Abstract
Attention-deficit/hyperactivity disorder (ADHD) is neurobehavioral disorder characterized by inattention, hyperactivity/impulsivity and impaired reward system function, such as delay aversion and low reward sensitivity. The pharmacological treatment for ADHD includes methylphenidate (MPH), or osmotic release oral system-MPH (OROS-MPH), which increases extrasynaptic dopamine and noradrenaline levels by blocking their reuptake. Although previous functional magnetic resonance imaging (fMRI) studies revealed that acute treatment with MPH alters activation of the nucleus accumbens during delay aversion in children and adolescents with ADHD, the effects a relatively long period of OROS-MPH treatment on delay aversion as well as reward sensitivity remain unclear. Thus, we evaluated brain activation with fMRI during a reward sensitivity paradigm that consists of high monetary reward and low monetary reward conditions before and after a 3-month treatment with OROS-MPH in 17 children and adolescents with ADHD (mean age, 13.3 ± 2.2) and 17 age- and sex-matched healthy controls (mean age, 13.0 ± 1.9). We found that before treatment there was decreased activation of the nucleus accumbens and thalamus in patients with ADHD during only the low monetary reward condition, which was improved to same level as those of the healthy controls after the treatment. The observed change in brain activity was associated with improved ADHD symptom scores, which were derived from Japanese versions of the ADHD rating scale-IV. These results suggest that treatment with OROS-MPH for a relatively long period is effective in controlling reward sensitivity in children and adolescents with ADHD.
Keywords: Attention deficit/hyperactivity disorder, Dopamine, Functional magnetic resonance imaging, Osmotic release oral system-methylphenidate, Reward sensitivity
Highlights
-
•
Attention deficit/hyperactivity disorder (ADHD) has low reward sensitivity.
-
•
Activity in thalamus and nucleus accumbens was decreased in low monetary reward.
-
•
Osmotic release oral system-methylphenidate (OROS-MPH) is a medication for ADHD.
-
•
Brain activity and ADHD symptoms were improved by 3-month treatment with OROS-MPH.
-
•
OROS-MPH treatment for long periods may change brain activity of pediatric ADHD.
1. Introduction
Attention deficit/hyperactivity disorder (ADHD) is an early onset neurobehavioral disorder characterized by symptoms of inattention, impulsivity, and/or hyperactivity (Biederman, 2005). Patients with ADHD exhibit difficulties in several domains of attentional and cognitive function: problem solving, planning, alertness, cognitive flexibility, sustained attention, response inhibition, and working memory (Angold et al., 1999; Biederman et al., 1991). Other domains involving affective components, such as motivation and delay aversion for rewards, are also affected (Biederman et al., 1993; Borland and Heckman, 1976; Morrison, 1980). The worldwide prevalence of children with ADHD is 3 to 5% (Solanto, 2001). Prospective follow-up studies estimate that about 50% of children with ADHD have symptoms that continue into adulthood and, when left untreated, are associated with substance abuse, depression, unemployment, and criminal offenses (Biederman et al., 2006a; Molina et al., 2009).
ADHD has long been viewed as a neurobiological disorder of the prefrontal cortex and its connections. Prefrontal cortex circuits relevant to ADHD include the dorsal fronto-striatal, orbitofronto-striatal, and fronto-cerebellar circuits (Durston et al., 2011). Functionally, the fronto-cerebellar circuit has also been implicated in ADHD, in particular in timing and building temporal expectations (Durston et al., 2007). The dorsal fronto-striatal connections have been linked to cognitive control, whereas loops between the ventral striatum and orbitofrontal cortex have been linked to reward and motivation (Alexander et al., 1986).
Studies using functional magnetic resonance imaging (fMRI) have reported decreased activation of the ventral striatum, in both adolescents and adults with ADHD, during reward anticipation (Plichta et al., 2009; Scheres et al., 2007; Ströhle et al., 2008), reflecting the neural processing associated with delay aversion for rewards in ADHD patients. However, a recent study demonstrated that while striatal activity during reward anticipation is not impaired in adolescents with ADHD, it was impaired during reward outcome, suggesting abnormal striatal sensitivity in ADHD (Paloyelis et al., 2012). Moreover, children with ADHD require stronger rewards to modify their behavior and learn faster when using direct reinforcement (Kollins et al., 1998), suggesting that neural responses in ADHD patients are decreased during low reward conditions. Therefore, ADHD patients may have decreased reward sensitivity, thereby impeding responses to smaller rewards. Conversely, increased reward sensitivity results in smaller rewards eliciting the same responses as larger rewards. fMRI studies of the neural substrates associated with reward sensitivity indicate that the extent of striatal activation is associated with reward magnitude (Izuma et al., 2008). While abnormal volume changes or atypical functional changes in frontal or striatal regions have been well established in ADHD children (Valera et al., 2007), little work has been conducted to investigate the neural substrates associated with reward sensitivity in the pediatric brain.
In the present study, we focused on the neural processing of reward sensitivity in ADHD children and adolescents between the ages of 10–16 years. There have been several studies using monetary gambling tasks in pediatric populations, including studies of healthy children (n = 19, mean age = 11.2) and adolescents (n = 17, mean age = 14.5) (Habib et al., 2012), ADHD patients (23 ADHD children vs. 20 healthy control children, age range = 7 to 12, mean ages of 9.6 and 9.1, respectively) (Luman et al., 2008), and early-onset schizophrenia (15 adolescents with schizophrenia vs. 25 healthy control adolescents, age range of 12 to 21) (Kester et al., 2006). Further, Habib et al. (2012) provided evidence that the ability to experience counterfactually mediated emotions, such as regret and relief, continues to develop during late childhood and adolescence. The present study stands in contrast to findings from other studies, in that we investigated brain activation with fMRI during a reward sensitivity paradigm that consists of high monetary reward (HMR) and low monetary reward (LMR) conditions. Hence, we hypothesized that this approach would be useful in revealing the dependence on reward magnitude of the neural substrates associated with reward sensitivity in children as well as in young adults (Izuma et al., 2008).
As the dopamine transporter is the main target for ADHD stimulant medication, molecular imaging studies, using single photon emission computed tomography and positron emission tomography (PET), initially focused on the role of this marker, leading to the finding that ADHD patients have increased dopamine transporter density (Spencer et al., 2005). Recent meta-analysis revealed that despite high inter-study heterogeneity, striatal dopamine transporter density in ADHD patients was significantly elevated by 14%, on average, compared to healthy controls (Fusar-Poli et al., 2012). Methylphenidate (MPH) constitutes the main first-line ADHD therapy (Wilens, 2008) and increases extrasynaptic dopamine and noradrenaline levels by blocking their reuptake (Zetterström et al., 1988). Therapeutic doses of MPH were found to produce more than 50% dopamine transporter blockade in healthy volunteers (Volkow et al., 1998), leading to increased striatal extracellular dopamine levels (Volkow et al., 2001). In adult ADHD patients, attenuated dopamine levels in the caudate were increased following an acute intravenous single dose MPH treatment (0.5 mg/kg) (Volkow et al., 2007). Consistent with this result, acute oral MPH treatment (0.3 mg/kg) of ADHD adolescents increases dopamine concentrations in the ventral striatum, which is associated with reduced symptom severity (Rosa-Neto et al., 2005). Functionally, many studies revealed improvements in neural responses during reward, attention, response inhibition, and resting state processing following MPH treatment in ADHD patients (for see review, Cortese et al., 2012; Paloyelis et al., 2007). With respect to reward tasks, reduced activation of the striatum in ADHD children and adolescents during reward processing was improved by acute MPH treatment (Rubia et al., 2009). The wealth of data supporting the short-term efficacy and safety of stimulants, such as MPH, in treating children and adolescents with ADHD (Fone and Nutt, 2005) is contrasted by a dearth of data on the long-term effects of MPH treatment on reward processing.
Research continues to suggest that osmotic release oral system-methylphenidate (OROS-MPH) lessens ADHD symptoms throughout the day and has greater adherence, thought to be attributable to the convenience of once-daily dosing (Abikoff et al., 2009; Chou et al., 2009; Kim et al., 2009). Japan had no approved pharmacological treatments for ADHD until the recent approval of OROS-MPH in 2007. In addition to OROS-MPH, the noradrenaline transport inhibitor atomoxetine was approved for use in Japan in 2009. Although it has been reported that OROS-MPH treatment improves executive function in children and adolescents with ADHD (Yang et al., 2011; Yildiz et al., 2011), only two studies have shown effects of OROS-MPH on cognitive functions in Japanese children and adolescents with ADHD (Monden et al., 2012; Sawada et al., 2010). Therefore, we need to accumulate adequate evidence of OROS-MPH treatment on cognitive function as well as reward processing in order to characterize the safety and efficacy of long-term treatments in children and adolescents with ADHD.
Studies of the effects of stimulant treatment have shown that acute MPH treatment improved activity in fronto-striatal circuits and the cerebellum during cognitive inhibition in ADHD (Epstein et al., 2007). However, maximal clinical responses to MPH are not observed until approximately 4 to 6 weeks of treatment (Biederman et al., 2006b; Spencer et al., 2005). A few studies have focused on the effects of relatively long periods of MPH treatment on cognitive function in ADHD. Schulz et al. (2012) showed that MPH treatment for 6 to 8 weeks in ADHD children normalized activities in response inhibition task-related regions, such as the motor and cingulate cortices. Konrad et al. (2007) reported that MPH treatment for 1 year in children with ADHD improved activation of brain regions involved in an executive attention task. Stoy et al. (2011) examined the effect of MPH treatment during childhood on differences in brain activation during reward processing using a monetary incentive delay task in adult ADHD. With OROS-MPH, although treatment for 6 weeks has been found to increase activity in brain regions related to attention (Bush et al., 2008), the effect of relatively long treatment periods on neural processes for reward has not been investigated yet. Here, we examined whether a 3-month OROS-MPH treatment is associated with stable changes in neural activity related to reward sensitivity in ADHD children and adolescents using a longitudinal evaluation encompassing the pre- to post-treatment period, with a concurrent comparison to normally developing children and adolescents.
2. Patients and methods
2.1. Participants
We recruited only healthy males and males with ADHD, to simplify and strengthen the analysis and because very few females were in the ADHD cohort at the Kumamoto University Hospital. Male children and adolescents with ADHD met the Diagnostic and Statistical Manual of Mental Disorders DSM-IV Text Revision (DSM-IV-TR) criteria (American Psychiatric Association, 2000). This narrow age range was chosen in an effort to maximize the similarity of the participants recruited who were able to provide independent informed assent, while minimizing variations in brain morphometry related to development or maturation. To exclude other psychiatric diagnoses, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) was administered by a licensed pediatric–psychiatric clinician.
The diagnosis of ADHD was confirmed in structured interviews with investigators using the ADHD behavior module of the Japanese Version of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Present and Lifetime Versions (K-SADS-PL-J) (Kaufman et al., 1997; Miyawaki et al., 2003). It was confirmed that K-SADS-PL-J scored well on Cohen's κ (ADHD = 1.00, disruptive behavior disorder = 0.91, anxiety disorders = 0.76, tic disorders = 0.75) for inter-rater reliability by the simultaneous interview method and had concurrent validity for ADHD, disruptive behavior disorder, and anxiety disorders using various evaluation scales (Miyawaki et al., 2003). As additional instruments for diagnoses and ADHD symptom severity, we used the Japanese versions of the ADHD Rating Scale-IV (ADHD-RS-IV-J) (Yamazaki, 2003), the Barratt impulsiveness scale (BIS) (Someya et al., 2001), strengths and difficulties questionnaires (SDQ) (Iizuka et al., 2010), a scale of intrinsic versus extrinsic motivation toward learning (IEML) (Sakurai and Takano, 1985), the effort–reward imbalance for learning model questionnaire (LERI) (Fukuda et al., 2010) and the Chalder fatigue scale (CFS) (Tanaka et al., 2008). Exclusion criteria for participants with ADHD were premature birth (gestation ≤ 36 weeks) and diagnosis of any bipolar, psychotic, obsessive–compulsive, or tic disorder at any point in their lifetime. Exclusion criteria for comparison participants were a diagnosis of any current DSM-IV axis I disorder at any point in their lifetime.
Participants who had any history of substance abuse, recent substance use, head trauma with loss of consciousness, epilepsy, significant fetal exposure to alcohol or drugs, perinatal or neonatal complications, neurological disorders, or medical conditions that could adversely affect growth and development were excluded. Additional exclusion criteria for both healthy and ADHD children and adolescents were full scale intelligence quotient (IQ) below 80, as measured by the Wechsler Intelligence Scale for Children — Third Edition (WISC-III) (Wechsler, 1991), or left-handedness according to the Edinburgh handedness inventory (Oldfield, 1971).
Twenty ADHD patients, aged 10–16 years, participated in the fMRI experiments, once before treatment (btADHD) and once after treatment (atADHD) with OROS-MPH for 3 months. Three out of 20 patients were not able to complete the first or second experiments, due to motion artifacts during the fMRI scans. Thus, we analyzed data from a total of 17 male patients.
To obtain data from age-matched healthy controls (HC), 17 healthy children and adolescents, aged 10–16 years, were recruited from the community, with school students targeted. Seventeen healthy age-matched male children and adolescents completed the experiments. Members of the control group participated once in the fMRI experiment. The physical and neuropsychological characteristics of the participants are shown in Table 1. Age, body mass index, and IQ score were well matched between the patient and control groups. The protocol was approved by the Ethics Committee of the Graduate School of Medical Sciences, Kumamoto University, and all participants and their parents gave written informed consent for participation in the study after the study procedures had been explained to them, according to the Declaration of Helsinki.
Table 1.
Physical and psychological characteristics of the participants.
| Healthy controls | ADHD patients | p Value | |
|---|---|---|---|
| Sample number, n | 17 | 17 | – |
| Years of age | 13.0 ± 1.9 | 13.3 ± 2.2 | 0.680 |
| BMI, m/kg2 | 18.9 ± 2.3 | 20.4 ± 3.6 | 0.134 |
| WISC-III | |||
| FIQ score | 100.5 ± 4.6 | 96.8 ± 11.9 | 0.231 |
| VIQ score | 101.5 ± 5.6 | 95.4 ± 11.5 | 0.055 |
| PIQ score | 99.1 ± 5.9 | 98.1 ± 14.1 | 0.777 |
BMI, body mass index; WISC-III, Wechsler Intelligence Scale for Children-Third Edition; FIQ, full scale intelligence quotient; VIQ, verbal IQ; PIQ, performance IQ.
Values are presented as mean ± SD. p Values were obtained using the Student's t-test.
2.2. Treatment of OROS-MPH for ADHD
In Japan, the Ministry of Health, Labour and Welfare approved OROS-MPH (Concerta®, Johnson & Johnson Co.) for treatment of ADHD in 2007, while other types of MPH have not been approved yet. Therefore, we investigated the effects of OROS-MPH in the present study. The ADHD patients were administered 18 to 36 mg (average dose, 26.5 ± 7.4 mg) OROS-MPH (0.5–1.2 mg/kg per day) for 3 months. We did not administer a placebo to either ADHD patients or healthy children and adolescents. The treatment protocol was also approved by the Ethics Committee of Kumamoto University.
2.3. Experimental paradigms for functional imaging
The fMRI experimental design is shown in Fig. 1. In the monetary reward condition, the participants performed a simple gambling task, which was a block-design version of the task used in a previous study (Izuma et al., 2008). They were encouraged to try to earn as much money as possible and were told that one session would be randomly chosen at the end of the experiment and that their earnings in that session would be given to them. In each trial (3 s), the participants were presented with three cards labeled as “A”, “B” or “C” and were asked to choose one card within 2 s by pressing a button with the right index, middle or ring finger, which spatially corresponded to the location of the cards. Immediately after the button press, the chosen card was highlighted with a thick white border, and the outcome was displayed for 1 s. If the participants did not press any button within the choice period (2 s), the card they had chosen in one previous trial was automatically chosen, and its outcome was displayed.
Fig. 1.
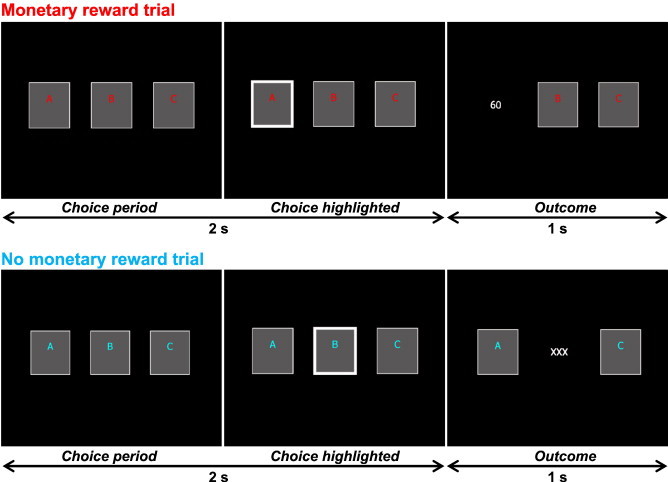
Time course of stimulus display sequences for the monetary reward trial (top) and the no monetary reward trial (bottom). In each monetary reward trial, participants were asked to choose one card within 2 s, and the outcome of the chosen card (0, 30, or 60 yen) was shown for 1 s. Each block consisted of eight monetary rewards or no-monetary reward trials (24 s). In each no-monetary reward trial, participants were similarly asked to choose one card, but the outcome was always “× × ×”, indicating no monetary reward.
When the letters on each card were written in red, the trial was a “monetary reward trial” in which each card was randomly associated with 0, 30, or 60 yen. Each condition consisted of eight trials (24 s). However, unknown to the participants, the total reward that they could earn in each condition was predetermined. In the HMR condition, they earned an average of 330 yen each (range = 270–390 yen), which was consistently higher than the expected value of the eight reward trials (240 yen). In the LMR condition, the participants earned an average of 150 yen each (range = 90–210 yen), which was consistently lower than the expected value. The participants knew that the expected value of the eight reward trials was 240 yen. However, they were not informed of the presence of the HMR and LMR conditions. They also participated in a no monetary reward (NMR) condition, indicated by blue letters, in which they chose one card, but the outcome presented was always “× × ×”, indicating that there was no monetary reward. The NMR condition, or a fixation rest condition (24 s), was always inserted between two reward conditions, so that the start and end of the reward manipulations could be clearly defined. For half of the participants, the colors (red and blue) used for the monetary reward and no monetary reward trials were switched to control for differences in activity related to visual processing of colors. All the participants completed a practice task for 2 min before scanning to ensure that all participants understood the task. We confirmed that during the practice task, all the participants choose one card within the choice period (2 s) at 100% accuracy (moving average of 8 trials). During scanning, they performed a total of four sessions for 6 min 24 s [4 trials (24 s per trial) for each of the four conditions (HMR, LMR, NMR, and fixation rest)] within which the HMR and LMR conditions were ordered differently, and the order of these four sessions was counterbalanced across participants. All participants were paid a fixed amount for their participation at the end of the experiment.
2.4. Functional MRI acquisition and analysis
All images were obtained using a 3-Tesla MR scanner (TRIO A Tim; Siemens, Erlangen, Germany) located at the Graduate School of Medical Sciences, Kumamoto University. For functional imaging, a series of 528 volumes (132 volumes per session) were acquired using interleaved T2-weighted, gradient echo, echo planar imaging (EPI) sequences. Each volume consisted of 44 transaxial slices with a thickness of 3.0 mm between slices, which included the entire cerebrum and cerebellum [repetition time (TR), 3000 ms; echo time (TE), 30 ms; flip angle (FA), 90°; field of view (FOV), 192 mm; in-plane matrix size, 64 × 64 pixels, voxel dimensions, 3.0 × 3.0 × 3.0 mm; slice gap, 0 mm]. Comfortable foam padding was tightly placed around the participant's head to minimize head movement. To acquire a fine structural whole-brain image, magnetization-prepared rapid-acquisition gradient-echo (MP-RAGE) images were obtained [TR, 1900 ms; TE, 4.62 ms; flip angle, 15°; FOV, 256 mm; one slab; number of slices per slab, 176; voxel dimensions, 1.0 × 1.0 × 1.0 mm].
The first four volumes acquired in each MRI session were discarded due to unsteady magnetization, and the remaining 128 volumes per session were used for analyses. Data were analyzed using the Statistical Parametric Mapping 5 package (The Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7.7.0 (Mathworks, Natick, MA). We realigned the EPI images with the time series and created a mean image. High-resolution whole-brain T1-weighted images were then co-registered with the mean image. This structural image was then normalized to the Montréal Neurological Institute (MNI) T1 image template (Evans et al., 1994), with the same parameters applied to all EPI images. The EPI images were spatially smoothed in three dimensions using an 8 mm full-width half-maximum Gaussian kernel.
Statistical analyses were performed at two levels. First, individual task-related activation was evaluated. We used a simple gambling task, which was a block-design version of the task used in a previous study (Izuma et al., 2008). In each task condition (i.e. HMR, LMR and NMR), the event onset was designated as the presentation of the first stimulus (choice period of three cards labeled as “A”, “B” or “C”, see Fig. 1) of the first trial in a block. In each trial (3 s), the participants were presented with three cards and were asked to choose one card within 2 s by pressing a button. After the button press, the chosen card was highlighted with a thick white border, and the outcome was displayed for 1 s. A block consisted of eight trials (8 trials × 3 s = 24 s) in each condition. Therefore, the duration of each event (= one block) was 24 s. Each task condition was repeated 4 times in one session. We modeled 3 regressors (HMR, LMR and NMR), which were convolved with a canonical hemodynamic response function to obtain the expected signal changes caused by the tasks. Regressors that were of no interest, such as the six realignment parameters that account for motion-related variance, were also included in the design model. The data were high-pass filtered with a cut-off period of 128 s to remove low-frequency signal drifts. An autoregressive model was used for whitening the residuals so as to meet the assumptions for application of a general linear model (GLM). The effect of each condition was evaluated with a GLM. The weighted sum of the parameters estimated in the individual analyses consisted of “contrast” images. Specifically, for each participant the following first level contrast images were generated: (HMR minus NMR) and (LMR minus NMR).
Second, the contrast images corresponding to each condition for each participant were used for group analyses with a random-effects model to obtain population inferences (Friston et al., 1999). We used a flexible factorial design that can compare the activities of reward level contrasts within (HMR minus NMR) and (LMR minus NMR), and between the btADHD and atADHD groups by repeated measures, as well as compare those between the HC and btADHD or atADHD groups by non-repeated measures. The resulting set of voxel values for each comparison constituted a statistical parametric map of t statistics [SPM(t)]. Significant signal changes for each contrast were assessed by means of t statistics on a voxel-by-voxel basis. The threshold for the SPM(t) for group analyses was set at p < 0.005 at the voxel level and p < 0.05 with a correction for multiple comparisons at the cluster level for the entire brain (Izuma et al., 2008).
Comparisons of the HMR and LMR conditions with the NMR condition (HMR or LMR minus NMR) were performed in order to obtain the activation pattern of the two types of reward task processing (Izuma et al., 2008) in each study group (HC, btADHD and atADHD). To specify the brain areas involved in reward sensitivity, we used the reward level contrast (HMR minus LMR) masked by the high reward contrast (HMR minus NMR) in each study group. To specify the brain areas involved in the treatment of OROS-MPH, we used the study group contrast (atADHD minus btADHD or HC) without masked images in the HMR and LMR conditions (HMR or LMR minus NMR). Anatomic localization of significant voxels within clusters was performed using the Wake Forest University (WFU) Pick-Atlas (Maldjian et al., 2003) and a probabilistic cytoarchitectonic map (Eickhoff et al., 2005).
The effects of task condition (HMR, LMR and NMR) or study group (HC, btADHD and atADHD) on the task performance (reaction time) in each task condition were analyzed using a two-way analysis of variance (ANOVA). A two-way repeated measures ANOVA was used to investigate the effects of task and study group on reaction time between the btADHD and atADHD groups. When statistically significant effects were found, intergroup differences were evaluated using the paired t-test or Student's t-test. All p values were two-tailed, and p values less than 0.05 were considered significant. These analyses were performed with the IBM SPSS 19.0 software package (SPSS Inc, Chicago, IL).
3. Results
3.1. Questionnaire results
The results for questionnaires are summarized in Table 2. The inattention, hyperactivity/impulsivity and total scores in ADHD-RS-IV-J, BIS, and QCD for the btADHD group were significantly higher than those of the HC. The IEML, LERI and CFS scores were similar between the two groups. In the ADHD patients, inattention, hyperactivity/impulsivity and total scores in ADHD-RS-IV-J, BIS, SDQ and IEML after treatment were improved in comparison with those before treatment. The LERI and CFS scores were not changed after treatment. Although the inattention, hyperactivity/impulsivity and total scores of ADHD-RS-IV-J and SDQ in the atADHD group were still higher than those of the HC group, BIS scores between the atADHD and HC groups were not different.
Table 2.
Effects of OROS-MPH treatment on questionnaire results in patients with ADHD.
| Healthy controls | ADHD patients |
||
|---|---|---|---|
| Before | After | ||
| ADHD-RS-IV-J | |||
| Inattention score | 4.2 ± 4.3 | 16.8 ± 4.4⁎⁎⁎ | 11.8 ± 6.3⁎⁎⁎,†† |
| Hyperactivity/Impulsivity score | 1.1 ± 1.3 | 7.3 ± 6.1⁎⁎⁎ | 3.5 ± 3.7⁎,† |
| Total score | 5.3 ± 5.4 | 24.1 ± 8.8⁎⁎⁎ | 15.3 ± 8.3⁎⁎⁎,†† |
| BIS score | 70.4 ± 6.9 | 77.8 ± 9.8⁎ | 65.7 ± 10.9††† |
| QCD score | 49.0 ± 7.6 | 33.9 ± 10.1⁎⁎⁎ | 39.4 ± 8.9⁎⁎,† |
| IEML score | 84.2 ± 14.9 | 75.5 ± 17.2 | 83.2 ± 15.5† |
| LERI score | 0.90 ± 0.24 | 1.05 ± 0.36 | 0.94 ± 0.25 |
| CFS score | 10.4 ± 5.8 | 13.4 ± 6.8 | 12.8 ± 5.9 |
ADHD-RS-IV-J, Japanese versions of the ADHD rating scale-fourth edition; BIS, Barratt impulsiveness scale; QCD, questionnaires for children with difficulties; IEML, scale of intrinsic versus extrinsic motivation toward learning; LERI, effort–reward imbalance for learning model questionnaire; CFS, Chalder fatigue scale. Values are presented as mean ± SD. ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001, significantly different from the corresponding values for the healthy adolescents (Student's t-test). †p < 0.05, ††p < 0.01, †††p < 0.001, significantly different from the corresponding values for ADHD patients before treatment with OROS-MPH (paired t-test).
3.2. Behavioral results
The results for reaction times are summarized in Table 3. In comparisons among the HC, btADHD and atADHD groups, two-way ANOVA revealed no significant main effects of task condition [F(2, 144) = 1.64, p = 0.198], study group [F(2, 144) = 0.54, p = 0.587] or interactions [F(2, 144) = 1.40, p = 0.967] on reaction time. In comparisons between the btADHD and atADHD groups, two-way repeated measures ANOVA revealed a significant main effect of task condition on reaction time [F(1, 16) = 14.05, p = 0.002], whereas no significant effects on reaction time were observed with the study group [F(1, 16) = 0.22, p = 0.647] or interactions [F(1, 16) = 0.76, p = 0.396]. These results indicate that the reaction times among the HC, btADHD and atADHD groups were not different.
Table 3.
Performance of monetary reward tasks before and after OROS-MPH treatment in patients with ADHD.
| Healthy controls | ADHD patients |
||
|---|---|---|---|
| Before | After | ||
| RT of HMR, ms | 593 ± 265 | 570 ± 214 | 573 ± 259 |
| RT of LMR, ms | 545 ± 243 | 520 ± 209 | 538 ± 299 |
| RT of NMR, ms | 544 ± 252 | 448 ± 97 | 488 ± 237 |
RT, reaction time; HMR, high monetary reward; LMR, low monetary reward; NMR, no monetary reward. Values are presented as mean ± SD.
3.3. Imaging results
Imaging results for the HMR and LMR conditions (HMR or LMR minus NMR) are shown in Fig. 2 and Tables 4 (HC), 5 (btADHD) and 6 (atADHD). In the HMR condition, activation of the right middle and medial frontal gyri, cingulate gyrus, inferior parietal lobule, precuneus, bilateral striatum (including the nucleus accumbens), thalamus and cerebellum was commonly observed in the HC group, as well as in the btADHD and atADHD groups (Fig. 2A, Tables 4–6). During the HMR condition, the right inferior frontal gyrus and insula were commonly activated in the HC and atADHD groups, but not in the btADHD group. The right middle frontal gyrus, cingulate gyrus, insula, caudate and cerebellum were also commonly activated during the LMR condition in the HC, btADHD and atADHD groups. Although activation of the right medial frontal gyrus, bilateral nucleus accumbens and thalamus during the LMR condition was observed in the HC and atADHD groups, this was not observed in the btADHD group (Fig. 2B, Tables 4–6).
Fig. 2.
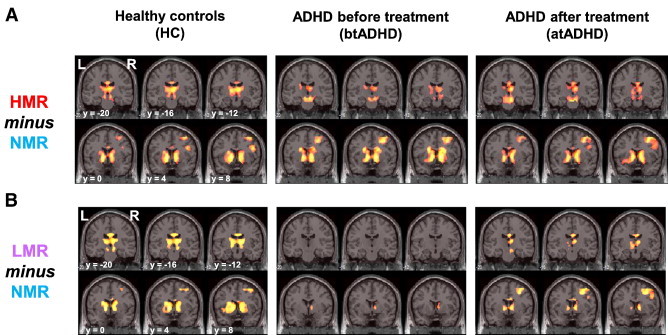
Statistical parametric maps of (A) high monetary reward (HMR minus NMR) and (B) low monetary reward (LMR minus NMR) in healthy controls (HC) and ADHD patients before (btADHD) and after treatment (atADHD) with OROS-MPH are shown. Right (R) and left (L) sides and y-axis (MNI coordinate) are indicated.
Table 4.
Activated brain regions associated with the high monetary reward (HMR) and low monetary reward (LMR) conditions in healthy controls.
| Brain region | Healthy control |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cluster |
Side | BA | MNI coordinate |
Z value | ||||
| Size | p Value | x | y | z | ||||
| HMR minus NMR | ||||||||
| Insula | 11,477 | < 0.001 | L | − 30 | 18 | − 2 | 6.44 | |
| R | 32 | 22 | 0 | 6.89 | ||||
| Caudate | L | − 10 | 6 | 0 | 5.64 | |||
| R | 12 | 8 | 4 | 5.18 | ||||
| Putamen | L | − 22 | 10 | 12 | 4.25 | |||
| R | 20 | 10 | 8 | 3.97 | ||||
| Nucleus accumbens | L | − 12 | 2 | − 2 | 6.08 | |||
| R | 14 | 8 | − 2 | 5.71 | ||||
| Thalamus | L | − 8 | − 20 | 12 | 4.07 | |||
| R | 6 | − 16 | 14 | 5.29 | ||||
| Cerebellum | L | − 8 | − 76 | − 28 | 4.47 | |||
| R | 6 | − 78 | − 22 | 3.92 | ||||
| Postcentral gyrus | 3015 | < 0.001 | R | 40 | 52 | − 36 | 52 | 3.24 |
| Inferior parietal lobule | R | 40 | 40 | − 52 | 38 | 6.28 | ||
| Superior parietal lobule | R | 7 | 38 | − 64 | 54 | 5.32 | ||
| Precuneus | L | 7 | − 6 | − 68 | 44 | 3.56 | ||
| R | 7 | 8 | − 66 | 44 | 5.65 | |||
| Middle frontal gyrus | 2152 | < 0.001 | R | 10 | 38 | 56 | 12 | 3.24 |
| R | 46 | 50 | 32 | 30 | 4.24 | |||
| R | 9 | 46 | 16 | 40 | 3.73 | |||
| R | 6 | 40 | 6 | 56 | 4.39 | |||
| Inferior frontal gyrus | R | 9 | 46 | 8 | 26 | 4.54 | ||
| Medial frontal gyrus | 1641 | 0.001 | R | 9 | 6 | 34 | 36 | 5.43 |
| Cingulate gyrus | L | 32 | − 8 | 16 | 48 | 5.11 | ||
| R | 32 | 6 | 22 | 44 | 4.86 | |||
| LMR minus NMR | ||||||||
| Inferior frontal gyrus | 8493 | < 0.001 | L | 47 | − 30 | 20 | − 2 | 4.61 |
| R | 47 | 32 | 20 | − 2 | 5.56 | |||
| Insula | L | − 30 | 18 | − 4 | 5.15 | |||
| R | 30 | 22 | 2 | 5.45 | ||||
| Caudate | L | − 10 | 6 | 0 | 3.94 | |||
| R | 14 | 10 | 6 | 4.54 | ||||
| Putamen | L | − 20 | 4 | 16 | 3.98 | |||
| R | 16 | 12 | 0 | 4.67 | ||||
| Nucleus accumbens | L | − 10 | 6 | − 2 | 4.21 | |||
| R | 12 | 4 | − 4 | 3.90 | ||||
| Thalamus | L | − 12 | − 12 | 8 | 4.24 | |||
| R | 12 | − 10 | 10 | 4.73 | ||||
| Cerebellum | 5259 | < 0.001 | L | − 8 | − 74 | − 26 | 4.67 | |
| R | 4 | − 76 | − 26 | 4.35 | ||||
| Medial frontal gyrus | 1257 | 0.004 | L | 9 | − 8 | 28 | 34 | 3.18 |
| R | 9 | 6 | 34 | 36 | 4.74 | |||
| Cingulate gyrus | L | 32 | − 10 | 16 | 48 | 4.30 | ||
| R | 32 | 6 | 22 | 44 | 4.26 | |||
| Middle frontal gyrus | 1014 | 0.013 | R | 46 | 46 | 36 | 22 | 3.60 |
| R | 9 | 50 | 28 | 36 | 3.81 | |||
| R | 6 | 40 | 8 | 54 | 3.43 | |||
NMR, no monetary reward; L, left; R, right; BA, Brodmann's area; MNI, Montréal Neurological Institute. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level.
Table 5.
Activated brain regions associated with the high monetary reward (HMR) and low monetary reward (LMR) conditions before treatment of ADHD patients.
| Brain region | ADHD (before treatment) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cluster |
Side | BA | MNI coordinate |
Z value | ||||
| Size | p Value | x | y | z | ||||
| HMR minus NMR | ||||||||
| Middle frontal gyrus | 20,145 | < 0.001 | R | 9 | 50 | 28 | 36 | 4.66 |
| R | 6 | 30 | 6 | 44 | 4.78 | |||
| Medial frontal gyrus | L | 6 | − 8 | 28 | 38 | 3.97 | ||
| R | 9 | 8 | 30 | 38 | 4.84 | |||
| Cingulate gyrus | R | 32 | 8 | 24 | 46 | 5.24 | ||
| Caudate | L | − 10 | 6 | 0 | 5.43 | |||
| R | 12 | 8 | 0 | 6.01 | ||||
| Putamen | L | − 20 | 10 | 12 | 3.89 | |||
| R | 22 | 10 | 10 | 4.17 | ||||
| Nucleus accumbens | L | − 10 | 0 | − 8 | 3.51 | |||
| R | 10 | 0 | − 10 | 4.02 | ||||
| Thalamus | L | − 24 | − 30 | 6 | 3.87 | |||
| R | 6 | − 20 | 14 | 3.84 | ||||
| Cerebellum | L | − 4 | − 74 | − 22 | 5.48 | |||
| R | 34 | − 66 | − 28 | 3.91 | ||||
| Inferior parietal lobule | 2532 | < 0.001 | R | 40 | 40 | − 46 | 42 | 4.07 |
| Precuneus | R | 7 | 10 | − 62 | 46 | 5.15 | ||
| Inferior parietal lobule | 1200 | 0.006 | L | 40 | − 42 | − 40 | 40 | 3.25 |
| Superior parietal lobule | L | 7 | − 28 | − 60 | 44 | 4.16 | ||
| Precuneus | L | 7 | − 16 | − 62 | 50 | 3.61 | ||
| LMR minus NMR | ||||||||
| Inferior parietal lobule | 4667 | < 0.001 | L | 40 | − 42 | − 42 | 42 | 3.44 |
| R | 40 | 44 | − 50 | 46 | 4.45 | |||
| Superior parietal lobule | L | 7 | − 30 | − 56 | 40 | 4.29 | ||
| R | 7 | 38 | − 66 | 52 | 4.20 | |||
| Precuneus | R | 7 | 10 | − 62 | 46 | 5.89 | ||
| Cerebellum | 2739 | < 0.001 | L | − 28 | − 62 | − 32 | 4.65 | |
| R | 28 | − 60 | − 28 | 3.67 | ||||
| Insula | 1352 | 0.003 | R | 30 | 22 | 2 | 5.46 | |
| Middle frontal gyrus | R | 10 | 32 | 48 | 2 | 2.60 | ||
| Caudate | R | 8 | 16 | 10 | 4.04 | |||
NMR, no monetary reward; L, left; R, right; BA, Brodmann's area; MNI, Montréal Neurological Institute. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at the voxel level.
Table 6.
Activated brain regions associated with the high monetary reward (HMR) and low monetary reward (LMR) conditions after treatment of ADHD patients.
| Brain region | ADHD (after treatment) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cluster |
Side | BA | MNI coordinate |
Z value | ||||
| Size | p Value | x | y | z | ||||
| HMR minus NMR | ||||||||
| Middle frontal gyrus | 20,217 | < 0.001 | R | 10 | 40 | 56 | − 6 | 4.87 |
| R | 46 | 36 | 32 | 22 | 4.09 | |||
| R | 9 | 50 | 30 | 34 | 5.53 | |||
| R | 6 | 30 | 6 | 46 | 4.92 | |||
| Medial frontal gyrus | R | 9 | 4 | 32 | 36 | 5.42 | ||
| Inferior frontal gyrus | R | 9 | 46 | 6 | 28 | 3.74 | ||
| Cingulate gyrus | R | 32 | 8 | 24 | 44 | 5.38 | ||
| Insula | R | 30 | 22 | 0 | 6.83 | |||
| Caudate | L | − 10 | 8 | 2 | 5.18 | |||
| R | 12 | 10 | 2 | 5.67 | ||||
| Putamen | L | − 18 | 10 | 10 | 3.11 | |||
| R | 20 | 12 | 10 | 2.99 | ||||
| Nucleus accumbens | L | − 10 | 0 | − 4 | 3.75 | |||
| R | 10 | 4 | − 8 | 3.54 | ||||
| Thalamus | L | − 12 | − 8 | 4 | 3.93 | |||
| R | 10 | − 4 | 4 | 5.08 | ||||
| Cerebellum | L | − 28 | − 64 | − 30 | 5.69 | |||
| R | 6 | − 76 | − 16 | 4.79 | ||||
| Inferior parietal lobule | 5233 | < 0.001 | R | 40 | 36 | − 58 | 44 | 5.47 |
| Superior parietal lobule | L | 7 | − 30 | − 56 | 42 | 4.92 | ||
| Precuneus | L | 7 | − 12 | − 66 | 46 | 3.93 | ||
| R | 7 | 8 | − 68 | 44 | 5.13 | |||
| LMR minus NMR | ||||||||
| Middle frontal gyrus | 2905 | < 0.001 | R | 6 | 30 | 6 | 44 | 5.08 |
| Medial frontal gyrus | R | 9 | 4 | 30 | 34 | 5.92 | ||
| Cingulate gyrus | R | 32 | 6 | 24 | 42 | 5.81 | ||
| Inferior parietal lobule | 2760 | < 0.001 | R | 40 | 36 | − 60 | 44 | 5.31 |
| Superior parietal lobule | R | 7 | 42 | − 46 | 36 | 5.31 | ||
| Precuneus | R | 7 | 8 | − 66 | 44 | 5.18 | ||
| Caudate | 2376 | < 0.001 | L | − 10 | 6 | 2 | 3.31 | |
| R | 14 | 4 | 20 | 2.83 | ||||
| Nucleus accumbens | L | − 10 | 4 | − 2 | 3.21 | |||
| R | 8 | 0 | − 2 | 3.21 | ||||
| Thalamus | L | − 6 | − 8 | 0 | 3.73 | |||
| R | 8 | − 6 | 4 | 4.47 | ||||
| Cerebellum | R | 4 | − 30 | − 30 | 2.76 | |||
| Inferior parietal lobule | 979 | 0.015 | L | 40 | − 40 | − 46 | 42 | 3.45 |
| Superior parietal lobule | L | 7 | − 28 | − 58 | 42 | 4.75 | ||
| Precuneus | L | 7 | − 12 | − 66 | 46 | 2.77 | ||
| Insula | 935 | 0.019 | R | 30 | 22 | 2 | 6.51 | |
NMR, no monetary reward; L, left; R, right; BA, Brodmann's area; MNI, Montréal Neurological Institute. The extent threshold was set at p = 0.05 with a correction for multiple comparisons at the cluster level for the entire brain. The height threshold was set at p = 0.005 (uncorrected) at voxel level.
In order to specify the brain areas involved in reward sensitivity, we examined task contrast (HMR minus LMR) in each study group (Fig. 3A). We found that although the activation level of each activated region (see Table 4) was not different between the LMR and HMR conditions in the HC group, activation of the bilateral nucleus accumbens was lower in the LMR than in the HMR condition in the btADHD group. In the atADHD group, differences in activation of the bilateral nucleus accumbens between the LMR and HMR conditions were not observed. No differences were found in each study group when the LMR and HMR conditions (LMR minus HMR) were compared.
Fig. 3.
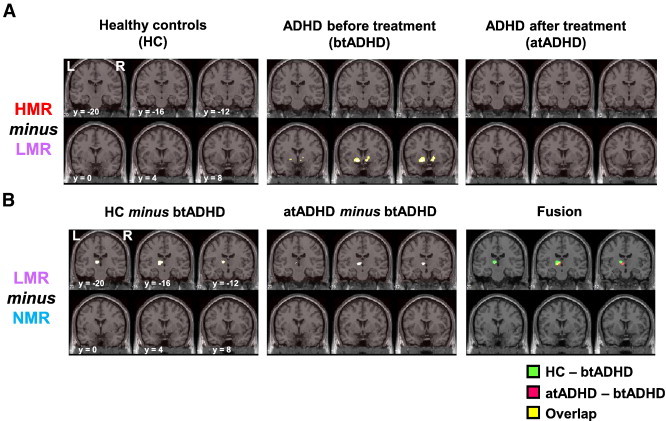
Statistical parametric maps of (A) reward sensitivity (HMR minus LMR) in healthy controls (HC) and ADHD patients before (btADHD) and after treatment (atADHD) with OROS-MPH and of (B) the effects of OROS-MPH treatment on brain activation during low monetary reward (HC minus btADHD and atADHD minus btADHD) are shown. Right (R) and left (L) sides and y-axis (MNI coordinate) are indicated.
To specify the brain areas affected by OROS-MPH treatment, we examined the study group contrast (atADHD minus btADHD or HC) in the HMR (HMR minus NMR) and LMR (LMR minus NMR) conditions (Fig. 3B). In the HMR condition, no differences were detected in the activation level of each activated region (see Tables 5, 6) between the atADHD and btADHD groups. However, in the LMR condition, thalamic activation was higher in the atADHD group than in the btADHD group. Thalamic activation during the LMR condition was also higher in the HC group than in the btADHD group. The strongly activated thalamic areas overlapped between the HC and atADHD groups. The activation levels in other regions were similar in the HC and atADHD groups, in both the HMR and LMR conditions. The results of the analysis of reward sensitivity using task contrast (HMR minus LMR) in the btADHD group (Fig. 3A) indicated that thalamic activation (x = − 20, y = − 18, z = 6) in the LMR condition tended to be lower than in the HMR condition (uncorrected p < 0.01, cluster size = 15 voxels).
We found that the ADHD-RS-IV-J, BIS, SDQ and IEML scores, as well as decreased activation of the nucleus accumbens and thalamus during the LMR condition before treatment, were improved after treatment in the ADHD patients. In order to clarify the effect of OROS-MPH treatment on the relationship between improved ADHD symptoms and brain activity during low reward processing, we performed correlation analyses between questionnaire delta scores (atADHD minus btADHD) and delta activation of the nucleus accumbens and thalamus (atADHD minus btADHD) (Table 7). In accordance with circular analysis (Kriegeskorte et al., 2009), we redefined the regions of interest for the nucleus accumbens and thalamus, based on a previous study that used the same monetary reward tasks (Izuma et al., 2008). This study showed activities, using the MNI-group coordinates, for the left (x = − 14, y = 10, z = − 4) and right (x = 12, y = 10, z = − 4) nucleus accumbens, as well as the thalamus (x = − 14, y = − 14, z = 14), in the (HMR minus NMR) condition. In the present study, activation of the left and right nucleus accumbens and thalamus were summarized with principal eigenvariates over all voxels within a radius of 4 mm, with group coordinates determined using the volume-of-interest tool in SPM5. The correlation analyses revealed that the delta activation of the left nucleus accumbens was negatively correlated with the delta of inattention score of ADHD-RS-IV-J. Likewise, a negative correlation was observed between the thalamic activation delta and delta scores for inattention of ADHD-RS-IV-J. Significant correlations were not observed between the activation deltas for the nucleus accumbens or thalamus and the deltas of other questionnaire scores.
Table 7.
Correlations between questionnaire scores and brain activation during the low monetary reward condition in ADHD patients.
| Delta score | Delta activation |
||
|---|---|---|---|
| Left NAcc | Right NAcc | Thalamus | |
| ADHD-RS-IV-J | |||
| Inattention | − 0.515⁎ | − 0.026 | − 0.488⁎ |
| Hyperactivity/impulsivity | − 0.020 | 0.308 | − 0.267 |
| Total | − 0.214 | 0.161 | − 0.431 |
| BIS | 0.282 | − 0.121 | 0.204 |
| QCD | − 0.158 | 0.217 | 0.017 |
| IEML | 0.268 | 0.304 | 0.128 |
ADHD-RS-IV-J, Japanese versions of the ADHD rating scale-fourth edition; BIS, Barratt impulsiveness scale; QCD, questionnaires for children with difficulties; IEML, scale of intrinsic versus extrinsic motivation toward learning; NAcc, nucleus accumbens. Delta score and activation were calculated by subtracted after treatment values from before treatment values. Pearson's coefficient values (r) are shown.
p < 0.05, significant correlation.
Although we investigated the effects of OROS-MPH dosage on activation of the nucleus accumbens and thalamus and questionnaire scores, significant differences were not observed among the dosage groups (18 mg, 27 mg and 36 mg) (Table 8).
Table 8.
Effects of OROS-MPH dosage on brain activity during the low monetary reward condition and questionnaire scores in ADHD patients.
| Delta activation or score | Dosage of OROS-MPH |
||
|---|---|---|---|
| 18 mg (n = 6) | 27 mg (n = 6) | 36 mg (n = 5) | |
| Brain region | |||
| Left NAcc | 0.27 ± 0.80 | 0.17 ± 0.68 | 0.72 ± 0.64 |
| Right NAcc | 0.30 ± 0.60 | 0.57 ± 0.89 | 0.49 ± 0.57 |
| Thalamus | 0.16 ± 0.79 | − 0.07 ± 0.80 | 1.22 ± 0.87 |
| ADHD-RS-IV-J | |||
| Inattention | − 2.5 ± 3.5 | − 4.3 ± 4.3 | − 8.8 ± 9.7 |
| Hyperactivity/impulsivity | − 3.0 ± 4.0 | − 3.8 ± 4.9 | − 5.0 ± 10.4 |
| Total | − 5.5 ± 5.5 | − 8.0 ± 7.1 | − 13.8 ± 18.5 |
| BIS | − 10.5 ± 3.8 | − 17.7 ± 7.6 | − 7.4 ± 2.7 |
| QCD | 10.0 ± 7.0 | 4.8 ± 15.9 | 1.0 ± 5.0 |
| IEML | 5.0 ± 12.3 | 8.5 ± 11.7 | 10.2 ± 17.6 |
OROS-MPH, osmotic release oral system-methylphenidate; NAcc, nucleus accumbens; ADHD-RS-IV-J, Japanese versions of the ADHD rating scale-fourth edition; BIS, Barratt impulsiveness scale; QCD, questionnaires for children with difficulties; IEML, scale of intrinsic versus extrinsic motivation toward learning. Delta score and activity were calculated by subtracting after treatment values from before treatment values. Values are presented as mean ± SD.
4. Discussion
Consistent with our results, a prior study showed that clinical improvement, in terms of inattention and hyperactivity/impulsivity in Japanese children and adolescents with ADHD lessened following OROS-MPH treatment for 2–3 months (Sawada et al., 2010). Although a few fMRI studies have shown the effects of relatively-long periods of MPH treatment on cognitive function, such as the attention task for children and adults with ADHD (Konrad et al., 2007; Stoy et al., 2011), there are no previous reports of the effects of OROS-MPH treatment on monetary reward processing in children and adolescents with ADHD. Thus, we are the first to demonstrate the possible effect of OROS-MPH treatment on the reward system in the brain of ADHD children and adolescents.
Fronto-striatal dysfunction is one of the pathophysiological hallmarks of ADHD (Cubillo et al., 2010). Previous fMRI studies on the effects of a gambling task have shown decreased activation of the ventral striatum in both adolescents and adults with ADHD during monetary reward processing (Plichta et al., 2009; Scheres et al., 2007; Ströhle et al., 2008). These studies evaluated brain activation during a delay while waiting for a reward (reward anticipation). However, the present study evaluated the neural substrates associated with reward sensitivity during reward outcome, as opposed to reward anticipation. Recently, Paloyelis et al. (2012) reported that although the striatal activity of adolescents with ADHD during reward anticipation was not altered in comparison to healthy adolescents, activity during a high monetary reward condition (5 £ = around 680 Japanese yen) was increased. In the present study, an increase in striatal activation during the HMR condition [average of 330 yen each (range = 270–390 yen)] in ADHD patients was not observed. In contrast to the HMR condition, striatal activation was decreased during the LMR condition [average of 150 yen each (range = 90–210 yen)]. These results suggest that elevated or reduced striatal activity reflects impairment of striatal sensitivity due to dysfunctional transfer of phasic dopamine release from the actual reward to its predicting stimulus, thereby resulting in impaired appraisal of motivational outcomes and subsequent behavioral adaptation (Tripp and Wickens, 2008).
Although a number of studies have reported that activation of the nucleus accumbens during monetary reward anticipation occurs (Dreher et al., 2007; Ernst et al., 2004; Kirsch et al., 2003; Knutson et al., 2001, 2005; Liu et al., 2007), a few studies have also reported an association between activation of the nucleus accumbens and reward outcome (Breiter et al., 2001; Izuma et al., 2008). Other brain regions have been suggested to play a role in reward outcome, including the orbitofrontal and medial prefrontal cortex (Dillon et al., 2008; Knutson et al., 2003; O'Doherty et al., 2003), the cingulate cortex (Knutson et al., 2003; Nieuwenhuis et al., 2005), the amygdala (Dreher et al., 2007; Hamann et al., 2004; Stoeckel et al., 2008), and the thalamus (Izuma et al., 2008; Rademacher et al., 2010; Thut et al., 1997). The present unexpected finding of pronounced thalamic activation associated with monetary reward adds to the results of prior imaging studies, which have shown recruitment of this structure during the processing of monetary reward (Bjork et al., 2004; Izuma et al., 2008; Martin-Soelch et al., 2003; Thut et al., 1997).
MPH treatment for ADHD patients increases extrasynaptic dopamine and noradrenaline levels by blocking reuptake (Zetterström et al., 1988). Acute oral MPH treatment of ADHD adolescents increases dopamine concentrations in the ventral striatum, with the dopamine concentrations positively correlated with improvement in symptom severity (Rosa-Neto et al., 2005). In contrast to acute MPH treatment in children and adolescents with ADHD, changes in dopaminergic transmission in each brain region following long-term MPH treatment are unclear. Although changes in thalamic dopamine concentrations following MPH treatment were not described in this study (Rosa-Neto et al., 2005), PET studies, using radiolabeled MPH, of healthy adults have shown that the availability of the dopamine transporter in the basal ganglia is the highest, and the second highest region is the thalamus, relative to the temporal insula, cingulate, orbitofrontal, frontal and occipital cortices (Wang et al., 1995). Therefore, MPH and OROS-MPH should theoretically increase dopamine transmission within the ventral striatum (including the nucleus accumbens) as well as the thalamus. It is possible that acute MPH treatment alters dopaminergic transmission only in the ventral striatum (Rosa-Neto et al., 2005). However, both MPH and OROS-MPH treatments, for a relatively long period in ADHD children and adolescents, may alter dopaminergic activity in both the ventral striatum and thalamus; thus, the role played by these regions in reward sensitivity processing may improve after treatment for 3 months.
Improved activation of the nucleus accumbens and thalamus during lower monetary reward processing were correlated with decreased inattention score of ADHD-RS-IV-J following OROS-MPH treatment in the present study. Previous findings have shown that cortico-striatal-thalamic circuits are associated with reward, and task demands trigger highly similar processes that have been linked to attentional control (Gitelman et al., 1999; Hopfinger et al., 2000; Krebs et al., 2012). In keeping with this notion, the reward mechanism appears to act by utilizing and modulating attentional processes that are typically employed in endogenous attentional control, as has been suggested (e.g., Pessoa and Engelmann, 2010). Thus, improvement of reward processing following OROS-MPH treatment may contribute to the improved attentional function involved in ADHD symptoms.
Although the effect of MPH treatment on ADHD children and adolescents has been shown, a relationship between MPH treatment and the risk for substance abuse in ADHD patients has also been discussed (Robbins, 2002). Lambert and Hartsough (1998) showed that cocaine abuse and nicotine abuse were associated with ADHD patients who had used stimulant medication in childhood in contrast to un-medicated ADHD patients. Conversely, a meta-analysis revealed a 1.9-fold reduction in the risk for substance use disorders in children and adolescents who were treated with stimulants, including MPH, compared with those who did not receive pharmacotherapy (Wilens et al., 2003). OROS-MPH has an immediate-release outer coating and a core that delivers MPH based on osmotic pressure (Markowitz et al., 2003). This technology combines the benefits of immediate-release MPH formulations (IR-MPH) with sustained drug release, where the time to maximum concentrations (Tmax) is approximately 6 to 8 h (Modi et al., 2000). In addition, the maximum plasma concentration (Cmax) for OROS-MPH is lower than IR-MPH (Modi et al., 2000). OROS-MPH shows effectiveness and side effects similar to those seen with IR-MPH (Weisler, 2007). In comparison with IR-MPH, the pharmacokinetic profile of OROS-MPH contributes to slower absorption and brain entry, as well as sustained dopamine receptor and transporter occupancies (Parasrampuria et al., 2007; Spencer et al., 2006). These results suggest that OROS-MPH has a lower abuse potential than IR-MPH. In the current study, although we could not directly evaluate the risk for substance abuse associated with MPH treatment in ADHD patients, we could compare activation levels of brain regions related to monetary reward processing between post-medication ADHD patients and healthy children and adolescents. In a previous fMRI study, an increase in the activation of the ventral striatum during monetary reward anticipation in cannabis users relative to the drug-naive controls was observed (Nestor et al., 2010). Buckholtz et al. (2010) noted that neurochemical and neurophysiological hyper-reactivity in the dopaminergic reward system, such as nucleus accumbens comprises a neural substrate for impulsive–antisocial behavior and substance abuse in psychopathy. In the present study, we did not observe hyper-activated brain regions (nucleus accumbens and thalamus) during both high and low monetary reward processing in ADHD patients after OROS-MPH treatment in comparison with healthy children and adolescents. These results suggest that OROS-MPH treatment for 3 months in ADHD children and adolescents may present a low risk for the development of substance abuse.
In conclusion, decreased reward sensitivity, based on decreased activation of the nucleus accumbens and thalamus during the low monetary reward condition, and ADHD symptoms were improved by OROS-MPH treatment for 3 months in ADHD children and adolescents. In addition, hyper-activated brain areas of ADHD patients, relative to the healthy children and adolescents, during monetary reward after OROS-MPH treatment were not observed. Further study is necessary to identify the extent of treatment effects of OROS-MPH in children and adolescents with ADHD using placebo trials. However, the present findings suggest that OROS-MPH treatment for a 3-month period is effective for children and adolescents with ADHD and that the current fMRI experiments are useful for the objective non-invasive evaluation of dopaminergic function following OROS-MPH treatment.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (B) and Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (KAKENHI: grant number 24300149 and 23650223 to A.T.). This work also was partially supported by a Grant-in-Aid for Scientific Research from Japan–U.S. Brain Research Cooperation Program (grant number 230201 to A.T.), as well as the Research and Education Program for Life Science, University of Fukui (grant number 210201 to A.T.), the Hayao Nakayama Foundation for Science & Technology and Culture (grant number H22-A053 to K.M.) and the RIKEN Special Postdoctoral Researchers Program (grant number K24232 to K.M.). We would like to thank Ms. Tomoko Yamaguchi and Ms. Kanako Tajima for their excellent technical assistance in participant recruitment and MRI data analyses, respectively. In addition, we would also like to thank Mr. Hiroki Arimura, Mr. Terumasa Takemaru, Mr. Motohira Mio, Ms. Hiroko Ueda, Ms. Niino Iseri, Ms. Eri Uchida and Ms. Saori Yasuoka for their excellent technical assistance with experimental preparation and Forte Science Communications for editorial help with the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abikoff H., Nissley-Tsiopinis J., Gallagher R., Zambenedetti M., Seyffert M., Boorady R., McCarthy J. Effects of MPH-OROS on the organizational, time management, and planning behaviors of children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:166–175. doi: 10.1097/CHI.0b013e3181930626. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th ed. The American Psychiatric Publishing; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision (DSM-IV-TR) [Google Scholar]
- Angold A., Costello E.J., Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biological Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Biederman J., Newcorn J., Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. The American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S.V., Spencer T., Wilens T., Norman D., Lapey K.A., Mick E., Lehman B.K., Doyle A. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. The American Journal of Psychiatry. 1993;150:1792–1798. doi: 10.1176/ajp.150.12.1792. [DOI] [PubMed] [Google Scholar]
- Biederman J., Mick E., Surman C., Doyle R., Hammerness P., Harpold T., Dunkel S., Dougherty M., Aleardi M., Spencer T. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2006;59:829–835. doi: 10.1016/j.biopsych.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Biederman J., Monuteaux M.C., Mick E., Spencer T., Wilens T.E., Silva J.M., Snyder L.E., Faraone S.V. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland B.L., Heckman H.K. Hyperactive boys and their brothers: a 25-year follow-up study. Archives of General Psychiatry. 1976;33:669–675. doi: 10.1001/archpsyc.1976.01770060013002. [DOI] [PubMed] [Google Scholar]
- Breiter H.C., Aharon I., Kahneman D., Dale A., Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Buckholtz J.W., Treadway M.T., Cowan R.L., Woodward N.D., Benning S.D., Li R., Ansari M.S., Baldwin R.M., Schwartzman A.N., Shelby E.S., Smith C.E., Cole D., Kessler R.M., Zald D.H. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience. 2010;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Spencer T.J., Holmes J., Shin L.M., Valera E.M., Seidman L.J., Makris N., Surman C., Aleardi M., Mick E., Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Archives of General Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Chou W.J., Chou M.C., Tzang R.F., Hsu Y.C., Gau S.S., Chen S.J., Wu Y.Y., Huang Y.F., Liang H.Y., Cheng H. Better efficacy for the osmotic release oral system methylphenidate among poor adherents to immediate-release methylphenidate in the three ADHD subtypes. Psychiatry and Clinical Neurosciences. 2009;63:167–175. doi: 10.1111/j.1440-1819.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI Studies. American Journal of Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Ecker C., Giampietro V., Taylor E., Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Holmes A.J., Jahn A.L., Bogdan R., Wald L.L., Pizzagalli D.A. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J.C., Schmidt P.J., Kohn P., Furman D., Rubinow D., Berman K.F. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Mulder M.J., Spicer J.A., Galvan A., Tottenham N., Scheres A., Xavier Castellanos F., van Engeland H., Casey B.J. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Durston S., van Belle J., de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;69:1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Epstein J.N., Casey B.J., Tonev S.T., Davidson M.C., Reiss A.L., Garrett A., Hinshaw S.P., Greenhill L.L., Glover G., Shafritz K.M., Vitolo A., Kotler L.A., Jarrett M.A., Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent–child dyads with ADHD. Journal of Child Psychology and Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., McClure E.B., Monk C.S., Munson S., Eshel N., Zarahn E., Leibenluft E., Zametkin A., Towbin K., Blair J., Charney D., Pine D.S. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Evans A.C., Kamber M., Collins D.L., MacDonald D. An MRI based probalistic atlas of neuroanatomy. In: Shorvon S.D., editor. Magnetic Resonance Scanning and Epilepsy. Plenum Press; New York: 1994. pp. 263–274. [Google Scholar]
- Fone K.C., Nutt D.J. Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Current Opinion in Pharmacology. 2005;5:87–93. doi: 10.1016/j.coph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J. How many subjects constitute a study? NeuroImage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Yamano E., Joudoi T., Mizuno K., Tanaka M., Kawatani J., Takano M., Tomoda A., Imai-Matsumura K., Miike T., Watanabe Y. Effort–reward imbalance for learning is associated with fatigue in school children. Behavioral Medicine. 2010;36:53–62. doi: 10.1080/08964281003774919. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Rubia K., Rossi G., Sartori G., Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. The American Journal of Psychiatry. 2012;169:264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Nobre A.C., Parrish T.B., LaBar K.S., Kim Y.H., Meyer J.R., Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Habib M., Cassotti M., Borst G., Simon G., Pineau A., Houdé O., Moutier S. Counterfactually mediated emotions: a developmental study of regret and relief in a probabilistic gambling task. Journal of Experimental Child Psychology. 2012;112:265–274. doi: 10.1016/j.jecp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Hamann S., Herman R.A., Nolan C.L., Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Iizuka C., Yamashita Y., Nagamitsu S., Yamashita T., Araki Y., Ohya T., Hara M., Shibuya I., Kakuma T., Matsuishi T. Comparison of the strengths and difficulties questionnaire (SDQ) scores between children with high-functioning autism spectrum disorder (HFASD) and attention-deficit/hyperactivity disorder (AD/HD) Brain & Development. 2010;32:609–612. doi: 10.1016/j.braindev.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kester H.M., Sevy S., Yechiam E., Burdick K.E., Cervellione K.L., Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophrenia Research. 2006;85:113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kim Y., Shin M.S., Kim J.W., Yoo H.J., Cho S.C., Kim B.N. Neurocognitive effects of switching from methylphenidate-IR to OROS-methylphenidate in children with ADHD. Human Psychopharmacology. 2009;24:95–102. doi: 10.1002/hup.1010. [DOI] [PubMed] [Google Scholar]
- Kirsch P., Schienle A., Stark R., Sammer G., Blecker C., Walter B., Ott U., Burkart J., Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. NeuroImage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B., Taylor J., Kaufman M., Peterson R., Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins S.H., Shapiro S.K., Newland M.C., Abramowitz A. Discriminative and participant-rated effects of methylphenidate in children diagnosed with attention deficit hyperactivity disorder (ADHD) Experimental and Clinical Psychopharmacology. 1998;6:375–389. doi: 10.1037/1064-1297.6.4.375. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Fink G.R., Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: results from a longitudinal functional MRI study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Roberts K.C., Song A.W., Woldorff M.G. The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex. 2012;22:607–615. doi: 10.1093/cercor/bhr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W.K., Bellgowan P.S., Baker C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert N.M., Hartsough C.S. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Liu X., Powell D.K., Wang H., Gold B.T., Corbly C.R., Joseph J.E. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. Journal of Neuroscience. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M., Oosterlaan J., Knol D.L., Sergeant J.A. Decision-making in ADHD: sensitive to frequency but blind to the magnitude of penalty? Journal of Child Psychology and Psychiatry. 2008;49:712–722. doi: 10.1111/j.1469-7610.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Markowitz J.S., Straughn A.B., Patrick K.S. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–1299. doi: 10.1592/phco.23.12.1281.32697. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C., Missimer J., Leenders K.L., Schultz W. Neural activity related to the processing of increasing monetary reward in smokers and nonsmokers. European Journal of Neuroscience. 2003;18:680–688. doi: 10.1046/j.1460-9568.2003.02791.x. [DOI] [PubMed] [Google Scholar]
- Miyawaki D., Suzuki F., Mamoto A., Takahashi K., Kiriike N. The reliability and validity of Japanese version of the schedule for affective disorders and schizophrenia for school-age children — present and lifetime version (K-SADS-PL) Japanese Journal of Child & Adolescent Psychiatry. 2003;197 (in Japanese) [Google Scholar]
- Modi N.B., Lindemulder B., Gupta S.K. Single- and multiple-dose pharmacokinetics of an oral once-a-day osmotic controlled-release OROS (methylphenidate HCl) formulation. Journal of Clinical Pharmacology. 2000;40:379–388. doi: 10.1177/00912700022009080. [DOI] [PubMed] [Google Scholar]
- Molina B.S., Hinshaw S.P., Swanson J.M., Arnold L.E., Vitiello B., Jensen P.S., Epstein J.N., Hoza B., Hechtman L., Abikoff H.B., Elliott G.R., Greenhill L.L., Newcorn J.H., Wells K.C., Wigal T., Gibbons R.D., Hur K., Houck P.R., MTA Cooperative Group The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monden Y., Dan H., Nagashima M., Dan I., Tsuzuki D., Kyutoku Y., Gunji Y., Yamagata T., Watanabe E., Mori M.Y. Right prefrontal activation as a neuro-functional biomarker for monitoring acute effects of methylphenidate in ADHD children: An fNIRS study. Neuroimage: Clinical. 2012;123:1147–1157. doi: 10.1016/j.nicl.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J.R. Adult psychiatric disorders in parents of hyperactive children. The American Journal of Psychiatry. 1980;137:825–827. doi: 10.1176/ajp.137.7.825. [DOI] [PubMed] [Google Scholar]
- Nestor L., Hester R., Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Slagter H.A., von Geusau N.J., Heslenfeld D.J., Holroyd C.B. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005;21:3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- O'Doherty J., Winston J., Critchley H., Perrett D., Burt D.M., Dolan R.J. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y., Mehta M.A., Kuntsi J., Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Review of Neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y., Mehta M.A., Faraone S.V., Asherson P., Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:722–732. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasrampuria D.A., Schoedel K.A., Schuller R., Gu J., Ciccone P., Silber S.A., Sellers E.M. Assessment of pharmacokinetics and pharmacodynamic effects related to abuse potential of a unique oral osmotic-controlled extended-release methylphenidate formulation in humans. Journal of Clinical Pharmacology. 2007;47:1476–1488. doi: 10.1177/0091270007308615. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. Embedding reward signals into perception and cognition. Frontiers in Neuroscience. 2010;4:17. doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Vasic N., Wolf R.C., Lesch K.P., Brummer D., Jacob C., Fallgatter A.J., Grön G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Rademacher L., Krach S., Kohls G., Irmak A., Gründer G., Spreckelmeyer K.N. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Robbins T.W. ADHD and addiction. Nature Medicine. 2002;8:24–25. doi: 10.1038/nm0102-24. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P., Lou H.C., Cumming P., Pryds O., Karrebaek H., Lunding J., Gjedde A. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. NeuroImage. 2005;25:868–876. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sakurai S., Takano S. A new self-report scale of intrinsic versus extrinsic motivation toward learning in children. Tsukuba Psychology Research. 1985;7:43–54. (in Japanese) [Google Scholar]
- Sawada M., Iida J., Ota T., Negoro H., Tanaka S., Sadamatsu M., Kishimoto T. Effects of osmotic-release methylphenidate in attention-deficit/hyperactivity disorder as measured by event-related potentials. Psychiatry and Clinical Neurosciences. 2010;64:491–498. doi: 10.1111/j.1440-1819.2010.02134.x. [DOI] [PubMed] [Google Scholar]
- Scheres A., Milham M.P., Knutson B., Castellanos F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Fan J., Bédard A.C., Clerkin S.M., Ivanov I., Tang C.Y., Halperin J.M., Newcorn J.H. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2012;69:952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto M.V. Oxford University Press; New York: 2001. Attention-Deficit/Hyperactivity Disorder. [Google Scholar]
- Someya T., Sakado K., Seki T., Kojima M., Reist C., Tang S.W., Takahashi S. The Japanese version of the Barratt Impulsiveness Scale, 11th version (BIS-11): its reliability and validity. Psychiatry and Clinical Neurosciences. 2001;55:111–114. doi: 10.1046/j.1440-1819.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Spencer T.J., Biederman J., Madras B.K., Faraone S.V., Dougherty D.D., Bonab A.A., Fischman A.J. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biological Psychiatry. 2005;57:1293–1300. doi: 10.1016/j.biopsych.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Spencer T.J., Biederman J., Ciccone P.E., Madras B.K., Dougherty D.D., Bonab A.A., Livni E., Parasrampuria D.A., Fischman A.J. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. The American Journal of Psychiatry. 2006;163:387–395. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- Stoeckel L.E., Weller R.E., Cook E.W., III, Twieg D.B., Knowlton R.C., Cox J.E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stoy M., Schlagenhauf F., Schlochtermeier L., Wrase J., Knutson B., Lehmkuhl U., Huss M., Heinz A., Ströhle A. Reward processing in male adults with childhood ADHD — a comparison between drug-naïve and methylphenidate-treated subjects. Psychopharmacology. 2011;215:467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- Ströhle A., Stoy M., Wrase J., Schwarzer S., Schlagenhauf F., Huss M., Hein J., Nedderhut A., Neumann B., Gregor A., Juckel G., Knutson B., Lehmkuhl U., Bauer M., Heinz A. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. NeuroImage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Fukuda S., Mizuno K., Imai-Matsumura K., Jodoi T., Kawatani J., Takano M., Miike T., Tomoda A., Watanabe Y. Reliability and validity of the Japanese version of the Chalder Fatigue Scale among youth in Japan. Psychological Reports. 2008;103:682–690. doi: 10.2466/pr0.103.3.682-690. [DOI] [PubMed] [Google Scholar]
- Thut G., Schultz W., Roelcke U., Nienhusmeier M., Missimer J., Maguire R.P., Leenders K.L. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Tripp G., Wickens J.R. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of child psychology and psychiatry and allied disciplines. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Gatley S.J., Logan J., Ding Y.S., Hitzemann R., Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. The American Journal of Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G., Fowler J.S., Logan J., Gerasimov M., Maynard L., Ding Y., Gatley S.J., Gifford A., Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. Journal of Neuroscience. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Newcorn J., Telang F., Solanto M.V., Fowler J.S., Logan J., Ma Y., Schulz K., Pradhan K., Wong C., Swanson J.M. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Wang G.J., Volkow N.D., Fowler J.S., Ding Y.S., Logan J., Gatley S.J., MacGregor R.R., Wolf A.P. Comparison of two pet radioligands for imaging extrastriatal dopamine transporters in human brain. Life Sciences. 1995;57:PL187–PL191. doi: 10.1016/0024-3205(95)02099-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1991. Manual for the Wechsler Intelligence Scale for Children. [Google Scholar]
- Weisler R.H. Review of long-acting stimulants in the treatment of attention deficit hyperactivity disorder. Expert Opinion on Pharmacotherapy. 2007;8:745–758. doi: 10.1517/14656566.8.6.745. [DOI] [PubMed] [Google Scholar]
- Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology. 2008;28:46–53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- Wilens T.E., Faraone S.V., Biederman J., Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Yamazaki K. Japanese Guideline for the Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder (ADHD) Jiho; Tokyo: 2003. ADHD-RS-IV Japanese versions; pp. 48–54. (in Japanese) [Google Scholar]
- Yang L., Cao Q., Shuai L., Li H., Chan R.C., Wang Y. Comparative study of OROS-MPH and atomoxetine on executive function improvement in ADHD: a randomized controlled trial. The International Journal of Neuropsychopharmacology. 2011;21:1–12. doi: 10.1017/S1461145711001490. [DOI] [PubMed] [Google Scholar]
- Yildiz O., Sismanlar S.G., Memik N.C., Karakaya I., Agaoglu B. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry and Human Development. 2011;42:257–269. doi: 10.1007/s10578-010-0212-3. [DOI] [PubMed] [Google Scholar]
- Zetterström T., Sharp T., Collin A.K., Ungerstedt U. In vivo measurement of extracellular dopamine and DOPAC in rat striatum after various dopamine-releasing drugs; implications for the origin of extracellular DOPAC. European Journal of Pharmacology. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]


