Abstract
Recent evidence suggests that immobilization of the upper limb for 2–3 weeks induces changes in cortical thickness as well as motor performance. In constraint induced (CI) therapy, one of the most effective interventions for hemiplegia, the non-paretic arm is constrained to enforce the use of the paretic arm in the home setting. With the present study we aimed to explore whether non-paretic arm immobilization in CI therapy induces structural changes in the non-lesioned hemisphere, and how these changes are related to treatment benefit. 31 patients with chronic hemiparesis participated in CI therapy with (N = 14) and without (N = 17) constraint. Motor ability scores were acquired before and after treatment. Diffusion tensor imaging (DTI) data was obtained prior to treatment. Cortical thickness was measured with the Freesurfer software. In both groups cortical thickness in the contralesional primary somatosensory cortex increased and motor function improved with the intervention. However the cortical thickness change was not associated with the magnitude of motor function improvement. Moreover, the treatment effect and the cortical thickness change were not significantly different between the constraint and the non-constraint groups. There was no correlation between fractional anisotropy changes in the non-lesioned hemisphere and treatment outcome. CI therapy induced cortical thickness changes in contralesional sensorimotor regions, but this effect does not appear to be driven by the immobilization of the non-paretic arm, as indicated by the absence of differences between the constraint and the non-constraint groups. Our data does not suggest that the arm immobilization used in CI therapy is associated with noticeable cortical thinning.
Keywords: Immobilization, Sensorimotor deprivation, Hemiparesis, Surface-based morphometry, Cortical thickness, Diffusion tensor imaging, Stroke, Learned non-use, Plasticity
1. Introduction
It is estimated that 85% of stroke survivors sustain upper limb hemiparesis (Thorngren and Westling, 1990) with 30–60% experiencing permanent impairments of motor function (van der Lee, 2003). The need to improve long-term motor outcome, and the challenges involved in this endeavor, has long been recognized. The discovery of adult brain plasticity, together with the emergence of positive evidence for motor function improvement through repetitive training and practice, has driven a paradigm shift in the treatment of motor deficits after stroke (French et al., 2007; Taub et al., 2002). One concept, constraint induced movement therapy (CI-therapy), has received particularly strong resonance in the field. This is evidenced by several systematic reviews (e.g. Nijland et al., 2011; Peurala et al., 2012; Sirtori et al., 2009), and multi-centered trials (e.g. EXCITE, Wolf et al., 2007; Wolf et al., 2010) which suggest sustainable improvements of upper limb function through CI-therapy or its derivatives (e.g. Page, 2007; Sterr and Freivogel, 2003).
The signature CI-therapy intervention comprises 6 h of daily training with the paretic arm while constraining the non-paretic arm with a splint–sling constraint for 90% of waking hours (Taub et al., 1993). This daily regime is provided for 10 consecutive days spread over two weeks. The concept of linking paretic arm practice with constraining the non-paretic arm is rooted in theoretical assumptions. Specifically, CI-therapy assumes that increased paretic arm use, induced by a combination of massed practice and changes to the behavioral tendency to disuse the paretic limb spontaneously, promotes functional reorganization of the brain and the recovery of function. Through the constraint of the non-paretic arm during the intervention period, CI-therapy further aims to break the behavioral contingencies that perpetuate the conditioned non-use of the paretic arm (Sterr et al., 2002; Taub et al., 1993). It is presumed that the constraint makes a large contribution to the sustained improvements in the everyday life setting (Taub, 1994).
Several studies have explored the functional and structural changes induced by CI therapy (e.g. Cope et al., 2010; Liepert, 2006; Liepert et al., 2000; Liepert et al., 1998; Mark et al., 2006; Sawaki et al., 2008; Wittenberg et al., 2003). These studies generally indicated some use-dependent changes in the reorganized neural systems controlling paretic arm movements (e.g. Sawaki et al., 2008), as well as changes in gray and white matter density (e.g. Gauthier et al., 2008). It is assumed that these changes are driven by the increased use of the paretic hand through the daily shaping training and the concurrent constraint of the non-paretic arm. However, the constraint not only facilitates paretic arm use but also reduces the sensory input and motor output of the non-paretic arm. One might therefore question whether the constraint causes neuroplastic changes for the paretic as well as the non-paretic arm. More specifically, a recent neuroimaging study on the effects of arm immobilization (Langer et al., 2012) suggests that immobilizing the upper limb for a period of 2–3 weeks causes cortical thinning in the sensorimotor hand area contralateral to the immobilized limb. At the same time function in the non-immobilized (non-dominant) hand improves. Presumably these structural and behavioral effects are caused by activity-dependent changes in the neural representations of the immobilized and non-immobilized hands respectively.
The findings by Langer et al. (2012) are potentially very important for the concept of CI therapy. They not only support the idea that skill transfer from one hand to the other is facilitated by constraining one limb, but also suggest that the structural characteristics of the non-lesioned hemisphere are changed by this measure. The interaction between homologous motor representations in the two hemispheres during recovery is complex, and different theories have been put forward to explain the role of interhemispheric facilitation on the prediction of outcome (e.g. Carter et al., 2010; Murase et al., 2004; Takeuchi and Izumi, 2012; van Meer et al., 2012; van Meer et al., 2010). The constraint element of CI therapy might well interfere with these processes, in particular when applied in the post-acute phase. Understanding the effects of the constraint on the non-lesioned hemisphere is therefore important.
Based on Langer's findings, one might further predict that wearing the constraint would induce a reduction of cortical thickness in the sensorimotor cortex through the short-term deprivation of the sensorimotor representation of the non-paretic limb. At the same time, however, it is possible that the increased use of the paretic arm might induce use-related changes of the ipsilateral hand representation, which may be manifested in a cortical thickness increase. The present study therefore sought to examine structural changes in the non-lesioned hemisphere of 31 patients with chronic stroke undergoing CI therapy with (N = 14) or without constraint (N = 17). Using the Freesurfer software we conducted a cortical thickness analysis using a whole brain as well as a hypothesis-driven region of interest cortical thickness analysis for the non-lesioned hemisphere. We assumed that cortical thickness would change with the intervention and that this change should be greater in those wearing the constraint. We further predicted that wearing the constraint would facilitate skill transfer, and hence expected stronger treatment effects in the constraint group. In addition, we reasoned that if treatment effects are greater in those wearing the constraint, and constraint-wearing affects cortical thickness, then a significant correlation between treatment benefit and cortical thickness should be found.
2. Material and methods
2.1. Participants
31 patients with moderate to severe chronic upper-limb hemiparesis of the left (N = 15) or the right (N = 16) arm following first ever stroke participated in the study. Hemiparesis was caused by unilateral mixed lesions (illustrated in Inline Supplementary Fig. S1 in Appendix A), as determined by visual inspection of a trained neurologist (Appendix A.3). Details are summarized in Table 1.
Inline Supplementary Figure S1.
Fig. S1.
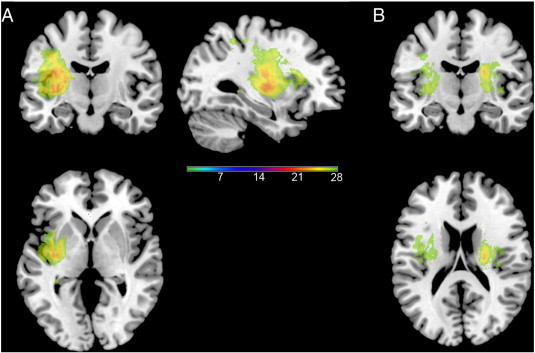
Lesioned areas in the 31 patients included. A. Lesioned areas after all right lesioned participants are flipped onto left hemisphere. B. Lesioned areas before right lesioned participants are flipped. In both cases, scale represents number of subjects with lesion in this area.
Table 1.
Participant demographics for cortical thickness analysis. Mean ± SEM. Only pre-morbid handedness significantly differed between groups.
| Total | Constrained | Un-constrained | p-Value | |
|---|---|---|---|---|
| No. of participants (n) | 31 | 14 | 17 | – |
| Age (years) | 57 ± 2 | 54 ± 3 | 59 ± 2 | 0.3 |
| Gender (M/F) | 20/11 | 11/3 | 9/8 | 0.3 |
| Paretic hand (R/L) | 16/15 | 9/5 | 7/10 | 0.3 |
| Pre-morbid handedness (R/L) | 24/7 | 8/6 | 16/1 | 0.03⁎ |
| Chronicity (mths) | 45 ± 8 | 51 ± 13 | 40 ± 10 | 0.5 |
| Hours of therapy (3/1.5) | 17/14 | 7/7 | 10/7 | 0.7 |
| Constraint (Y/N) | 15/16 | – | – | – |
| Lesion side (R/L) | 15/16 | 5/9 | 10/7 | 0.3 |
| Lesion location (subcortical/cortico-subcortical) | 18/13 | 6/8 | 12/5 | 0.2 |
p < .05.
31 patients with moderate to severe chronic upper-limb hemiparesis of the left (N = 15) or the right (N = 16) arm following first ever stroke participated in the study. Hemiparesis was caused by unilateral mixed lesions (illustrated in Inline Supplementary Fig. S1 in Appendix A), as determined by visual inspection of a trained neurologist (Appendix A.3). Details are summarized in Table 1.
Inline Supplementary Fig. S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.05.005.
Participants were recruited via General Practitioners (GP's), hospitals and online support communities. Patients were screened for cognitive and emotional problems in a clinical interview conducted by trained psychologists. Clinical levels of depression, seizures within 6 months prior to the study, a mini-mental state examination (MMSE) < 24, and severe aphasia were exclusion criteria. The minimum motor criterion for participation comprised the ability to produce a voluntary movement with any part of the hand no matter how small. Patients who exceeded Taub's criterion of 20° wrist- and 10° finger extension were excluded.
The study was approved by the local NHS Ethics Committee and the Ethics Committee of the University of Surrey. Written informed consent was obtained prior to participation, along with GP's assent for participation. Financial reimbursement was given for travel cost and accommodation when necessary.
2.2. Intervention
All patients received two weeks of modified CI therapy with or without the non-paretic arm constraint for a period of two weeks. Patients were advised to wear the constraint during their waking hours for the whole fortnight except for situations and activities that were excluded in the treatment contract. Shaping training for the paretic hand was given for either 3 or 1.5 h a day during weekdays (10 days in total), leading to four subgroups, 3 h with constraint (n = 7), 3 h without constraint (n = 10), 1.5 h with constraint (n = 7), and 1.5 h without constraint (n = 7). Group allocation was randomized. To analyze the effects of constraint wearing on cortical thickness, the 3 h and 1.5 h subgroups were collapsed for the two constraint conditions respectively.
2.3. Assessment of motor ability
The Wolf Motor Function Test (WMFT, (Wolf et al., 2001)) and the Motor Activity Log (MAL, (Lum et al., 2006)) were acquired before and after the intervention to assess changes in the functional ability and the everyday use of the paretic arm. WMFT data was also acquired for the non-paretic arm. From these tests four outcome parameters where extracted, Time Taken (TT) and Functional Ability Score (FAS) in the WMFT, and Amount of Use (AoU) and Quality of Movement (QoM) in the MAL. Treatment benefit was determined as the difference between pre- and post-treatment scores (Fig. 1).
Fig. 1.
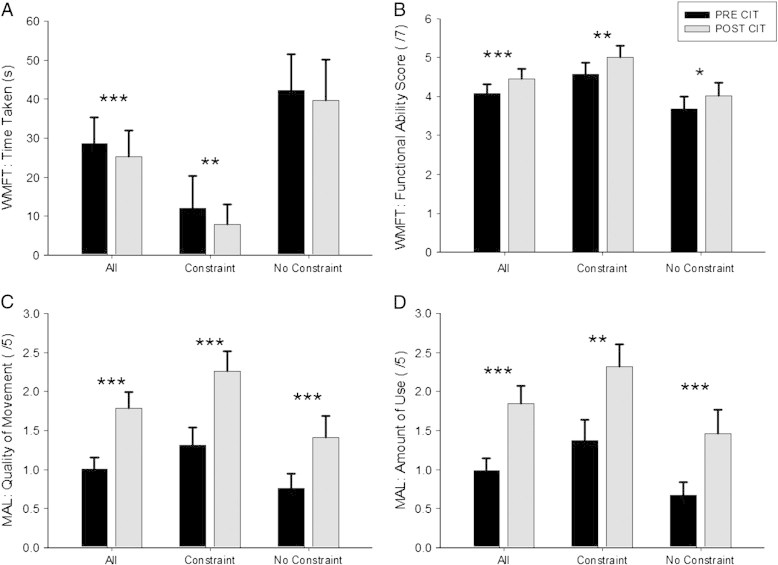
Motor ability scores pre and post interventions (CI therapy) for all subjects, those who used a constraint, and those who did not (no constraint). A: Wolf Motor Function Test (WMFT): time taken (s); B: WMFT: functional ability score (max. = 7); C: Motor Activity Log (MAL): quality of movement (max. = 5); D: MAL: amount of use (max. = 5). *** p < .001; ** p < .01; * p < .05.
One sample t-tests were calculated for all behavioral scores except for WMFT TT, which required a non-parametric rank based test (Wilcoxon signed rank test) because of the way items that could not be performed are scored (designated as 120 s, which creates an outlier). Independent samples t-tests were used to determine the effect of constraint on treatment outcome.
2.4. MRI image acquisition
A 3 T Siemens Trio scanner (Erlangen, Germany) equipped with an array head coil was used to acquire high-resolution T1-weighted images with an MPRAGE (magnetization prepared rapid acquisition gradient echo pulse) sequence with repetition time (TR) = 1830 ms, echo time (TE) = 4.43 ms, inversion time = 1100 ms, flip angle = 11°, field of view = 256 mm, 176 slices, voxel size = 1 × 1 × 1 mm3, and in-plane matrix = 256 × 256. These T1-weighted images were acquired before and after CI-therapy (days 1 and 15).
Diffusion-weighted images (DWI) were acquired with a single-shot diffusion-weighted echo-planar imaging sequence, with diffusion gradients along 12 directions (b0 = 0, 1 image and b1 = 1000 s/mm2, 12 images) and TR = 8900 ms, TE = 100 ms, number of averages = 4, 55 slices, voxel size = 2.5 × 2.5 × 2.5 mm3, and in-plane matrix = 88 × 128. As the DWI image acquisition is very noisy, this data was only acquired in a subset of 20 patients comfortable in taking part (see Table 2), and only at one time point after CI-therapy (day 15).
Table 2.
Participant demographics for DTI analysis. Mean ± SEM. Only pre-morbid handedness significantly differed between groups.
| Total | Constrained | Un-constrained | p-Value | |
|---|---|---|---|---|
| No. of participants (n) | 20 | 9 | 11 | – |
| Age (years) | 58 ± 2 | 55 ± 4 | 60 ± 2 | 0.3 |
| Gender (M/F) | 11/9 | 7/2 | 4/7 | 0.06 |
| Paretic hand (R/L) | 10/10 | 5/4 | 5/6 | 0.7 |
| Pre-morbid handedness (R/L) | 17/3 | 6/3 | 11/0 | 0.04⁎ |
| Chronicity (mths) | 46 ± 11 | 56 ± 19 | 38 ± 12 | 0.4 |
| Hours of therapy (3/1.5) | 8/12 | 3/6 | 5/6 | 0.6 |
| Constraint (Y/N) | 9/11 | – | – | – |
| Lesion side (R/L) | 10/10 | 4/5 | 6/5 | 0.7 |
| Lesion location (subcortical/cortico-subcortical) | 12/8 | 5/4 | 7/4 | 0.7 |
p < .05.
The T1-weighted images and DWI images from subjects with lesions in the right hemisphere were flipped in the L/R direction, in order to ensure that the non-lesioned hemisphere was on the right side for all subjects.
2.5. Cortical thickness analysis
Structural images of each patient were processed for surface-based analysis using the standard recon-all pipeline of the Freesurfer 5.0 suite (see http://surfer.nmr.mgh.harvard.edu for details). The pipeline is based on cortical surface modeling, spherical coordinate space morphing, multi-subject registration based on surface curvature, and automated segmentation of cortical regions. Note that these steps are only possible in the absence of lesions. For this reason the Freesurfer analysis was confined to non-lesioned hemisphere. Primary analysis of the non-lesioned hemisphere was performed on the estimated cortical volume at each vertex of the mesh. Region of interest (ROI) analysis was then undertaken using cortical volume within the pre- and postcentral gyri. The boundaries of these ROIs were based on the Desikan atlas (Desikan et al., 2006). Detailed information about recon-all pipeline theory, algorithms and implementation is available at Fischl and Dale (2000), Fischl et al. (1999a), and Fischl et al. (1999b).
2.6. Statistical analysis of cortical thickness and ROI data
Significant differences in whole brain cortical thickness after therapy (post minus pre CIT) were compared for the constraint and the non-constraint group using the General Linear Model embedded in the QDEC (Query, Design, Estimate and Contrast) interface of Freesurfer v5.1.0 (http://surfer.nmr.mgh.harvard.edu), with group as a predictor and thickness as response. The cluster-corrected significance level was set to 5% and Monte Carlo null hypothesis simulations were applied. Based on Langer et al. (2012) a subsequent region of interest (ROI) analysis was performed to determine the effect of constraint on the volume differences (post–pre CI therapy) of the precentral and postcentral gyri, using independent samples t-tests. Spearman's rho correlations were used to assess the association between cortical volume in these ROIs, and treatment benefits in the WMFT and the MAL. All tests are two tailed unless otherwise reported.
2.7. Fractional anisotropy (FA) analysis
FA analysis was conducted in a similar fashion to Langer et al. (2012) on the subset of 20 patients where DWI images were acquired. These methods are described in Appendix A.1. In brief, a voxel-wise regression analysis was conducted to determine whole brain FA differences (lesioned and non-lesioned hemisphere) between groups. In addition, a ROI analysis was performed using a probabilistic cortico-spinal tract (CST) mask generated by combining individual patient's tract data into one image (using a seed area in the midbrain and termination area in the precentral gyrus).
3. Results
3.1. Therapy effects
Significant improvements in all behavioral measures were observed after therapy for all participants (N = 31; MAL–AoU (t(30) = 5.8, p < .001); MAL–QoM: (t(30) = 7.6, p < .001); WMFT–FAS (t(30) = 4.3, p < .001); WMFT–TT (z = − 3.3; p = .001)). These treatment benefits were also evident when the two constraint groups were tested separately (with constraint (N = 14): MAL–AoU (t(13) = 3.7, p = .003); MAL–QoM: (t(13) = 6.0, p < .001); WMFT–FAS (t(13) = 3.2, p = .007); WMFT–TT (z = − 3.3; p = .001); without constraint (N = 17): MAL–AoU (t(16) = 4.5, p < .001); MAL–QoM: (t(16) = 4.9, p < .001); WMFT–FAS (t(16) = 2.9, p = .011)). An exception was WMFT TT, which showed no treatment effect in the non-constraint group (z = − 1.6; p = .102). Importantly, treatment outcome was not affected by the constraint condition as indicated by insignificant group differences for MAL–AoU (t(29) = 0.5, p = .6), MAL–QoM: (t(29) = 1.4, p = .2); WMFT–FAS (t(29) = 0.5, p = .6; and WMFT–TT (t(29) = − 0.2, p = .8)).
Significant treatment effects were also found when the participants were split into those receiving 3 h and those receiving 1.5 h of therapy. A detailed description of these effects is provided in Appendix A.2 and summarized in Inline Supplementary Fig. S2.
Inline Supplementary Figure S2.
Fig. S2.
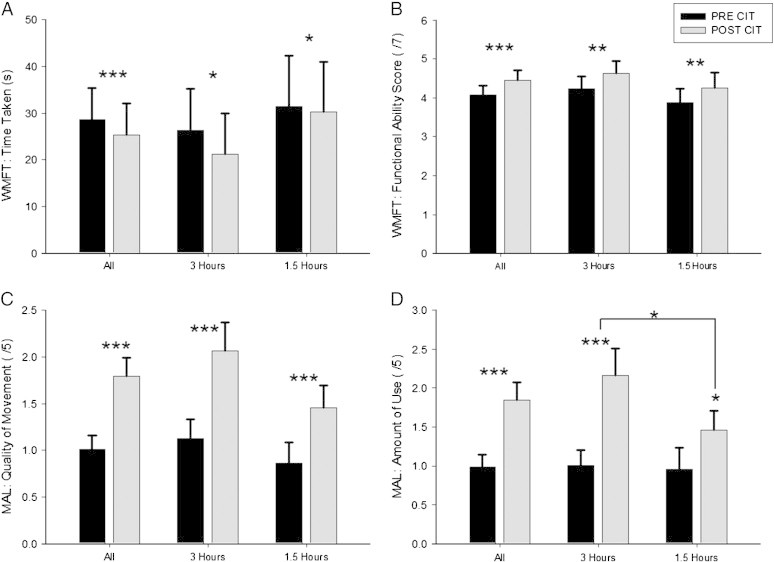
Motor ability scores pre and post interventions (CI therapy) for all subjects, those who had 3 h of therapy and those who had 1.5 h of therapy. A: Wolf Motor Function Test (WMFT): time taken (s); B: WMFT: functional ability score (max. = 7); C: Motor Activity Log (MAL): quality of movement (max. = 5); D: MAL: amount of use (max. = 5). *** p < .001; ** p < .01; * p < .05.
Significant treatment effects were also found when the participants were split into those receiving 3 h and those receiving 1.5 h of therapy. A detailed description of these effects is provided in Appendix A.2 and summarized in Inline Supplementary Fig. S2.
Inline Supplementary Fig. S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.05.005.
3.2. Cortical thickness analysis
Non-lesioned hemisphere analysis across all participants, corrected for multiple comparisons, revealed an increase in cortical thickness after therapy with a cluster peak centered over the precentral gyrus (M1, MNI: 36.5, − 14.5, 60.0, z = 2.0). This cluster also indicated an increase in thickness over the post-central gyrus (S1), and the middle frontal gyrus (see Fig. 2). Region of interest analysis of M1 and S1 revealed a significant increase of cortical volume in the post-central gyrus (S1; t(30) = 3.0, p = .005) but not the pre-central central gyrus (M1; t(30) = 1.5, p = 0.1).
Fig. 2.
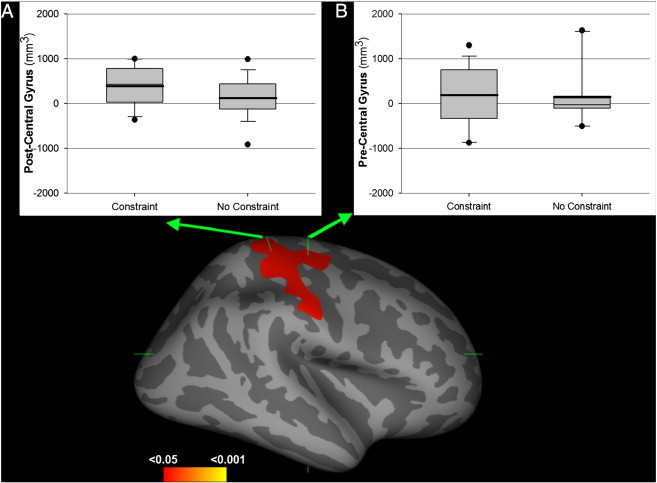
Cortical thickness and volume changes after therapy. The main picture depicts the area showing significant cortical thickness change after therapy (non-lesioned hemisphere analysis). A, B. Region of interest analysis for the post-central (A) and pre-central (B) gyrus cortical volumes, split by constraint condition.
The increase in cortical volume observed in the post-central gyrus was not significantly different for the constraint and the non-constraint groups (t(29) = 1.7, p = .097) or the hours of treatment received (t(29) = 0.1, p = .9). Furthermore, these cortical volume changes were not significantly associated with treatment benefit (MAL–AoU (ρ(31) = − 0.01, p = .9); MAL–QoM: (ρ(31) = − 0.08, p = .7); WMFT–FAS (ρ(31) = − 0.12, p = .5); WMFT–TT (ρ(31) = − 0.001, p = 1)).
3.3. Fractional anisotropy analysis
The whole brain analysis revealed no correlation between treatment effect and FA in the non-lesioned hemisphere (p < 0.05, corrected for multiple comparisons). No difference in FA was found between the constraint and the non-constraint groups for either the lesioned or the non-lesioned hemisphere (p < 0.05, corrected for multiple comparisons). However, FA within the lesioned hemisphere showed significant associations with the motor ability scales. These are reported in detail in Appendix A.3, and summarized in Fig. 1C.
4. Discussion
In the present study we examined the impact of constraining the non-paretic arm during CI therapy on structural characteristics of the non-lesioned hemisphere in chronic low functioning stroke patients. The study was inspired by a recent publication showing an immobilization-induced reduction in cortical thickness in the hand region of the contralateral primary sensorimotor cortex, as well as a transfer of motor skills to the non-constrained hand. In contrast to these findings, the present study suggested an increase in cortical thickness in the hand area of the contralesional sensorimotor cortex in patients partaking in CI therapy. This effect was equally present in patients who wore the constraint and those who did not. In addition, functional ability and motor performance improved significantly with the CI therapy intervention but the treatment effect was similar in the constraint and the non-constraint groups. Together these data suggest that CI therapy, applied with or without the constraint, improves motor function and also induces an increase in the cortical thickness of the hand area of the primary somatosensory cortex in the non-lesioned hemisphere. These data complement the existing literature on the efficacy of CI therapy and expand present knowledge on the structural changes occurring in the non-lesioned hemisphere. Critically, the data suggests that cortical thickness in the contralesional hemisphere is not altered by the constraint condition.
At first glance the present findings stand in contrast to the results reported by Langer et al. (2012), since we found no specific effect of constraint on cortical thickness while the Langer findings would have predicted that constraining the non-paretic arm induces cortical thinning within the sensorimotor cortex controlling the constrained extremity. However, the immobilization interventions in the two studies are not necessarily comparable. Langer's participants had broken their dominant arm, and therefore had this arm in a cast for 2–3 weeks without interruption. In contrast, the immobilization in CI therapy is intermittent in the sense that the constraint is worn only during the day, and only in the situations that are safe for the patient. The intensity of the immobilization and hence the intensity of the sensorimotor deprivation are therefore substantially less intensive in CI therapy than the continuous immobilization provided by a cast. Moreover, because of the fairly poor baseline motor ability of the patients in the present study, the constraint was worn on average 4.3 h per day according to patients' self-reports. It is therefore not entirely surprising that no significant differences in cortical thickness were found between the constraint and the non-constraint groups. Most likely the intensity of the immobilization was too weak to generate the sustained sensorimotor deprivation which induces the cortical thinning observed following cast immobilization. A further factor countering cortical thinning in the contralesional hemisphere is the possible recruitment of this hemisphere for the control of the paretic arm (Bosnell et al., 2011; Lindenberg et al., 2010; O'Shea et al., 2007; Takatsuru et al., 2009). As a result, this hemisphere is subject to enhanced functional activation, which possibly nullifies the effects of the sensorimotor deprivation associated with wearing the constraint.
Interestingly, the present study found that CI therapy, whether it was provided with or without a constraint, induced an increase in cortical thickness of the sensorimotor cortex of the contralesional hemisphere. This is a remarkable finding as it suggests that structural tissue changes occur ipsilateral to the hemiparetic hand in response to the shaping training of the paretic arm. We speculate that this increase in cortical thickness reflects use-dependent structural changes, such as an increase in synapses, apical dendrites, axonal spines, or indeed glia cells, that are likely to underlie the functional reorganization observed for the non-lesioned hemisphere through EEG, TMS and fMRI studies (e.g. Fridman et al., 2004; Gerloff et al., 2006; Lotze et al., 2006).
In addition to cortical thickness we also explored the relationship of motor ability and fractional anisotropy, in line with the procedures used by Langer et al. (2012). This analysis revealed no differences between the groups or associations with treatment benefit. This suggests that the changes in cortical thickness are unlikely to be driven by detectable changes in axonal connections.
According to the theory, the constraint condition in CI therapy is an important driver for increased everyday use of the affected arm, and hence a presumed contributor to treatment success. It is therefore surprising that treatment benefit was not different between the constraint and the non-constraint conditions. However, there are several aspects to the study that might have contributed to the absence of a constraint effect in treatment efficacy. Firstly, our patients had moderate to severe hemiparesis. Their motor function improved significantly with intervention, however, in absolute terms the improvements were relatively small. It is possible that the additional treatment benefit obtained by constraining the non-paretic arm is too small to be detected with the sample size. On the other hand, it is also possible that the poor residual ability of the patients made wearing the constraint difficult outside the intervention setting, which may have caused poor adherence with the protocol. Indeed, the subjective estimates of the time the constraint was worn varied considerably (1.2 to 11.9 h per day), and, with a mean of 3.6 h, on average much lower than the 90% waking time instruction of standard CI therapy. It is therefore plausible that the absence of significant constraint effects for treatment benefits or cortical thickness changes can be explained by the limited amount of time the constraint was actually worn. Although the group size was small and relatively heterogeneous in relation to group size, gender and lesion location, only prior handedness differed significantly between the constraint and un-constrained groups.
To the best of our knowledge no published work so far has assessed the effects of constraint wearing in CI therapy on cortical thickness. Given the evidence for cortical thinning following upper-limb immobilization in healthy volunteers, investigating the effect of the constraint on the intact hemisphere is important. With the present study we had the opportunity to explore this topic as a first step. However, our groups are small and a mediating effect of more or less intensive shaping training cannot be excluded. Specifically designed studies in larger less heterogeneous groups are needed to fully understand whether and how constraining the non-paretic arm changes structural brain parameters, and how these changes might be linked to treatment outcome.
4.1. Conclusion
Paretic arm training in CI therapy is associated with increased cortical thickness in the contralesional primary sensorimotor area of patients with chronic low-functioning stroke. The finding suggests that the brain changes associated with CI therapy are not only confined to a functional reorganization but also include structural changes. The data does not provide support for the notion that constraining the non-paretic arm reduces cortical thickness in the non-lesioned hemisphere. Rather the data shows that cortical thickness increases independent of the constraint. Because of the methodological constraints of surface-based morphology, the present study could only assess structural changes in the non-lesioned hemisphere. Future research will aim to assess the effects of CI therapy, and indeed paretic arm constraint, in both, the lesioned and the non-lesioned hemispheres.
Acknowledgments
This study was supported by the Medical Research Council grant awarded to AS [Grant ID: 61501]. JS acknowledges the support of the FAPESP Brazil.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Supplementary material.
References
- Bosnell R.A., Kincses T., Stagg C.J., Tomassini V., Kischka U., Jbabdi S., Woolrich M.W., Andersson J., Matthews P.M., Johansen-Berg H. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabilitation and Neural Repair. 2011;25:607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.R., Astafiev S.V., Lang C.E., Connor L.T., Rengachary J., Strube M.J., Pope D.L., Shulman G.L., Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Annals of Neurology. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope S.M., Liu X.C., Verber M.D., Cayo C., Rao S., Tassone J.C. Upper limb function and brain reorganization after constraint-induced movement therapy in children with hemiplegia. Developmental Neurorehabilitation. 2010;13:19–30. doi: 10.3109/17518420903236247. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French B., Thomas L.H., Leathley M.J., Sutton C.J., McAdam J., Forster A., Langhorne P., Price C.I., Walker A., Watkins C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews. 2007:CD006073. doi: 10.1002/14651858.CD006073.pub2. [DOI] [PubMed] [Google Scholar]
- Fridman E.A., Hanakawa T., Chung M., Hummel F., Leiguarda R.C., Cohen L.G. Reorganization of the human ipsilesional premotor cortex after stroke. Brain: A Journal of Neurology. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Gauthier L.V., Taub E., Perkins C., Ortmann M., Mark V.W., Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke: A Journal of Cerebral Circulation. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C., Bushara K., Sailer A., Wassermann E.M., Chen R., Matsuoka T., Waldvogel D., Wittenberg G.F., Ishii K., Cohen L.G., Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain: A Journal of Neurology. 2006;129:791–808. doi: 10.1093/brain/awh713. [DOI] [PubMed] [Google Scholar]
- Langer N., Hanggi J., Muller N.A., Simmen H.P., Jancke L. Effects of limb immobilization on brain plasticity. Neurology. 2012;78:182–188. doi: 10.1212/WNL.0b013e31823fcd9c. [DOI] [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2006;19:41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Liepert J., Bauder H., Miltner W.H.R., Taub E., Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Liepert J., Miltner W., Bauder H., Sommer M., Dettmers C., Taub E., Weiller C. Motor cortex plasticity during constraint-induced movement therapy in stroke patients. Neuroscience Letters. 1998;250:5–8. doi: 10.1016/s0304-3940(98)00386-3. [DOI] [PubMed] [Google Scholar]
- Lindenberg R., Renga V., Zhu L.L., Nair D., Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M., Markert J., Sauseng P., Hoppe J., Plewnia C., Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum P.S., Uswatte G., Taub E., Hardin P., Mark V.W. A telerehabilitation approach to delivery of constraint-induced movement therapy. Journal of Rehabilitation Research and Development. 2006;43:391–400. doi: 10.1682/jrrd.2005.02.0042. [DOI] [PubMed] [Google Scholar]
- Mark V.W., Taub E., Morris D.M. Neuroplasticity and constraint-induced movement therapy. Europa Medicophysica. 2006;42:269–284. [PubMed] [Google Scholar]
- Murase N., Duque J., Mazzocchio R., Cohen L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Annals of Neurology. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Nijland R., Kwakkel G., Bakers J., van Wegen E. Constraint-induced movement therapy for the upper paretic limb in acute or sub-acute stroke: a systematic review. International Journal of Stroke: Official Journal of the International Stroke Society. 2011;6:425–433. doi: 10.1111/j.1747-4949.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- O'Shea J., Johansen-Berg H., Trief D., Gobel S., Rushworth M.F. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Page S.J. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: a kinematic study of motor control mechanisms. Neurorehabilitation and Neural Repair. 2007;21:574. doi: 10.1177/1545968307308498. (author reply 574–575) [DOI] [PubMed] [Google Scholar]
- Peurala S.H., Kantanen M.P., Sjogren T., Paltamaa J., Karhula M., Heinonen A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials. Clinical Rehabilitation. 2012;26:209–223. doi: 10.1177/0269215511420306. [DOI] [PubMed] [Google Scholar]
- Sawaki L., Butler A.J., Leng X., Wassenaar P.A., Mohammad Y.M., Blanton S., Sathian K., Nichols-Larsen D.S., Wolf S.L., Good D.C., Wittenberg G.F. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabilitation and Neural Repair. 2008;22:505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirtori V., Corbetta D., Moja L., Gatti R. Constraint-induced movement therapy for upper extremities in patients with stroke. Cochrane Database of Systematic Reviews (4), CD004433. 2009 doi: 10.1002/14651858.CD004433.pub2. [DOI] [PubMed] [Google Scholar]
- Sterr A., Freivogel S. Motor-improvement following intensive training in low-functioning chronic hemiparesis. Neurology. 2003;61:842–844. doi: 10.1212/wnl.61.6.842. [DOI] [PubMed] [Google Scholar]
- Sterr A., Freivogel S., Schmalohr D. Neurobehavioral aspects of recovery: assessment of the learned nonuse phenomenon in hemiparetic adolescents. Archives of Physical Medicine and Rehabilitation. 2002;83:1726–1731. doi: 10.1053/apmr.2002.35660. [DOI] [PubMed] [Google Scholar]
- Takatsuru Y., Fukumoto D., Yoshitomo M., Nemoto T., Tsukada H., Nabekura J. Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:10081–10086. doi: 10.1523/JNEUROSCI.1638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N., Izumi S. Maladaptive plasticity for motor recovery after stroke: mechanisms and approaches. Neural Plasticity. 2012;2012:359728. doi: 10.1155/2012/359728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E. Overcoming learned nonuse a new approach to treatment in physical medicine. In: Carlson J.G., Seifert A.R., Birbaumer N., editors. Clinical Applied Psychophysiology. Plenum Press; New York: 1994. pp. 185–220. [Google Scholar]
- Taub E., Miller N.E., Novack T.A., Cook E.W., Fleming W.C., Nepomuceno C.S., Connell J.S., Crago J.E. Technique to improve chronic motor deficit after stroke. Archives of Physical Medicine and Rehabilitation. 1993;74:347–354. [PubMed] [Google Scholar]
- Taub E., Uswatte G., Elbert T. New treatments in neurorehabilitation founded on basic research. Nature Reviews. Neuroscience. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- Thorngren M., Westling B. Rehabilitation and achieved health quality after stroke. A population-based study of 258 hospitalized cases followed for one year. Acta Neurologica Scandinavica. 1990;82:374–380. doi: 10.1111/j.1600-0404.1990.tb03320.x. [DOI] [PubMed] [Google Scholar]
- van der Lee J.H. Constraint-induced movement therapy: some thoughts about theories and evidence. Journal of Rehabilitation Medicine: Official Journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2003;41–45 doi: 10.1080/16501960310010133. [DOI] [PubMed] [Google Scholar]
- van Meer M.P., Otte W.M., van der Marel K., Nijboer C.H., Kavelaars A., van der Sprenkel J.W., Viergever M.A., Dijkhuizen R.M. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32:4495–4507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer M.P., van der Marel K., Wang K., Otte W.M., El Bouazati S., Roeling T.A., Viergever M.A., Berkelbach van der Sprenkel J.W., Dijkhuizen R.M. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg G.F., Chen R., Ishii K., Bushara K.O., Eckloff S., Croarkin E., Taub E., Gerber L.H., Hallett M., Cohen L.G. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabilitation and Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- Wolf S.L., Catlin P.A., Ellis M., Archer A.L., Morgan B., Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke: A Journal of Cerebral Circulation. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- Wolf S.L., Newton H., Maddy D., Blanton S., Zhang Q., Winstein C.J., Morris D.M., Light K. The Excite trial: relationship of intensity of constraint induced movement therapy to improvement in the Wolf motor function test. Restorative Neurology and Neuroscience. 2007;25:549–562. [PubMed] [Google Scholar]
- Wolf S.L., Thompson P.A., Winstein C.J., Miller J.P., Blanton S.R., Nichols-Larsen D.S., Morris D.M., Uswatte G., Taub E., Light K.E., Sawaki L. The Excite stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke: A Journal of Cerebral Circulation. 2010;41:2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


