Abstract
The addition of low, nondepleting doses of rabbit antithymocyte globulin (ATG) to human peripheral blood mononuclear cells has been shown to expand functional CD4+CD25+FoxP3+ regulatory T cells (Tregs) in vitro. This report is the first to elucidate the exact cellular mechanisms of ATG-mediated Treg expansion. CD4+ T cells require monocytes, but not other antigen presenting cell subsets, to be present in coculture to expand Tregs. However, T cells do not require direct cell–cell contact with monocytes, suggesting the importance of soluble factors. Moreover, ATG initially “reprograms” CD4+ T cells, but not monocytes, and induces STAT3 and STAT5 signaling in CD4+ cells. These reprogrammed CD4+ T cells subsequently secrete GM-CSF and IL-10 only in case of intact STAT3 signaling, which in turn promote the generation of tolerogenic CD14+CD11c+ dendritic cells characterized by enhanced IL-10 and decreased IL-12 production. Treg expansion following ATG treatment is accompanied by enhanced gene expression of both GM-CSF and Bcl-2, but not TGF-β, in peripheral blood mononuclear cells. These results demonstrate that ex vivo expansion of human Tregs by ATG is due to its ability to reprogram CD4+ T cells in a STAT3-dependent but TGF-β-independent manner, leading to the generation of monocyte-derived dendritic cells with a tolerogenic cytokine profile.
Keywords: Monocytes, rabbit antithymocyte globulin, regulatory T cells, tolerogenic DC
Introduction
Regulatory T cells (Tregs) are a population of CD4+ T cells characterized by the ability to suppress immune responses to self and foreign antigens, either by direct suppression of target cells via cell–cell contact, or by secretion of soluble suppressor mediators (1–6). Tregs can either develop centrally in the thymus (natural Tregs), or be induced peripherally under defined conditions of activation and antigen presentation (induced Tregs; Refs. 2,6,7). Tregs are essential for the induction and maintenance of immunological tolerance, and play an important role in regulating the immune response in various immune-mediated conditions, including allergic and autoimmune disease, infection, malignancy and organ transplantation (8–13). Therefore, harnessing the regulatory potential of Tregs represents a promising strategy to dampen detrimental immune reactions to self- and allo-antigens (14). In this context, several “biologicals” have been studied in an attempt to identify agents capable of enhancing Treg generation and/or improving their suppressive function (15).
Antithymocyte globulin (ATG) is a polyclonal antibody which is generated upon injection of human thymocytes into rabbits (Thymoglobulin) or horses (Atgam). At higher doses, ATG effectively depletes T cells and is therefore used as an immunosuppressive drug in the induction therapy of kidney transplant recipients and for the treatment of graft-versus-host disease. Although Tregs appear to be relatively resistant to ATG-mediated T-cell depletion and can suppress the recovery of T cells with an effector phenotype (16,17), our group was the first to report that the addition of low, nondepleting doses of rabbit ATG (Thymoglobulin) to peripheral blood mononuclear cells (PBMCs) can expand human CD4+CD25+FoxP3+ Tregs with suppressive properties in vitro (18). These results were later confirmed by several other investigators (19,20).
Recently, a Canadian group reported that ATG-expanded CD4+CD25+FoxP3+ generated from purified CD4+ T cells lack suppressive properties, and that transient FoxP3 expression is due to cell activation (21). Given the importance of various soluble factors such as TGF-β and IL-2 (22–24), but particularly antigen presenting cells/dendritic cells (APCs/DCs) in Treg expansion (25–29), we set out to elucidate the exact cellular mechanisms of ATG-mediated expansion of human Tregs ex vivo. We focused on the potential involvement of APC subsets, their interactions with CD4+ T cells and the functional consequences of the interaction of these immune cell subsets with ATG.
Materials and Methods
Cell preparation
Peripheral blood from healthy volunteers was collected in heparinized tubes after informed consent; PBMCs were isolated by standard Ficoll density gradient centrifugation. CD4+ T cells were enriched by magnetic activated cell sorting (MACS) (negative selection), using the CD4+ isolation Kit II (MiltenyiBiotec, Auburn, CA, USA), resulting in a purity of 88.5 ± 3% (n = 10), as assessed by flow cytometry. CD19 or CD14 Microbeads (MiltenyiBiotec) were used for depletion of B cells (0.3 ± 0.2% after depletion; n = 4) or monocytes (0.4 ± 0.4% after depletion, n = 5), respectively. Monocytes were enriched by magnetic activated cell sorting (MACS) (positive selection), using CD14 MicroBeads (MiltenyiBiotec), resulting in a purity of 98 ± 2% (n = 8).
Antibodies
Thymoglobulin (Genzyme, Cambridge, MA, USA), an ATG preparation generated by injection of human thymocytes into rabbits, was used at a concentration of 10 μg/mL. Purified rabbit polyclonal IgG (rbt IgG), at the same concentration, was used as a control.
Cell cultures
To investigate the involvement of APCs in ATG-mediated Treg expansion, unselected PBMCs, purified CD4+ T cells or B-cell-depleted/monocyte-depleted PBMCs were treated for 24 h with ATG or Rbt IgG. To identify the cell type primarily targeted by ATG, CD4+ and CD14+ cells were separately treated with ATG or Rbt Ig, washed twice with phosphate buffered saline (PBS) to remove ATG/Rbt IgG, and cocultured with treated or untreated CD14+ and CD4+ cells, respectively, for another 24 h. To determine the dominant mechanism of T cell-APC interaction, some coculture experiments were performed using Transwell inserts (pore size 0.4 μm; Corning Inc., NY, USA), inhibiting direct cell–cell interaction, but allowing cell–cell crosstalk via humoral factors.
In some experiments, the role of IL-10, GM-CSF, ILT-4, STAT3 and STAT5 was investigated by using specific neutralizing antibodies against IL-10, GM-CSF, ILT-4 (eBioscience, San Diego, CA, USA) or inhibitors of STAT3 and STAT5 (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
All cell cultures were performed under standard conditions (37°C; 5% CO2), using 24-well plates (Costar 3524, Corning Inc.) with 1 × 106 cells per cell type in 1 mL RPMI 1640 medium (Cambrex, Bioscience, Walkersville, MD) supplemented with 10% heat-inactivated human serum. After the incubation period, cells were collected, washed twice and analyzed by flow cytometry. In some experiments, cell culture supernatants were collected for further analysis by LUMINEX (Millipore, Billerica, MA, USA) and/or cells were recovered for further investigation by gene expression or phosphoprotein analysis (see later).
Flow cytometry
Treg staining was performed by surface staining with anti-human CD4-APC and anti-human CD25-PE (BD Bioscience, San Jose, CA, USA; eBioscience). Cells were then washed twice, resuspended in 1 mL of cold Fix/Perm Buffer (eBioscience), and incubated at 4°C overnight to permeabilize the cells. After washing twice with 2 mL of Permeabilization Buffer, intracellular FoxP3-staining was performed using anti-human FoxP3-FITC (PCH101; eBioscience). Tregs are shown as the percentage of CD25+FoxP3+ cells after gating on CD4+ T cells.
Monocyte surface staining was performed using anti-human fluorochrome-labeled monoclonal antibodies from BD Bioscience (CD14, CD11c, CD80, CD86, HLA-DR, CD123), eBioscience (ILT4, DC-SIGN) and Miltenyi-Biotech (BDCA2).
Luminex assay
Cytokine levels in cell culture supernatants were measured using a fluorescence-bead-based antibody sandwich immunoassay (LUMINEX, Millipore) according to the manufacturer’s instructions. Plates were analyzed using Upstate Bead View Software (Version 1.0.4.23259).
Gene expression analysis
PBMCs and isolated CD4+ T cells were incubated with ATG or Rbt Ig and recovered after 2, 4, 8, 12 and 24 h. RNA was extracted using Stratagene Absolutely RNA Microprep kit (Stratagene, La Jolla, CA, USA) and used for RT reaction. Resulting cDNA was utilized in a real-time PCR using primers for GM-CSF, TGF-β, Bcl-2, GATA3, RORC and Hes1 genes.
Intracellular signaling experiments
Purified CD4+ T cells were incubated with ATG or Rbt Ig, recovered at different time points (0.5, 1, 2 h) and lysed to permit protein extraction. The extracted protein was then utilized in a fluorescence-bead-based antibody sandwich immunoassay (LUMINEX, Millipore) of the principal components of different intracellular pathways (STAT3, STAT5A/B, P38, IκBα, P70 S6 kinase, Erk MAPK1/2, JNK and CREB), performed in accordance with the manufacturer’s instructions.
Statistical analysis
Student’s t-test was used for comparison of means between two groups. A p value < 0.05 was considered as statistically significant. Data were expressed as mean ± standard error of the mean.
Results
The presence of monocytes is essential for ATG-mediated Treg expansion
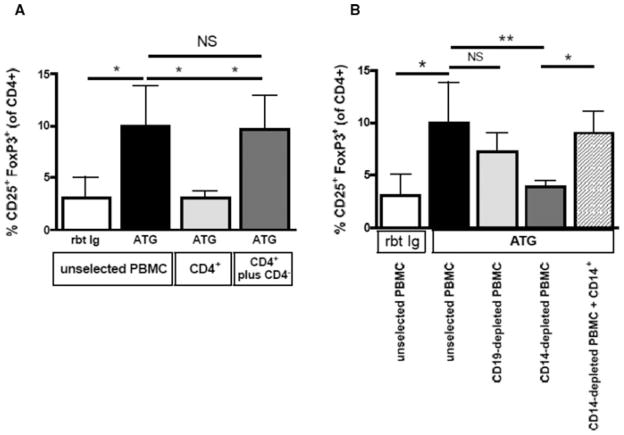
As previously shown, treating unselected PBMCs with low dose ATG results in Treg expansion within 24 h. However, Treg expansion was not observed when isolated CD4+ T cells alone were treated with ATG. The addition of CD4− cells, including APCs, to purified CD4+ T cells restored the ability of ATG to expand Tregs, indicating a crucial role of APCs in ATG-mediated Treg expansion (Figure 1A).
Figure 1. ATG-mediated Treg expansion is dependent on the presence of monocytes.
(A) ATG expands Tregs from unselected PBMCs but is not able to expand Tregs from isolated CD4+ T cells. (B) To determine the APC type required for Treg expansion, PBMCs were depleted of either CD19+ B cells or CD14+ monocytes before ATG treatment. Although B-cell depletion did not affect ATG-mediated Treg expansion, monocyte depletion abrogated it, indicating that monocytes are essential for ATG-mediated Treg expansion. Data are representative of four independent experiments. NS, not significant; *p < 0.05, **p < 0.01.
To identify the particular type of APC required for ATG-mediated Treg expansion, PBMCs were depleted of either CD19+ B cells or CD14+ monocytes before ATG treatment. Although ATG-mediated Treg expansion was preserved in B cell-depleted PBMCs, monocyte-depleted PBMCs did not show Treg expansion. When CD14+ monocytes were added back to previously CD14+-depleted PBMCs, ATG-mediated Treg expansion was restored to the same degree as that seen in unselected PBMCs (Figure 1B). Rbt Ig did not expand Tregs in any of these experiments (data not shown).
ATG induces gene expression of GM-CSF and Bcl-2, but not TGF-β
We tested whether ATG influences the expression of genes previously described to be critical for the development, differentiation and function of Tregs, Th17 and Th2 cells and monocyte-derived dendritic cells (Mo-DCs). When compared to Rbt Ig-treated PBMCs, ATG-treated PBMCs showed marked upregulation of both GM-CSF (relative expression compared to GAPDH gene expression (RE) after 4 h 0.66 ± 0.12 vs. 0.10 ± 0.05; n = 4/4; p = 0.04; Figure 2) and anti-apoptotic Bcl-2 (RE after 8 h 23.05 ± 1.67 vs. 2.90 ± 0.30; n = 4/4; p < 0.01; Figure 2) genes as early as 2 h after treatment, whereas the gene encoding for TGF-β, an established inducer of Tregs, was not upregulated at any studied time point (Figure 2). Increased GM-CSF gene expression was similarly seen in isolated CD4+ T cells (RE after 24 h 1.81 ± 0.15 vs. 0.22 ± 0.11; n = 4/4; p < 0.01). The gene expression of GATA3, RORC and transcription regulator Hes-1 was not affected by ATG (data not shown).
Figure 2. ATG induces GM-CSF and Bcl-2 gene expression in PBMCs.
Human PBMCs were incubated with ATG or Rbt Ig for 0 to 24 h. Cells were recovered at 0, 2, 4, 8, 12 and 24 h, followed by RNA extraction. The gene profile was assessed by quantitative Taqman PCR. In contrast to Rbt Ig, ATG treatment markedly induced expression of GM-CSF and anti-apoptotic Bcl-2 gene, occurring as early as 2 h after culture, whereas gene expression of TGF-β, an established inducer of Tregs, was not increased upon ATG treatment. Data are shown as relative gene expression compared to the expression of the housekeeping GAPDH gene and are representative of three independent experiments (n = 3). *p < 0.05, **p < 0.01.
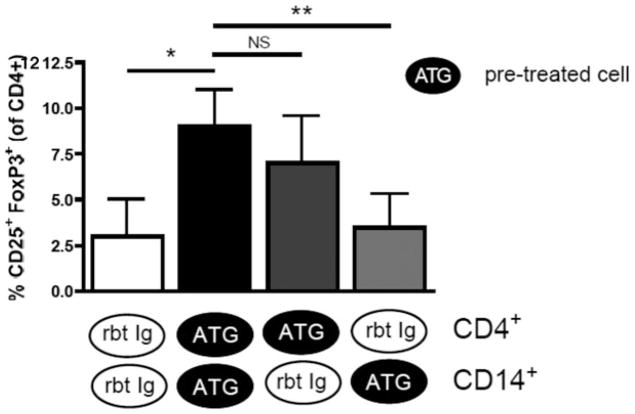
Treg expansion requires initial direct activation of CD4+ T cells by ATG, but not its immediate presence
To determine whether ATG-CD4+ or ATG-CD14+ interactions are primarily required during ATG-mediated Treg expansion, we separately treated CD4+ T cells and CD14+ monocytes with ATG or Rbt Ig over 24 h, washed them twice to remove ATG/Rbt Ig and cocultured the pretreated CD4+ and CD14+ cells for an additional 24 h in the absence of ATG/Rbt Ig. When both CD4+ and CD14+ cells were pretreated with ATG, marked Treg expansion was observed compared to Rbt Ig-treated cells, indicating that ATG-mediated Treg expansion requires cell activation by ATG, but is not dependent on its immediate presence (Figure 3). To identify the cell type primarily targeted by ATG, we pretreated CD4+ T cells and CD14+ cells separately with ATG or Rbt Ig for 24 h, washed them twice, and cocultured them with untreated CD14+ and CD4+ T cells, respectively, for another 24 h in the absence of ATG/Rbt Ig. Treg expansion was preserved when ATG-treated CD4+ T cells were cocultured with untreated CD14+ cells, but not vice versa, and never when either cell type was pre-treated with control Rbt Ig (Figure 3). These data demonstrate the need for direct activation of CD4+ T cells by ATG, but not the immediate presence of ATG during Treg expansion.
Figure 3. ATG-mediated Treg expansion requires initial CD4+ T-cell activation by ATG, but not its immediate presence.
Purified CD4+ and CD14+ T cells were separately treated with ATG or Rbt Ig for 24 h, washed twice to remove ATG or Rbt Ig and cocultured together for another 24 h without any further treatment. No Treg expansion occurred when both cell types were pretreated with rbt Ig, but Tregs expanded significantly when both cell types were separately pretreated with ATG, indicating that no immediate ATG presence is necessary for Treg expansion. To detect whether CD4+ and/or CD14+ cells require activation by ATG to mediate Treg expansion, both cell types were independently treated for 24 h with ATG or Rbt Ig, washed and cocultured with CD14+ or CD4+ cells, respectively, for another 24 h in the absence of additional treatment. Treg expansion occurred when CD4+ T cells were pretreated with ATG, but not when CD14+ cells were pretreated with ATG. As expected, no Treg expansion occurred in any of the experiments with Rbt Ig. These results indicate that ATG-mediated Treg expansion requires activation of CD4+ T cells by ATG but not its immediate presence in the Treg-generating culture. Data are representative of four independent experiments (n = 3). NS, not significant; *p < 0.05.
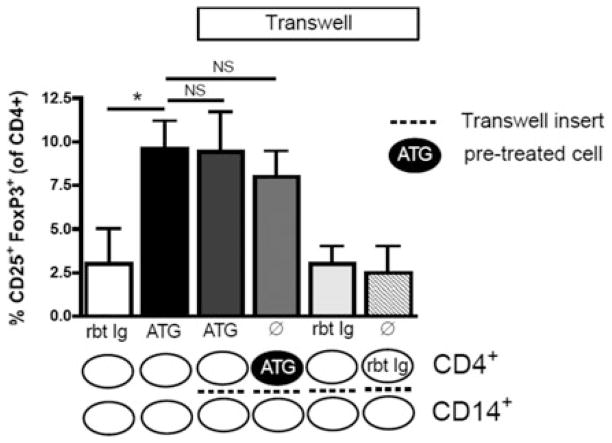
CD4+ T cells crosstalk with monocytes via soluble factors
To further investigate the nature of the interaction between T cells and APCs during ATG-mediated Treg expansion, CD4+ and CD14+ cells were cocultured in the presence of ATG with or without Transwell inserts, which prevent direct contact between CD4+ and CD14+ cells, but allow cell–cell crosstalk via soluble factors. Compared to cell cultures without Transwell inserts, Treg expansion was maintained when CD4+ and CD14+ cells were separated by Transwell inserts (Figure 4). Similarly, ATG-activated CD4+ T cells co-cultured with, but separated from untreated monocytes showed marked Treg expansion (Figure 4). No Treg expansion occurred with Rbt Ig treatment or when Rbt Ig-treated CD4+ T cells were used (Figure 4). These data indicate that no direct contact between T cells and monocytes is necessary for Treg expansion, and that the crosstalk between these cells is mediated by soluble factors.
Figure 4. ATG-mediated Treg expansion does not require direct T cell-monocyte contact.
When CD4+ T cells were cocultured with CD14+ monocytes in the presence of ATG, although separated by a Transwell insert, Treg expansion was similar to that seen in cultures without Transwells, indicating that no direct T cell-APC contact is necessary for ATG-mediated Treg expansion. Coculture of ATG-pretreated CD4+ T cells with CD14+ monocytes also showed similar Treg expansion despite the presence of a Transwell insert and the absence of additional ATG treatment. Rbt Ig or rbt Ig-pretreated CD4+ did not result in any Treg expansion. These results indicate that no direct T cell-APC contact is necessary for ATG-mediated Treg expansion, and that ATG-mediated Treg expansion is based on soluble factors. Data are representative of four independent experiments (n = 3). NS, not significant; *p < 0.05.
ATG “reprograms” CD4+ T cells
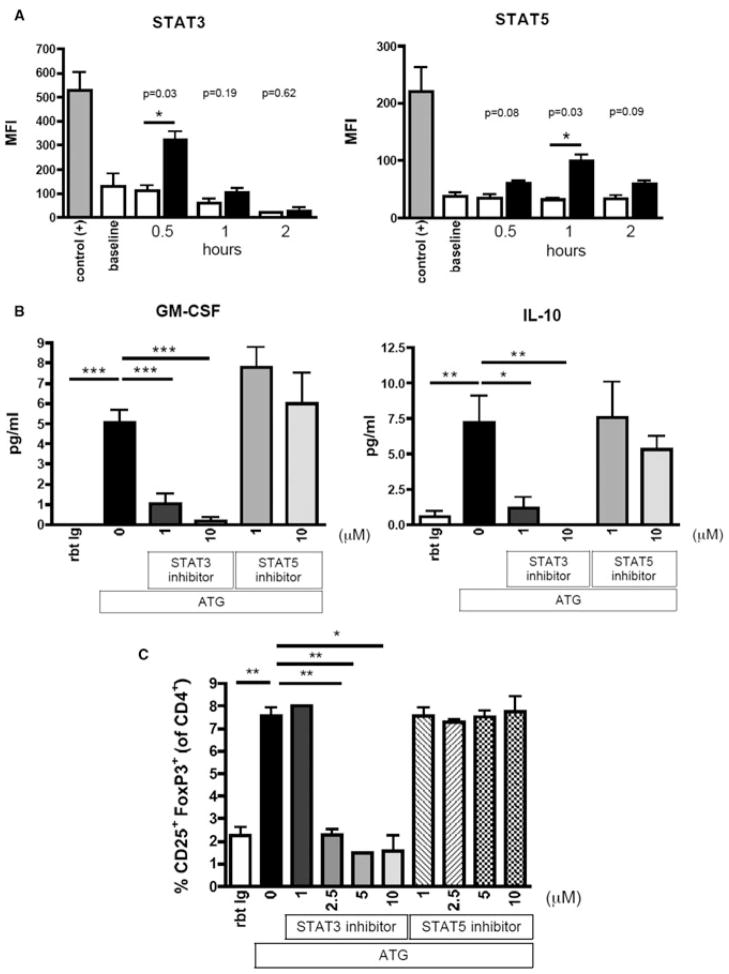
Next, we explored the functional consequences of the ATG-CD4+ T-cell interaction. Levels of GM-CSF and IL-10 were moderately increased in the supernatants of isolated CD4+ T cells treated with low dose ATG (10 μg/mL), but were almost absent in the supernatants of ATG-treated monocytes. Intriguingly, levels of these cytokines were markedly more increased when CD4+ and CD14+ cells were cultured together (Figure 5). Next, we assessed the influence of ATG on intracellular signaling pathways essential for Treg/Th17 development and differentiation. Compared to Rbt Ig-treated CD4+ T cells, cell lysates from ATG-treated CD4+ T cells showed increased amounts of phosphorylated STAT3 and STAT5a/b, indicating that ATG induces intracellular signaling via these proteins (Figure 6A). In contrast, ATG did not influence the phosphorylation of any other key signaling protein tested (P-38, IκB-α, P70 S6 kinase, MAPK1/2, JNK, CREB).
Figure 5. ATG-mediated Treg expansion is accompanied by increased release of GM-CSF and IL-10.
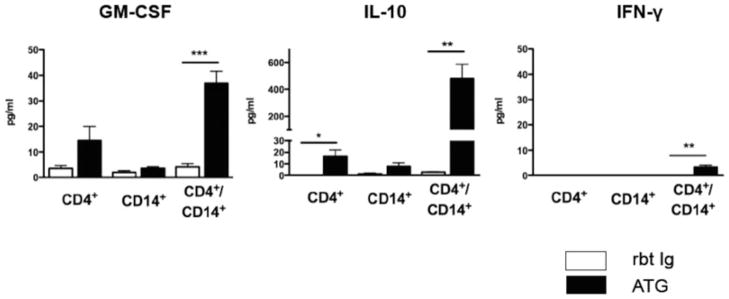
CD4+ and CD14+ cells were incubated separately or in coculture over 48 h and treated with ATG or Rbt Ig (10 μg/mL). Although ATG moderately increased GM-CSF and IL-10 in CD4+ cells alone and barely had any effect on CD14+ cells, levels of both GM-CSF and IL-10 were markedly increased when CD4+ and CD14+ cells were cultured together. Data are representative of three independent sets of experiments (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 6. Intact STAT3 signaling is critical for ATG-mediated reprogramming of CD4+ T cells and subsequent ATG-mediated Treg expansion.
(A) Purified CD4+ T cells were incubated with ATG or Rbt Ig in serum-free medium over 0.5, 1 or 2 h. Cells were lysed and 5 μg of total protein used to analyze intracellular signaling pathways using LUMINEX technology. ATG-treatment significantly increased the amount of phosphorylated STAT3 and STAT5, indicating that ATG induces intracellular signaling via these proteins. Data show mean fluorescence intensities and is representative of three independent experiments (n = 3). (B) ATG induces production of GM-CSF and IL-10 by CD4+ T cells. Inhibition of STAT3 signaling abolishes both GM-CSF and IL-10 production, whereas STAT5 inhibition does not impair it. Data are representative of three independent experiments (n = 3). (C) STAT3 inhibition but not STAT5 inhibition impairs ATG-mediated Treg expansion. Data are representative of three independent experiments (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001.
Next, we tested whether intact STAT3 or STAT5 signaling is required for the observed cytokine profile of CD4+ T cells after ATG treatment. When STAT3 signaling was blocked using a specific STAT3 inhibitor, cytokine production of ATG-activated CD4+ T cells was significantly abrogated, whereas inhibition of STAT5 signaling did not impair it (Figure 6B). To elucidate the functional role of STAT3 signaling in ATG-mediated Treg expansion, we inhibited STAT3 or STAT5 in cultures with ATG-mediated Treg expansion. Although blocking STAT3 signaling abolished ATG-mediated Treg expansion, inhibition of STAT5 did not show any effects on it (Figure 6C).
These data point to a functional role of STAT3 for ATG-mediated Treg expansion.
Reprogrammed CD4+ T cells induce Mo-DCs with a tolerogenic cytokine profile
To further investigate the critical requirement for monocytes, we analyzed the phenotypical changes of CD14+ monocytes during ATG-mediated Treg expansion. Compared to 24-h treatment with Rbt Ig, both CD14+CD11c+ Mo-DCs and CD14−CD11c+ cells significantly increased following ATG treatment (CD14+CD11c+: 6.5 ± 3.6 vs. 1 ± 0.5%; p < 0.0001; n = 12; CD14−CD11c+: 1.2 ± 0.6% vs. 0.3 ± 0.1%; p = 0.0001; n = 12). No differences were found in the frequencies of BDCA2+ plasmocytoid DCs or in the expression of CD123 (data not shown). Following ATG treatment, Mo-DCs displayed marked upregulation of maturation markers CD80 (24 ± 18% vs. 7 ± 4%; p < 0.01; n = 8), CD86 (41 ± 21% vs. 13 ± 5%; p < 0.01; n = 8) and human leukocyte antigen (HLA)–DR (8 ± 5% vs. 1.6 ± 1%; p < 0.01, n = 8), as well as upregulation of inhibitory immunoglobulin like transcript 4 (ILT4; 25 ± 12% vs. 4.5% ± 4%; p < 0.01; n = 8), when compared to treatment with Rbt Ig.
These data were also confirmed by costaining CD14+ cells with the more DC-specific CD209 (DC-SIGN; Figure S1).
To assess the particular cytokine profile of CD14+ monocytes during ATG-mediated Treg expansion, CD14+ monocytes were cocultured with CD4+ T cells (separated by Transwell inserts) in the presence of either ATG or Rbt Ig for 24 h. Moreover, to test whether GM-CSF or IL-10 produced by CD4+ T cells upon ATG treatment further influences the subsequent cytokine production by monocytes, we used neutralizing antibodies against IL-10 or/ and GM-CSF (10 μg/mL) in additional cultures. After 24 h, the CD14+ cells were thoroughly washed three times with PBS to remove any remaining antibodies, and recultured in the presence of lipopolysaccharide (LPS) (100 ng/mL) for 48 h. The supernatants of these cultures were then used in Luminex multicytokine assays. CD14+ cells which were exposed to CD4+ T cells and ATG showed increased levels of IL-10 and decreased levels of IL-12 compared to CD14+ cells cocultured with CD4+ T cells in the presence of Rbt Ig (Figure 7). The addition of neutralizing anti-IL-10 to the primary ATG coculture abolished the ATG-induced monocytic IL-10 production and partially restored the IL-12 production, thereby reverting the tolerogenic cytokine profile induced by ATG. GM-CSF blockade also decreased ATG-induced IL-10 production by monocytes, but did not affect IL-12 production (Figure 7).
Figure 7. Reprogrammed CD4+ T cells induce DC with tolerogenic cytokine profiles.
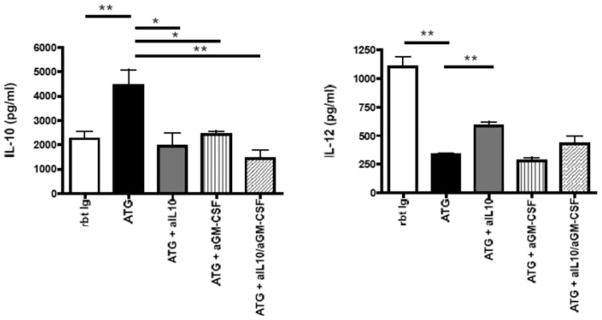
To assess the cytokine profile of monocytes during ATG-mediated Treg expansion, CD14+ monocytes cells were cocultured with CD4+ T cells separated by Transwell inserts, in the presence of either ATG or Rbt Ig. To test the effects of IL-10 and GM-CSF produced by CD4+ T cells on the further cytokine profile of monocytes, neutralizing antibodies against IL-10 and/ or GM-CSF were used in some cultures. After 24 h, monocytes were recovered, washed three times with PBS and recultured for 48 h with LPS (100 ng/mL). Monocytes initially cultured with CD4+ T cells and ATG showed increased production of IL-10 and decreased production of IL-12 when compared to monocytes incubated with CD4+ T cells and Rbt Ig. Blockade of both IL-10 and/ or GM-CSF in the initial coculture abolished further ATG-induced IL-10 production by monocytes. Blockade of IL-10 but not GM-CSF also resulted in partially restored IL-12 production. Data shown are representative of at least three independent experiments (n = 4). *p < 0.05, **p < 0.01.
Taken together, these data show that ATG-treated CD4 T cells induce the generation of Mo-DCs with a tolerogenic cytokine profile.
Discussion
Our group and several other investigators have previously demonstrated that Tregs can be expanded from human PBMCs with low doses of ATG, mainly due to the induction of adaptive Tregs (18–20). Crucially, these Tregs have been shown to be functional, capable of suppressing the autologous immune response in vitro, both to CD3/CD28-mAb stimulation (19) and to alloantigen, as demonstrated in a mixed lymphocyte reaction (18,20). In this study, we investigated the key cellular mechanisms of ATG-mediated Treg expansion, the potential involvement of APCs and the consequences of the interaction of ATG with these immune cell subsets. We first demonstrated that ATG-mediated expansion of Tregs is critically dependent on the presence of APCs, as Treg expansion did not occur when CD4+ T cells alone were incubated with ATG, but was restored when CD4− cells, which include APCs, were added back to cocultures. Further experiments identified CD14+ monocytes as the critical APC subset for ATG-mediated Treg expansion, as Treg expansion did not occur in CD14-depleted PBMCs. The presence of monocytes and their subsequent conversion to tolerogenic DCs (tDCs) seems to be crucial for the expansion of functional Tregs. These results may explain the findings in a recent study demonstrating the absence of any suppressive effects of ATG-expanded Tregs (21). The authors attribute their observation to transient rather than stable FoxP3 expression, which has previously been shown to be a marker of cell activation rather than regulation. Notably, they utilized purified CD4+ T cells instead of unselected PBMCs for Treg expansion ex vivo, and indeed, we did not observe induction of FoxP3+ Tregs when purified CD4+ T cells were exposed to nondepleting doses of ATG in the absence of monocytes.
ATG (Thymoglobulin) is a polyclonal antibody generated upon injection of human thymocytes into rabbits. Thus, it contains numerous antibodies directed against many different T-cell epitopes (30–32), as well as molecules expressed by APC subsets (33). Alternate treatment of CD4+ or CD14+ cells with ATG and subsequent incubation with treated or untreated counterpart cells revealed that ATG primarily targets CD4+ T cells, as Treg expansion did not occur when CD14+ cells only were initially exposed to ATG. Furthermore, Treg expansion occurred when previously ATG-treated CD4+ T cells were cocultured with untreated CD14+ monocytes in the absence of ATG. This indicates that ATG-mediated Treg expansion requires initial activation of CD4+ T cells by ATG, but not its immediate presence; however, the presence of CD14+ monocytes, for which the direct exposure to ATG is not relevant, is essential. These observations may be also important for the development of novel strategies for ex vivo expansion of Tregs, given that the isolation of rare Tregs is cumbersome.
As we showed that CD4+ T cells are the primary target of ATG, we further investigated the ATG-induced changes of various genes and phosphoproteins that have been reported to be associated with the generation of tDC and Tregs. We observed an ATG-induced increase of both GM-CSF and Bcl-2 genes, whereas the genes of RORC and GATA3, markers of Th17/Th2 differentiation, the transcription regulator Hes1 and TGF-β were not affected. Moreover, we observed increased phososphorylation of STAT3 and STAT5 during ATG treatment in CD4+ T cells, while ATG treatment had no effect on the phosphorylation of other key intracellular signaling pathways. Increased expression of the GM-CSF gene in CD4+ T cells was also confirmed by increased levels of GM-CSF protein in the supernatants of ATG-treated CD4+ T cells. The anti-apoptotic Bcl-2 gene has recently been shown to be induced by GM-CSF in a STAT5-dependent manner (34) but has not yet been directly linked to Tregs. It is possible that it could potentially contribute to ATG-mediated effects on Treg generation by promoting Treg survival. Interestingly, ATG affected neither gene expression of TGF-β, nor its production by CD4+ or CD14+ cells, indicating that ATG-mediated Treg expansion is independent of TGF-β.
Our data also indicate increased phososphorylation of STAT3 and STAT5 during ATG treatment in CD4+ T cells, whereas ATG treatment had no effect on the phosphorylation of other key intracellular signaling pathways. Subsequent studies revealed that inhibition of STAT3 resulted both in the abrogation of ATG-induced production of IL-10 and GM-CSF by CD4+ T cells, and eventually in the abolishment of ATG-mediated Treg expansion, whereas the inhibition of STAT5 did not effect either of these processes. STAT5 and STAT3 have, heretofore, been considered as reciprocal regulators of Treg versus Th17 differentiation, with STAT5 promoting Treg generation and function and inhibiting Th17 generation, and STAT3 having the opposite effect (35). However, there is growing evidence that the balance of Treg/Th17 differentiation is more complex, and that STAT3 signaling, which occurs at an early, mutual stage of the Treg/Th17 differentiation pathway, can promote Treg generation and function (36,37). Our data show that the induction of STAT3 by ATG has functional relevance for ATG-mediated Treg expansion, whereas the induction of STAT5 does not seem to be relevant for this process. Whether the increase of these factors is a direct effect of ATG or a result of the ATG-induced cytokine milieu will require further investigation.
Experiments with Transwell inserts, whereby CD4+ and CD14+ cells were separated during coculture and subsequently treated with ATG, revealed that no direct cell–cell contact between these cells is necessary for ATG-mediated Treg expansion. Therefore, we analyzed the cytokine profile of CD4+ and CD14+ cells after ATG treatment, both separately and in coculture. CD4+ T cells, reprogrammed by treatment with ATG, demonstrated increased production of both GM-CSF and IL-10, an effect not seen when monocytes were separately treated with ATG. Intriguingly, the production of these cytokines was strongly increased in the presence of both cell types in cocultures. This synergistic effect is supportive of the requirement for both cell subsets for Treg expansion with low-dose ATG, and may be due to enhanced, self-perpetuating paracrine secretion of these cytokines. Interestingly, CD14+ monocytes collected after coculture with CD4+ T cells and ATG showed increased levels of IL-10 and decreased levels of IL-12 after stimulation with LPS when compared to CD14+ monocytes, which were cocultured with CD4+ T cells and control Ig. This indicates that CD14+ monocytes acquire a tolerogenic cytokine profile during ATG-mediated Treg expansion. Moreover, we show that blocking of IL-10 and/ or GM-CSF during ATG-activation of CD4+ T cells at least partially reverts this tolerogenic cytokine profile, emphasizing a positive feedback mechanism, with IL-10 produced by ATG-activated CD4+ T cells enhancing the subsequent IL-10 production by monocytes.
Together with the observed generation of Mo-DCs, these results suggest that ATG promotes the differentiation of tDCs from CD14+ monocytes (38,39). Although the tolerogenicity of these cells has been frequently, but not unequivocally, associated with their low expression of maturation markers, we noted the differentiation of Mo-DC with increased expression of maturation markers in the context of ATG-mediated Treg expansion. This is in accordance with the conclusion of several authors that the cytokine profile, rather than maturation status, is much more indicative of the tolerogenic properties of DCs (40–42). These data are also consistent with the ability of IL-10 and GM-CSF to promote the differentiation of Treg-inducing tDCs (43–49). The increased production of IL-10 by CD4+ and CD14+ cells is of particular interest, as IL-10 skews GM-CSF-/IL-4-induced MoDC toward a tolerogenic phenotype (50). However, despite clear evidence that monocytes are a requisite for ATG-mediated expansion of functional Tregs, and that CD4+ T cells reprogrammed by ATG induce the generation of Mo-DCs with a tolerogenic cytokine profile, our data does not directly establish if such newly generated Mo-DC are indispensable for ATG-mediated Treg expansion. As ATG induces the generation of both Tregs and Mo-DC within a dynamic process of only 24 h in cocultures, it is difficult to exclusively target this particular cell type.
In summary, our data provide novel insights into the mechanisms of the generation of functional human CD4+CD25+FoxP3+ Tregs by ATG. These results may pave the way for the development of novel therapeutic strategies to exploit Tregs for the treatment of immune-mediated diseases and prevention of allograft rejection.
Supplementary Material
Acknowledgments
O.B. is funded by Research Fellowship Grants from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the American Society of Transplantation (AST). N.N. received support from the Clinical Trials in Organ Transplantation (CTOT) cooperative research program through the NIH Grant U01 AI 063623 and Genzyme.
Abbreviations
- APC
Antigen-Presenting Cell
- ATG
Anti Thymocyte Globulin
- DC
Dendritic Cell
- GM-CSF
Granulocyte Macrophage Colony-Stimulating Factor
- IL
Interleukin
- ILT-4
Ig-Like Transcript 4
- LPS
Lipopolysaccharide
- MACS
Magnetic Activated Cell Sorting
- Mo-DC
Monocyte-Derived Dendritic Cell
- PBMC
Peripheral Blood Mononuclear Cell
- PBS
Phosphate Buffered Saline
- Rbt IgG
Rabbit Immunoglobuling
- RT
Reverse Transcriptase
- STAT
Signal Transducers and Activators of Transcription
- TGF
Transforming Growth Factor
- Treg
Regulatory T Cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article
Figure S1: ATG induces CD14+DC-SIGN+ monocyte-derived dendritic cells in the presence of CD4+ T cells.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Yong Z, Chang L, Mei YX, Yi L. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17:120–129. doi: 10.1016/j.trim.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 7.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid Y. Regulatory T cells and infection: A dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 9.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 10.Boros P, Bromberg JS. Human FOXP3+ regulatory T cells in transplantation. Am J Transplant. 2009;9:1719–1724. doi: 10.1111/j.1600-6143.2009.02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 13.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JH, Koenen HJ, Hilbrands LB, Joosten I. Immunotherapy with regulatory T cells in transplantation. Immunotherapy. 2009;1:855–871. doi: 10.2217/imt.09.45. [DOI] [PubMed] [Google Scholar]
- 15.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: A critical evaluation! Clin J Am Soc Nephrol. 2009;4:1661–1669. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 16.D’Addio F, Yuan X, Habicht A, et al. A novel clinically relevant approach to tip the balance toward regulation in stringent transplant model. Transplantation. 2010;90:260–269. doi: 10.1097/tp.0b013e3181e64217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minamimura K, Gao W, Maki T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol. 2006;176:4125–4132. doi: 10.4049/jimmunol.176.7.4125. [DOI] [PubMed] [Google Scholar]
- 18.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: Induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25high FOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sewgobind VD, van der Laan LJ, Kho MM, et al. Characterization of rabbit antithymocyte globulins-induced CD25+ regulatory T cells from cells of patients with end-stage renal disease. Transplantation. 2010;89:655–666. doi: 10.1097/TP.0b013e3181c9cc7a. [DOI] [PubMed] [Google Scholar]
- 21.Broady R, Yu J, Levings MK. ATG-induced expression of FOXP3 in human CD4+ T cells in vitro is associated with T-cell activation and not the induction of FOXP3(+) T regulatory cells. Blood. 2009;114:5003–5006. doi: 10.1182/blood-2009-04-214437. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura A, Wakabayashi Y, Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-beta. J Biochem. 2010;147:781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008;13:1440–1446. doi: 10.2741/2773. [DOI] [PubMed] [Google Scholar]
- 25.Belkaid Y, Oldenhove G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 28.Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacy-toid DC. Eur J Immunol. 2010;40:2667–2676. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roman E, Shino H, Qin FX, Liu YJ. Cutting edge: Hematopoietic-derived APCs select regulatory T cells in thymus. J Immunol. 2010;185:3819–3823. doi: 10.4049/jimmunol.0900665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebellato LM, Gross U, Verbanac KM, Thomas JM. A comprehensive definition of the major antibody specificities in polyclonal rabbit antithymocyte globulin. Transplantation. 1994;57:685–694. doi: 10.1097/00007890-199403150-00010. [DOI] [PubMed] [Google Scholar]
- 31.Michallet MC, Preville X, Flacher M, Fournel S, Genestier L, Revillard JP. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins. Transplantation. 2003;75:657–662. doi: 10.1097/01.TP.0000053198.99206.E6. [DOI] [PubMed] [Google Scholar]
- 32.Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194–1200. [PubMed] [Google Scholar]
- 33.Leitner J, Grabmeier-Pfistershammer K, Majdic O, Zlabinger G, Steinberger P. Interaction of antithymocyte globulins with dendritic cell antigens. Am J Transplant. 2011;11:138–145. doi: 10.1111/j.1600-6143.2010.03322.x. [DOI] [PubMed] [Google Scholar]
- 34.Choi JK, Kim KH, Park H, Park SR, Choi BH. Granulocyte macrophage-colony stimulating factor shows anti-apoptotic activity in neural progenitor cells via JAK/STAT5-Bcl-2 pathway. Apop-tosis. 2011;16:127–134. doi: 10.1007/s10495-010-0552-2. [DOI] [PubMed] [Google Scholar]
- 35.Wei L, Laurence A, O’Shea JJ. New insights into the roles of Stat5a/b and Stat3 in T cell development and differentiation. Semin Cell Dev Biol. 2008;19:394–400. doi: 10.1016/j.semcdb.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallandre JR, Brillard E, Crehange G, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: Implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 37.Kong LY, Wei J, Sharma AK, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillet-Hladky S, de Carvalho CM, Bernaud J, Bendahou C, Bloy C, Rigal D. Rabbit antithymocyte globulin inhibits monocyte-derived dendritic cells maturation in vitro and polarizes monocyte-derived dendritic cells towards tolerogenic dendritic cells expressing in-doleamine 2,3-dioxygenase. Transplantation. 2006;82:965–974. doi: 10.1097/01.tp.0000235549.47976.d0. [DOI] [PubMed] [Google Scholar]
- 39.Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, Daniel V. An-tithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. 2007;83:485–497. doi: 10.1097/01.tp.0000251975.81281.22. [DOI] [PubMed] [Google Scholar]
- 40.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: Cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 41.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 42.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol. 2011;89:235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Dawicki W, Zhang X, Town J, Gordon JR. Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol. 2010;185:5003–5010. doi: 10.4049/jimmunol.0903446. [DOI] [PubMed] [Google Scholar]
- 45.Khatri I, Alexander C, Brandenburg K, et al. Induction of tolerogenic vs immunogenic dendritic cells (DCs) in the presence of GM-CSF is regulated by the strength of signaling from monophosphoryl lipid A (MPLA) in association with glutathione and fetal hemoglobin gamma-chain. Immunol Lett. 2009;124:44–49. doi: 10.1016/j.imlet.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a–dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheatem D, Ganesh BB, Gangi E, Vasu C, Prabhakar BS. Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol. 2009;131:260–270. doi: 10.1016/j.clim.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 49.Sheng JR, Li LC, Ganesh BB, Prabhakar BS, Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.