Abstract
Major Depressive Disorder (MDD) has been associated with biased processing and abnormal regulation of negative and positive information, which may result from compromised coordinated activity of prefrontal and subcortical brain regions involved in evaluating emotional information. We tested whether patients with MDD show distributed changes in functional connectivity with a set of independently derived brain networks that have shown high correspondence with different task demands, including stimulus salience and emotional processing. We further explored if connectivity during emotional word processing related to the tendency to engage in positive or negative emotional states. In this study, 25 medication-free MDD patients without current or past comorbidity and matched controls (n = 25) performed an emotional word-evaluation task during functional MRI. Using a dual regression approach, individual spatial connectivity maps representing each subject's connectivity with each standard network were used to evaluate between-group differences and effects of positive and negative emotionality (extraversion and neuroticism, respectively, as measured with the NEO-FFI). Results showed decreased functional connectivity of the medial prefrontal cortex, ventrolateral prefrontal cortex, and ventral striatum with the fronto-opercular salience network in MDD patients compared to controls. In patients, abnormal connectivity was related to extraversion, but not neuroticism. These results confirm the hypothesis of a relative (para)limbic–cortical decoupling that may explain dysregulated affect in MDD. As connectivity of these regions with the salience network was related to extraversion, but not to general depression severity or negative emotionality, dysfunction of this network may be responsible for the failure to sustain engagement in rewarding behavior.
Keywords: Depression, Extraversion, Functional magnetic resonance imagint, Salience network, Whole-brain functional connectivity
Highlights
-
•
We studied whole-brain functional connectivity in MDD during an emotional task.
-
•
We used a set of independent template networks, corresponding to various task demands.
-
•
We showed lower connectivity of reward related regions with a salience network in MDD.
-
•
Lower salience connectivity specifically related to extraversion in patients
-
•
Results may reflect vulnerability for MDD via a circuit vital for rewarding behavior.
1. Introduction
Failure to adaptively process emotional information is one of the major components of Major Depressive Disorder (MDD). Compromised capacity to adaptively appraise emotional stimuli may contribute to getting ‘stuck in the rut’, a recent reformulation of MDD as an inability to regulate the depressive state resulting from a tendency to engage in, and an inability to disengage from a negative mood state, instead of a disorder of a negative mood state per se (Holtzheimer and Mayberg, 2011). At the same time, anhedonia, a fundamental aspect of MDD, may in large part reflect an inability to engage in positive emotional appraisal and may further contribute to the inability to regulate the depressive state. Animal research and neuroimaging studies with humans indicate that adaptive appraisal of emotional stimuli and regulation of emotional states depends on intact interactions between (para)limbic and subcortical brain structures and dorsal and lateral prefrontal cortical (PFC) areas (Ochsner et al., 2004; Phillips et al., 2003; Wager et al., 2008). A reduction in the coordinated engagement of such neural systems during emotional processing is one possible mechanism underlying MDD. The interconnections of regions involved in evaluation of potentially salient information and those involved in cognitive appraisal and top-down control may be particularly important for predicting the capacity of individuals to effectively regulate incoming emotional information and emotional states.
So far, functional magnetic resonance imaging (fMRI) studies that have specifically tested for the connectivity of (para)limbic and prefrontal regions in MDD have mainly used seed-based analysis approaches. These studies have suggested abnormal connectivity of the amygdala (Anand et al., 2005; Carballedo et al., 2011; Chen et al., 2008; Cullen et al., 2009; Lui et al., 2011), thalamus (Anand et al., 2005; Chen et al., 2008; Cullen et al., 2009; Lui et al., 2011), frontal regions (Lemogne et al., 2009; Lui et al., 2011; Sheline et al., 2010) including the anterior cingulate cortex (ACC); (Anand et al., 2005; Carballedo et al., 2011; Cullen et al., 2009; Lui et al., 2011; Sheline et al., 2010) and parietal regions (Bluhm et al., 2009; Sheline et al., 2010; Zhou et al., 2010), during emotional paradigms (Anand et al., 2005; Chen et al., 2008; Lemogne et al., 2009) and without any externally cued task (the so called resting state; Bluhm et al., 2009; Cullen et al., 2009; Greicius et al., 2007; Lui et al., 2011; Sheline et al., 2010). Although these studies support the hypothesis of abnormal (para)limbic–prefrontal connectivity in MDD, such approaches only allow to test for connectivity based upon a limited number of seed regions (e.g., the amygdala), potentially missing important information about more broadly interconnected regions throughout the brain.
In this study we tested whether MDD patients showed different patterns of connectivity in distributed networks during a controlled emotional processing task compared with controls and examined whether these differences were related to individual differences in the propensity to engage in positive and negative affective behavior. As choosing a seed region or set of seed-templates may be biased when the seed is based at least partially on the data being analyzed (Kriegeskorte et al., 2009), a problem that is particularly pronounced in clinical fMRI studies that compare seed-based connectivity across groups, we used a set of completely independently-derived independent component networks (ICNs). These ICNs have been derived from a resting state study using healthy individuals (Beckmann et al., 2005) and show strong correspondence to a set of spatial components estimated in a meta-analysis of the BrainMap database, which consists of activation maps from nearly 30,000 subjects engaged in a wide variety of tasks (Smith et al., 2009). Our template ICNs therefore depict interconnected networks of brain regions that co-activate when particular categories of tasks or cognitive processes are being performed (Smith et al., 2009), including perception, motor processing, cognition, and emotional processing. We hypothesized that prefrontal and limbic connectivity with the template networks, specifically those found previously to become active during emotion tasks, would be reduced in a group of individuals with MDD compared to a sample of matched healthy controls.
2. Materials & methods
2.1. Participants
Twenty-five right-handed medication-free patients with a half-year diagnosis of MDD (16 female; age range: 20–52), with no past or present diagnosis of comorbid anxiety disorders were selected from the Netherlands Study of Depression and Anxiety (NESDA Penninx et al., 2008; Appendix A) neuroimaging study. Eighteen patients were medication naïve and seven patients reported to have used anti-depressive medication before participating to the NESDA but had already ceased taking medication on their own prior to enrolment. Three of the 7 patients who had previously used anti-depressive medication were medication free for at least 3 weeks before scanning and the remainder for at least 6 weeks. Diagnoses according to Diagnostic and Statistical Manual of Mental Disorders — Fourth Edition (DSM-IV) algorithms were established using the structured Composite International Diagnostic Interview (CIDI; Robins et al., 1988)-lifetime version 2.1, administered by a trained interviewer.
Twenty-five right-handed medication-free patients with a half-year diagnosis of MDD (16 female; age range: 20–52), with no past or present diagnosis of comorbid anxiety disorders were selected from the Netherlands Study of Depression and Anxiety (NESDA Penninx et al., 2008; Appendix A) neuroimaging study. Eighteen patients were medication naïve and seven patients reported to have used anti-depressive medication before participating to the NESDA but had already ceased taking medication on their own prior to enrolment. Three of the 7 patients who had previously used anti-depressive medication were medication free for at least 3 weeks before scanning and the remainder for at least 6 weeks. Diagnoses according to Diagnostic and Statistical Manual of Mental Disorders — Fourth Edition (DSM-IV) algorithms were established using the structured Composite International Diagnostic Interview (CIDI; Robins et al., 1988)-lifetime version 2.1, administered by a trained interviewer.
The control group consisted of 25 age, sex, handedness, scan-center and education matched healthy control participants also recruited through NESDA, who were currently free of, and had never met criteria for depressive or anxiety disorders or any other axis-I disorder, and were not taking any psychotropic drugs. Exclusion for all participants in this analysis were: 1) a history of major internal or neurological disorder, 2) dependency or recent abuse (past year) of alcohol and/or drugs, 3) hypertension, 4) left handedness, 5) general MR-contraindications.
2.2. Task paradigm
We employed an event-related subject-paced word-classification paradigm (Daselaar et al., 2003), programmed in E-prime software (SPSS Inc. and IL, USA.). Forty positive, 40 negative, 40 neutral study words, and 40 baseline trials were presented in a pseudo-randomized order in 20 blocks of eight words. Each block consisted of two negative words, two positive words, two neutral words, and two control words. Words were matched for length (ranging from three to twelve letters) and frequency of use. The task was paced by the subject, but each word was presented with a maximum duration of 5 s. Subjects had to indicate whether they thought the word presented was positive, negative, or neutral to them (response options were displayed at the bottom of the screen). Control words were ‘≪left’, ‘≪middle≫’, and ‘right≫’ and participants were instructed to press the corresponding button. This task was part of a word recognition paradigm (van Tol et al., 2012). Therefore, the task started and ended with three filler words to protect for the ‘primacy–recency’ memory effect.
2.3. Procedure
Patients and controls fulfilled an initial baseline measurement, which included establishment of diagnosis using the CIDI and a wide range of psychosocial measures including a demographic interview, current and life-time psychopathology interview, personal history information, current depressive and anxiety state questionnaires (including the Inventory of Depressive Symptomatology (IDS) (Rush et al., 1986) and Beck's Anxiety Inventory (BAI); Beck et al., 1988), and personality measures (NEO Five Factor Inventory [NEO FII]; Costa and McGrae, 1992). After an average interval of eight weeks, patients and controls were included for the MRI session in one of the three participating centers, the Leiden University Medical Center (LUMC), Academic Medical Center (AMC) Amsterdam, or University Medical Center Groningen (UMCG). The Ethical Review Boards of each center approved this study. All participants provided written informed consent.
Severity of depression and anxiety on the day of scanning was again assessed using Dutch versions of the BAI (Beck et al., 1988) the Montgomery Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979), and the IDS (Rush et al., 1986). The word paradigm was performed as part of a larger functional imaging session (Demenescu et al., 2011; van Tol et al., 2011; van Tol et al., 2012; Veer et al., 2010).
2.4. Image acquisition
Imaging data were acquired using Philips 3 T MR-systems (Best, The Netherlands) located at the LUMC, AMC, and UMCG, equipped with a SENSE-8 (LUMC and UMCG) and a SENSE-6 (AMC) channel head coil, respectively. For each subject, echo-planar images (EPI) were obtained using a T2*-weighted gradient echo sequence (repetition time [TR] = 2300 ms, echo time [TE] = 30 ms [UMCG: TE = 28 ms], matrix size: 96 × 96 [UMCG: 64 × 64], 35 axial slices [UMCG: 39 slices], interleaved acquisition, 2.29 × 2.29 mm in-plane resolution [UMCG: 3 × 3 mm], 3 mm slice thickness). EPI's were scanned parallel to the anterior–posterior commissure plane. Scan session length was dependent on the subjects' pace of completing the task (i.e. response time in classifying words and baseline assignments) and varied between 128 and 240 volumes (Mean = 166.3, SD = 20.6). Anatomical imaging included a sagittal 3D gradient-echo T1-weighted sequence (TR = 9 ms, TE = 3.5 ms; matrix 256 × 256; voxel size: 1 × 1 × 1 mm; 170 slices).
2.5. Statistical analysis
Imaging data acquired during the word-classification task were processed using FSL software (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl; Smith et al., 2004). Preprocessing of the fMRI images was carried out using FEAT (FMRI Expert Analysis Tool), implemented in MELODIC Version 3.10. The following processing steps were applied: motion correction, slice timing correction, non-brain removal, spatial smoothing using a Gaussian kernel of 5 mm full width at half minimum, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 150 s). Next, the functional images were registered to MNI-152 standard space (T1-standard brain averaged over 152 subjects; Montreal Neurological Institute, Montreal, QC, Canada) using a two-step registration from functional to high-resolution structural T1-image (rigid body, 6 degrees of freedom) to MNI-template (affine registration, 12 degrees of freedom). Normalized 4D datasets were resampled to 4 mm isotropic voxels, a voxel size slightly larger than the largest native voxel size from any of the datasets and matching preprocessing steps in previous work from our group (Veer et al., 2010).
Resampled and normalized datasets from both MDD patients and HCs were then entered into a dual regression analysis (Beckmann et al., 2009; Filippini et al., 2011; Khalili-Mahani et al., 2012) using the set of eight standard independent component networks (ICNs) as described in detail in Beckmann et al. (2005). In the dual regression procedure, two stages of multiple linear regression analyses are performed. 1) A time series is estimated for each ICN template per subject, using the subject's functional data (normalized to unit variance) as the predicted variable and the set of spatial ICNs as predictors; 2) A subject-specific weighted spatial map is ‘regressed’ using the time series created in Holtzheimer and Mayberg (2011) as predictors of the participant's functional data. This dual regression analysis approach generates statistical maps that show the connectivity of a subject specific network to the template network of interest. These resulting spatial maps represent an unbiased measure of the degree to which BOLD signal fluctuations in each voxel covary with each ICN time series for each subject separately. More simply, each participant's spatial map for a given ICN can be considered a voxelwise map of the strength of functional connectivity with that ICN. The spatial maps can be used in voxelwise analysis to assess differences between groups in connectivity strength to the template ICN. The dual regression method following ICA has proven its sensitivity to detect connectivity differences following alcohol and morphine intake (Khalili-Mahani et al., 2012) and in young carriers of the APOE4 gene relative to non-carriers (Filippini et al., 2011).
To assess main effects across both groups of functional connectivity within each ICN, individual spatial maps were included in a one-sample t-test performed separately for each ICN. To assess group differences in functional connectivity within each ICN, individual spatial maps were compared in a one-way ANOVA performed separately for each ICN, with scan site added as a covariate (coded as two dummy covariates). All results are reported at p < .05, family wise error corrected for multiple comparisons using permutation tests (# permutations = 5000) with cluster-mass thresholding (voxelwise threshold: t = 2.3; Hayasaka and Nichols, 2003). We exported significant clusters to SPSS to test for effects age, sex, illness severity and variations in personality scores related to tendencies to engage in positive or negative affect, extraversion and neuroticism, respectively. Scores were derived from the extraversion and neuroticism subscales of the NEO-FFI (Costa and McGrae, 1992), that have shown strong correlations with positive affect and negative affect as measured with the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). Neuroticism has been defined as the tendency to experience negative emotions and psychological distress in response to negative/stressful events and has been strongly associated with MDD (r = .60) (Rosellini and Brown, 2011), whereas extraversion is thought to reflect the degree of positive emotionality, sociability, and approach related behavior (Spielberg et al., 2011) and has been negatively and uniquely associated with MDD (r = -.33) and social anxiety disorder (r = -.59), and not with other affective disorders (Rosellini and Brown, 2011).
3. Results
3.1. Clinical characteristics and behavioral results
Table 1 lists sample characteristics and between group statistics. No site × diagnosis effects occurred (χ2 = 0, p = 1), and no systematic difference in clinical characteristics was observed between patients included at the different sites (IDS_T1: F2,24 = .83, p = .45; IDS_T2: F2,24 = .017, p = .98; MADRS_T2: F2,24 = 1.22, p = .32). No group difference was observed in the number of volumes acquired during the self-paced task (F1,49 = .69, p = .41).
Table 1.
Sample characteristics.
| MDD (n = 25) |
HC(n = 25) |
Between-group statistics |
|||||
|---|---|---|---|---|---|---|---|
| χ2 | U | F | p | ||||
| Female | n | 16 | 17 | .09 | .78 | ||
| AMC/LUMC/UMCG | n | 8/10/7 | 8/10/7 | .00 | 1 | ||
| Age | Years; mean (SD) | 33.9 (9.9) | 37.2 (10) | 1.39 | .24 | ||
| Education | Years; mean (SD) | 12.9 (2.6) | 13.8 (2.3) | 1.31 | .26 | ||
| Recurrent MDD | N | 15 | – | – | |||
| Age of onset | Years; mean (SD) | 25 (11) | – | – | |||
| MADRS | Mean (SD) | 14.4 (10.2) | .9 (1.7) | 60 | < .001 | ||
| Range | 0–33 | 0–6 | – | ||||
| IDS_T2 | Mean (SD) | 19.2 (11.9) | 3.8 (3.3) | 62 | < .001 | ||
| Range | 2–39 | 0–11 | – | ||||
| IDS_T1 | Mean (SD) | 28.5 (10.5) | 5.3 (2.8) | 38.5 | < .001 | ||
| Range | 2–47 | 0.10 | – | ||||
| BAI | Mean (SD) | 8.5 (7) | 2.3 (2.2) | 107 | < .001 | ||
| Range | 0–26 | 0–8 | – | ||||
| VAS | Mean (SD) | 24.8 (20.4) | 24 (22.3) | 293 | .89 | ||
| Range | 0–65 | 0–80 | – | ||||
| # volumes | Mean (SD) | 168.8 (18.8) | 163.9 (22.3) | .69 | .41 | ||
| Range | 128–207 | 138–240 | – | ||||
| Classification behavior | |||||||
| # words_pos | Mean (SD) | 38.5 (10.5) | 45.3 (9.5) | 6.08 | .02 | ||
| # words_neg | Mean (SD) | 40 (2.5) | 40.1 (6.4) | .01 | .93 | ||
| # words_neu | Mean (SD) | 47.1 (11.0) | 39.2 (9.6) | 7.22 | .01 | ||
| rt pos | Sec; mean (SD) | 1.52 (.33) | 1.36 (.28) | 3.18 | .08 | ||
| rt neg | Sec; mean (SD) | 1.28 (.27) | 1.27 (.44) | .01 | .93 | ||
| rt neu | Sec; mean (SD) | 1.66 (.39) | 1.55 (.36) | .96 | .33 | ||
| NEO-FFI score s | |||||||
| Extraversion | Mean (SD) | 34 (7.3) | 42.9 (6.5) | 20.81 | < .001 | ||
| Neuroticism | Mean (SD) | 40.4 (8.1) | 25 (4.3) | 70.72 | < .001 | ||
| Openness | Mean (SD) | 32.4 (5.7) | 31.7 (4.8) | .23 | .63 | ||
| Conscientiousness | Mean (SD) | 35.2 (7.5) | 41.8 (3.9) | 14.98 | < .001 | ||
| Agreeableness | Mean (SD) | 42 (4.8) | 45.6 (5) | 6.39 | .02 | ||
MDD: Major Depressive Disorder; HC: healthy controls; AMC: Academic Medical Center, University of Amsterdam; LUMC: Leiden University Medical Center; UMCG: University Medical Center Groningen; MADRS: Montgomery Åsberg Rating Scale; IDS: Inventory of Depressive Symptomatology; BAI: Beck Anxiety Inventory; VAS: Visual Analogue Scale; T1: time of the Netherlands Study of Depression and Anxiety baseline interview; T2: time of Magnetic Resonance Imaging session; # volumes: number of volumes acquired during the word classification task; # words_pos/neg/neu: number of words classified as positive, negative, or neutral; rt pos/neg/neu; response time of classifying positive/negative/neutral words; U: Mann–Whitney U non-parametric test statistic; χ2: chi-square test statistic; F: one-way ANOVA statistic.
MDD showed higher depression scores and anxiety scores, and higher scores on neuroticism, and agreeableness than controls, but lower scores on extraversion, and conscientiousness (Table 1). Between the NESDA interview (T1) and the MRI-session (T2) depressive symptom ratings decreased in MDD (IDS, paired-sample t-test: t24 = 4.05,p = .001). At the time of scanning, eight MDD patients had IDS scores indicative of the remitted state. Within the MDD group, neuroticism correlated positively with IDS score both at T1 and T2, whereas IDS was inversely correlated with extraversion (IDS_T1 — neuroticism: r = .63, p = .001; IDS_T2 — neuroticism: r = .47, p = .03; IDS_T1 — extraversion: r = − .55, p = .004; IDS_T2 — extraversion: r = − .49, p = .03; all two-tailed). An interaction of valence classification and diagnosis was observed (F[1,2; 57.66] = 6.05, p = .013, ŋ2 = .11), resulting from the MDD patients indicating less words as ‘positive’ and more words as ‘neutral’ than controls. Within MDD neutral and positive word classification was unrelated to IDS scores, extraversion and neuroticism (rkendall's tau < |.11|, p > .64). No main effect of diagnosis and no interaction of diagnosis × valence occurred on response times (F < 2.05, p > .14).
3.2. Functional connectivity results
Overall, mean spatial maps over both groups (i.e., one-sample t-tests per component) closely matched the template ICNs, although the components often included additional regions compared with the templates (see Appendix B).
Overall, mean spatial maps over both groups (i.e., one-sample t-tests per component) closely matched the template ICNs, although the components often included additional regions compared with the templates (see Appendix B).
Comparison of spatial maps between the two groups showed decreased connectivity in MDD compared to controls of bilateral medial PFC, nucleus accumbens, and right orbitofrontal cortex (OFC) extending into the caudate nucleus, with a template network comprising the ventrolateral-PFC, dorsolateral-PFC, ACC, medial-PFC, cerebellum, and cuneus (Fig. 1). This fronto-opercular template network had the strongest loading on emotion-related tasks in the BrainMap database (Smith et al., 2009) and has previously been described as the salience network (Seeley et al., 2007). No significant between-group differences were observed for the other networks.
Fig. 1.
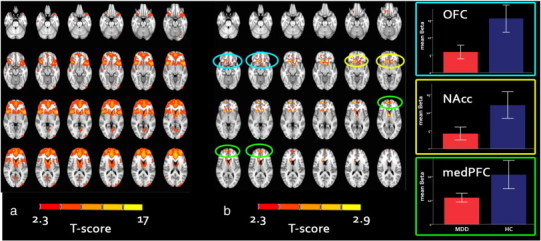
Salience network across patients and controls and between group differences.
a) Mean component across patients and controls together; b) differences between MDD and HC within the salience network showing decreased medial PFC, caudate nucleus, and nucleus accumbens connectivity in the MDD group. Results are displayed at p < .05, family wise error corrected.t > 2.3. Blue circles highlight lower connectivity in the orbitofrontal cortex (OFC), the yellow circles highlight lower connectivity of the nucleus accumbens (NAcc), and the green circles highlight lower connectivity in the medial prefrontal cortex (medPFC) with the salience network in MDD patients compared with controls; Right figures: mean and 95% confidence interval plots showing the mean connectivity strength in the OFC (upper plot), NAcc (middle plot), and medPFC (bottom plot).
3.3. Effects of illness severity and affect
We exported the individual beta-values of the total cluster and 8 sub-clusters to SPSS to test for possible confounds and relations with clinical variables and task behavior (Table 2; the main cluster was separated into sub-clusters based on whether each voxel's six directly neighboring voxels were also correlated, thus allowing us to make sure in post hoc tests that results pertained to all anatomically discrete sub-regions of the larger cluster). A one-way MANOVA confirmed the voxel-wise analysis across all sub-clusters, with lower values in MDD than in controls (F[8,41] = 2.66, p = .019). Adding age, education, sex, history of medication use, and number of volumes did not change the result (F[8,36] = 2.43, p = .033). In a previous study, we reported on decreased gray matter in inferior frontal gyrus and rostral ACC ((van Tol et al., 2010), consistent with meta-analytic results of (Bora et al., 2012)), both implicated in the salience network. However, volumetric difference could not account for the difference in connectivity with the salience network between MDD patients and controls, if anything making the group difference stronger (F[8,34] = 3.40, p = .006). Within MDD, connectivity of the whole medial PFC-striatal regions within the salience network was not explained by depression severity (IDS_T2: β = − .04, p = .86), change in IDS score between T1 and T2 ([∆ IDS = (IDS_T1 − IDS_T2) / IDS_T1]; β = .26, p = .23), or years since first episode (β = .36, p = .16) with age added to the model.
Table 2.
Effect of diagnosis on connectivity with the salience network: MDD < HC.
| Center of gravity (MNI) |
||||||
|---|---|---|---|---|---|---|
| Location | x | y | z | No. of voxels | T-value | |
| R | Orbitofrontal cortex, extending into caudate nucleus | 15 | 22 | − 2 | 131 | 2.83 |
| L | Nucleus accumbens | − 12 | 22 | − 8 | 68 | 2.51 |
| R | Medial prefrontal gyrus, frontal poles | 7 | 59 | 5 | 66 | 2.60 |
| R | Frontal pole/ventrolateral prefrontal gyrus | 22 | 50 | − 11 | 7 | 2.34 |
| L | Medial/superior prefrontal gyrus | − 20 | 45 | − 1 | 3 | 2.65 |
| R | Ventral caudate nucleus | 8 | 20 | − 2 | 1 | 2.56 |
| R | Putamen | 20 | 12 | − 6 | 1 | 2.35 |
| R | Medial prefrontal gyrus, subgenual anterior cingulate cortex | 4 | 20 | − 18 | 1 | 2.33 |
Total cluster showing decreased connectivity in MDD as compared to healthy controls. Coordinates indicate center of sub-clusters. Location: hemisphere; L = left hemisphere; R = right hemisphere; MNI: Montreal Neurological Institute coordinate system. Results are reported at p < .05, family wise error corrected for multiple comparisons using permutation tests (# permutations = 5000) of cluster-mass (voxelwise threshold: t = 2.3). Voxel size: 4 × 4 × 4 mm.
Within MDD, connectivity of the fronto-striatal areas with the salience network was positively predicted by extraversion scores (βE = .4, p = .049, R2 = .16), an effect that became stronger when neuroticism (N) was added to the model (βE = .64, p = .012; ∆R2 = .10, n.s.). Adding variations on other NEO-FFI scales (i.e. openness, conscientiousness, and agreeableness) and sex did not affect the predictive value of extraversion (βE = .80, p = .008). Finally, we added depression severity scores at the time of scanning to the model (IDS_T2), but this left the results unchanged as well (βE = .77, p = .014). Neuroticism did not predict abnormal connectivity in MDD (βN = .002, p = .99, R2 < .001); with extraversion added to the model: (βN = .39, p = .11; ∆R2 = .25, p < .05). Finally, connectivity of the total fronto-striatal cluster with the salience network was not predicted by word classification behavior (all β < .13, p > .55).
In healthy controls, extraversion and neuroticism were not predictive of fronto-striatal connectivity (βE = − .26, p = .21, R2 = .07; with neuroticism added to the model: βE = − .29, p = .20; ∆R2 = .005, n.s.; βN = .03, p = .91, R2 = .001; with extraversion added to the model: βN = − .08, p = .73, ∆R2 = .07, n.s.).
4. Discussion
In this study we investigated whole-brain functional connectivity in MDD during execution of an emotional word evaluation task using a subset of eight independently derived independent component networks. Consistent with previous studies (Anand et al., 2005; Cullen et al., 2009), we demonstrated decreased functional connectivity of the medial PFC, putamen, OFC, caudate nucleus, and ventral striatum (including the nucleus accumbens) with a fronto-opercular ‘salience’ network (Seeley et al., 2007) in medication-free MDD as compared with healthy control participants. Results were unexplained by age, education, sex, and brain volume of regions implicated in the neuroanatomical profile of MDD. Moreover, within MDD, functional connectivity of the fronto-striatal regions was unrelated to illness severity, but positively related to extraversion scores, which are strongly related to the tendency to engage in behavior that enhances positive affect. No differences in functional connectivity were observed within any other network.
These results confirm the hypothesis that abnormal intrinsic connectivity of cortical and subcortical and (para)limbic regions during emotional processing is part of the functional pathophysiology of MDD. Our results indicate that decreased coupling of medial prefrontal and striatal regions with the salience network might not be a state phenomenon of MDD. Instead it appears as a phenomenon that continues into the (newly) remitted phase, possibly representing a dimension that does not correlate with clinical recovery as measured with standard depression severity measurements. This dimension may relate to the propensity to engage in positive emotions (Cooper et al., 2000) and activities that promote social attention (Ashton et al., 2002), as connectivity in these regions was found to be uniquely related to the personality trait extraversion. Therefore, decreased connectivity of the fronto-striatal regions with the salience network may represent a long-lasting or trait characteristic of diminished capacity to engage in positive events and thoughts in a manner that can enhance positive mood states.
Importantly, the decreased subcortical–cortical coupling was observed with the salience network, a network that has been found to specifically correlate with subjective emotional ratings (Seeley et al., 2007). Furthermore, the salience network is the only network of the Smith et al. (2009) study that showed a high load on emotional processes and tasks in a large scale meta-analysis of the BrainMap database (Smith et al., 2009), but also loads on sensory perception, including pain perception, working memory, explicit memory, cognition, and action inhibition (Smith et al., 2009). The fronto-opercular salience network mainly comprises the dorsal anterior cingulate cortex, bilateral ventral lateral prefrontal cortices, and bilateral insula. These regions have been associated with emotional conflict resolution (Kerns et al., 2004), inhibitory control and mood regulatory processes (Johnstone et al., 2007), and the consciousness processing of affect (Craig, 2009). The insula is considered an important hub for processing salient events for action to be initiated, including calling on attentional resources (together with the anterior cingulate cortex) and regulating autonomic activity in reaction to salient stimuli (Menon, 2011). Disruption in this circuit therefore most likely leads to abnormalities in affective state regulation, and has previously been associated with MDD during rest (Cullen et al., 2009; Horn et al., 2010; Mayberg et al., 1999; Sheline et al., 2010; Veer et al., 2010).
Regions that were found to show abnormal connectivity with the salience network have been implicated in circuitry responsible for behavioral and affect regulation in neuropsychiatric disorders, including the anterior cingulate-, orbitofrontal-, and dorsolateral prefrontal circuitry (Mega and Cummings, 1994). It has been suggested that the medial PFC may play an important role in moderating visceral processes related to emotions (Price and Drevets, 2010). The nucleus accumbens has been repeatedly associated with reward processing and impaired motivation (Mega and Cummings, 1994). A failure to sustain activation in this region during up-regulation of positive emotional states has been linked to anhedonia in MDD patients (Heller et al., 2009). The medial OFC has been implicated in approach related behavior, reward anticipation, and fear-extinction (Milad and Rauch, 2007), and has numerous projections to visceral-motor structures that are critical to modulate behavior and emotional expression (Milad and Rauch, 2007; Price, 2003; Price and Drevets, 2010). Previously, activation in the medial OFC was found to be positively correlated with extraversion during reward processing (Mobbs et al., 2005). Finally, the dorsolateral caudate nucleus and frontal pole have been implicated in a circuitry related to the integration of prefrontal and subcortical activity for executive control (Mega and Cummings, 1994). Together, the present results indicate compromised functioning of a distributed network involved in behavioral modulation and -expression, and reward processing in MDD.
In this study, we included a well-characterized sample of right-handed, medication free MDD patients without any past or present comorbid psychopathology. Moreover, we used a new approach to study functional connectivity during the execution of an emotional word classification task. We used template ICNs to create subject specific component maps, based on the time series that best fit the weighted templates using the dual regression approach (Beckmann et al., 2009). These template components have been found to be highly consistent and have been independently detected in a number of populations (e.g., Alzheimer's disease (Rombouts et al., 2009)), MDD (Veer et al., 2010), and healthy controls (Beckmann et al., 2005). Overall, use of the template ICNs and the dual regression approach resulted in highly consistent components across groups that strongly corresponded to the template ICNs (Appendix B). The mean across-group ICNs also included some additional brain regions not in the template ICNs, possibly reflecting regions that show coupling with the template ICNs during the emotional word encoding task (as compared to the resting state), though such additional regions could also be the result of greater statistical sensitivity in the current sample and thus precludes an in-depth interpretation. A strength of the current approach is that it is not limited to standard fMRI task paradigms with associated restrictions on the timing and number of trials, but can be used to study sustained engagement of brain networks over the duration of a task involving multiple trials and even trial types. Another advantage is that the template-ICN dual regression method allows for the study of connectivity across groups and tasks in a standardized and unbiased way, which is crucial for studies involving comparisons between clinical and non-clinical samples. Using template ICNs should increase reproducibility of effects and comparability across studies, due to the independence of the ICN templates in reference to the data under study.
In this study, we included a well-characterized sample of right-handed, medication free MDD patients without any past or present comorbid psychopathology. Moreover, we used a new approach to study functional connectivity during the execution of an emotional word classification task. We used template ICNs to create subject specific component maps, based on the time series that best fit the weighted templates using the dual regression approach (Beckmann et al., 2009). These template components have been found to be highly consistent and have been independently detected in a number of populations (e.g., Alzheimer's disease (Rombouts et al., 2009)), MDD (Veer et al., 2010), and healthy controls (Beckmann et al., 2005). Overall, use of the template ICNs and the dual regression approach resulted in highly consistent components across groups that strongly corresponded to the template ICNs (Appendix B). The mean across-group ICNs also included some additional brain regions not in the template ICNs, possibly reflecting regions that show coupling with the template ICNs during the emotional word encoding task (as compared to the resting state), though such additional regions could also be the result of greater statistical sensitivity in the current sample and thus precludes an in-depth interpretation. A strength of the current approach is that it is not limited to standard fMRI task paradigms with associated restrictions on the timing and number of trials, but can be used to study sustained engagement of brain networks over the duration of a task involving multiple trials and even trial types. Another advantage is that the template-ICN dual regression method allows for the study of connectivity across groups and tasks in a standardized and unbiased way, which is crucial for studies involving comparisons between clinical and non-clinical samples. Using template ICNs should increase reproducibility of effects and comparability across studies, due to the independence of the ICN templates in reference to the data under study.
Despite a number of methodological strengths, some limitations should be mentioned. In this study, we pooled data from different scanning sites which might have introduces some unmodeled variance. However, we were careful in matching patient group and control group on site characteristics, and ensured that clinical differences did not occur between patients scanned at different locations. Moreover, we added variations in scanning site to our model, and results did not differ from the model where site was not added (post-hoc). Second, the NEO-FFI was not administered at the day of scanning, but on average, 2 months before during the NESDA baseline measurement. However, increasing the time between two measures will tend to decrease the correlation between them, with larger decreases for less reliable measures, thus once again leading if anything to an underestimate of effects. Nevertheless, the administration of the NEO-FFI and a formal measure of positive and negative affect (for example the PANAS; Watson et al., 1988) and an additional measurement of anhedonia on the day of scanning would be preferable in future studies to maximize sensitivity.
In this study, we have demonstrated, using an unbiased whole-brain functional connectivity approach, decreased connectivity of medial prefrontal regions and ventral subcortical and paralimbic regions in MDD during the execution of an emotional word evaluation task with a distributed salience network. Connectivity of these regions correlated specifically with a propensity to engage in behavior that enhances positive affect, and not with negative affect. We therefore propose that this inability to effectively regulate positive mood states may constitute a second path that contributes to getting ‘stuck in the rut’ (Holtzheimer and Mayberg, 2011). Future studies should test this hypothesis, in addition to investigating the specificity and generalizability of our results to different MDD sub-samples and different tasks. Given the power of this technique to compare distributed patterns of connectivity in an unbiased way, future studies should also examine treatment-related changes of connectivity with the salience network, specifically treatments probing positive affect.
Funding
This work was supported by the NESDA infrastructure (www.nesda.nl), which is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Healthcare (IQ healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute)). S.A.R.B. Rombouts is the recipient of a (VIDI) grant from The Netherlands Organization for Scientific Research (NWO, grant number917 863 68). The Erasmus staff exchange program is acknowledged for making the collaboration between M.J. van Tol and T. Johnstone possible.
Disclosures
M.J. van Tol, I.M. Veer, S.A.R.B. Rombouts, D.J. Veltman, M.A. van Buchem, F.G. Zitman, and I.T. Johnstone report no financial interests or potential conflicts of interest. A. Aleman received an investigator-initiated unrestricted research grant from Brystol-Myers Squibb and speakers bureau honoraria from AstraZeneca, Brystol-Myers Squibb, GlaxoSmithKline and Janssen. N.J.A. van der Wee received speaking fees from Eli Lilly and Wyeth; and served on advisory panels of Eli LIlly, Pfizer, Wyeth and Servier.
The following are the supplementary data related to this article.
Descriptives of the NESDA Neuroimaging Sample.
Mean spatial maps over all subjects and comparison with template independent components.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2013.05.012.
Acknowledgment
The infrastructure for the NESDA (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Healthcare (IQ healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos Institute)). S.A.R.B. Rombouts is the recipient of a (VIDI) grant from The Netherlands Organization for Scientific Research (NWO, grant number 917 863 68). The Erasmus staff exchange program is acknowledged for making the collaboration between M.J. van Tol and T. Johnstone possible. Development of analysis techniques were supported by BBSRC grant BB/H011021/1 to T. Johnstone. We thank Carien van Reekum for helpful suggestions during the preparation of this manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Marie-José van Tol, Email: m.j.van.tol@umcg.nl, mariejosevantol@gmail.com.
Tom Johnstone, Email: i.t.johnstone@reading.ac.uk.
References
- Anand A., Li Y., Wang Y., Wu J., Gao S., Bukhari L., Mathews V.P., Kalnin A., Lowe M.J. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Ashton M.C., Lee K., Paunonen S.V. What is the central feature of extraversion? Social attention versus reward sensitivity. Journal of Personality and Social Psychology. 2002;83:245–252. [PubMed] [Google Scholar]
- Beck A.T., Epstein N., Brown G., Steer R.A. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C., Filippini N., Smith S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage. 2009;47:S148. [Google Scholar]
- Bluhm R., Williamson P., Lanius R., Theberge J., Densmore M., Bartha R., Neufeld R., Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry and Clinical Neurosciences. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Bora E., Harrison B.J., Davey C.G., Yucel M., Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal–pallidal–thalamic circuits in major depressive disorder. Psychological Medicine. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Carballedo A., Scheuerecker J., Meisenzahl E., Schoepf V., Bokde A., Moller H.J., Doyle M., Wiesmann M., Frodl T. Functional connectivity of emotional processing in depression. Journal of Affective Disorders. 2011;134:272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Chen C.H., Suckling J., Ooi C., Fu C.H., Williams S.C., Walsh N.D., Mitterschiffthaler M.T., Pich E.M., Bullmore E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Cooper M.L., Agocha V.B., Sheldon M.S. A motivational perspective on risky behaviors: the role of personality and affect regulatory processes. Journal of Personality. 2000;68:1059–1088. doi: 10.1111/1467-6494.00126. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McGrae R.R. Psychological Assessment Resources; FL: 1992. Revised NEO personality inventory and NEO five-factor inventory. [Google Scholar]
- Craig A.D. How do you feel—now? The anterior insula and human awareness. Nature Review Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cullen K.R., Gee D.G., Klimes-Dougan B., Gabbay V., Hulvershorn L., Mueller B.A., Camchong J., Bell C.J., Houri A., Kumra S., Lim K.O., Castellanos F.X., Milham M.P. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S.M., Veltman D.J., Rombouts S.A., Raaijmakers J.G., Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Demenescu L.R., Renken R., Kortekaas R., van Tol M.J., Marsman J.B., van Buchem M.A., van der Wee N.J., Veltman D.J., den Boer J.A., Aleman A. Neural correlates of perception of emotional facial expressions in outpatients with mild-to-moderate depression and anxiety: multicenter fMRI study. Psychological Medicine. 2011;41:2253–2264. doi: 10.1017/S0033291711000596. [DOI] [PubMed] [Google Scholar]
- Filippini N., Ebmeier K.P., MacIntosh B.J., Trachtenberg A.J., Frisoni G.B., Wilcock G.K., Beckmann C.F., Smith S.M., Matthews P.M., Mackay C.E. Differential effects of the APOE genotype on brain function across the lifespan. NeuroImage. 2011;54:602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S., Nichols T.E. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Shackman A.J., Light S.N., Peterson M.J., Kolden G.G., Kalin N.H., Davidson R.J. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer P.E., Mayberg H.S. Stuck in a rut: rethinking depression and its treatment. Trends in Neurosciences. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D.I., Yu C., Steiner J., Buchmann J., Kaufmann J., Osoba A., Eckert U., Zierhut K.C., Schiltz K., He H., Biswal B., Bogerts B., Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression — the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal–subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns J.G., Cohen J.D., MacDonald A.W., III, Cho R.Y., Stenger V.A., Carter C.S. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N., Zoethout R.M., Beckmann C.F., Baerends E., de Kam M.L., Soeter R.P., Dahan A., van Buchem M.A., van Gerven J.M., Rombouts S.A. Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Human Brain Mapping. 2012;33:1003–1018. doi: 10.1002/hbm.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., Le B.G., Mayberg H., Volle E., Bergouignan L., Lehericy S., Allilaire J.F., Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4:305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S., Wu Q., Qiu L., Yang X., Kuang W., Chan R.C., Huang X., Kemp G.J., Mechelli A., Gong Q. Resting-state functional connectivity in treatment-resistant depression. The American Journal of Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Liotti M., Brannan S.K., McGinnis S., Mahurin R.K., Jerabek P.A., Silva J.A., Tekell J.L., Martin C.C., Lancaster J.L., Fox P.T. Reciprocal limbic–cortical function and negative mood: converging PET findings in depression and normal sadness. The American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mega M.S., Cummings J.L. Frontal–subcortical circuits and neuropsychiatric disorders. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007;1121:546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Hagan C.C., Azim E., Menon V., Reiss A.L. Personality predicts activity in reward and emotional regions associated with humor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16502–16506. doi: 10.1073/pnas.0408457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Penninx B.W., Beekman A.T., Smit J.H., Zitman F.G., Nolen W.A., Spinhoven P., Cuijpers P., De Jong P.J., Van Marwijk H.W., Assendelft W.J., Van Der M.K., Verhaak P., Wensing M., De G.R., Hoogendijk W.J., Ormel J., Van D.R. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. International Journal of Methods in Psychiatric Research. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Price J.L. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L.N., Wing J., Wittchen H.U., Helzer J.E., Babor T.F., Burke J., Farmer A., Jablenski A., Pickens R., Regier D.A. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rombouts S.A., Damoiseaux J.S., Goekoop R., Barkhof F., Scheltens P., Smith S.M., Beckmann C.F. Model-free group analysis shows altered BOLD FMRI networks in dementia. Human Brain Mapping. 2009;30:256–266. doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosellini A.J., Brown T.A. The NEO five-factor inventory: latent structure and relationships with dimensions of anxiety and depressive disorders in a large clinical sample. Assessment. 2011;18:27–38. doi: 10.1177/1073191110382848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.J., Giles D.E., Schlesser M.A., Fulton C.L., Weissenburger J., Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Research. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De L.M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De S.N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Miller G.A., Engels A.S., Herrington J.D., Sutton B.P., Banich M.T., Heller W. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. NeuroImage. 2011;54:661–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc., IL, USA.
- van Tol M.J., van der Wee N.J., van den Heuvel O.A., Nielen M.M., Demenescu L.R., Aleman A., Renken R., van Buchem M.A., Zitman F.G., Veltman D.J. Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry. 2010;67:1002–1011. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- van Tol M.J., van der Wee N.J., Demenescu L.R., Nielen M.M., Renken R., Aleman A., van Buchem M.A., Zitman F.G., Veltman D.J. fMRI correlates of visuospatial planning in depression and anxiety disorders. Acta Psychiatrica Scandinavica. 2011;124:273–284. doi: 10.1111/j.1600-0447.2011.01702.x. [DOI] [PubMed] [Google Scholar]
- van Tol M.J., Demenescu L.R., van der Wee N.J., Nielen M.M., den Boer J.A., Kortekaas R., Renken R., Zitman F.G., van Buchem M.A., Aleman A., Veltman D.J. Emotional word encoding and recognition in depression and anxiety disorders. Biological Psychiatry. 2012;71:593–602. doi: 10.1016/j.biopsych.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., Ferrarini L., Milles J., Veltman D.J., Aleman A., van Buchem M.A., van der Wee N.J., Rombouts S.A. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience. 2010;4 doi: 10.3389/fnsys.2010.00041. (pii: 41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Yu C., Zheng H., Liu Y., Song M., Qin W., Li K., Jiang T. Increased neural resources recruitment in the intrinsic organization in major depression. Journal of Affective Disorders. 2010;121:220–230. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Descriptives of the NESDA Neuroimaging Sample.
Mean spatial maps over all subjects and comparison with template independent components.


