Abstract
Introduction
Over the last years, evidence has accumulated that rolandic epilepsy (RE) is associated with serious cognitive comorbidities, including language impairment. However, the cerebral mechanism through which epileptiform activity in the rolandic (sensorimotor) areas may affect the language system is unknown. To investigate this, the connectivity between rolandic areas and regions involved in language processing is studied using functional MRI (fMRI).
Materials and methods
fMRI data was acquired from 22 children with rolandic epilepsy and 22 age-matched controls (age range: 8–14 years), both at rest and using word-generation and reading tasks. Activation map analysis revealed no group differences (FWE-corrected, p < 0.05) and was therefore used to define regions of interest for pooled (patients and controls combined) language activation. Independent component analysis with dual regression was used to identify the sensorimotor resting-state network in all subjects. The associated functional connectivity maps were compared between groups at the regions of interest for language activation identified from the task data. In addition, neuropsychological language testing (Clinical Evaluation of Language Fundamentals, 4th edition) was performed.
Results
Functional connectivity with the sensorimotor network was reduced in patients compared to controls (p = 0.011) in the left inferior frontal gyrus, i.e. Broca's area as identified by the word-generation task. No aberrant functional connectivity values were found in the other regions of interest, nor were any associations found between functional connectivity and language performance. Neuropsychological testing confirmed language impairment in patients relative to controls (reductions in core language score, p = 0.03; language content index, p = 0.01; receptive language index, p = 0.005).
Conclusion
Reduced functional connectivity was demonstrated between the sensorimotor network and the left inferior frontal gyrus (Broca's area) in children with RE, which might link epileptiform activity/seizures originating from the sensorimotor cortex to language impairment, and is in line with the identified neuropsychological profile of anterior language dysfunction.
Abbreviations: RE, rolandic epilepsy; ICA, independent component analysis
Keywords: Rolandic epilepsy, Language impairment, Resting-state fMRI, Independent component analysis, Resting-state networks, Sensorimotor/rolandic network
Graphical abstract
Highlights
► Using fMRI, it was demonstrated that the motor and language system are integrated. ► In rolandic epilepsy, functionally connectivity with the rolandic network is locally decreased. ► These findings provide an physiological explanation of language impairment in RE.
1. Introduction
Rolandic epilepsy (RE) is an idiopathic focal epilepsy of childhood with typical onset at age 7–10 years (Loiseau and Duché, 1989; Panayiotopoulos et al., 2008). The epileptic focus is mostly located in the inferior part of the rolandic area (i.e. the pre- and postcentral gyri), seizures are relatively mild and typically nocturnal, and involve hemifacial spasms and speech arrest (Loiseau and Duché, 1989; Panayiotopoulos et al., 2008). Furthermore, spontaneous remission of seizures is typically seen during adolescence. Given these characteristics, RE is classically considered a benign condition and is also known as benign (rolandic) epilepsy (of childhood) with centro-temporal spikes (BECTS), which is, however, insensitive to the distress inflicted on the children and their families by these events.
Recently RE has been associated with a variety of visuomotor, neuropsychological and cognitive comorbidities (Deltour et al., 2007, 2008; Kavros et al., 2008), of which language impairment is one of the most prominent (Clarke et al., 2007; Jocic-Jakubi and Jovic, 2006; Liasis et al., 2006; Lillywhite et al., 2009; Lundberg et al., 2005; Monjauze et al., 2005). It was recently suggested that the language impairments may be present before the onset of seizures (Overvliet et al., 2011) and may persist after seizure remission (Kanemura and Aihara, 2009; Monjauze et al., 2011). In this light, in RE, the prevention of language impairment might be considered of higher priority than seizure control.
Cognitive impairment risk has been associated with interictal epileptiform discharges in pediatric epilepsy including RE (Massa et al., 2001; Nicolai et al., 2007; Overvliet et al., 2010), but the underlying mechanism remains to be elucidated. Neuropsychological testing and functional MRI (fMRI) suggest anterior language dysfunction in RE (Lillywhite et al., 2009; Yuan et al., 2006), however a better understanding of the functional circuits linking (epileptiform activity in) the rolandic areas with language areas/dysfunction seems of major importance in this context.
In the current study, we aim to link epileptiform activity/seizures originating from the rolandic cortex with language impairment in children with RE using fMRI. We employed independent component analysis (ICA) to segment resting-state fMRI data from a group of children with RE and age-matched controls into distinct functional networks (Beckmann et al., 2005; Calhoun et al., 2009). ICA is a robust data-driven method, allows the study of functional organization on the whole brain level, and precludes the a priori definition of (a sparse set of) regions on interest (Cole et al., 2010). From the ICA output, we selected the network with maximum involvement of the bilateral pre- and postcentral gyri and, given the location of the epileptic focus, hypothesize that this rolandic network is impaired in RE. To infer on abnormalities associated with language impairment, we investigated rolandic network functional connectivity in language-mediating regions of interest derived from task fMRI (word-generation and reading tasks). To relate our findings to language performance, neuropsychological language testing was performed (Clinical Evaluation of Language Fundamentals, 4th edition).
2. Methods
2.1. Study population
Twenty-two children with a clinical diagnosis of RE (6 girls) were selected at our specialized epilepsy referral center (see selection criteria below). The age at seizure onset was (mean ± SD) 7.5 ± 2.3 years and the age at testing 11.4 ± 1.8 years, half of the subjects (11/22) had ongoing seizures (at least 1 seizure over the 6 months prior to scanning). Two children were left handed and 1 was ambidextrous. For comparison, 22 age-matched controls were included (11 girls, age 10.3 ± 1.7 years, 2 left handed). For further characteristics, see Table 1.
Table 1.
Subject characteristics. Note that age at epilepsy onset, epilepsy duration, and seizure frequency are difficult to accurately establish given the mild and typically nocturnal nature of the seizures, which may lead to delayed diagnosis and underestimation of the number of seizures.
| Subject characteristics | RE | Controls |
|---|---|---|
| N | 22 | 22 |
| Age [y] | 11.4 ± 1.8 | 10.3 ± 1.7 |
| Age at epilepsy onset [y] | 7.5 ± 2.3 | n.a. |
| Epilepsy duration [y] | 2.4 ± 2.0 | n.a. |
| Seizure frequency [per y] | 2.3 ± 1.6 | n.a. |
| Gender (male/female) | 16/6 | 11/11 |
| Handedness (r/l/ambidexter) | 19/2/1 | 20/2/0 |
| Number of AEDs (0/1/> 1) | 12/5/5 | n.a. |
N, number; AED, anti epileptic drug; n.a., not applicable. Notation: mean ± SD.
The study was approved by the medical ethics committees of both participating institutions and written informed consent was obtained from the participating children's parents and/or care-givers.
2.2. Selection criteria
Patient selection was based on criteria concerning seizure semiology and EEG as described in the literature (Berroya et al., 2005; Panayiotopoulos et al., 2008). EEG criteria include the presence of spike and slow wave complexes occurring as individual paroxysms or in repetitive clusters with a maximum in the mid temporal and/or central electrodes and with a temporal-frontal dipole field. Additional independent central, mid temporal, parietal or occipital spike wave foci in the same or other hemisphere were allowed. To exclude severe cases (Landau–Kleffner syndrome (LKS) or LKS-like), interictal epileptiform activity was required to be present < 85% of the time during non-REM sleep. With respect to seizure semiology, seizures with anarthria, hemiclonia involving the face and/or unilateral extremities, or secondarily generalized seizures were considered. In case of poorly observed nocturnal seizures (3 cases), post ictal signs of a generalized seizure or confirmation of post-ictal hemiparesis were sufficient for inclusion in case of otherwise typical EEG.
The children with RE underwent neuropsychological testing using the Wechsler Intelligence Score for Children, third edition, Dutch version (WISC-III, http://www.pearsonclinical.nl/tests), and all had a full-scale IQ > 70. None of the healthy controls had (a history of) dyslexia, learning disorders or neurological/psychiatric disorders, or attended special education. Children were excluded if they had dental braces (MRI quality), were somewhat afraid in the scanner, or had structural brain abnormalities on conventional MRI.
2.3. Magnetic resonance imaging
Imaging was performed on a 3 T MRI systems (Philips Achieva, Best, the Netherlands) using an 8-element receive-only head coil. Both resting-state and task fMRI data was acquired; resting-state data was acquired before task data. In addition, structural MRI was performed for anatomical reference and involved a T1-weighted scan with the following settings: 3D fast spoiled gradient echo sequence, echo time/repetition time/inversion time (TE/TR/TI) 3.8/8.3/1022 ms, 1 × 1 × 1 mm3 resolution, and acquisition time 8 min.
Functional MRI involved a T2*-weighted blood oxygen level dependent (BOLD) sequence comprising 195 dynamic acquisitions at TR 2 s, resulting in a total dynamic acquisition time of 6.5 min. Other settings included: single-shot echo planar imaging (EPI) sequence, TE 35 ms, 2 × 2 mm2 in plane resolution, and 4 mm axial slices.
Task fMRI involved a word-generation and a reading task. A standard block design was used and comprised six 30 s task blocks interleaved with 30 s baseline blocks. Each paradigm started and ended with a baseline block.
The word-generation task involved visual presentation of a letter (U-N-K-A-E-P) interleaved with presentation of an asterisk for visual fixation. Subjects were instructed to covertly generate as many words as possible starting with the presented letter.
In the reading task, text with semantic meaning (task block) was interleaved with nonsense text (baseline). To ensure continuous reading, the text was refreshed 3 times per block (i.e. every 10 s). Each text frame consisted of 4 lines and for the meaningful text, only the first 2 lines of each frame were essential for text continuity (short story).
2.4. fMRI preprocessing
fMRI preprocessing was performed using SPM8 (Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/). First, the dynamic series were realigned to correct for head movement using rigid body transformations (rotation and translation) and aligned to the structural scan. Next, the structural scan was segmented into gray matter, white matter, and cerebrospinal fluid, and registered into Montreal Neurological Institute (MNI) standard space. This normalization was subsequently also applied to the realigned dynamic series to generate movement corrected normalized fMRI data suitable for group analysis.
2.5. Activation mapping
Task-related dynamic series were smoothed using a Gaussian kernel of full width at half maximum (FWHM) 6 mm and analyzed using general linear models (GLMs) in SPM8. The task block design was convolved with a canonical hemodynamic response function (HRF) to model the task-related BOLD response. In addition, the movement parameters from the realignment step were used as regressors of no interest to model residual movement.
Significant activations were assessed using t-contrasts of task regressors; word-generation was contrasted versus baseline and meaningful text versus nonsense text. Voxel-wise effects were corrected for multiple comparisons using family wise error (FWE) p-value adjustment at p < 0.05.
A standard random-effects analysis was used for group analysis. As no significant group differences were found (p > 0.05, FWE corrected), pooled activation maps (patients and controls combined) were generated. Spherical regions of interest (ROIs) of language activation with a radius of 10 mm were defined at local maxima of pooled activation. In case of unilateral activation, a contralateral homotopic ROI was defined by mirroring with respect to the median plane.
2.6. Resting-state independent component analysis
Preprocessed resting-state dynamic series were spatio-temporally filtered and subjected to group ICA using FSL's MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components, v3.10, FMRIB's Software Library, Oxford, UK; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC). Filtering involved Gaussian smoothing (FWHM 5 mm) and temporal filtering at a 100 s (0.01 Hz) high pass filter cut off. Group ICA involved temporal concatenation of all resting-state datasets (patients as well as controls) and assessment of independent time series and associated spatial maps (BOLD related resting-state networks and noise induced artifactual maps). The number of components was estimated from the data using the build-in Bayesian approach.
Since resting-state networks represent distributed synchronization of spontaneous fluctuations in neuronal activity, they represent across subjects as similar spatial patterns (comparable neuronal circuits), but different time series. FSL's dual regression was used (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/DualRegression), which employs this observation to map pooled independent component (IC) maps to the individual subject level in a two step approach (Beckmann et al., 2009; Filippini et al., 2009). First, for each subject, the resting-state data was regressed against the pooled IC maps for each dynamic scan (spatial regression) to obtain subject specific time series. Second, these IC time series were used to calculate subject specific spatial maps (temporal regression), which were normalized by the residual noise to represents the functional connectivity with the corresponding network (Z-statistic).
The IC maps were visually inspected to select the network of maximum overlap with the bilateral pre- and postcentral gyri (i.e. the rolandic areas). A GLM framework using FSL's randomize (v2.8) and 5000 permutations was used to calculate the corresponding group networks (p < 0.05, cluster corrected). Furthermore, for each subject the functional connectivity with this network (Z-statistic) was assessed for each language ROI (see Fig. 1) and compared between patients and controls using two-tailed Student's t-tests; results were corrected for multiple comparisons using a false discovery rate (FDR) of 10%. Following Voets et al. (2012), this analysis was not confined to ROIs within the rolandic network to allow the detection of connectivity abnormalities beyond the network boundaries.
Fig. 1.
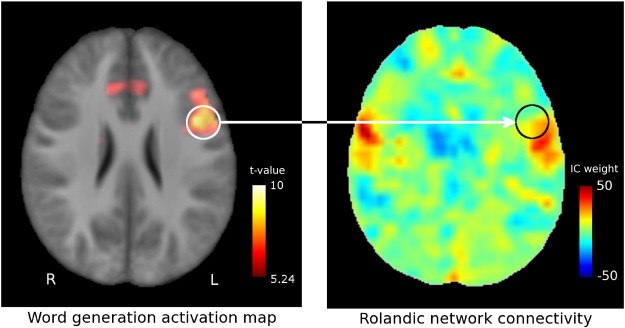
Region of interest rolandic network connectivity. The left inferior frontal gyrus is selected as a region of interest from the pooled word-generation activation map (left) and used to extract subject-specific values from the rolandic connectivity maps (right), which represent the degree of similarity between the voxel time series and the rolandic independent component (weight of the rolandic independent component in a full independent component fit to the data). Color bars represent pooled activation level (t-contrast, corrected for family-wise error (FWE) at p < 0.05) and rolandic connectivity for a representative subject, respectively. Images are normalized to MNI-space.
For completeness and consistency, the same approach was applied to the task data to check for activation differences on the ROI level.
2.7. Language assessment
All children underwent the Clinical Evaluation of Language Fundamentals, fourth version (CELF-4), Dutch edition (Semel et al., 2010). The CELF-4 is the gold standard for language assessment in children and provides several age-corrected measures for language performance (Paslawski, 2005). The core language score was assessed in all subjects to check for language impairment. To infer the specific impairment profile, in the patient group specific metrics such as expressive and receptive language index were also assessed.
Outcomes were compared between groups and to norm scores (mean ± SD: 100 ± 15) using 2-tailed Student's t-tests (p < 0.05). Furthermore, within all language ROIs it was tested for associations between rolandic network connectivity and language metrics using Spearman's correlation (p < 0.05).
3. Results
3.1. Activation mapping
Pooled (patients and controls combined) activation maps for the word-generation and reading tasks and associated ROIs are given in Fig. 2. Task-related BOLD signal fluctuations (i.e. activation) were found in the anterior cingulate cortex, bilateral insular regions, and the left inferior prefrontal cortex. Reading task activation was seen in the bilateral mid temporal gyri and posteriorly displayed a left predominance. See Table 2 for more information on these 9 language-mediating regions of interest.
Fig. 2.
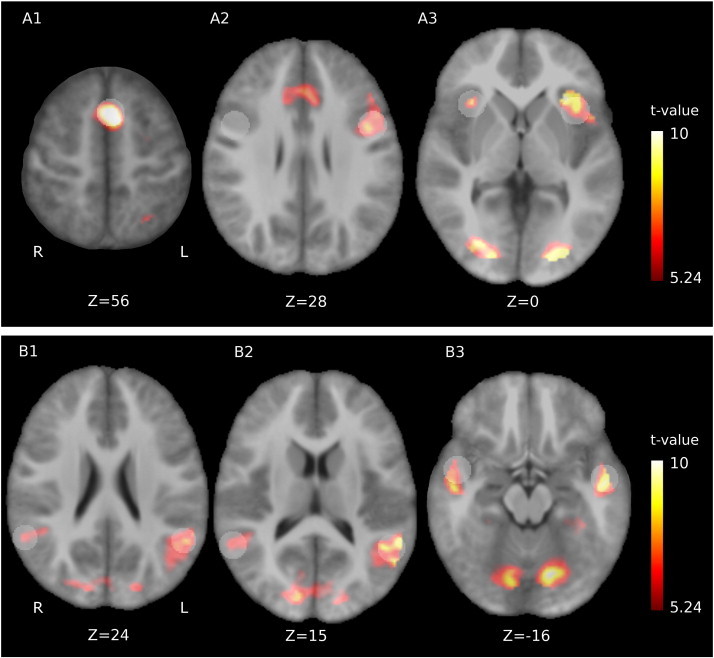
Activation maps. Pooled activation maps (i.e. patients and controls combined) for A: word-generation and B: reading. In word-generation, the anterior cingulate cortex (A1), left inferior prefrontal cortex (A2) and bilateral insular regions (A3) were activated. Reading induced activity in posterior (B1–2) and anterior (B3) bilateral mid temporal regions. Z-values indicate MNI slice coordinates, the colorbar gives the activation t-statistic and activation maps are given for p < 0.05 (family-wise error (FWE) corrected). Regions of interest are overlaid in transparent white, the one in the right inferior prefrontal cortex (A2) was constructed by mirroring with respect to the median plane. Z-values indicate axial MNI coordinates.
Table 2.
Regions of interest (ROIs) for activation in reading and word-generation. MTG: mid temporal gyrus; ant: anterior; post: posterior; R: right; L: left; ACC: anterior cingulate cortex; IFG: inferior frontal gyrus.
| Task ROIs | Description | MNI-coordinate [mm] | t-Value (ROI mean) |
|---|---|---|---|
| Word gen | ACC | (− 2,14,54) | 8.7 |
| IFG L | (− 48,7,26) | 6.8 | |
| IFG R | (48,7,26)a | 1.0 | |
| Insula L | (− 38,15,0) | 4.8 | |
| Insula R | (34,20,3) | 2.5 | |
| Reading | MTG R ant | (50,0,− 18) | 2.6 |
| MTG R post | (58,− 50,18) | 3.9 | |
| MTG L ant | (− 52,− 6,− 18) | 3.8 | |
| MTG L post | (− 52,− 52,18) | 6.2 |
Constructed from the contralateral (activation-based) ROI by mirroring with respect to the median plane.
In both tasks, activation was also found in the occipital lobes, due to the visual task presentation.
3.2. Rolandic network differences
Pooled probabilistic group ICA identified 34 independent components; the component identified as the rolandic network is depicted in Fig. 3 (p < 0.05, uncorrected). This network covered bilateral pre- and postcentral gyri (sensorimotor areas), but in addition included perisylvian (among which superior temporal) regions, as well as bilateral cerebellar and medial regions. Furthermore, the involvement of a left prefrontal region is suggested (see arrowheads), which was absent at the right.
Fig. 3.
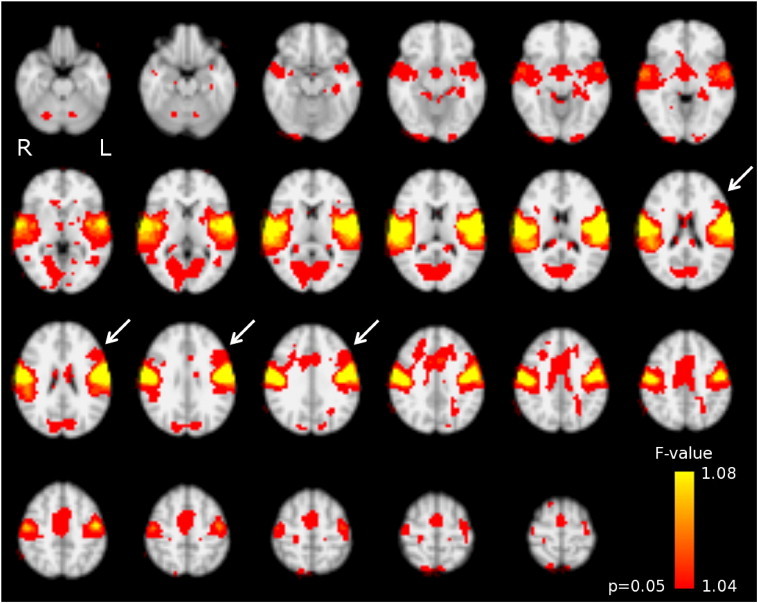
Rolandic resting-state network as identified using pooled group independent component analysis. This involves the bilateral sensorimotor areas (pre- and postcentral gyrus), superior temporal, cerebellar, and medial regions. Note the involvement of a left inferior prefrontal region (arrowheads), which is absent at the right. Colorbar: voxel-wise F-value for the test on the relevance of the rolandic independent component (IC) to the full-IC fit of the pooled data concatenated over time. Results are normalized to MNI-space.
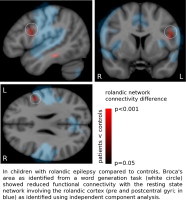
Upon further investigation, this region corresponded to the left inferior frontal gyrus showing activation for the word-generation task, and had significantly reduced rolandic network connectivity in patients compared to controls (ROI centered at MNI coordinate − 48,7,26 mm; p = 0.011, FDR corrected), see Fig. 4.
Fig. 4.
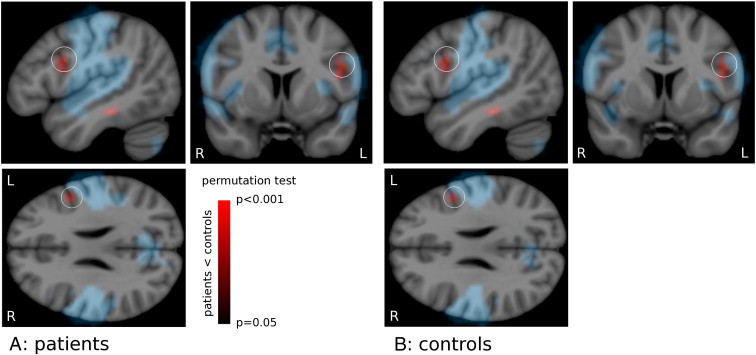
Local reduction of rolandic network connectivity. The rolandic network of A) controls and B) patients (blue). Rolandic network functional connectivity is reduced in patients compared to controls (red colorbar; identical overlay in A and B) outside (but directly adjacent to) the network itself. This local reduction of connectivity coincides with a region of activation for word-generation (white circle; p = 0.011). See text for details. Maps generated by permutation testing (N = 5000, p < 0.05); rolandic network cluster corrected. Colorbar: p-value for the group difference. Results are normalized to MNI-space.
No other ROI-wise reductions in rolandic network connectivity were found, nor were any ROIs identified in which rolandic network connectivity was increased.
Also with respect to the activation maps, no ROI-wise differences were found in the respective ROIs for neither the word-generation nor the reading task.
3.3. Language assessment
As expected, patients had lower core language scores than controls (95 ± 18 vs 105 ± 11, respectively; p = 0.03). Furthermore, patients scored below the norm (100 ± 15) on language content index (88 ± 18; p = 0.01) and receptive language index (86 ± 19; p = 0.005). Also a trend of reduced language working memory was found (92 ± 18; p = 0.09).
3.4. Association between connectivity and language metrics
No associations were found between language metrics and functional connectivity for any of the language ROIs.
4. Discussion
In this study, we attempted to link the rolandic nature of RE (given the location of the epileptic focus) with the growing insight that language impairment may be an inherent trait of this type of epilepsy. We used functional MRI to study the rolandic network in association with language areas in children with RE employing resting-state and language task fMRI in a task-informed resting-state analysis. We hypothesized that the rolandic network is disturbed in RE and investigated whether the aberrant regions co-localize with well-known language areas as identified by task fMRI. Furthermore, we correlated rolandic network connectivity within these language areas with language metrics as assessed by neuropsychological testing.
4.1. Major findings
The rolandic network was successfully identified from resting state data using group ICA. This network extended beyond the sensorimotor cortex to include superior temporal regions and also seemed to involve a left inferior prefrontal region. This region was identified as Broca's area using activation maps of a word generation task, and displayed significantly reduced rolandic network connectivity in patients compared to controls. No associations were found between rolandic network connectivity and neuropsychological metrics of language performance in any of the language task ROIs (reading and word generation).
4.2. Functional (sub)networks
The network we refer to as “rolandic” resembles one of a limited set of canonical resting-state networks typically referred to as the sensorimotor network (Beckmann et al., 2005; Cole et al., 2010). However, this naming conveys a functional interpretation, which we deemed inappropriate in the current context since it was selected based purely on involvement of the rolandic areas, but actually was more extensive. Generally, even though close similarities exist between resting-state networks and task-derived activation patterns, their correspondence is not one-to-one, which complicates comprehensive and complete functional descriptions of resting-state networks (Smith et al., 2009). For this reason, we found it more adequate to adopt an anatomical-descriptive naming interpretable in the context of the pathology under study (rolandic network and RE, respectively). Indeed, the rolandic network as identified in this study was demonstrated to be not purely of primary sensorimotor nature, but included perisylvian regions and also seemed to extend into the left inferior frontal gyrus (Broca's area). Similarly, in recent work on hierarchical clustering of resting state networks based on similarity of characteristic time series, the rolandic and superior temporal regions were clustered into a so-called centro-temporal module (Doucet et al., 2011, 2012). In general, separation or merging of functional networks not only depends on intrinsic functional architecture, but is also determined by the dimensionality of the IC decomposition. More fine-grained functional parcellations can be constructed by increasing the number of components, but this will put additional demands on data quality (Cole et al., 2010). The Bayesian dimensionality estimation we employed indicated 34 components to be supported by our data (resting-state acquisitions of 6.5 min at TR = 2 s), which is a typical order for the relatively short resting-state acquisitions (5–10 min) presently reported in literature (Doucet et al., 2011; Greicius et al., 2003; Voets et al., 2012). Our results indicate that at this level of detail, superior temporal, rolandic and possibly left inferior frontal regions cluster within the same functional network, although this finding does not preclude their separation into more specific sub-networks at higher-dimensional analysis. Furthermore, it is known that functional specialization of neuronal networks is progressively established during maturation and is not yet completed at the age range under study (8–14 years; (Joseph et al., 2012; Rubia, in press)).
4.3. Connectivity differences beyond functional networks
Note that although the involvement of the left inferior frontal gyrus in the rolandic network was suggested by the uncorrected pooled results (Fig. 3), this was not confirmed by the (cluster-corrected) group maps (Fig. 4, in blue). Indeed, Tomasi et al. performed a resting-state study of the language network in adults (970 subjects), in which involvement of the rolandic areas could not be demonstrated (Tomasi and Volkow, 2012). However, we were able to demonstrate significantly reduced connectivity with the rolandic network in patients compared to controls in the left inferior frontal gyrus on the ROI level, i.e. outside (but directly adjacent to) the rolandic network. The finding of aberrant connectivity beyond the functional network under investigation is not uncommon; for example the recruitment of the right frontal lobe into the left-lateralized language network has been described in left-sided temporal lobe epilepsy (Waites et al., 2006). Recently Voets et al. also reported connectivity abnormalities in temporal lobe epilepsy beyond the resting-state networks under investigation, among others in an “extended sensorimotor network”, incidentally also involving temporal regions (Voets et al., 2012).
4.4. Functional integration of motor and language networks
The reduced integration of Broca's area into the rolandic network might be of relevance for understanding language impairment in RE. Indeed, an extensive body of literature exists on the relevance of the motor system for language (Cappa and Pulvermuller, 2012; Meister et al., 2003; Pulvermuller et al., 2006). Remarkably, this integration goes beyond the (somewhat trivial) relevance of the (pre)motor cortex in the coordination of complex articulatory movement. For example, using fMRI it has been shown that the perception of speech sounds most strongly activates the superior temporoparietal cortex (Wernicke's area), but shows differential activation dependent on the specific articulator involved in the motor cortex (lips and tongue area, respectively) (Pulvermuller et al., 2006). Thus, a shared neuronal substrate was demonstrated for speech production (motor system) and comprehension (language system). Furthermore, motor neuron disease (MND) has been associated with a specific deficit in the processing of verbs, suggesting that the motor and language symptoms are due to the same selective neurodegenerative process, spreading along functionally integrated networks (Bak and Chandran, 2012). In completely different fields of research (linguistics), the motor theory of speech perception and theories on the gestural origin of language are well established (Galantucci et al., 2006; Johannesson, 1950). In line with this, the relevance of gestures in speech perception has recently been demonstrated using EEG (Obermeier et al., 2012). Taken together, the concept of moto-lingual integration seems key in explaining language impairment in RE.
4.5. Relation with language performance
No associations were found between rolandic network connectivity and neuropsychological metrics of language performance in any of the language ROIs. Possibly reduced functional connectivity between Broca's area and the rolandic network is not very sensitive to dysfunction within the language system itself. Another possible explanation is that as the CELF-4 assesses the language profile as a whole, its specificity to impairments in Broca's area might have been insufficient. We employed the CELF-4 since it is one of the few tests that is developed for and well-validated in children (Paslawski, 2005). Furthermore, the impaired language content index found in RE signifies problems in semantic development and sentence formulation and construction, which have been linked to the inferior prefrontal cortex (Broca's expressive language area) (Rogalsky and Hickok, 2011). In addition, the left inferior frontal gyrus has been associated with working memory, which is in line with the trend of reduced language working memory we found in RE (Rogalsky and Hickok, 2011). The impairment with respect to receptive language index hints at problems in posterior language function (Wernicke's receptive language area). However, the view that the language network can be strictly segregated in expressive anterior and receptive posterior regions is difficult to maintain given recent evidence (D'Ausilio et al., 2012; Pulvermuller et al., 2006). For example, the inferior prefrontal cortex has also been associated with language comprehension (Pulvermuller et al., 2006). Hence, the CELF-4 results are consistent with anterior language dysfunction, which has been described in RE before (Lillywhite et al., 2009).
4.6. Future research
Future studies would benefit from the inclusion of more precisely characterized patients. For example, in the present cohort the duration of epilepsy was quite variable (SD of 2.4 years on a mean of 2). Narrowing our inclusion based on such measures, however, would not have made much sense because they are difficult to accurately estimate. Due to the mild and typically nocturnal nature of RE, there might be a considerable (and variable) lag between the diagnosis and the first occurrence of seizures (Overvliet et al., 2011). To our knowledge, there is no accurate alternative for the child's or parents' report to determine the actual age at onset, which makes adequate patient characterization especially challenging. Furthermore, the onset of (subclinical) epileptiform activity rather than full-blown seizures might actually be more relevant in the context of language impairment in RE. This is supported by the finding that in RE families, seizure-free siblings of children with RE are at increased risk for language impairment (Clarke et al., 2007), and also compromises timely inclusion in scientific studies. Related to this is the question of whether the language impairments may persist after spontaneous seizure remission. Recent findings suggest that this is the case, however, this matter deserves further research (Monjauze et al., 2011). Finally, in our experience, it is hard to recruit large cohorts of RE patients, even at a specialized epilepsy referral center, compromising statistical power of the analyses. We expect this to be caused by the fact that an important fraction of children with RE only receive primary care. Our study adds to the growing body of evidence that RE is not merely a benign condition. In line with this, we hope that primary caregivers will become more readily inclined to refer a child under suspicion of epilepsy to specialized care. Especially in the case of RE this might too often still not be the case (Hughes, 2010).
5. Conclusion
The functional connectivity between the resting-state network involving the rolandic regions and the left inferior frontal gyrus (Broca's area) was reduced in RE. This functional decoupling might be key in understanding RE-typical language impairment, and is in line with the identified neuropsychological profile of anterior language dysfunction.
Acknowledgments
We would like to acknowledge Esther Peeters for data acquisition, Marc Geerlings for continuous hardware and software support, and all children and their parents for participating.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Bak T.H., Chandran S. What wires together dies together: verbs, actions and neurodegeneration in motor neuron disease. Cortex. 2012;48:936–944. doi: 10.1016/j.cortex.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S. OHBM annual meeting, San Francisco. 2009. Group comparison of resting-state FMRI data sing multi-subject ICA and dual regression. [Google Scholar]
- Berroya A.M., Bleasel A.F., Stevermuer T.L., Lawson J., Bye A.M. Spike morphology, location, and frequency in benign epilepsy with centrotemporal spikes. Journal of Child Neurology. 2005;20:188–194. doi: 10.1177/08830738050200030401. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa S.F., Pulvermuller F. Cortex special issue: language and the motor system. Cortex. 2012;48:785–787. doi: 10.1016/j.cortex.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Clarke T., Strug L.J., Murphy P.L., Bali B., Carvalho J., Foster S., Tremont G., Gagnon B.R., Dorta N., Pal D.K. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case–control study. Epilepsia. 2007;48:2258–2265. doi: 10.1111/j.1528-1167.2007.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ausilio A., Bufalari I., Salmas P., Fadiga L. The role of the motor system in discriminating normal and degraded speech sounds. Cortex. 2012;48:882–887. doi: 10.1016/j.cortex.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Deltour L., Barathon M., Quaglino V., Vernier M.P., Despretz P., Boucart M., Berquin P. Children with benign epilepsy with centrotemporal spikes (BECTS) show impaired attentional control: evidence from an attentional capture paradigm. Epileptic Disorders. 2007;9:32–38. doi: 10.1684/epd.2007.0066. [DOI] [PubMed] [Google Scholar]
- Deltour L., Querné L., Vernier-Hauvette M.P., Berquin P. Deficit of endogenous spatial orienting of attention in children with benign epilepsy with centrotemporal spikes (BECTS) Epilepsy Research. 2008;79:112–119. doi: 10.1016/j.eplepsyres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Doucet G., Naveau M., Petit L., Delcroix N., Zago L., Crivello F., Jobard G., Tzourio-Mazoyer N., Mazoyer B., Mellet E., Joliot M. Brain activity at rest: a multiscale hierarchical functional organization. Journal of Neurophysiology. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- Doucet G., Naveau M., Petit L., Zago L., Crivello F., Jobard G., Delcroix N., Mellet E., Tzourio-Mazoyer N., Mazoyer B., Joliot M. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. NeuroImage. 2012;59:3194–3200. doi: 10.1016/j.neuroimage.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., Matthews P.M., Beckmann C.F., Mackay C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci B., Fowler C.A., Turvey M.T. The motor theory of speech perception reviewed. Psychonomic Bulletin and Review. 2006;13:361–377. doi: 10.3758/bf03193857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R. Benign epilepsy of childhood with centrotemporal spikes (BECTS): to treat or not to treat, that is the question. Epilepsy & Behavior. 2010;19:197–203. doi: 10.1016/j.yebeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Jocic-Jakubi B., Jovic N.J. Verbal memory impairment in children with focal epilepsy. Epilepsy & Behavior. 2006;9:432–439. doi: 10.1016/j.yebeh.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Johannesson A. The gestural origin of language; evidence from six ‘unrelated’ languages. Nature. 1950;166:60–61. doi: 10.1038/166060a0. [DOI] [PubMed] [Google Scholar]
- Joseph J.E., Swearingen J.E., Clark J.D., Benca C.E., Collins H.R., Corbly C.R., Gathers A.D., Bhatt R.S. The changing landscape of functional brain networks for face processing in typical development. NeuroImage. 2012;63:1223–1236. doi: 10.1016/j.neuroimage.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura H., Aihara M. Growth disturbance of frontal lobe in BCECTS presenting with frontal dysfunction. Brain & Development. 2009;31:771–774. doi: 10.1016/j.braindev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Kavros P.M., Clarke T., Strug L.J., Halperin J.M., Dorta N.J., Pal D.K. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008;49:1570–1580. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- Liasis A., Bamiou D.E., Boyd S., Towell A. Evidence for a neurophysiologic auditory deficit in children with benign epilepsy with centro-temporal spikes. Journal of Neural Transmission. 2006;113:939–949. doi: 10.1007/s00702-005-0357-6. [DOI] [PubMed] [Google Scholar]
- Lillywhite L.M., Saling M.M., Harvey A.S., Abbott D.F., Archer J.S., Vears D.F., Scheffer I.E., Jackson G.D. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–2284. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Loiseau P., Duché B. Benign childhood epilepsy with centrotemporal spikes. Cleveland Clinic Journal of Medicine. 1989;56:S17–S22. doi: 10.3949/ccjm.56.s1.17. (discussion S40-12) [DOI] [PubMed] [Google Scholar]
- Lundberg S., Frylmark A., Eeg-Olofsson O. Children with rolandic epilepsy have abnormalities of oromotor and dichotic listening performance. Developmental Medicine and Child Neurology. 2005;47:603–608. [PubMed] [Google Scholar]
- Massa R., de Saint-Martin A., Carcangiu R., Rudolf G., Seegmuller C., Kleitz C., Metz-Lutz M.N., Hirsch E., Marescaux C. EEG criteria predictive of complicated evolution in idiopathic rolandic epilepsy. Neurology. 2001;57:1071–1079. doi: 10.1212/wnl.57.6.1071. [DOI] [PubMed] [Google Scholar]
- Meister I.G., Boroojerdi B., Foltys H., Sparing R., Huber W., Töpper R. Motor cortex hand area and speech: implications for the development of language. Neuropsychologia. 2003;41:401–406. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- Monjauze C., Tuller L., Hommet C., Barthez M.A., Khomsi A. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain and Language. 2005;92:300–308. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Monjauze C., Broadbent H., Boyd S.G., Neville B.G., Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52:e79–e83. doi: 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- Nicolai J., van der Linden I., Arends J.B., van Mil S.G., Weber J.W., Vles J.S., Aldenkamp A.P. EEG characteristics related to educational impairments in children with benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2007;48:2093–2100. doi: 10.1111/j.1528-1167.2007.01203.x. [DOI] [PubMed] [Google Scholar]
- Obermeier C., Dolk T., Gunter T.C. The benefit of gestures during communication: evidence from hearing and hearing-impaired individuals. Cortex. 2012;48:857–870. doi: 10.1016/j.cortex.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M., Besseling R.M., Vles J.S., Hofman P.A., Backes W.H., van Hall M.H., Klinkenberg S., Hendriksen J., Aldenkamp A.P. Nocturnal epileptiform EEG discharges, nocturnal epileptic seizures, and language impairments in children: Review of the literature. Epilepsy & Behavior. 2010;19:550–558. doi: 10.1016/j.yebeh.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M., Aldenkamp A.P., Klinkenberg S., Vles J.S.H., Hendriksen J. Impaired language performance as a precursor or consequence of Rolandic epilepsy? Journal of the Neurological Sciences. 2011;304:71–74. doi: 10.1016/j.jns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos C.P., Michael M., Sanders S., Valeta T., Koutroumanidis M. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain. 2008;131:2264–2286. doi: 10.1093/brain/awn162. [DOI] [PubMed] [Google Scholar]
- Paslawski C. The Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4): a review. Canadian Journal of School Psychology. 2005;20:129–134. [Google Scholar]
- Pulvermuller F., Huss M., Kherif F., del Prado Moscoso, Martin F., Hauk O., Shtyrov Y. Motor cortex maps articulatory features of speech sounds. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C., Hickok G. The role of Broca's area in sentence comprehension. Journal of Cognitive Neuroscience. 2011;23:1664–1680. doi: 10.1162/jocn.2010.21530. [DOI] [PubMed] [Google Scholar]
- Rubia K., in press. Functional brain imaging across development. European Child & Adolescent Psychiatry. (http://www.ncbi.nlm.nih.gov/pubmed/22729957) [DOI] [PMC free article] [PubMed]
- Semel E., Wiig E.H., Secord W.A. 3rd ed. Pearson; Amsterdam: 2010. Clinical Evaluation of Language Fundamentals 4, Nederlandse versie. [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Resting functional connectivity of language networks: characterization and reproducibility. Molecular Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets N.L., Beckmann C.F., Cole D.M., Hong S., Bernasconi A., Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135:2350–2357. doi: 10.1093/brain/aws137. [DOI] [PubMed] [Google Scholar]
- Waites A.B., Briellmann R.S., Saling M.M., Abbott D.F., Jackson G.D. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Annals of Neurology. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Yuan W., Szaflarski J.P., Schmithorst V.J., Schapiro M., Byars A.W., Strawsburg R.H., Holland S.K. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47:593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


