further labeled by horseradish peroxidase-conjugated avidin complex (Vector Laboratories, CA) and finally visualized by DAB (Vector Laboratories) as previously described (40).
Elispot Assays
The cellular immune response was investigated on POD5. Recipient spleens were harvested and splenocytes were isolated and freshly used. Elispot assays using 1×105 mitomycin-inhibited donor splenocytes as stimulator cells and 1×106 recipient splenocytes as responder cells were performed according to the manufacturer’s protocol (BD Biosciences, CA) using IFN-γ and IL-4-coated plates. Duplicates or quadruplicates were done for each animal. Spots were counted automatically by an ELISPOT plate reader (CTL, St. Louis, OH) for scanning and analyzing.
DSA Assay
On POD28, sera from recipient mice were decomplemented by heating to 56°C for 30 min. Equal amounts of sera and donor splenocyte suspensions (5×106/ml) were incubated for 30 min at 37°C. Bound allogeneic IgG antibodies were labelled with the FITC-conjugated goat anti-mouse IgG F(ab’)2 fragment (Sigma-Aldrich) and analyzed by flow cytometry (BD Bioscience).
KCa3.1 mRNA-Expression
mRNA was extracted and purified from tracheal transplants using the RNeasy Mini Kit (Qiagen). Semi-quantitative RT-PCR was conducted using iQ™ CYBR® Green Supermix (Bio-Rad) and a Stratagene MX3000p cycler. Primer sequences were as follows: mKCa3.1: F, GTGGCCAAGCTGTACATGA; R, GCCACAGTGTGTCTGTGAGG; mGAPDH: F, CAATGAATACGGCTACAGCAAC; R, AGGGAGATGCTCAGTGTTGG. Expression levels were normalized to the house keeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reference gene by calculating the ΔCt values (ΔCt = Ct(target) – Ct(GAPDH)) and the ratios to GAPDH (ratio = 2^-ΔCt). Results were expressed as percentage of GAPDH (% GAPDH).
Proliferation Assay in vitro
Proliferation assays were performed by stimulating 1×105 splenocytes from either C57Bl/6J WT or KCa3.1−/− mice with 5µg/ml ConA (Sigma-Aldrich). Cells were incubated with different concentrations of TRAM-34 in flat-bottom 96 well plates for 48 hours. [3H]-TdR incorporation was measured, normalized to ConA-activated controls, and the background was subtracted.
Statistical analysis
Data are presented as mean ± standard deviation. Comparisons between groups were performed by analysis of variance (ANOVA) between groups with Fisher’s Least Significant Difference (LSD) post hoc tests. Probability values (p) smaller than 0.05 were considered significant. Statistical analysis was performed using the SPSS statistical software package 17.0 for Windows (SPSS Inc., Chicago, IL).
Supplementary Material
Acknowledgements
The authors thank Christiane Pahrmann for her technical support and Ethicon (Norderstedt, Germany) for providing the sutures. Special thanks to the UKE Microscopy Imaging Facility (umif) at the University Hospital Hamburg-Eppendorf (Bernd Zobiak) for their help and equipment support.
Funding
S.S. received funding from the Deutsche Forschungsgemeinschaft (DFG) (SCHR992/3-1; SCHR992/4-1) and the International Society for Heart and Lung Transplantation (ISHLT). H.W. was funded by the National Institute of Health (RO1 GM076063).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
none
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-seventh official adult lung and heart-lung transplant report--2010. The Journal of Heart and Lung Transplantation. 29(10):1104. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Sundaresan S, Trulock EP, Mohanakumar T, Cooper JD, Patterson GA. Prevalence and outcome of bronchiolitis obliterans syndrome after lung transplantation. The Annals of Thoracic Surgery. 1995;60(5):1341. doi: 10.1016/0003-4975(95)00751-6. [DOI] [PubMed] [Google Scholar]
- 3.Paradis I, Yousem S, Griffith B. Airway obstruction and bronchiolitis obliterans after lung transplantation. Clin Chest Med. 1993;14(4):751. [PubMed] [Google Scholar]
- 4.Burke C, Baldwin J, Morris A, et al. Twenty-eight cases of human heart-lung transplantation. The Lancet. 1986;327(8480):517. doi: 10.1016/s0140-6736(86)90881-0. [DOI] [PubMed] [Google Scholar]
- 5.Griffith BP, Paradis IL, Zeevi A, et al. Immunologically mediated disease of the airways after pulmonary transplantation. Ann Surg. 1988;208(3):371. doi: 10.1097/00000658-198809000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehler AKS, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest. 1998;114(5):1411. doi: 10.1378/chest.114.5.1411. [DOI] [PubMed] [Google Scholar]
- 7.Adams BF, Brazelton T, Berry GJ, Morris RE. The role of respiratory epithelium in a rat model of obliterative airway disease. Transplantation. 2000;69(4):661. doi: 10.1097/00007890-200002270-00031. [DOI] [PubMed] [Google Scholar]
- 8.George Chandy K, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends in Pharmacological Sciences. 2004;25(5):280. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutman GA, Chandy KG, Adelman JP, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55(4):583. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- 10.Wulff H, Gutman GA, Cahalan MD, Chandy KG. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J Biol Chem. 2001;276(34):32040. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- 11.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231(1):59. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rus H, Pardo CA, Hu L, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci U S A. 2005;102(31):11094. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanger CM, Rauer H, Neben AL, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276(15):12249. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 14.Ghanshani S, Wulff H, Miller MJ, et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275(47):37137. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 15.Khanna R, Chang MC, Joiner WJ, Kaczmarek LK, Schlichter LC. hSK4/hIK1, a Calmodulin-binding KCa Channel in Human T Lymphocytes. Journal of Biological Chemistry. 1999;274(21):14838. doi: 10.1074/jbc.274.21.14838. [DOI] [PubMed] [Google Scholar]
- 16.Jensen BS, Hertz M, Christophersen P, Madsen LS. The Ca2+-activated K+ channel of intermediate conductance:a possible target for immune suppression. Expert Opin Ther Targets. 2002;6(6):623. doi: 10.1517/14728222.6.6.623. [DOI] [PubMed] [Google Scholar]
- 17.Chou CC, Lunn CA, Murgolo NJ. KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev Mol Diagn. 2008;8(2):179. doi: 10.1586/14737159.8.2.179. [DOI] [PubMed] [Google Scholar]
- 18.Kohler R, Wulff H, Eichler I, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108(9):1119. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- 19.Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol. 2008;28(6):1084. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyama K, Wulff H, Chandy KG, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118(9):3025. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grgic I, Kiss E, Kaistha BP, et al. Renal fibrosis is attenuated by targeted disruption of K(Ca)3.1 potassium channels. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14518. doi: 10.1073/pnas.0903458106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wulff H, Knaus HG, Pennington M, Chandy KG. K+ channel expression during B cell differentiation: implications for immunomodulation and autoimmunity. J Immunol. 2004;173(2):776. doi: 10.4049/jimmunol.173.2.776. [DOI] [PubMed] [Google Scholar]
- 23.Wulff H, Calabresi PA, Allie R, et al. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. The Journal of Clinical Investigation. 2003;111(11):1703. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reznik SI, Jaramillo A, SivaSai KSR, et al. Indirect Allorecognition of Mismatched Donor HLA Class II Peptides in Lung Transplant Recipients with Bronchiolitis Obliterans Syndrome. American Journal of Transplantation. 2001;1(3):228. doi: 10.1034/j.1600-6143.2001.001003228.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu KC, Jaramillo As, Mendeloff EN, et al. Concomitant allorecognition of mismatched donor HLA class I,Äì and class II,Äìderived peptides in pediatric lung transplant recipients with bronchiolitis obliterans syndrome. The Journal of Heart and Lung Transplantation. 2003;22(1):35. doi: 10.1016/s1053-2498(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 26.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. 1998;65(5):648. doi: 10.1097/00007890-199803150-00008. [DOI] [PubMed] [Google Scholar]
- 27.Sekine Y, Yasufuku K, Heidler KM, et al. Monocyte chemoattractant protein-1 and RANTES are chemotactic for graft infiltrating lymphocytes during acute lung allograft rejection. American Journal of Respiratory Cell and Molecular Biology. 2000;23(6):719. doi: 10.1165/ajrcmb.23.6.3825. [DOI] [PubMed] [Google Scholar]
- 28.Schmid-Antomarchi H, Schmid-Alliana A, Romey G, et al. Extracellular ATP and UTP control the generation of reactive oxygen intermediates in human macrophages through the opening of a charybdotoxin-sensitive Ca2+-dependent K+ channel. Journal of Immunology. 1997;159(12):6209. [PubMed] [Google Scholar]
- 29.Hertz MI, Henke CA, Nakhleh RE, et al. Obliterative bronchiolitis after lung transplantation - A fibroproliferative disorder associated with platelet-derived growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10385. doi: 10.1073/pnas.89.21.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oyaizu T, Okada Y, Shoji W, et al. Reduction of recipient macrophages by gadolinium chloride prevents development of obliterative airway disease in a rat model of heterotopic tracheal transplantation. Transplantation. 2003;76(8):1214. doi: 10.1097/01.TP.0000088672.48259.F1. [DOI] [PubMed] [Google Scholar]
- 31.Smith CR, Jaramillo As, Duffy BF, Mohanakumar T. Airway epithelial cell damage mediated by antigen-specific T cells: implications in lung allograft rejection. Human Immunology. 2000;61(10):985. doi: 10.1016/s0198-8859(00)00175-0. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima J, Ono M, Takeda M, Kawauchi M, Furuse A, Takizawa H. Role of costimulatory molecules on airway epithelial cells acting as alloantigen-presenting cells. Transplantation Proceedings. 1997;29(4):2297. doi: 10.1016/s0041-1345(97)00334-5. [DOI] [PubMed] [Google Scholar]
- 33.Di L, Srivastava S, Zhdanova O, et al. Inhibition of the K(+) channel KCa3.1 ameliorates T cell-mediated colitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1541. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau CL, Palmer SM, Posther KE, et al. Influence of panel-reactive antibodies on posttransplant outcomes in lung transplant recipients. The Annals of Thoracic Surgery. 2000;69(5):1520. doi: 10.1016/s0003-4975(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 35.Mauck KA, Hosenpud JD. The bronchial epithelium: A potential allogeneic target for chronic rejection after lung transplantation. Journal of Heart and Lung Transplantation. 1996;15(7):709. [PubMed] [Google Scholar]
- 36.Kuo E, Bharat A, Shih J, et al. Role of Airway Epithelial Injury in Murine Orthotopic Tracheal Allograft Rejection. The Annals of Thoracic Surgery. 2006;82(4):1226. doi: 10.1016/j.athoracsur.2006.03.122. [DOI] [PubMed] [Google Scholar]
- 37.Qu N, de Vos P, Schelfhorst M, de Haan A, Timens W, Prop J. Integrity of Airway Epithelium Is Essential Against Obliterative Airway Disease in Transplanted Rat Tracheas. The Journal of Heart and Lung Transplantation. 2005;24(7):882. doi: 10.1016/j.healun.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Ikonen TS, Brazelton TR, Berry GJ, Shorthouse RS, Morris RE. Epithelial re-growth is associated with inhibition of obliterative airway disease in orthotopic tracheal allografts in non-immunosuppressed rats. Transplantation. 2000;70(6):857. doi: 10.1097/00007890-200009270-00002. [DOI] [PubMed] [Google Scholar]
- 39.Deuse T, Schrepfer S, Reichenspurner H, et al. Techniques for experimental heterotopic and orthotopic tracheal transplantations - When to use which model? Transpl Immunol. 2007;17(4):255. doi: 10.1016/j.trim.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Chen YJ, Raman G, Bodendiek S, O'Donnell ME, Wulff H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. Journal of Cerebral Blood Flow and Metabolism. 2011;31(12):2363. doi: 10.1038/jcbfm.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu L, Mullen K, Allie R, Calabresi P. Blockade of Kv1.3 potassium channels inhibits granzyme B production in human CD8+T lymphocytes. Clinical Immunology. 2006;119:S45. [Google Scholar]
- 42.Ataga KI, Orringer EP, Styles L, et al. Dose-escalation study of ICA-17043 in patients with sickle cell disease. Pharmacotherapy. 2006;26(11):1557. doi: 10.1592/phco.26.11.1557. [DOI] [PubMed] [Google Scholar]
- 43.Ataga KI, Smith WR, De Castro LM, et al. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood. 2008;111(8):3991. doi: 10.1182/blood-2007-08-110098. [DOI] [PubMed] [Google Scholar]
- 44.Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends. Expert Rev Clin Pharmacol. 2010;3(3):385. doi: 10.1586/ecp.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140(2):502. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 46.Jaramillo A, Fernández FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T. Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatric Transplantation. 2005;9(1):84. doi: 10.1111/j.1399-3046.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- 47.Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late sequelae in human heart-lung transplantation. Chest. 1984;86(6):824. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 48.Cairn J, Yek T, Banner NR, Khaghani A, Hodson ME, Yacoub M. Time-related changes in pulmonary function after conversion to tacrolimus in bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2003;22(1):50. doi: 10.1016/s1053-2498(02)00548-x. [DOI] [PubMed] [Google Scholar]
- 49.Groetzner J, Wittwer T, Kaczmarek I, et al. Conversion to sirolimus and mycophenolate can attenuate the progression of bronchiolitis obliterans syndrome and improves renal function after lung transplantation. Transplantation. 2006;81(3):355. doi: 10.1097/01.tp.0000195781.02268.5e. [DOI] [PubMed] [Google Scholar]
- 50.Gottlieb J, Szangolies J, Koehnlein T, Golpon H, Simon A, Welte T. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2008;85(1):36. doi: 10.1097/01.tp.0000295981.84633.bc. [DOI] [PubMed] [Google Scholar]
- 51.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med. 2003;167(9):1271. doi: 10.1164/rccm.200205-410OC. [DOI] [PubMed] [Google Scholar]
- 52.Morrell MR, Despotis GJ, Lublin DM, Patterson GA, Trulock EP, Hachem RR. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(4):424. doi: 10.1016/j.healun.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 53.Si H, Heyken W-T, Wölfle SE, et al. Impaired Endothelium-Derived Hyperpolarizing Factor-Mediated Dilations and Increased Blood Pressure in Mice Deficient of the Intermediate-Conductance Ca2+-Activated K+ Channel. Circulation Research. 2006;99(5):537. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 54.Hele DJYM, Belvisi MG. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2001;2(3):169. doi: 10.1186/rr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hua X, Deuse T, Tang-Quan KR, Robbins RC, Reichenspurner H, Schrepfer S. Heterotopic and orthotopic tracheal transplantation in mice used as models to study the development of obliterative airway disease. J Vis Exp. 2010;(35) doi: 10.3791/1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci U S A. 2000;97(14):8151. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichenspurner H, Soni V, Nitschke M, et al. Obliterative airway disease after heterotopic tracheal xenotransplantation: pathogenesis and prevention using new immunosuppressive agents. Transplantation. 1997;64(3):373. doi: 10.1097/00007890-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 58.Velotta JB, Deuse T, Peter C, et al. 330: Effects of the JAK3-Inhibitor R348 on Different Cell Types Involved in Obliterative Airway Disease. The Journal of Heart and Lung Transplantation. 2009;28(2, Supplement 1):S180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.