Abstract
Background
Despite suppression of the human immunodeficiency virus type 1(HIV-1) load by highly active antiretroviral therapy (HAART), recovery of CD4+ T cell counts can be impaired. We investigated whether this impairment may be associated with hyporesponsiveness of T cells to γ-chain (γc) cytokines known to influence T cell homeostasis.
Methods
The responsiveness of T cells to interleukin (IL)-2, IL-7, and IL-15 was determined by assessing cytokine-induced phosphorylation of the signal transducer and activator of transcription 5 (STAT5) in peripheral T cells obtained from 118 HIV-positive subjects and 13 HIV-negative subjects.
Results
The responsiveness of T cells to interleukin (IL)-7 but not to IL-2 or IL-15 was lower among HIV-positive subjects than among HIV-negative subjects. Among subjects with viral load suppression, the degree of IL-7 responsiveness (1) correlated with naive CD4+ T cell counts and was a better immune correlate of the prevailing CD4+ T cell count than were levels of human leukocyte antigen-DR1 or programmed death-1, which are predictors of T cell homeostasis during HIV infection; and (2) was greater in subjects with complete (i.e., attainment of ≥500 CD4+ T cells/mm3 ≥5 years after initiation of HAART) versus incomplete immunologic responses. The correlation between plasma levels of IL-7 and CD4+ T cell counts during HAART was maximal in subjects with increased IL-7 responsiveness.
Conclusions
Responsiveness of T cells to IL-7 is associated with higher CD4+ T cell counts during HAART and thus may be a determinant of the extent of immune reconstitution.
INTRODUCTION
T cell homeostasis, the ability of the immune system to maintain normal T cell counts after transient periods of depletion or expansion, is significantly compromised during human immunodeficiency virus (HIV) type 1 (HIV-1) infection [1]. T cell homeostasis requires the presence of interleukin (IL)-2, IL-7, and IL-15, the 3 major common γ-chain (γc) cytokines [2, 3]. For example, in HIV- negative lymphopenic individuals, γc cytokine-dependent proliferation of naive T cells occurs as part of a compensatory process known as “lymphopenia-induced homeostatic proliferation” [4–6]. However, for unknown reasons, during HIV-1 infection, this compensatory response frequently fails to prevent exhaustion of the T cell pool [1]. On the basis of the lymphotrophin hypothesis [7], impaired T cell homeostasis during HIV-1 infection might be a consequence of cytokine deprivation. However, this is unlikely to be a major contributory factor, because high circulating levels of IL-2 and IL-7 are observed in HIV-positive subjects [4, 8, 9]. Another possible contributory factor is the reduced expression of cytokine receptors, because the T cells of HIV-positive subjects have lower levels of IL-2 and IL-7 receptors than do those of HIV-negative subjects [10, 11]. However, experimental evidence in animal models and untreated HIV- positive subjects indicates that IL-7 receptor expression is not the sole determinant of T cell homeostasis [12–14].
These findings suggest that perhaps it is the ability of T cells to respond to γc cytokines, rather than the levels of these cytokines and their receptors per se, that serves as a determinant of T cell homeostasis during HIV infection. As an extension of this premise, in the present study, we considered the possibility that the hyporesponsiveness of T cells to γc cytokines may underlie the impaired T cell homeostasis observed in up to 30% of HIV-positive patients who have a muted recovery of CD4+ T cell counts, despite highly active antiretroviral therapy (HAART)-induced viral load (VL) suppression [15–17].
To examine this possibility, the intracellular activity of phosphoepitopes was used in this study as a “readout” of the ability of peripheral T cells to respond to γc cytokines. This biochemical readout is a novel means of uncovering signaling pathways that are perturbed during disease and predicting response to therapies [18]. For example, specific phosphoprotein-signaling profiles predict the response to cancer chemotherapy [19]. Because Janus kinase-dependent phosphorylation of the signal transducer and activator of transcription 5 (STAT5) is a common signaling event that occurs rapidly after ligation of γc cytokines to their receptors [20], we used the abundance of phosphorylated STAT5 (pSTAT5) present after ex vivo stimulation of T cells with IL-2, IL-7, or IL-15 as a marker of the degree to which T cells respond to γc cytokines. We assessed constitutive and cytokine-induced pSTAT5 levels in a large group of HIV-positive subjects whose CD4+ T cell counts and plasma HIV-1 levels from the time of initiation of HAART were known. Other host factors, such as levels of T cell activation ([21], assessed in the present study in terms of expression of HLA-DR), programmed death-1 (PD-1) [22], and CCR5 [23, 24], are known to influence T cell homeostasis during untreated HIV infection and/or immune recovery during HAART. In addition, plasma IL-7 levels influence immune recovery [25]. Therefore, to determine the relative importance of these factors in immune recovery, we determined, for the same group of HIV-positive subjects, the association of levels of constitutive and cytokine-induced pSTAT5; levels of receptors for IL-2, IL-7, and IL-15; and HLA-DR, PD-1, CCR5, and plasma IL-7 levels with CD4+ T cell counts during HAART.
SUBJECTS AND METHODS
Study subjects
A total of 118 HIV-positive individuals who were monitored at the Wilford Hall Medical Center (WHMC), San Antonio, Texas, were evaluated (figure 1A). These subjects were part of the US Military’s Department of Defense HIV Natural History Study. The clinical characteristics of these subjects are presented in table 1. Subjects with VL suppression were defined as subjects receiving HAART who had undetectable plasma HIV-1 RNA levels (<50 copies/mL) on the day of assessment of pSTAT5 levels. Subjects with complete and incomplete immunologic responses were defined as those subjects with VL suppression who, at ≥5 years after initiation of HAART, attained CD4+ cell counts of ≥500 and <500 cells/mm3, respectively [15]. Additional details regarding the overall WHMC cohort are described elsewhere [26–28]. A control group of 13 healthy adult HIV-negative volunteers was examined. Fully informed consent was provided by all of the volunteers. Approval was given by the institutional review board at the participating institutions.
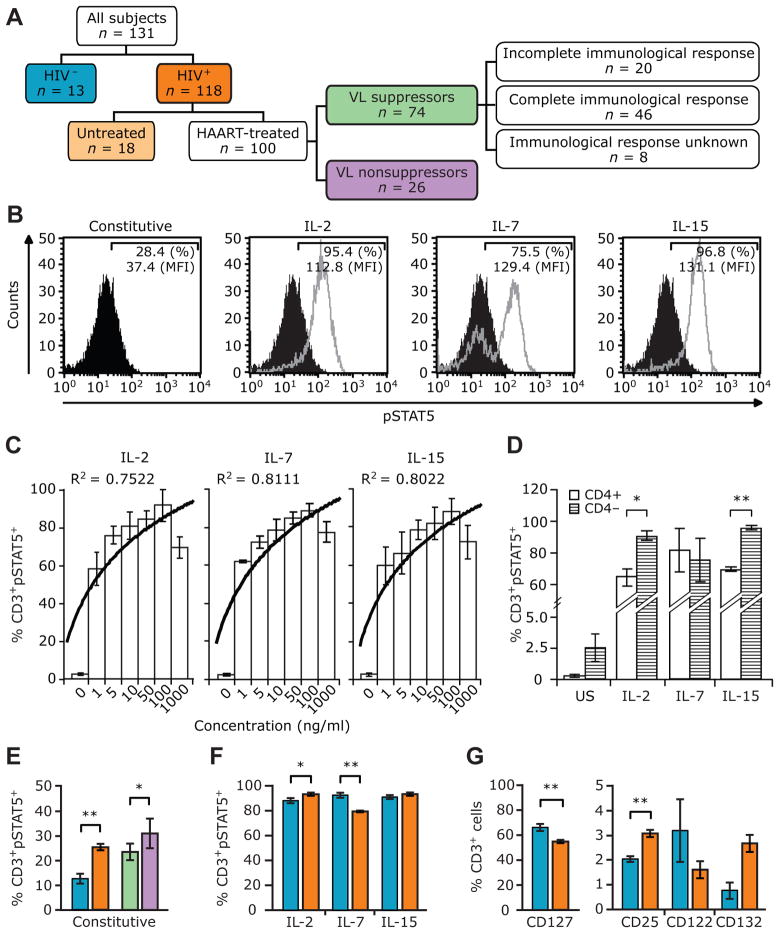
Figure 1.
Constitutive and γ-chain (γc) cytokine-induced phosphorylated signal transducer and activator of transcription 5 (pSTAT5) levels in HIV-positive and HIV-negative subjects. A, Study groups. Unless otherwise indicated, the color codes used in this panel are used in the remainder of the panels in this figure and in figure 2. B, Representative histograms of constitutive (black) and cytokine-induced (gray) pSTAT5 levels in an HIV-positive subject. Percentages denote the percentages of CD3+ T cells that were also positive for pSTAT5. Mean fluorescence intensity (MFI) values for pSTAT5 present in CD3+ T cells are also shown. Interleukin (IL)-2, IL-7, or IL-15 responsiveness is used interchangeably with IL-2-, IL-7-, or IL-15-induced pSTAT5 and responsiveness of T cells to the indicated cytokines. C, Percentages of CD3+ pSTAT5+ cells after stimulation with increasing concentrations of IL-2, IL-7, or IL-15. Histograms and error bars denote the mean levels and 95% confidence intervals, respectively, of constitutive (denoted by “0”) and cytokine-induced pSTAT5 in CD3+ T cells. The trend line denotes a logarithmic curve fit measured and shown as R2 values. Data are from 4 different HIV-negative subjects. D, Percentages of CD3+ pSTAT5+ cells in CD4+ and CD4− (i.e., CD8+) T cells after stimulation with 100 ng of the indicated cytokines per milliliter. US, unstimulated. Data are from 3 different HIV-negative individuals, and similar results were obtained when gating on CD8+ and CD8− T cells (data not shown). E–G, Histograms and error bars denote the mean levels and 95% confidence intervals, respectively, of constitutive (E) and cytokine-induced pSTAT5 (F) and cytokine receptors (G) in the indicated study groups. P values were calculated using Student’s t test. *P<.05; **P<.01. P = .06, for the difference in CD132 expression between HIV-positive and HIV-negative subjects. Data in panels E and F are for 118 HIV-positive and 13 HIV-negative subjects, except in panel F, in which the data for IL-15 are derived for 110 HIV-positive subjects. Data in panel G are for 104 HIV-positive and 13 HIV-negative subjects, with the exception of data for CD25, which are for 97 HIV-positive subjects.
Table 1.
Characteristics of the human immunodeficiency virus (HIV)–positive study subjects.
| Characteristic | Subjects with VLSa (n = 74) | Subjects with NVLSb (n = 26) | Untreated subjects (n = 18) | Overall Pc | P,d VLS vs. NVLS groups |
|---|---|---|---|---|---|
| Age at recruitment, years | 28.55 ± 0.57 | 33.06 ± 1.45 | 33.55 ± 2.17 | .020 | .006 |
| Male, % of subjects | 96 | 100 | 100 | .387 | .294 |
| Race/ethnicity, no. (%) of subjects | |||||
| European American | 48 (64.8) | 13 (50.0) | 6 (33.3) | .020 | .156 |
| African American | 25 (33.8) | 13 (50.0) | 12 (66.6) | ||
| CD4+ T cell count, cells/mm3 | |||||
| Pre-HAART,e mean | 399.6 ± 23.6 | 479.5 ± 50.1 | NA | .189 | .189 |
| Same dayf | 667.5 ± 39.5 | 413.9 ± 41.8 | 640.5 ± 65.3 | .001 | <.001 |
| Meang | 656.2 ± 34.4 | 399.7 ± 38.8 | 616.7 ± 54.0 | <.001 | <.001 |
| VL, copies/mL | |||||
| Same dayf | <50 | 13,805.8 ± 4475.2 | 15,363.3 ± 5005.2 | <.001 | <.001 |
| Meang,h | 81.3 ± 21.8 | 11,080.0 ± 3147.8 | 14,643.8 ± 3562.4 | <.001 | <.001 |
| Duration of HAART, years | 8.19± 0.48 | 7.18 ± 0.32 | NA | .087 | 087 |
| Moderate- or high-risk GRG,i no.(%) of subjects | 29(392) | 9(346) | 4(22.2) | .316 | .645 |
NOTE. Data are the mean value −1-standard error, unless otherwise indicated. One subject with VLS was Hispanic American. GRG, genetic risk group; HAART, highly active antiretroviral therapy; NA, not applicable; NVLS, no VL suppression: VL, viral load; VLS. VL suppression.
HAART-treated subjects with an undetectable VL on the day of assessment of phosphorylated signal transducer and activator of transcription 5 (pSTAT5).
HAART-treated subjects with a detectable VL on the day of assessment of pSTAT5.
Significance of overall differences between subjects with VLS, subjects with NVLS, and untreated HIV-positive subjects, as assessed by Kruskal-Wallis test, for continuous variables, and by x2 test for categorical variables.
Significance values determined by the Mann-Whitney U test, for continuous variables, and by x2 test, for categorical variables, for differences between subjects with VLS and those with NVLS.
Mean of all CD4− T cell count measurements during the 1 year before initiation of HAART.
CD4+ T cell count or VL on the day of assessment of pSTAT5.
Mean CD4− T cell count or VL is the mean of the measurements during the 1 year before assessment of pSTAT5.
Subjects with a low or high VL on the day of assessment of pSTAT5 also had a low or high VL in the 1 year before assessment of pSTAT5; this finding indicates that VL suppression status is relatively stable during HAART in the subjects studied.
Based on CCL3L1 dose and CCR5 genotypes.
Cell preparation
Peripheral blood mononuclear cells (PBMCs) were isolated by Histopaque gradient separation, washed twice with sterile saline, and resuspended in serum-free RPMI 1640 medium supplemented with L-glutamine.
Flow cytometric analysis
Surface expression of CD25, CD122, CD127, and CD132 in CD3+ T cells was determined using standard protocols. Freshly isolated PBMCs were incubated in the presence of Fc blocking reagent and were washed before staining. Appropriate isotype controls were included in all assays. Expression of HLA-DR and CCR5 in CD4+ T cells and PD-1 in CD3+ T cells was also determined. The proportion of naive CD4+ T cells was estimated using the percentage of gated CD4+ CD45RO− CCR7+ cells. Phosphoprotein staining was performed using standard protocols [19, 29] (figure 1B). For our studies, we selected a cytokine dose of 100 ng/mL for stimulation, because this appeared to be the most optimal concentration (figure 1C). Because, in accordance with a recent study [30], the mean levels of IL-7-induced pSTAT5 fluorescence intensity in T cells did not differ between HIV-positive and HIV-negative individuals, the pSTAT5 levels reported in the present study correspond to the percentage of T cells that were positive for pSTAT5 (%CD3+pSTAT5+) after cytokine stimulation. Initiation of HAART occurred 8 years (mean interval ± interquartile range [IQR], 8.27 ± 4.36 years) before assessment of pSTAT5 and PD-1 levels. CCR5 and HLA-DR levels were assessed 6–12 months before pSTAT5 levels were assessed. Antibodies were obtained from BD Pharmingen.
ELISA
IL-7 levels were measured in plasma samples obtained on the same day that pSTAT5 levels were assessed. The sensitivity cutoff of the assay was 4 pg/mL (R&D Systems).
Statistical analyses
The prevailing CD4+ T cell counts are defined as the mean of the CD4+ T cell measurements determined in the 1 year before assessment of pSTAT5 levels. Linear generalized estimating equations (GEEs) were used to model the time trends of CD4+ T cell counts. Where appropriate, we used the Mann-Whitney U test and Student’s t test to determine between-group differences. Correlations were quantified non-parametrically by use of Spearman’s coefficients. Associations between the biomarkers evaluated and outcome variables were assessed by multivariate linear regression. All statistical analyses were conducted using Stata software (version 7.0; Stata).
RESULTS
Characteristics of the study cohort
HIV-positive individuals were categorized in terms of receipt of HAART, attainment of HAART-induced VL suppression, and immunologic responses, according to criteria described elsewhere [15] (figure 1A). Compared with subjects without VL suppression, subjects with VL suppression were younger and had significantly higher CD4+ T cell counts at the time of assessment of pSTAT5 levels (table 1). These 2 patient groups did not differ according to sex, race/ethnicity, pre-HAART CD4+ cell counts, length of treatment, or distribution of the CC chemokine ligand 3-like 1 (CCL3L1)- and CCR5-based genetic risk groups (table 1), genetic factors that have been shown to also influence CD4+ T cell recovery during HAART [26, 27].
Cytokine receptor and pSTAT5 levels in HIV-negative and HIV-positive subjects
In this study, responsiveness of T cells to γc cytokines was defined as the proportion of peripheral T cells that express pSTAT5 after stimulation with IL-2, IL-7, or IL-15 (figure 1B). We observed that stimulation of T cells with increasing concentrations of IL-2, IL-7, or IL-15 was associated with a stepwise increase in pSTAT5 levels, with maximal effects observed with a dose of 100 ng/mL (figure 1C). After stimulation with IL-7, there were no differences in pSTAT5 levels in CD4+ or CD4− (i.e., CD8+) T cells (figure 1D). However, pSTAT5 levels were higher in CD4− T cells than in CD4+ T cells after stimulation with IL-2 or IL-15 (figure 1D).
Comparative analyses of HIV-negative and HIV-positive subjects revealed a significant influence of HIV-1 infection on levels of constitutive and γc cytokine-induced pSTAT5, as well as levels of cytokine receptors (figure 1E–G). Previous studies have demonstrated that HIV induces STAT-dependent signaling pathways [31, 32]. In the present study, we confirmed these previous observations that constitutive levels of pSTAT5 were higher in the T cells of HIV-positive subjects than in those of HIV-negative subjects and that they were also higher among subjects without VL suppression than among subjects with VL suppression (figure 1E). In addition, there was a positive correlation between the extent of viral replication, as reflected by the VL, and the constitutive pSTAT5 level (table 2). Compared with HIV-negative subjects, HIV-positive subjects had higher levels of CD25 and IL-2-induced pSTAT5, reduced levels of CD127 and IL-7-induced pSTAT5, and a trend toward higher expression of CD132 (figure 1F and 1G). By contrast, levels of CD122 and IL-15-induced pSTAT5 were similar in HIV-positive and HIV-negative subjects (figure 1F and 1G).
Table 2.
Correlations between viral load or CD4+ T cell count and levels of phosphorylated signal transducer and activator of transcription 5 (pSTAT5) and cytokine receptors.
| Parametera | Viral loadb | CD4+ T cell countb | ||
|---|---|---|---|---|
| ρ | P | ρ | P | |
| %CD3+pSTAT5+ | ||||
| Constitutive (n = 118) | 0.23 | .011 | −0.22 | .014 |
| IL-2 induced (n = 118) | −0.24 | .007 | −0.05 | .544 |
| IL-7 induced (n = 118) | −0.62 | <.001 | 0.46 | <.001 |
| IL-15 induced (n = 110) | −0.18 | .049 | −0.09 | .324 |
| Receptor | ||||
| CD25 (n = 97) | −0.23 | .021 | 0.16 | .10 |
| CD122 (n = 104) | 0.23 | .019 | −0.18 | .055 |
| CD132 (n = 104) | 0.32 | .001 | −0.19 | .044 |
| CD127 (n = 104) | −0.50 | <.001 | 0.38 | <.001 |
NOTE. Data show Spearman correlations (ρ and P values) between the levels of constitutive and cytokine-induced pSTAT5 and the viral load and CD4+ T cell counts. These analyses include all subjects, regardless of the treatment status. %CD3+pSTAT5+, percentage of CD3+ cells that express pSTAT5; ρ, Spearman’s rank correlation coefficient.
The number of subjects from whom the data were derived is shown in parentheses.
Mean CD4+ T cell counts and viral load measurements in the 1 year before the date of assessment of the parameters listed in this table.
HIV suppression and IL-7 responsiveness
Although the aforementioned data indicated that HIV infection results in perturbations in levels of γc cytokine responsiveness and receptor expression, they did not provide insights into whether HIV preferentially targeted specific γc cytokine-dependent signaling pathways. To examine this possibility, we determined the influence of the degree of viral replication and its suppression by HAART on levels of γc cytokine-induced pSTAT5 and cytokine receptor expression. Although associations between the VL and the levels of these parameters were detected (table 2 and figure 2A and 2B), the strongest associations were for the IL-7-IL-7R (CD127) axis. Statistically, there were very strong negative correlations between the VL and levels of CD127 and IL-7-induced pSTAT5 (table 2). In addition, compared with subjects without VL suppression, subjects with VL suppression had higher pSTAT5 levels after stimulation with IL-7 but not after stimulation with IL-2 or IL-15 (figure 2A). Furthermore, subjects with VL suppression had higher levels of CD127, whereas levels of IL-2 and IL-15 receptors—namely, IL-2R (CD25), IL-2/IL-15R (CD122), the common γc cytokine receptor (CD132)—were similar between subjects with and without VL suppression (figure 2B).
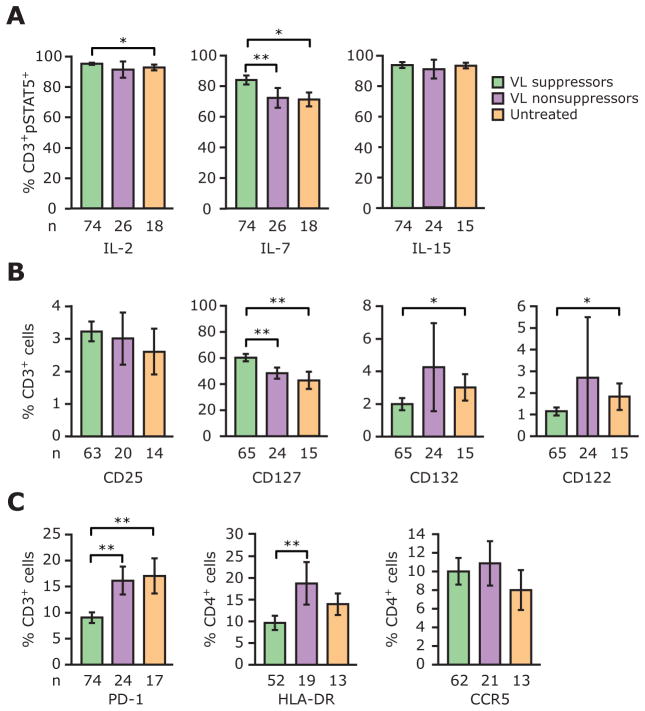
Figure 2.
Levels of phosphorylated signal transducer and activator of transcription 5 (pSTAT5) and cytokine receptors differ among subjects with viral load (VL) suppression, those without VL suppression, and untreated HIV-positive subjects. Histograms and error bars denote the mean percentage and 95% confidence interval, respectively, of CD3+ or CD4+ cells that express the indicated parameters, which are γ-chain (γc) cytokine-induced pSTAT5 (%CD3+ pSTAT5+ T cells) (A), cytokine receptors (B), and programmed death-1 (PD-1), HLA-DR, and CCR5 (C). P values were derived using Student’s t test.*P<.05;**P<.01. n, no. of subjects from whom the data were derived.
These findings suggest that HIV infection may preferentially target IL-7 responsiveness and IL-7 receptor levels. Predictably, because the VL in untreated subjects was similar to that in subjects without VL suppression (table 1), we observed that the levels of cytokine receptors and γc cytokine-induced pSTAT5 noted in subjects without VL suppression approximated those found in untreated subjects (figure 2A and 2B).
Two findings underscored the fact that the aforementioned associations were unlikely to be spurious and that the group of subjects in the present study provided sufficient statistical power to detect the relevant immunologic phenotypes present during HIV disease. First, levels of HLA-DR and PD-1 were higher in subjects without VL suppression and in untreated subjects than in subjects with VL suppression (figure 2C). Second, univariate analyses in the entire group of HIV-positive subjects revealed that subjects with higher levels of PD-1 and HLA-DR had lower prevailing CD4+ T cell counts (table 3). These data are consistent with previous reports indicating that (1) an increased VL is associated with higher levels of PD-1 and HLA-DR, and (2) enhanced expression of HLA-DR and PD-1 is associated with negative effects on T cell homeostasis (i.e., lower CD4+ T cell counts) [21, 22].
Table 3.
Association between interleukin (IL)–7 responsiveness and CD4+ T cell counts.
| Group, parameter [number of subjects]a | Univariateb | Multivariate | ||
|---|---|---|---|---|
| Coefficientc | P | Coefficientc | P | |
| All HIV-positive subjects | ||||
| T cell activation (%CD4+HLA-DR+) [n = 84] | −12.86 | <.001 | −7.33 | .088 |
| PD-1 expression (%CD3+PD-1+) [n = 115] | −17.75 | <.001 | −13.7 | .015 |
| CCR5 expression (%CD4+CCR5+) [n = 96] | −4.24 | .505 | −1.21 | .839 |
| Average VLd [n = 118] | −0.01 | .007 | 0.001 | .957 |
| IL-7 responsiveness (%CD3+pSTAT5+) [n = 118] | 5.96 | .001 | 5.01 | .006 |
| IL-7 plasma level [n = 118] | −0.14 | .838 | −0.49 | .426 |
| Subjects with VL suppression | ||||
| T cell activation % C D 4+HLA-DR+) [n = 52] | −16.9 | .016 | −11.2 | .144 |
| PD-1 expression (%CD3+PD-1+)[n = 74] | −17.65 | .022 | −9.90 | .328 |
| CCR5 expression (%CD4+CCR5+) [n = 62] | −15.8 | .017 | −2.98 | .744 |
| IL-7 responsiveness %CD3+pSTAT5+) [n = 74] | 8.18 | .002 | 6.04 | .039 |
| IL-7 plasma level [n = 74] | −0.26 | .719 | −0.46 | .518 |
NOTE. CD4+ T cell counts are the mean of all CD4+ T cell count measurements in the 1 year before the date of assessment of phosphorylated signal transducer and activator of transcription 5 (pSTAT5) levels. The number of subjects in the multivariate model conducted for all HIV-positive subjects and for subjects with VL suppression was 84 and 52, respectively. HIV, human immunodeficiency virus; PD-1, programmed death-1; pSTAT5, phosphorylated signal transducer and activator of transcription 5; VL, viral load.
In the univariate analyses.
Univariate analyses were conducted using each of the indicated variables as a separate predictor of the mean CD4+ T cell count, whereas multivariate analyses were conducted using a linear regression model that used all these predictors simultaneously in a single model.
Regression coefficient estimated from univariate or multivariate regression models.
Mean VL during the 1 year before the date of assessment of pSTAT5 levels.
Thus, the juxtaposition of findings from 2 comparisons— namely, HIV-positive subjects versus HIV-negative subjects (figure 1E–1G) and subjects with VL suppression versus subjects without VL suppression (figure 1E and figure 2)—suggested the following pathogenic model: high viral replication during HIV-1 infection induces high constitutive levels of pSTAT5. These high levels, along with an abnormal pattern of cytokine receptor expression, may desensitize pSTAT5-dependent signaling pathways for further cytokine stimulation, as is reflected by the negative correlations between VL and cytokine-induced pSTAT5 levels (table 2). We had anticipated that suppression of viral replication by HAART would be associated with changes in all the pSTAT5 and cytokine receptor profiles. However, we found this to be true only for the perturbations in the IL-7/CD127 axis, because suppression of viral replication was associated with higher levels of CD127 and IL-7-induced pSTAT5, but levels of CD25, CD122, and CD132, as well as those of IL-2- and IL-15-induced pSTAT5, were similar between subjects with and without VL suppression. Taken together, these findings indicated specificity between the extent of viral replication and perturbations in IL-7-dependent signaling pathways.
IL-7 responsiveness, CD127 expression, and CD4 T cell count
For the overall study cohort, the following correlations were observed for the prevailing CD4+ T cell counts: (1) there were negative and positive correlations with constitutive and IL-7-induced pSTAT5 levels, respectively; (2) there were no correlations with IL-2- or IL-15-induced pSTAT5 levels or IL-2/and IL-15 receptor levels; and (3) among the cytokine receptors, the strongest correlation was with CD127 expression, which was a positive correlation (table 2).
CD127 expression and IL-7 responsiveness
The following findings suggested that CD127 and IL-7-induced pSTAT5 were tracking similar biological processes: there was a strong inverse correlation between the VL and levels of both CD127 and IL-7- induced pSTAT5 (table 2). The levels of these 2 parameters were lower in subjects without VL suppression than in those with VL suppression (figure 2A and 2B), and they both correlated positively with CD4+ T cell counts and with each other (table 2 and figure 3A). Thus, one possibility was that the negative association between the VL and IL-7-induced pSTAT5 levels was simply a reflection of the coincident negative association between VL and CD127 expression. However, there are 2 reasons that this was unlikely. First, although there was a strong correlation between CD127 and IL-7-induced pSTAT5 levels, we found that one-half of the variation in IL-7-induced pSTAT5 levels could not be attributed to CD127 expression (ρ correlation, 0.749; 1 − R2 = 0.44). Second, in a multivariate regression model, IL-7 responsiveness during HIV disease was associated with CD4+ T cell counts, even after controlling for the effects of CD127 (figure 3A).
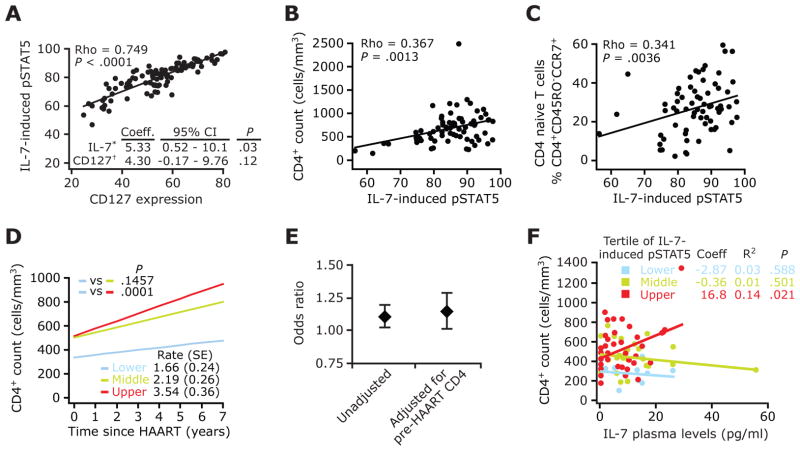
Figure 3.
Responsiveness of T cells to interleukin (IL)-7 is associated with prevailing CD4+ T cell counts during HAART and modulates the influence of plasma IL-7 on immune recovery. A, Scatterplots depicting pairwise correlations between IL-7 responsiveness and CD127 expression in all HIV-positive subjects. Inset, A multivariate linear regression model in which the CD4+ T cell count was the outcome variable and levels of IL-7-induced phosphorylated signal transducer and activator of transcription 5 (pSTAT5) (IL-7*) and CD127 (CD127†) were the predictors. Coeff., regression coefficient; CI, confidence interval. Data are derived from 102 HIV-positive subjects. B and C, Scatterplots show the correlation between IL-7-induced pSTAT5 levels and total (B) and naive (C) CD4+ T cell counts in subjects with viral load (VL) suppression. A–C, Numbers in the upper left denote Spearman’s ρ coefficient and significance. Data in panels B and C are for 74 and 69 subjects with VL suppression, respectively. D, CD4+ T cell trajectories from the time of initiation of HAART in subjects with VL suppression, according to the upper, middle, and lower tertiles of IL-7-induced pSTAT5 levels. The overall monthly rate of CD4+ T cell gains (± standard error [SE]), as estimated by linear generalized estimating equations, is shown at the bottom right. P values denote differences in rates of increases in CD4+ T cell counts between tertiles of IL-7-induced pSTAT5, with the lower tertile (blue) used as the reference group. The number of subjects and the number of CD4+ T cell count measurements (shown in parentheses) in the lower, middle, and upper tertiles of IL-7-induced pSTAT5 levels were 13 (186), 27 (418), and 34 (541), respectively. E, Results of nested logistic regression analyses in which the likelihood of a complete immunologic response associated with responsiveness of T cells to IL-7 (IL-7-induced pSTAT5 levels were used as a continuous variable) was computed before and after adjustment for the pre-HAART CD4+ T cell count. Diamonds and error bars denote the odds ratio and 95% CI, respectively, of having a complete immunologic response. Data are derived from 66 subjects with VL suppression. F, Association between plasma IL-7 levels and prevailing CD4+ T cell counts within each tertile of IL-7-induced pSTAT5. IL-7-induced pSTAT5 levels were categorized into tertiles, and the correlation between IL-7 levels and CD4+ T cell counts among subjects categorized as belonging in these tertiles is shown. Linear regression was used to predict the mean CD4+ T cell count based on endogenous plasma IL-7 levels in subjects with VL suppression. Numbers correspond to the Spearman ρ coefficient (Coeff.) and the P value for each tertile of IL-7-induced pSTAT5 levels. R2 indicates the amount of variance in CD4+ T cell count that is explained by plasma IL-7 levels within each tertile of IL-7 responsiveness. Numbers of subjects in the lower, middle, and upper tertiles of IL-7-induced pSTAT5 levels were 13, 27, and 34, respectively.
IL-7 responsiveness and CD4 T cell recovery
We next sought to distinguish whether the higher levels of IL-7-induced pSTAT5 observed in subjects with VL suppression, compared with subjects without suppression, were merely an epiphenomenon occurring in parallel to virologic control or whether IL-7 responsiveness was associated with the extent of CD4+ recovery, independent of viral suppression. In addition, we assessed whether the association between IL-7 responsiveness and CD4+ T cell recovery was independent of plasma levels of IL-7 and parameters that are known to have a negative influence on CD4+ T cell homeostasis during HIV infection and/or during HAART, such as levels of HLA-DR [21, 33], PD-1 [22], and CCR5 [23, 34].
To assess the association between the prevailing CD4+ T cell counts and immune recovery during HAART, we first determined whether the prevailing CD4+ T cell count was reflective of the historical changes in CD4+ T cell counts from the time of initiation of HAART. We found that, among those who received HAART, the prevailing CD4+ T cell count closely tracked the prior trajectory of CD4+ T cell counts from the time of initiation of HAART until the day of assessment of T cell responsiveness (see the Appendix, which appears only in the electronic version of the Journal). We categorized the prevailing CD4+ T cell counts into 5 strata, and we found that HAART-treated subjects who, at the time of assessment of pSTAT5 levels, had the highest prevailing CD4+ T cell counts, had gained an average of 4 CD4+ T cells per month from the time of initiation of HAART, whereas those who had the lowest prevailing CD4+ T cell counts had lost an average of 1 cell per month (see the Appendix, which appears only in the electronic version of the Journal). We therefore reasoned that, in our study population, the prevailing CD4+ T cell count may serve as a proxy for changes in the CD4+ T cell counts of subjects who received HAART. The following 5 findings demonstrated an association between the responsiveness of T cells to IL-7 and immunologic recovery that was independent of several parameters known to have an influence on immune reconstitution.
First, among subjects with VL suppression, higher levels of IL-7-induced pSTAT5 correlated positively with higher total and naive CD4+ T cell counts (figure 3B and 3C). No association was found between IL-7-induced pSTAT5 and memory CD4+ T cell counts (data not shown).
Second, a positive correlation between IL-7 responsiveness and the prevailing CD4+ T cell count was detected in subjects with VL suppression, as well as in all subjects, when the effects of the VL were accounted for in multivariate models (table 3).
Third, although univariate analyses showed that higher levels of HLA-DR and PD-1 were negatively associated with CD4+ T cell counts, multivariate analyses revealed that only IL-7 responsiveness was independently associated with the prevailing CD4+ T cell count (table 3).
Fourth, CD4+ T cell trajectories from the time of initiation of HAART differed according to tertiles of IL-7-induced pSTAT5 levels (figure 3D). The mean monthly increase in CD4+ T cell counts in subjects with VL suppression was estimated to be 3.5, 2.2, and 1.6 cells/mm3 for subjects in the upper, middle, and lower tertiles of IL-7-induced pSTAT5 (figure 3D).
Fifth, the extent of IL-7 responsiveness was higher in those subjects who were treated with HAART and were categorized as having a complete immunologic response than in those who were categorized as having an incomplete immunologic response; the latter group constituted 30% of the study population, a frequency that is consistent with that found in previous studies [15, 16].
Levels of IL-7-induced pSTAT5, but not levels of IL-7, PD-1, CCR5, or HLA-DR, differed between subjects with complete and incomplete immunologic responses (table 4). Differences in IL-7 responsiveness among these groups of subjects were not confounded by differential lengths of follow-up: the mean duration of follow-up (± standard deviation) was 7.57 ± 2.45 years for subjects with a complete response versus 6.76 ± 3.01 years for subjects with an incomplete response (P = .2507, for the difference between groups). By logistic regression analysis, we found that subjects with higher IL-7 responsiveness were more likely to be categorized as having a complete immunologic response, after adjustment for pre-HAART CD4+ T cell counts (figure 3E), which are a known predictor of the extent of immune reconstitution [15–17] and are the only other parameter that was strongly associated with CD4+ T cell recovery in the group of subjects with VL suppression studied herein (table 4).
Table 4.
Distinguishing characteristics of subjects with complete and incomplete immunologic responses (IRs)
| Parameter | Complete IR | Incomplete IR | P |
|---|---|---|---|
| PD-1 expression (%CD3+PD-1+)a | 8.59 ± 0.55b | 10.18 ± 1.15 | .264 |
| T cell activation (%CD4+ HLA-DR+)c | 8.61 ± 0.67 | 12.94 ± 2.45 | .093 |
| CCR5 expression (%CD4+CCR5+)b | 9.41 ± 0.77 | 12.10 ± 1.77 | .191 |
| IL-7 plasma levela | 17.46 ± 8.88 | 9.91 ± 2.84 | .706 |
| Age,a years | 33.46 ± 1.11 | 31.95 ± 1.67 | .503 |
| Pre-HAART CD4+ T cell count a,d,e | 477.75 ± 23.55 | 218.47 ± 26.12 | <.001 |
| IL-7-induced pSTAT5a | 86.7± 0.70 | 77.13 ± 4.78 | .005 |
NOTE. Data are the mean ± standard error. Subjects with complete and incomplete immunologic responses (IRs) were subjects who attained viral load suppression and who, at 5≥years after initiation of highly active antiretroviral therapy (HAART), had attained CD4+ cell counts of ≥500 and <500 cells/mm3, respectively. The length of follow-up did not differ between subjects with complete and incomplete IRs (see main text). IL, interleukin; PD-1, programmed death-1; pSTAT5, phosphorylated signal transducer and activator of transcription 5.
Forty-six subjects with complete IR; 20 subjects with incomplete IR.
Forty subjects with complete IR; 17 subjects with incomplete IR.
Thirty-four subjects with complete IR; 14 subjects with incomplete IR.
Expressed as the number of cells per cubic millimeter.
Mean of all CD4+ T cell count measurements during the 1 year before initiation of HAART.
It is noteworthy that, in the aforementioned analyses, plasma levels of IL-7 did not appear to influence CD4+ T cell recovery (table 3). However, in light of the observation that there was an association between IL-7 responsiveness and CD4+ T cell counts during HAART, we considered the possibility that the previously ascribed positive influence of IL-7 on T cell homeostasis [4, 35–38] might be primarily apparent in the subset of subjects who also have evidence of high IL-7 responsiveness. Consistent with this possibility, a positive correlation between plasma IL-7 levels and CD4+ T cell counts was detected in subjects with VL suppression who had high but not low or intermediate levels of IL-7-induced pSTAT5 (figure 3F). We found that, among subjects with VL suppression who had high responsiveness to IL-7 ex vivo, every unit (1 pg/mL) of plasma levels of IL-7 was associated with 17 more CD4+ T cells (figure 3F).
DISCUSSION
We found that, among subjects with HAART-induced VL suppression, there was a strong association between the responsiveness of T cells to IL-7 (but not to IL-2 or IL-15) and prevailing CD4+ T cell counts, which, in this study, were defined as the average of all CD4+ T cell counts noted in the 1 year before assessment of pSTAT5 levels. This association might have relevance regarding the extent of immune reconstitution during HAART, because there was a strong positive correlation between prevailing CD4+ T cell counts and the rate of change in CD4+ T cell counts from the time of initiation of HAART until the time of assessment of pSTAT5 levels. Persistent T cell activation is a major determinant of impaired CD4+ T cell recovery [33], and accordingly, in univariate analyses, we found that a high T cell activation status was associated with lower prevailing CD4+ T cell counts. However, when several parameters known to influence immune recovery were included as covariates in a multivariate analysis, only IL-7 responsiveness remained significantly associated with prevailing CD4+ T cell counts. Furthermore, subjects categorized as having a complete or incomplete immunologic response differed according to IL-7 responsiveness but not to activation status or levels of PD-1, CCR5, or plasma IL-7. Finally, IL-7 responsiveness predicted prevailing CD4+ T cell counts after adjustment for the pre-HAART CD4+ T cell count, which is the only other parameter that distinguished subjects with a complete response from those with an incomplete response. Taken together, these data suggested that the responsiveness of T cells to IL-7 may help promote and/or maintain CD4+ T cell recovery in subjects who attain HAART-induced VL suppression.
This inference is consistent with the viewpoint that, among the γc cytokines, IL-7 is most influential in regulating the proliferation and survival of CD4+ T cells [35, 36]. In addition, even though many γc cytokines participate in lymphopenia-induced homeostatic proliferation, only IL-7 appears to be an absolute requirement for this process in vivo [37, 38]. The specificity of IL-7-dependent signaling pathways in facilitating CD4+ T cell reconstitution was underscored by our findings that surface levels of CD127 but not CD25, CD122, or CD132 were significantly higher in subjects with VL suppression.
Furthermore, the positive correlation between IL-7-induced pSTAT5 levels and the frequency of naive CD4+ T cells in subjects with VL suppression suggests that the high IL-7 responsiveness observed in subjects with a complete immunologic response may underlie the previous observation that such individuals have increased numbers of circulating naive CD4+ T cells and thymic emigrants [39–41]. Our results have 3 major implications. First, intersubject differences in IL-7 responsiveness, as assessed by cytokine-induced pSTAT5 levels, may serve as a novel basis for the significant heterogeneity in the extent of immune recovery noted during HAART. A limitation of our study is that it is based on a cross-sectional study design, and studies are currently under way to determine whether hyporesponsiveness of T cells to IL-7 before HAART predicts impaired immune recovery during therapy.
Second, our findings have relevance for the quest to identify biomarkers that predict therapeutic outcomes. The observation that the correlation between IL-7 and CD4+ T cell counts was most evident in those who also had high IL-7 responsiveness suggests that assessing cytokine and cytokine receptor levels without accounting for the functional cytokine-dependent intracellular signaling pathways may convey limited predictive capacity.
Third, our findings suggest that the IL-7-CD127 axis might be an important therapeutic target for enhancing immunologic reconstitution. In this respect, IL-7 is being evaluated as a coadjuvant to HAART [42]. Because intersubject differences in increases in CD4+ T cell counts are observed after administration of recombinant IL-7 [43], assessment of IL-7 responsiveness might help to identify subjects in whom this immune-based therapy might have the greatest salutary effect.
Supplementary Material
Acknowledgments
Financial support: Veterans Administration Center on AIDS and HIV Infection, South Texas Veterans Health Care System; National Institutes of Health (NIH; MERIT award R37046326 to S.K.A.). S.K.A. is a recipient of the Elizabeth Glaser Scientist Award and the Burroughs Wellcome Clinical Scientist Award in Translational Research. Support for the Wilford Hall Medical Center cohort was provided by the Infectious Disease Clinical Research Program (IDCRP) of the Uniformed Services University of the Health Sciences (USUHS). The IDCRP is a Department of Defense triservice program executed through the USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine, in collaboration with the Department of Health and Human Services/NIH/National Institute of Allergy and Infectious Diseases/Division of Clinical Research through Interagency Agreement HU0001–05-2–0011.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Navy, Air Force, or the Department of Defense.
We are indebted to the subjects who enrolled in these studies and made this work possible. We thank S. Wegner and other members of the Infectious Disease Clinical Research Program for critical support of this work; G. Crawford for invaluable programmatic support at University of Texas Health Science Center, San Antonio; members of the Ahuja laboratory for critical reading of the manuscript; and W. Bradley for technical assistance. We also thank L. Song and U. Aluyen for outstanding dedication to the graphic work and A. S. Ahuja for forbearance.
Footnotes
Potential conflicts of interest: none.
References
- 1.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 2.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 3.Marrack P, Bender J, Hildeman D, et al. Homeostasis of alpha beta TCR+ T cells. Nat Immunol. 2000;1:107–11. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 4.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 5.Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–5. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Boyman O, Kim HO, et al. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khaled AR, Durum SK. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat Rev Immunol. 2002;2:817–30. doi: 10.1038/nri931. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 9.Orsilles MA, Pieri E, Cooke P, Caula C. IL-2 and IL-10 serum levels in HIV-1-infected patients with or without active antiretroviral therapy. Apmis. 2006;114:55–60. doi: 10.1111/j.1600-0463.2006.apm_108.x. [DOI] [PubMed] [Google Scholar]
- 10.Vingerhoets J, Bisalinkumi E, Penne G, et al. Altered receptor expression and decreased sensitivity of T-cells to the stimulatory cytokines IL-2, IL-7 and IL-12 in HIV infection. Immunol Lett. 1998;61:53–61. doi: 10.1016/s0165-2478(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 11.Rethi B, Fluur C, Atlas A, et al. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. Aids. 2005;19:2077–86. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 12.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive Expression of IL-7 Receptor {alpha} Does Not Support Increased Expansion or Prevent Contraction of Antigen-Specific CD4 or CD8 T Cells following Listeria monocytogenes Infection. J Immunol. 2008;180:2855–62. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 13.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–5. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leone A, Lehrman G, Jain M, Keiser P, Picker L, Villinger F, Sodora D. HAART Therapy in HIV+ Patients Increases IL-7 Responsiveness of Peripheral T Cells ex vivo (Abstract# 455). 14th Conference on Retroviruses and Oportunistic Infections (CROI); Los Angeles. February 25–28, 2007.2007. [Google Scholar]
- 15.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–72. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 16.Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4:255–62. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 17.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 18.Perez OD, Nolan GP. Phospho-proteomic immune analysis by flow cytometry: from mechanism to translational medicine at the single-cell level. Immunol Rev. 2006;210:208–28. doi: 10.1111/j.0105-2896.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 19.Irish JM, Hovland R, Krutzik PO, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118:217–28. doi: 10.1016/j.cell.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 21.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–95. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 22.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 23.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. Aids. 2001;15:1627–34. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 24.Gervaix A, Nicolas J, Portales P, et al. Response to treatment and disease progression linked to CD4+ T cell surface CC chemokine receptor 5 density in human immunodeficiency virus type 1 vertical infection. J Infect Dis. 2002;185:1055–61. doi: 10.1086/339802. [DOI] [PubMed] [Google Scholar]
- 25.Correa R, Resino S, Munoz-Fernandez MA. Increased interleukin-7 plasma levels are associated with recovery of CD4+ T cells in HIV-infected children. J Clin Immunol. 2003;23:401–6. doi: 10.1023/a:1025325718213. [DOI] [PubMed] [Google Scholar]
- 26.Ahuja SK, Kulkarni H, Catano G, et al. CCL3L1-CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV-1-infected individuals. Nat Med. 2008;14:413–20. doi: 10.1038/nm1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dolan MJ, Kulkarni H, Camargo JF, et al. CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV-AIDS pathogenesis via viral entry-independent mechanisms. Nat Immunol. 2007;8:1324–1336. doi: 10.1038/ni1521. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez E, Kulkarni H, Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–40. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 29.Perez OD, Krutzik PO, Nolan GP. Flow cytometric analysis of kinase signaling cascades. Methods Mol Biol. 2004;263:67–94. doi: 10.1385/1-59259-773-4:067. [DOI] [PubMed] [Google Scholar]
- 30.Lee AW, Sharp ER, O’Mahony A, et al. Single cell, phosphoepitope-specific analysis demonstrates cell type- and pathway-specific dysregulation of Jak/STAT and MAPK signaling associated with in vivo HIV-1 infection. J Virol. 2008 doi: 10.1128/JVI.01582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohler JJ, Tuttle DL, Coberley CR, Sleasman JW, Goodenow MM. Human immunodeficiency virus type 1 (HIV-1) induces activation of multiple STATs in CD4+ cells of lymphocyte or monocyte/macrophage lineages. J Leukoc Biol. 2003;73:407–16. doi: 10.1189/jlb.0702358. [DOI] [PubMed] [Google Scholar]
- 32.Bovolenta C, Camorali L, Lorini AL, et al. Constitutive activation of STATs upon in vivo human immunodeficiency virus infection. Blood. 1999;94:4202–9. [PubMed] [Google Scholar]
- 33.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 34.Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 35.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 36.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 38.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marziali M, De Santis W, Carello R, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. Aids. 2006;20:2033–41. doi: 10.1097/01.aids.0000247588.69438.fd. [DOI] [PubMed] [Google Scholar]
- 40.Benveniste O, Flahault A, Rollot F, et al. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. J Infect Dis. 2005;191:1670–9. doi: 10.1086/429670. [DOI] [PubMed] [Google Scholar]
- 41.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001;15:1749–56. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 42.ClinicalTrials.gov, a service of the U.S. National Institutes of Health. Available at: http://www.clinicaltrials.gov/
- 43.Rosenberg SA, Sportes C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother (1997) 2006;29:313–9. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.